Abstract
Inflammatory bowel disease is a chronic, relapsing, and tissue-destructive disease. Resveratrol (3,4,5-trihydroxy-trans-stilbene), a naturally occurring polyphenol that exhibits beneficial pleiotropic health effects, is recognized as one of the most promising natural molecules in the prevention and treatment of chronic inflammatory disease and autoimmune disorders. In the present study, we investigated the effect of resveratrol on dextran sodium sulfate (DSS)-induced colitis in mice and found that it effectively attenuated overall clinical scores as well as various pathological markers of colitis. Resveratrol reversed the colitis-associated decrease in body weight and increased levels of serum amyloid A, tumor necrosis factor-α, interleukin (IL-6), and IL-1β. After resveratrol treatment, the percentage of CD4+ T cells in mesenteric lymph nodes (MLN) of colitis mice was restored to normal levels, and there was a decrease in these cells in the colon lamina propria (LP). Likewise, the percentages of macrophages in MLN and the LP of mice with colitis were decreased after resveratrol treatment. Resveratrol also suppressed cyclooxygenase-2 (COX-2) expression induced in DSS-exposed mice. Colitis was associated with a decrease in silent mating type information regulation-1 (SIRT1) gene expression and an increase in p-inhibitory κB expression and nuclear transcription factor-κB (NF-κB) activation. Resveratrol treatment of mice with colitis significantly reversed these changes. This study demonstrates for the first time that SIRT1 is involved in colitis, functioning as an inverse regulator of NF-κB activation and inflammation. Furthermore, our results indicate that resveratrol may protect against colitis through up-regulation of SIRT1 in immune cells in the colon.
The etiology and pathogenesis of two major forms of inflammatory bowel disease (IBD), Crohn's disease (CD) and ulcerative colitis, are poorly understood (Podolsky, 2002). It is widely held that human IBD is multifactorial and caused by immunologic, environmental, and genetic factors. More recently, it has been suggested that human IBD may be the result of abnormalities of the immune system or normal gut flora (MacDonald et al., 2000). It has also been suggested that colitis in mice may be due to an overall autoimmune dysregulation or an imbalance in T cells (Holländer et al., 1995). It has been shown that intestinal colon mucosa of CD patients are dominated by T cells producing inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)-α (Fiocchi, 1998).
The polyphenolic phytoalexin, resveratrol [(RES) 3,5,4′-trihydroxy-trans-stilbene], is a naturally occurring stilbene found in peanuts, grapes, and red wine that exerts several biological activities (Gholam et al., 2007). It has been shown to extend the life span of yeast and mice and regulates tumor growth and oxidation (de la Lastra and Villegas, 2005). It also reduces both acute and chronic chemically induced edema (Jang et al., 1997) and lipopolysaccharide-induced airway inflammation (Birrell et al., 2005). The anti-inflammatory mechanism of resveratrol is not completely understood, but reductions in the expression and activity of cyclooxygenase (COX)-1 and COX-2 have been reported (Martin et al., 2006). Resveratrol also modulates early inflammation in colitis, but its effects during chronic colitis remain undetermined (Martin et al., 2004).
Silent information regulator-1 (SIRT1) is a member of the class III group of histone deacetylases collectively called sirtuins. SIRT1 is homologous to the yeast Sir2 gene, which has been implicated in chromatin silencing, cell survival, and aging (North and Verdin, 2004). SIRT members are considered be nuclear sensors of redox signaling. Recent studies have documented a function of SIRT in genetic control of aging (Michan and Sinclair, 2007). Overexpression of SIRT orthologs has been found to increase the life span of Caenorhabditis elegans and Drosophila, indicating that SIRT family members function in longevity (Tissenbaum and Guarente, 2001). It has also been shown that SIRT1 prevents neuronal degeneration and protects cardiomyocytes from oxidative stress-mediated cell damage (Pillai et al., 2005). Resveratrol activates SIRT1, a longevity gene, extending the life span of diverse species.
Nuclear transcription factor κB (NF-κB) is a key regulator of inducible expression of many genes involved in immune and inflammatory response in the gut. NF-κB-induced cytokines contribute to the stimulation, activation, and differentiation of immune cells, thus perpetuating inflammation. Many established drugs are known to mediate, at least in part, anti-inflammatory effects of inflammation score via inhibition of NF-κB activity. Proinflammatory cytokines and bacterial pathogens activate NF-κB, mostly through inhibitory κB (IκB) kinase-dependent phosphorylation and degradation of IκB proteins. NF-κB p65 has been shown to be critically important in chronic inflammatory diseases. Inhibition of NF-κB activation has been suggested as an anti-inflammatory strategy in IBD (Neurath et al., 1996). Recent studies have demonstrated that SIRT1 inhibits NF-κB transcription by directly deacetylating the RelA/p65 protein at lysine 310 (Yeung et al., 2004).
Conventional treatment of colitis can reduce periods of active disease and help maintain remission, but these treatments often bring marginal results and the disease becomes refractory. Antibody therapy has some precedence in the treatment of colitis. Administration of anti-TNF-α antibody in mice (Powrie et al., 1994), infliximab in humans (Mouser and Hyams, 1999), and a CXCR3 ligand in IL-10(−/−) mice (Singh et al., 2003a) have been shown to inhibit the progression of colitis. We have also shown that mucosal CD4+ T cells elevated in active disease can be abrogated by anti-CXCL10 antibody (Ab) treatment (Singh et al., 2008b). It is unfortunate that the side effects associated with these treatments could result in adverse reactions or poor responses by the patients, thereby limiting their clinical use (Mouser and Hyams, 1999).
For this reason, many colitis sufferers turn to unconventional treatments in the hope of abating symptoms of active disease. It is estimated that 40% of IBD patients use some form of megavitamin therapy or herbal or dietary supplement (Head and Jurenka, 2004). Due to the strong anti-inflammatory and antioxidant properties of resveratrol, we hypothesized that resveratrol may be highly effective against colitis. In this study, we provide results indicating that orally administered resveratrol ameliorates DSS-induced colitis in mice. Although resveratrol was found to act through multiple pathways, we found that mice with colitis had decreased expression of the SIRT1 gene in the colon and a consequent up-regulation of NF-κB activation. Resveratrol treatment reversed these effects.
Materials and Methods
Animals.
Female C57BL/6 mice aged 8 to 12 weeks were purchased from The Jackson Laboratory (Bar Harbor, ME). The animals were housed and maintained in microisolator cages under conventional housing conditions at the South Carolina School of Medicine animal facility. Experimental groups consisted of six mice each and the study was repeated three times.
Acute Colitis Induced by Dextran Sulfate Sodium.
Acute colitis was induced by using dextran sulfate sodium (DSS) as described elsewhere (Dieleman et al., 1998). In brief, 8-week-old C57BL/6 mice received either water or drinking water containing 3% DSS (MP Biomedicals, LLC, Aurora, OH) (ad libitum) for 7 days followed by water cycle alone for 7 days. The body weight of mice was monitored every day from day 0 at the start of resveratrol treatment. Mice received either 100 μl of 10, 50, and 100 mg/kg b.wt. dose of resveratrol by oral gavage as described previously (Singh et al., 2007) or saline on alternate days, starting with the day mice received DSS (ad libitum). Resveratrol, a purified compound with the molecular formula C14H12O3, was obtained from Sigma-Aldrich (St. Louis, MO). The Certificate of Origin indicated that it was originally purified and extracted from the bushy knotweed plant and was found to be greater than 99% pure by both gas chromatography and thin-layer chromatography. At the end of the experiments blood was collected and colon samples were washed with phosphate-buffered saline (PBS), cut longitudinally, formalin fixed, and paraffin embedded.
Cell Isolation.
Spleens and mesenteric lymph nodes (MLN) from individual mice were mechanically dissociated, respectively, and red blood cells were lysed with lysis buffer (Sigma-Aldrich). Single-cell suspensions of spleen and MLN were passed through a sterile wire screen (Sigma-Aldrich). Cell suspensions were washed twice in RPMI 1640 (Sigma-Aldrich) and stored in media containing 10% fetal bovine serum on ice until used after 1 to 2 h. The small intestine/colon was cut into 1-cm stripes and stirred in PBS containing 1 mM EDTA at 37°C for 30 min. The cells from intestinal lamina propria (LP) were isolated as described previously (Singh et al., 2003a). In brief, the LP was isolated by digesting intestinal tissue with collagenase type IV (Sigma-Aldrich) in RPMI 1640 (collagenase solution) for 45 min at 37°C with moderate stirring. After each 45-min interval, the released cells were centrifuged and stored in complete media, and mucosal pieces were replaced with fresh collagenase solution at least two times. LP cells were further purified by using a discontinuous Percoll (Pharmacia, Uppsala, Sweden) gradient collecting at the 40 to 75% interface. Lymphocytes were maintained in complete medium, which consisted of RPMI 1640 supplemented with 10 ml/l nonessential amino acids (Mediatech, Washington, DC), 1 mM sodium pyruvate (Sigma-Aldrich), 10 mM HEPES (Mediatech), 100 U/ml penicillin, 100 μg/ml streptomycin, 40 μg/ml gentamicin (Elkins-Sinn, Inc., Cherry Hill, NJ), 50 μM mercaptoethanol (Sigma-Aldrich), and 10% fetal calf serum (Atlanta Biologicals, Norcross, GA).
Flow Cytometry Analysis.
Cells from the spleen, MLN, and LP were freshly isolated as described above for each experimental group. For three to four color FACS cell surface antigens staining, cells were preblocked with Fc receptors for 15 min at 4°C. The cells were washed with FACS staining buffer (PBS with 1% bovine serum albumin) and then stained with phycoerythrin-Cy5 (Cy-Chrome)-, fluorescein isothiocyanate-, or phycoerythrin-conjugated anti-CD3 (145-2C11), -CD4 (H129.19), -CD8 (LY-2 53–6.7), -CD11b (M1/70), -CD11c (HL3), and/or -CD69, -CD62L (BD Pharmingen, San Diego, CA) for 30 min with occasional shaking at 4°C. The cells were washed two times with FACS staining buffer and thoroughly resuspended in BD Cytofix/Cytoperm (BD Pharmingen) solution for 20 min. The cells were washed again two times with BD perm/wash solution after keeping it 10 min at 4°C. For intracellular cytokines, resuspended fixed permeabilized cells were stained with predetermined allophycocyanin fluorochrome-conjugated anti-cytokine antibody (TNF-α, IFN-γ for 30 min at 4°C in the dark). Lymphocytes were then washed thoroughly with FACS staining buffer and analyzed by flow cytometry (FC 500; Beckman Coulter, Fort Collins, CO).
Cytokine Quantitation by Luminex Analysis.
T helper cell-derived cytokines IL-1β, IL-6, IFN-γ, and TNF-α in the serum were determined by Beadlyte mouse multicytokine detection system kit (Bio-Rad Laboratories, Hercules, CA). Filter bottom enzyme-linked immunosorbent assay (ELISA) plates (Bio-Rad Laboratories) were rinsed with 100 μl of bio-plex assay buffer and removed by using a Millipore Multiscreen Separation Vacuum Manifold System (Millipore Corporation, Billerica, MA) set at 5 mmHg. Analyte beads in assay buffer were added into wells followed by 50 μl of serum or standard solution and incubated for 30 min at room temperature with continuous shaking (at setting number 3) using a Lab-Line Instrument Titer Plate Shaker (Lab-Line Instruments, Inc., Melrose, IL). The filter bottom plates were washed as described previously and centrifuged at 300g for 30 s. Subsequently, 50 μl of anti-mouse IFN-γ, IL-1β, IL-6, or TNF-α Ab-biotin reporter solution was added in each well followed by incubation with continuous shaking for 30 min followed by centrifugation and washing. Next, 50 μl of streptavidin-phycoerythrin solution was added and incubated with continuous shaking for 10 min at room temperature. Bio-Plex assay buffer (125 μl) was added, and Beadlyte readings were measured using a Luminex System (Luminex Corporation, Austin, TX) and calculated using Bio-Rad Bio-plex software. The cytokine Beadlyte assays were capable of detecting >5 pg/ml for each analyte.
Acute-Phase (Serum Amyloid A) ELISA.
Serum amyloid A (SAA) level was determined by ELISA (BioSource International, Camarillo, CA). In brief, 50 μl of SAA-specific mAb solution was used to coat microtiter strips to capture SAA. Serum samples and standards were added to wells and incubated for 2 h at room temperature. After washing in the assay buffer, the horseradish peroxidase (HRP)-conjugated anti-SAA mAbs solution was added and incubated for 1 h at 37°C. After washing, 100 μl of tetramethylbenzidine (BioSource) substrate solution was added. The reaction was stopped after incubation for 15 min at room temperature. After the stop solution was added, the plates were read at an optical density of 450 nm.
Histology.
Colon was preserved by using 10% buffer neutral formalin followed by 4% paraformaldehyde and embedded in paraffin. Fixed tissues were sectioned at 6 μm and stained with hematoxylin and eosin for microscopic examination. Intestinal lesions were multifocal and of variable severity. Grades were given to intestinal sections that took into account the number of lesions as well as severity. A score (0–4) was given based on the established criteria described previously (Singh et al., 2003a). The summation of these scores provided a total colonic disease score per mouse. The summation of these disease scores provided a total colonic disease score that could range from 0 to 4 with grade 1 lesions in proximal, middle, and distal colon segments.
SIRT1, COX1, and COX2 mRNA Expression.
For reverse transcription-polymerase chain reaction (RT-PCR) primer design, mouse mRNA sequences for COX-1 and COX-2 mRNAs were obtained from the National Institutes of Health-National Center for Biotechnology. Primers were designed by using the Beacon 2.0 computer program to generate 342- and 361-base pair fragments of COX-1 and COX-2, respectively. Thermodynamic analysis of the primers was conducted by using the following computer programs: Primer Premier and MIT Primer III (MIT, Boston, MA). The resulting primer sets were compared against the entire mouse genome to confirm specificity and to ensure that they flanked mRNA splicing regions. To detect the expression of COX-1, COX-2 in LP cells harvested from colon of mice (as described under Materials and Methods), sets of mouse COX-1-specific forward (5′-ACAGG ATGAACAGTCTACCCACC-3′) and reverse (5′-GTAGGAATCAGAACAGATGCTGA-3′) primers, and COX-2-specific forward (5′-ACCATTTGAACTATTCTACCAGC-3′) and reverse (5′-AGTCGGCCTGGGATGGCATCAG-3′) primers were used. In brief, total RNAs from spleen, MLN, and colon LP lymphocytes were harvested, and cDNAs were synthesized as described previously (Singh et al., 2007). PCR was performed for 30 cycles using the following conditions: 30 s at 95°C (denaturing temperature), 40 s at 54°C (annealing temperature), and 60 s at 72°C (extension temperature), with a final incubation at 72°C for 10 min for SIRT1. However, the annealing temperature for COX-1 and COX-2 was 57°C. The PCR products, generated from mouse gene primer pairs, were normalized against PCR products generated from mouse 18S (215 base pairs) forward (5′-GCCCGAGCCGCCTGGATAC-3′) and reverse (5′-CCGGCGGGTCATGGGAATA AC-3′) primers after electrophoresis on 1.5% agarose gel and visualization with UV light. The band intensity of PCR products was determined by using the Bio-Rad image analysis system (Bio-Rad Laboratories). The -fold increase mRNA in each tissue sample was evaluated by RT-PCR analysis using the Bio-Rad Icycler and software (Bio-Rad Laboratories).
Western Blot Analysis for SIRT1 and p-IκBα-Ser32 Expression.
Immunoblotting was performed as described previously (Singh et al., 2007). The cells were suspended in radioimmunoprecipitation assay lysis buffer (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) for p-IκBα-Ser32 and SIRT1 analysis. Cell lysates for SIRT1 and p-IκBα-Ser32 were prepared by freezing and thawing, and the protein concentration was measured by using a standard Bradford assay (Bio-Rad Laboratories). The protein concentration for p-IκBα-Ser32 and SIRT1 was measured using standard BCA protein assay kit (Pierce, Rockford, IL). The proteins were fractionated in 12% SDS-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes by using a dryblot apparatus (Bio-Rad Laboratories). The membrane was incubated in blocking buffer (SIRT1; 5% milk and 0.05% Tween 20, p-IκBα-Ser32; 1% milk, 1% bovine serum albumin, and 0.05% Tween 20) for 1 h at room temperature. This process was followed by incubation in mouse SIRT1- and -p-Iκβα-Ser32-specific (1:200; Santa Cruz Biotechnology, Inc.) primary antibody and β-actin (1:5000; Sigma-Aldrich) primary antibody at 4°C overnight. HRP-conjugated secondary Ab was used at 1:2000 dilutions (Cell Signaling Technology Inc., Danvers, MA). The membrane was then washed three times (10–15 min) with washing buffer (PBS + 0.2% Tween 20) and incubated for 1 h in HRP-conjugated secondary antibody (Cell Signaling Technology Inc.) in blocking buffer. The membranes were then washed several times and incubated in developing solution (equal volume of solution A and B; ECL Western Blotting Detection Reagents; GE Healthcare, Little Chalfont, Buckinghamshire, UK), and signal was detected by using Chemi Doc System (Bio-Rad Laboratories). Densitometry analyses of the Western blots were done by using Chemi Doc Software (Bio-Rad Laboratories).
Electrophoretic Mobility Shift Assay.
The double-stranded oligonucleotide probes corresponding to wild-type or mutant NF-κB motifs were synthesized. The sequences of hairpin oligonucleotide probes are described as follows: wild-type NF-κB probe, forward probe containing NF-κB motif 5′-ACAGGAGAAAGGTGTTTCCCTTGACTGC-3′ and reverse probe containing NF-κB motif 5′-GCAGTCAAGGGAAACACCTTTCTCCTGT-3′; mutant NF-κB probe, forward probe containing mutant NF-κB motif 5′-ACAGGAGAAAGGTGTTTAAATTGACTGC-3′ and reverse probe containing mutant NF-κB motif 5′-GCAGTCAATTTAAACACCTTTCTCCTGT-3′.
Preparation of Nuclear Extracts from LP Lymphocyte Cells (Colon).
To prepare nuclear extract for electrophoretic mobility shift assay, lamina propria lymphocyte cells from colon of mice induced with water (control) or DSS and treated with resveratrol were harvested. All subsequent steps were done on ice following the protocol described earlier (Singh et al., 2007). In brief, the cell pellet was suspended in 200 μl of buffer A [10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, and 0.5 mM dithiothreitol] and lysed by passing through a 28-gauge needle four times. The nuclei were then pelleted by centrifugation for 10 s, and the supernatant was aspirated. The crude nuclei preparation was then extracted by adding 120 μl of buffer C [20 mM HEPES, (pH 7.9), 25% (v/v) glycerol, 420 mM KCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, and 0.5 mM phenylmethylsulfonyl fluoride] and incubating for 15 min on ice. One hundred twenty microliters of buffer D [20 mM HEPES (pH 7.9), 0.2 mM EDTA, 0.5 mM phenylmethylsulfonyl fluoride, and 0.5 mM dithiothreitol] was added and then centrifuged for 10 min at 15,000 rpm. The supernatant was harvested, snap-frozen in liquid nitrogen, and stored at −80°C. The protein concentration was determined using the BCA protein determination kit from Pierce, using albumin as a protein standard.
Generation of Double-Stranded NF-κB Oligo Probes and Radiolabeling.
Equal concentration of forward and reverse oligo probes of wild-type and mutant NF-κB were incubated first at 95°C for 3 min and then allowed to cool down at room temperature in a standard thermal cycler. The double-stranded wild-type and mutant NF-κB oligonucleotide probes were 5′-end-labeled by mixing 1 to 5 pmol of oligonucleotide with 10 μCi of [γ-32P]ATP (MP Biomedicals, LLC) and 8 units of T4 polynucleotide kinase (New England Biolabs, Ipswich, MA) in 1× PNK buffer and incubating for 1 h at 37°C. After incubation, the end-labeled oligonucleotides were purified from free ATP by passing over a NICK column (Amersham Biosciences). The 3 to 5 μg of nuclear proteins was mixed with 1 μl of radiolabeled oligonucleotide (50,000 cpm) in a reaction mix containing 1 μl of binding buffer [10 mM Tris, 1 mM EDTA, 1 mM dithiothreitol, 100 mM KCl, 10% (v/v) glycerol] and 1 μg of poly(dI-dC) (Amersham Biosciences) as a nonspecific inhibitor in a final volume of 25 μl. These reaction mixtures were incubated for 30 min at 25°C. The samples were resolved on a 6% polyacrylamide gel in Tris borate EDTA that had been prerun for 30 min. The gels were dried and exposed to X-ray film. For the specific and nonspecific competition analyses, equimolar amounts of the cold hairpin oligonucleotide competitors were added to the binding reaction before the addition of the labeled oligonucleotide probes.
Statistics.
The data were expressed as the mean ± S.E.M. and compared using a two-tailed paired Student's t test or an unpaired Mann-Whitney U test. The results were analyzed using the Statview II statistical program (Abacus Concepts, Inc., Berkeley, CA) and Microsoft Excel (Microsoft, Seattle, WA). Single-factor analyses of variance were used to evaluate groups. Results were considered statistically significant if p values were <0.05 between the control and experimental groups.
Results
Effect of Resveratrol on DSS-Induced Colitis in Mice.
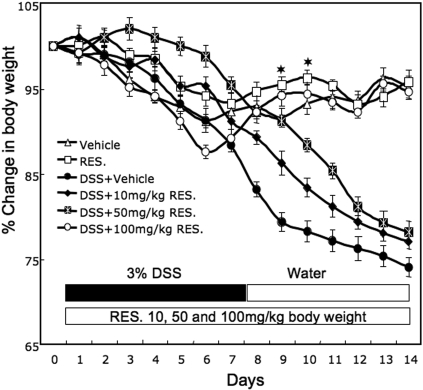
We used the following groups of mice in our study. The control group consisted of C57BL/6 mice given no treatment; the RES designated group received resveratrol alone suspended in 100 μl of distilled water (oral gavage); mice in the DSS+vehicle group were given DSS alone (3%) in drinking water; DSS+RES mice received a combination of DSS in drinking water and resveratrol by oral gavage. DSS administration by drinking water for 7 days induced acute colitis in C57BL/6 mice, as shown by significant weight loss, diarrhea, ruffled fur, and occasional bleeding. DSS-induced colitis caused an approximately 15 to 20% reduction in initial body weight. The weight of mice continued to decline throughout the study (Fig. 1). When resveratrol was administered into DSS-treated mice at 10, 50, and 100 mg/kg b.wt., a dose-dependent improvement in body weight was noted; however, complete recovery from DSS-induced decrease in body weight was seen only at dose of 100 mg/kg b.wt. (Fig. 1). Throughout the study, mice receiving resveratrol alone showed no significant alterations in the various parameters such as inflammation score, inflammatory cytokine, and SAA levels when compared with control mice. Thus, we primarily compared results between DSS+vehicle versus DSS+RES groups.
Fig. 1.
Change in body weight of mice after DSS induction and resveratrol treatment. C57BL/6 mice were given no treatment (Δ, control), resveratrol (100 mg/kg b.wt.) alone suspended in 100 μl of distilled water by oral gavage (□, RES), DSS alone (3%) in drinking water (●, DSS+vehicle), or a combination of DSS and resveratrol (DSS+RES) at 10 (⧫), 50 (☒), and 100 (○) mg/kg b.wt. of resveratrol for 14 days. After 7 days, DSS was replaced with a water cycle (ad libitum) for another 7 days. The body weight of the mice was recorded daily. Changes in body weight were expressed as a percentage of the weight minus the change in weight divided by the weight before the onset of colitis at day 1 (current weight-weight of previous day/day 1 weight). The statistical significance between values of each group was assessed by Student's t test. Data represent the mean of three experiment involving six mice per group. Asterisks indicate statistically significance difference (P < 0.01) between DSS+vehicle and DSS+100 mg/kg RES-treated mice.
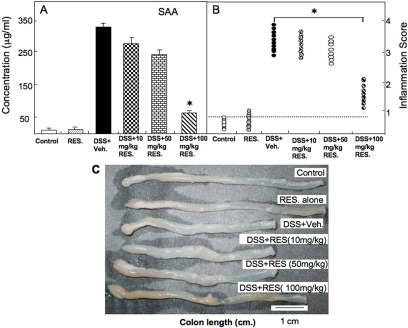
Resveratrol Prevented Elevations of SAA and Inflammation Scores and Restored Colon Length after Colitis.
We have previously shown that SAA levels are indicators of the progression of colitis (Singh et al., 2003a). SAA concentrations in the DSS+vehicle group were elevated significantly compared with concentrations in untreated control mice. In contrast, mice in the DSS+resveratrol group had markedly decreased SAA levels compared with mice in the DSS+vehicle group. The effect of resveratrol was dose- dependent with highest levels of SAA inhibition seen at 100 mg/kg (Fig. 2A). Moreover, the results suggested the utility of using SAA as an indicator of acute colitis in the DSS-induced colitis model. When we histologically scored the progression of colitis, we found that the mean scores of mice in the DSS+resveratrol group were significantly lower than those of mice given DSS+vehicle (Fig. 2B). In these studies as well, only the highest dose of resveratrol (100 mg/kg) was effective (Fig. 2B). DSS-induced animals had shortened colons (Fig. 2C) compared with those of control mice. Most importantly, treatment with resveratrol at 100, but not 10 or 50 mg/kg b.wt., significantly increased colon length in the DSS-administered group (Fig. 2C). These results clearly indicated that resveratrol at a dose of 100 mg/kg attenuated SAA levels, increased colon length, and diminished inflammation in mice with DSS-induced colitis. Therefore, in all subsequent experiments, we used 100 mg/kg b.wt. of resveratrol.
Fig. 2.
Effect of resveratrol on changes in SAA levels, colitis scores, and colon lengths in mice with DSS-induced colitis. In mice treated with DSS and resveratrol, as described in the legend for Fig. 1, the persistence or improvement of colitis was monitored by evaluating SAA levels (A), inflammation scores (B), and colon lengths (C). Asterisks indicate statistically significant differences (P < 0.01) between groups of six mice treated with DSS+vehicle and DSS+resveratrol.
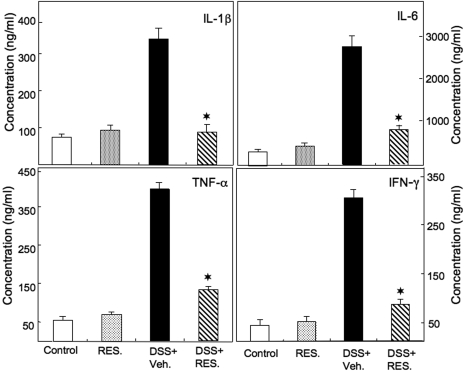
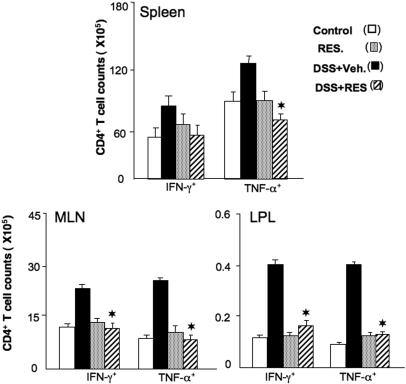
Resveratrol Ameliorates Serum Inflammatory Cytokine Increases Associated with Colitis.
TNF-α, IL-6, and IL-1β are often overproduced during inflammatory diseases, including IBD (Powrie et al., 1994). Resveratrol treatment decreased IFN-γ, TNF-α, IL-6, and IL-1β levels in the serum of mice with acute colitis (Fig. 3). To determine the effect of resveratrol on the production of Th1 cytokines (e.g., IFN-γ and TNF-α) expressed during colitis, we enumerated IFN-γ- and TNF-α-expressing CD4+ T cells. We observed a significant decline in the number of CD4+ T cells expressing IFN-γ or TNF-α in the spleen, MLN, and LP of mice given DSS+resveratrol compared with mice treated with DSS+vehicle (Fig. 4). Taken together, these findings clearly indicated that resveratrol treatment decreases systemic, inductive MLN and effector LP mucosal sites of CD4+ T cells that express TNF-α and IFN-γ, restoring the numbers of these cells to those found in naive mice or mice treated with resveratrol alone.
Fig. 3.
Resveratrol-mediated reduction of serum levels of IL-1β, IL-6, TNF-α, and IFN-γ in DSS-induced colitis. Colitis was induced in mice that were exposed to vehicle or 100 mg/kg b.wt. of resveratrol as described in the legend to Fig. 1. Serum cytokines were measured 14 days after the DSS induction of colitis by ELISA assay. The data presented are the mean concentrations from six mice ± S.E.M. in serum. Asterisks indicate statistically significance difference (P < 0.01) between DSS+vehicle and DSS+RES-treated mice.
Fig. 4.
Changes in the number of CD4+ T cells expressing IFN-γ and TNF-α in mice with colitis mice given resveratrol treatment. Splenic, MLN, and LP lymphocytes were isolated from the four groups of C57BL/6 mice as described in the legend to Fig. 1. Changes in the numbers of CD4+ T cells expressing IFN-γ and TNF-α were determined by flow cytometry and expressed as the total number of cells/mice ± S.E.M. Data shown are from a representative experiment; three independent experiments involving six mice/group yielded similar results. Asterisks indicate statistically significant differences (P < 0.01) between groups treated with DSS+vehicle versus DSS+resveratrol (100 mg/kg).
Resveratrol Modulates the Severity of Colitis.
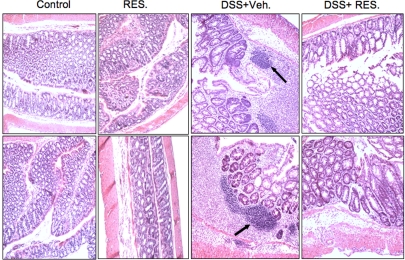
Mice given resveratrol had significant reductions in intestinal inflammation (Fig. 5). The mean histological scores of mice with severe colitis that were given DSS+vehicle were significantly higher than the scores of mice treated with DSS+resveratrol (Figs. 2B and 5). The pathologic changes associated with colitis included transmural necrosis, edema, and diffuse leukocyte infiltrates (polymorphonuclear leukocytes, lymphocytes, and eosinophils) in the colon. The architecture of the crypts was distorted, and the lamina propria was thickened in the area of distorted crypts. Most importantly, the number of these infiltrates was significantly reduced after resveratrol treatment (Fig. 5). After resveratrol treatment, mice showed marked improvement in the characteristic intestinal inflammation associated with DSS-induced acute colitis.
Fig. 5.
Histological characterization of DSS-induced colitis after resveratrol treatment. Histological sections of colons from the four groups of mice were presented (as described in Fig. 1). DSS+vehicle-treated mice showed significant lymphocyte infiltration and distortion of glands, whereas DSS+resveratrol-treated mice showed colon lumen having markedly decreased lymphocyte infiltration. Other pathologic changes during DSS-induced colitis included diffuse leukocyte infiltrates, distorted crypts, and thickening of the LP in the area of distorted crypts in the colon. These changes were significantly reversed in DSS+resveratrol (100 mg/kg) groups.
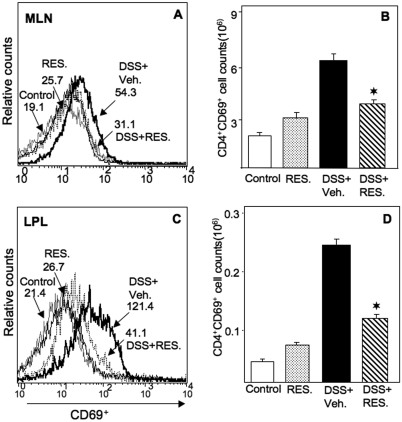
Resveratrol Reduces Effector CD4+ T Cells from MLN and LP.
Previous studies from our laboratory have demonstrated that resveratrol causes apoptosis in activated T cells and to a lesser extent in naive T cells (Singh et al., 2007). To determine whether resveratrol has different effects on effector T cells than it does on regulatory T cells, we assessed surface phenotype and cell numbers in both MLNs and LPs. DSS+resveratrol treatment significantly reduced the number of activated T cells in both MLN and LP (CD4+ CD69+) compared with numbers in the DSS+vehicle group (Fig. 6, A–D). The CD4+ T cell population bearing CD62L was unaffected by resveratrol treatment (data not shown). These results are consistent with the notion that resveratrol targets rapidly proliferating T-cell populations and reduces activated T- cell populations in the MLN and LP.
Fig. 6.
Effect of resveratrol on effector T cells in MLNs and LPs after DSS-induced colitis. MLN and LP lymphocytes were isolated from the four groups of mice as described in Fig. 1 and stained for CD4+CD69+ T cells. Changes in the mean fluorescent intensity of CD69+ expressed by CD4+ T cells from MLNs and LPs were compared from various groups (A and C). The number (mean of six samples ± S.E.M.) of CD69-expressing CD4+ T cells from each group was counted, as shown in B and D. Asterisks indicate statistically significant differences; i.e., P < 0.01 between DSS+vehicle and the DSS+resveratrol (100 mg/kg)-treated group.
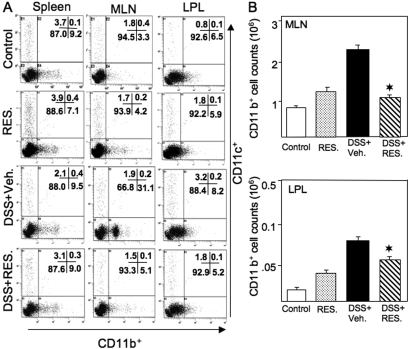
Resveratrol Modulates Macrophages in DSS-Induced Colitis.
Large populations of macrophages are present in normal intestinal mucosa. These are important for host immunological, inflammatory, and T-cell responses to luminal antigens. To determine the effect of resveratrol on macrophages during colitis progression, we examined changes in the percentage and number of macrophages after DSS induction of colitis and after resveratrol treatment. Macrophage numbers were significantly increased in MLNs and LPs after DSS+vehicle induction; these cells in both locations were significantly reduced after DSS+resveratrol treatment (Fig. 7, A and B). The only significant change in the dendritic (CD11c+) cell populations in any of the groups tested was a slight increase in these cells in the LPs of mice given DSS+vehicle. These findings not only indicate that DSS induction considerably increases the percentage of macrophages in the MLN and LP but also suggest that resveratrol reverses these increases.
Fig. 7.
Resveratrol reduced DSS-induced macrophages in MLN and LP. Splenic, MLN, and LP lymphocytes were isolated from the four groups of mice (as described in Fig. 1 legend) and stained for CD11b+ and CD11c+ markers using flow cytometry. The numbers in the bottom right quadrant indicate the total percentage of CD11b+ cells; numbers in the upper left quadrant indicate the total percentage of CD11c+ cells (A). The numbers (mean of six samples ± S.E.M.) of total CD11b+ T cells from MLN and LP in each group were counted (B). Data are shown from a representative of three independent experiments. Resveratrol was used at 100 mg/kg. Asterisks indicate statistically significance difference (P < 0.01) between DSS+vehicle and DSS+RES-treated mice.
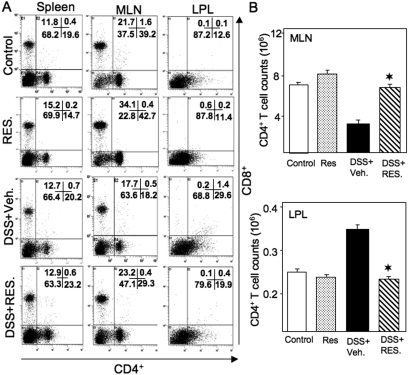
Resveratrol Modulates CD4+ T Cells during Colitis.
We analyzed T cells in the spleen, MLN, and LP by flow cytometry. Although we did not notice any major changes in CD4+ and CD8+ T cells in the spleen, the distribution of these cells changed significantly in the MLN and LP. CD4+ T cells made up 39.2% of cells in the MLN and 12.6% of cells in the LP in control mice (Fig. 8A). After the induction of colitis with DSS+vehicle, the percentage of CD4+ T cells in MLN decreased to 18.2% of the total cells. This decrease was reversed to 29.3% in MLNs after DSS+resveratrol treatment. On the other hand, lymphocytes from normal LPs contained 12.6% CD4+ T cells. After DSS+vehicle induction, lymphocytes increased to 29.6%. After DSS+resveratrol treatment, the percentage of CD4+ T cells in the LP decreased to 19.9%, slightly higher than the normal level (Fig. 8A). Likewise, CD8+ T cells comprised 21.7% of all cells in the MLN of control mice. DSS+vehicle treatment significantly decreased the percentage of CD8+ T cells to 17.7% in the MLN, whereas DSS+resveratrol treatment increased the percentage of CD8+ T cells in the MLN to 23.2% (Fig. 8A). The changes in the percentages of both CD4+ and CD8+ T cells correspond well with the total number of cells in each group (Fig. 8B).
Fig. 8.
Resveratrol modulates DSS-induced CD4+ and CD8+ T cells during colitis. Splenic, MLN, and LP lymphocytes were isolated from the four groups of C57BL/6 mice (as shown in Fig. 1 legend) and stained for CD4+ and CD8+ T cells using flow cytometry. The numbers in the bottom right quadrant indicate the total percentage of CD4+ T cells (A); the numbers in the upper left quadrant indicate the total percentage of CD8+ T (A) cells. The number (mean of six samples ± S.E.M.) of total CD4+ T cells from MLN and LP in each group was counted (B). Data are shown from a representative of three independent experiments. Resveratrol was used at 100 mg/kg. Asterisks indicate statistically significance difference (P < 0.01) between DSS+vehicle and DSS+RES-treated mice.
Together, these findings indicate that resveratrol treatment considerably increased the percentage of CD4+ T-helper lymphocytes in the MLN while decreasing the percentage of such cells in the LP. Moreover, resveratrol treatment substantially decreased the loss (or emigration) of CD4+ T lymphocytes from the LP and increased the percentage of CD4+ T lymphocytes in the MLN after DSS-induced colitis.
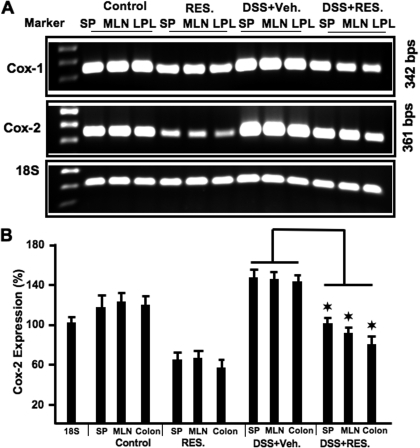
Resveratrol Suppresses COX-2 Expression.
COX-1 and COX-2 are expressed at varying levels in different tissues and are important in physiological homeostasis and inflammation. COX-2 is induced under various inflammatory conditions, including inflammatory bowel disease, and in mouse models of colitis (Fukata et al., 2006). To further quantify the effect of resveratrol on inflammatory markers, we used RT-PCR analysis to examine COX-1 and COX-2 expression in T cells from spleens, MLN, and LP of each group of mice. The levels of both COX-1 and COX-2 expression decreased significantly in resveratrol-treated mice compared with controls. Likewise, in DSS+resveratrol-treated mice, there were significant decreases in COX-1 and COX-2 levels in all lymphoid organs tested compared with DSS+vehicle-treated mice (Fig. 9, A and B). The effect of resveratrol on COX-1 was modest, whereas that on COX-2 was more potent.
Fig. 9.
Effect of resveratrol on COX1 and COX-2 expression in DSS-induced colitis. A, splenic, MLN, and LP lymphocytes were isolated from the four groups of C57BL/6 mice (as described in Fig. 1 legend) and analyzed for COX-1 and COX-2 expression by RT-PCR. B, expression. The 18S housekeeping gene was used as a positive control. Expression levels were compared with 18S and normalized. Data from multiple experiments on COX-2 expression are shown as mean ± S.E.M. Asterisks indicate statistically significant differences (P < 0.01) between groups treated with DSS+vehicle versus DSS+resveratrol (100 mg/kg).
Role of SIRT1 Gene Expression during Colitis and the Effect of Resveratrol.
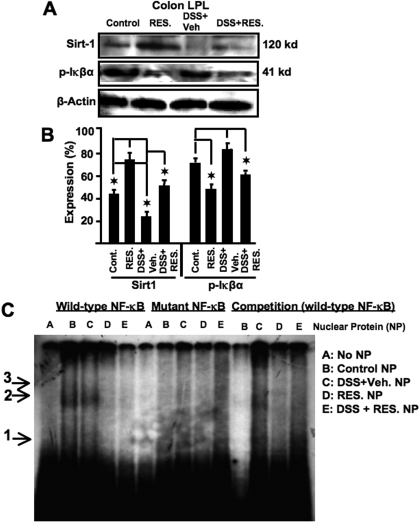
Increasing evidence shows positive effects of resveratrol and SIRT1 activation in several age-related disorders (Pearson et al., 2008). We next investigated the potential function of SIRT1 in the colitis model. To this end, we used Western blot analysis to examine SIRT1 expression in cells from the LPs of mice with colitis. The cells from LPs of control mice expressed significant levels of SIRT1, and that expression was significantly increased after resveratrol treatment (Fig. 10, A and B). In DSS+vehicle-treated mice, levels of SIRT1 expression decreased significantly during acute colitis, a change that was reversed by resveratrol treatment. These results indicated that SIRT1 is down-regulated in LP cells during colitis and that resveratrol treatment increases SIRT1 expression.
Fig. 10.
Resveratrol up-regulates SIRT1 and down-regulates p-IκBα activity at the effector site of colitis. Colon LP lymphocytes were isolated from various groups of mice (as described in Fig. 1 legend) and analyzed for p-IκBα and SIRT1 expression (A and B) by Western blot and electrophoretic mobility shift assay analysis of NF-κB motif (C). Double-stranded wild-type and mutant NF-κB oligonucleotide probes were generated. Nuclear proteins (3–5 μg) generated from colon LP lymphocytes of various groups of mice (as described previously) were used in each reaction. Radiolabeled (32P) wild-type NF-κB or mutant NF-κB probes were either directly used or used after incubation with nuclear protein. Arrow 1 shows wild-type and mutant NF-κB probe DNA bands. Arrows 2 and 3 indicate NF-κB-nuclear protein complexes. Data from multiple experiments have been depicted as mean ± S.E.M. (B). The 18S housekeeping gene was used as the positive control. The expression levels were compared with 18S and normalized. Asterisks indicate statistically significant differences (P < 0.01) between groups treated with DSS+vehicle versus DSS+resveratrol (100 mg/kg).
Inverse Correlation between SIRT1 and p-IκBα Activity.
In the pathogenesis of IBD, dysregulation of cytokine production and signaling mechanisms of intestinal epithelial cells, lymphocytes, and macrophages has been implicated. NF-κB is considered to be a key factor in the pathogenesis of IBD. Moreover, an inverse correlation between SIRT1 and NF-κB activation has been reported (Pfluger et al., 2008). As an index of NF-κB activation, we measured the levels of p-IκBα expression in LP cells. Resveratrol caused a significant decrease in p-IκBα expression in LP cells in both control and induced-colitis mice (Fig. 10, A and B). Overall, there was an inverse correlation between SIRT1 and p-IκBα expression. To confirm that the phosphorylation of p-IκBα led to nuclear translocation of NF-κB, we did a gel shift assay with nuclear extracts. As shown in Fig. 10C, we observed an NF-κB-specific band in nuclear extracts from lamina propria lymphocytes of control and DSS-treated mice, which disappeared when mutant or cold NF-κB oligomers were added. The translocation of NF-κB in control mice may be due to activation of LP by the gut microflora. Note that resveratrol treatment caused a significant decrease in nuclear translocation of NF-κB in both groups of mice.
Discussion
There has been significant progress toward the treatment of IBD in recent years. However, available treatments often produce side effects, leaving patients with no other options. Therefore, alternative treatment modalities are needed. In this study, we show that the oral administration of resveratrol, an ingredient primarily found in red grapes, reverses DSS-induced colitis in mice. We have found that resveratrol treatment resolves colitis, corrects weight loss, and reduces local and systemic SAA, IL-6, IL-1β, IFN-γ, and TNF-α levels. Our results suggest that increases in the number of mucosal CD4+ T cells and macrophages during acute colitis can be mitigated by resveratrol treatment. Moreover, resveratrol elevates SIRT1 gene expression and down-regulates p-IκBα as well as COX-2 expression in the colons of mice with colitis. Taken together, these results also establish for the first time that the antiaging gene, SIRT1, has a significant function in colitis, having been decreased in the LPs of mice with colitis and up-regulated after resveratrol treatment, with consequent decrease in NF-κB activity.
CD4+ T cells have a major role in the induction of IBD, and much of the intestinal damage caused by this disease is a result of T cell-mediated injury (Elson et al., 1996). We have previously shown that adoptive transfer of CXCR3+CD4+ T cells results in colitis in T-cell receptor (β × δ)−/− mice (Singh et al., 2003b). The number of CD4+ T cells in the LP represents the majority of lymphocytes increased in DSS-treated mice compared with control mice. These cells were attenuated by the administration of resveratrol.
In the present study, we have shown that majority of CD4+ T cells express TNF-α and IFN-γ (Fig. 4) and that the number of these cells significantly declined in MLN and LP after resveratrol treatment. These results confirmed our earlier findings that the numbers of CD4+ T cells in spleens, MLN, and LP represent the majority of lymphocytes expressing inflammatory cytokines during colitis (Singh et al., 2008a). This finding is also in agreement with the findings of other studies using DSS-induced colitis models that showed the potentiation of CD4+ T cells in colitis (Dieleman et al., 1998; Shintani et al., 1998; Grose et al., 2001; Ogawa et al., 2004). The present results imply that intestinal inflammation is driven by the presence of CD4+ T cells and potentiated by Th1 and inflammatory cytokines, which can be abrogated by resveratrol treatment.
In this study, resveratrol treatment reversed the increase in the percentages of activated CD4+ T cells in MLN and LP during colitis, clearly indicating that resveratrol has potent anti-inflammatory effects that parallel the reduction in colitis-induced T-cell expansion. This observation is supported by our findings that the percentage of colitis-induced CD69+ cells was reduced (Fig. 6). The preferential reduction in effector T-cell response was supported by decreased TNF-α and IFN-γ in LP, with concomitant reductions in systemic IL-1β and IL-6 (Fig. 3). We have previously reported that resveratrol induces apoptosis in activated T cells (Singh et al., 2007). Thus, the decrease in activated T cells during colitis may result from apoptosis induction by resveratrol.
Along with TNF-α, sustained acute-phase responses are also associated with both human IBD and murine colitis (Berg et al., 1996). The activated macrophages increase IL-6 and IL-1β expression in patients with IBD (Casini-Raggi et al., 1995). IFN-γ has a critical role in the induction and progression of colitis (Parronchi et al., 1997). In the present study, we demonstrated that expression of local and/or systemic TNF-α, IL-6, IL-1β, and IFN-γ was decreased by resveratrol treatment in mice after DSS induction of colitis. These results are in agreement with previously published in vivo and in vitro findings that resveratrol reduces the level of inflammatory cytokines and inflammatory cell infiltrates in the colon (Martin et al., 2006).
In active IBD, there is increased turnover and activation of monocytes (Mee and Jewell, 1980), which are the source of intestinal macrophages. In the present study, there was a significant increase in CD11b+ macrophages in both MLN and LP in DSS-induced mice compared with normal mice (Fig. 7). This result is in agreement with other studies using the DSS-induced model of colitis, which showed increased macrophages during colitis (Shibata et al., 2007). Moreover, we found that this increase in CD11b+ cells was reversed to the normal level after resveratrol treatment.
We also determined changes in the expression of COX-1 and COX-2 in DSS-induced colitis. Previous studies have shown (Li et al., 2002) increases in COX-2 expression in trinitrobenzenesulfonic acid (TNBS)-induced colitis in rats, which were reduced by resveratrol treatment (Martin et al., 2006). In this study, we found that expression of COX-2, but not COX-1, was significantly increased in spleens, MLN, and LP cells during DSS-induced colitis. Resveratrol caused a marked decrease in COX-2 and, to a modest extent, COX-1, as in the TNBS model of colitis in rats (Martin et al., 2004).
SIRT1 is considered a longevity factor, because overexpression of this deacetylase increases the life span of many species tested (Tissenbaum and Guarente, 2001). SIRT1 activation suppresses gene transcription and promotes cell survival by deacetylating targets such as histones, NF-κB, p53, and KU70 (Blander and Guarente, 2004). It has been reported that, once activated, SIRT1 can act as a sensor of decreased calorie intake (Picard et al., 2004). The role of SIRT1 in colitis has not been investigated previously. It is interesting that SIRT1 expression was significantly decreased in the LP cells of mice with colitis and that resveratrol treatment caused a significant increase in SIRT1 expression in these mice. It was striking that these data correlated with a significant increase in p-IκBα and an increase in NF-κB nuclear translocation. In contrast, treating mice with resveratrol reversed these effects in LP cells. These results suggest that activation of LP cells during colitis may down-regulate SIRT1 and thereby promote NF-κB activation. Resveratrol, by enhancing SIRT1 expression, may down-regulate NF-κB activation and thereby decrease the inflammation triggered by DSS. A similar lower induction of cytokines by down-modulation of NF-κB activity by SIRT1 has been reported in high-fat-diet-induced metabolic damage (Pfluger et al., 2008). Thus, resveratrol, by inhibiting NF-κB activation, may promote the induction of apoptosis in activated immune cells (Singh et al., 2007).
More recently, it has been reported that low doses of resveratrol (1 mg/kg/day) induced SIRT3 and SIRT7 but not SIRT1 in DSS-induced colitis in rat (Larrosa et al., 2009). The noninduction of SIRT1 in this rat model can be explained by two possibilities. First, resveratrol was administered for 25 days, with DSS exposure only during the last 5 days. Thus, the rats had been preconditioned with resveratrol, whereas we administered resveratrol on the day of DSS exposure. Second, for SIRT1 expression, we used purified LP lymphocytes, whereas Larrosa et al. (2009) used the distal part of the colon. Additional studies are necessary to determine whether the levels of SIRT1, -3, and -7 in the colon are influenced by the dose and length of exposure to resveratrol.
In an earlier study, we noted that 100 to 250 mg/kg b.wt. dose of resveratrol was necessary to prevent inflammation in experimental autoimmune encephalomyelitis model (Singh et al., 2007). In the current study, we observed that a similar dose of 100 mg/kg b.wt. was necessary to effectively ameliorate DSS-induced colitis. In other experimental models as well, resveratrol has been used at high concentrations such as 500, 1000, and 1500 mg/kg b.wt., for 10 days, to inhibit tumor growth in BALB/c mice in a dose-dependent manner (Liu et al., 2003) or 100 mg/kg b.wt. to delay tumorigenesis in rats (Bhat and Pezzuto, 2001). Furthermore, the dose that we have used is feasible to achieve in humans because the human equivalent dose of 100 mg/kg in mouse is 486 mg, considering an average human weight of 60 kg. Currently, there are several nutraceutical companies selling purified resveratrol in 500-mg quantities in capsule form. Thus, our doses reflect the potential pharmacological/dietary supplement dose to treat colitis rather than the concentrations achievable through normal consumption of food containing resveratrol.
In summary, our studies suggest that resveratrol acts as an anti-inflammatory agent by targeting multiple pathways, including the SITR1 gene, which has not been previously investigated with regard to its association with colitis. The present study suggests that administering DSS to mice triggers Th1 cells (CD4+ T cells) and macrophages to emigrate from MLN to the LP and increase TNF-α and IL-6 cytokine production, which potentially leads to the development of acute colitis. Resveratrol treatment suppressed all indicators of inflammation including cytokines and Th1 cells, as well as expression of COX-2 and activation of NF-κB, thereby ameliorating colitis. We found, for the first time, that colitis induction may down-regulate SIRT1 and promote both NF-κB activation and consequent cytokine production in the colon and that resveratrol may reverse these effects by up-regulating SIRT1. We propose that such a mechanism may be critical in down-regulating the inflammation associated with colitis. Our studies also suggest that SIRT1 may be a novel therapeutic target against colitis.
This work was supported in part by the National Institutes of Health National Institute of Allergy and Infectious Diseases Extramural Activities [Grants IH-AI053703, AI058300]; the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES09098]; the National Institutes of Health National Institute on Drug Abuse [Grant DA016545]; the National Institutes of Health National Heart, Lung, and Blood Institute [Grant HL058641]; and the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant PO1-AT003961].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.160838.
- IBD
- inflammatory bowel disease
- CD
- Crohn's disease
- IL
- interleukin
- TNF
- tumor necrosis factor
- RES
- resveratrol
- COX
- cyclooxygenase
- SIRT1
- silent information regulator-1
- NF-κB
- nuclear transcription factor κB
- IκB
- inhibitory κB
- Ab
- antibody
- DSS
- dextran sulfate sodium
- PBS
- phosphate-buffered saline
- MLN
- mesenteric lymph nodes
- LP
- lamina propria
- FACS
- fluorescence-activated cell sorter
- IFN-γ
- interferon γ
- ELISA
- enzyme-linked immunosorbent assay
- SAA
- serum amyloid A
- HRP
- horseradish peroxidase
- RT-PCR
- reverse transcription-polymerase chain reaction
- TNBS
- trinitrobenzenesulfonic acid.
References
- Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. (1996) Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest 98:1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KP, Pezzuto JM. (2001) Resveratrol exhibits cytostatic and antiestrogenic properties with human endometrial adenocarcinoma (Ishikawa) cells. Cancer Res 61:6137–6144 [PubMed] [Google Scholar]
- Birrell MA, McCluskie K, Wong S, Donnelly LE, Barnes PJ, Belvisi MG. (2005) Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-κB-independent mechanism. FASEB J 19:840–841 [DOI] [PubMed] [Google Scholar]
- Blander G, Guarente L. (2004) The Sir2 family of protein deacetylases. Annu Rev Biochem 73:417–435 [DOI] [PubMed] [Google Scholar]
- Casini-Raggi V, Kam L, Chong YJ, Fiocchi C, Pizarro TT, Cominelli F. (1995) Mucosal imbalance of IL-1 and IL-1 receptor antagonist in inflammatory bowel disease. A novel mechanism of chronic intestinal inflammation. J Immunol 154:2434–2440 [PubMed] [Google Scholar]
- de la Lastra CA, Villegas I. (2005) Resveratrol as an anti-inflammatory and anti-aging agent: mechanisms and clinical implications. Mol Nutr Food Res 49:405–430 [DOI] [PubMed] [Google Scholar]
- Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. (1998) Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol 114:385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Beagley KW, Sharmanov AT, Fujihashi K, Kiyono H, Tennyson GS, Cong Y, Black CA, Ridwan BW, McGhee JR. (1996) Hapten-induced model of murine inflammatory bowel disease: mucosa immune responses and protection by tolerance. J Immunol 157:2174–2185 [PubMed] [Google Scholar]
- Fiocchi C. (1998) Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology 115:182–205 [DOI] [PubMed] [Google Scholar]
- Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, et al. (2006) Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology 131:862–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholam PM, Flancbaum L, Machan JT, Charney DA, Kotler DP. (2007) Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol 102:399–408 [DOI] [PubMed] [Google Scholar]
- Grose RH, Howarth GS, Xian CJ, Hohmann AW. (2001) Expression of B7 costimulatory molecules by cells infiltrating the colon in experimental colitis induced by oral dextran sulfate sodium in the mouse. J Gastroenterol Hepatol 16:1228–1234 [DOI] [PubMed] [Google Scholar]
- Head K, Jurenka JS. (2004) Inflammatory bowel disease. Part II: Crohn's disease–pathophysiology and conventional and alternative treatment options. Altern Med Rev 9:360–401 [PubMed] [Google Scholar]
- Holländer GA, Simpson SJ, Mizoguchi E, Nichogiannopoulou A, She J, Gutierrez-Ramos JC, Bhan AK, Burakoff SJ, Wang B, Terhorst C. (1995) Severe colitis in mice with aberrant thymic selection. Immunity 3:27–38 [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220 [DOI] [PubMed] [Google Scholar]
- Larrosa M, Yañéz-Gascón MJ, Selma MV, González-Sarrías A, Toti S, Cerón JJ, Tomás-Barberán F, Dolara P, Espín JC. (2009) Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J Agric Food Chem 57:2211–2220 [DOI] [PubMed] [Google Scholar]
- Li ZG, Hong T, Shimada Y, Komoto I, Kawabe A, Ding Y, Kaganoi J, Hashimoto Y, Imamura M. (2002) Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis 23:1531–1536 [DOI] [PubMed] [Google Scholar]
- Liu HS, Pan CE, Yang W, Liu XM. (2003) Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World J Gastroenterol 9:1474–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald TT, Monteleone G, Pender SL. (2000) Recent developments in the immunology of inflammatory bowel disease. Scand J Immunol 51:2–9 [DOI] [PubMed] [Google Scholar]
- Martín AR, Villegas I, La Casa C, de la Lastra CA. (2004) Resveratrol, a polyphenol found in grapes, suppresses oxidative damage and stimulates apoptosis during early colonic inflammation in rats. Biochem Pharmacol 67:1399–1410 [DOI] [PubMed] [Google Scholar]
- Martín AR, Villegas I, Sánchez-Hidalgo M, de la Lastra CA. (2006) The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. Br J Pharmacol 147:873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee AS, Jewell DP. (1980) Monocytes in inflammatory bowel disease: monocyte and serum lysosomal enzyme activity. Clin Sci (Lond) 58:295–300 [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. (2007) Sirtuins in mammals: insights into their biological function. Biochem J 404:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouser JF, Hyams JS. (1999) Infliximab: a novel chimeric monoclonal antibody for the treatment of Crohn's disease. Clin Ther 21:932–942 [DOI] [PubMed] [Google Scholar]
- Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, Strober W. (1996) Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med 2:998–1004 [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. (2004) Sirtuins: Sir2-related NAD-dependent protein deacetylases. Genome Biol 5:224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. (2004) Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol 110:55–62 [DOI] [PubMed] [Google Scholar]
- Parronchi P, Romagnani P, Annunziato F, Sampognaro S, Becchio A, Giannarini L, Maggi E, Pupilli C, Tonelli F, Romagnani S. (1997) Type 1 T-helper cell predominance and interleukin-12 expression in the gut of patients with Crohn's disease. Am J Pathol 150:823–832 [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab 8:157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. (2008) Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A 105:9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney MW, Guarente L. (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai JB, Isbatan A, Imai S, Gupta MP. (2005) Poly(ADP-ribose) polymerase-1-dependent cardiac myocyte cell death during heart failure is mediated by NAD+ depletion and reduced Sir2alpha deacetylase activity. J Biol Chem 280:43121–43130 [DOI] [PubMed] [Google Scholar]
- Podolsky DK. (2002) The current future understanding of inflammatory bowel disease. Best Pract Res Clin Gastroenterol 16:933–943 [DOI] [PubMed] [Google Scholar]
- Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. (1994) Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity 1:553–562 [DOI] [PubMed] [Google Scholar]
- Shibata W, Maeda S, Hikiba Y, Yanai A, Ohmae T, Sakamoto K, Nakagawa H, Ogura K, Omata M. (2007) Cutting edge: the IkappaB kinase (IKK) inhibitor, NEMO-binding domain peptide, blocks inflammatory injury in murine colitis. J Immunol 179:2681–2685 [DOI] [PubMed] [Google Scholar]
- Shintani N, Nakajima T, Okamoto T, Kondo T, Nakamura N, Mayumi T. (1998) Involvement of CD4+ T cells in the development of dextran sulfate sodium-induced experimental colitis and suppressive effect of IgG on their action. Gen Pharmacol 31:477–481 [DOI] [PubMed] [Google Scholar]
- Singh NP, Hegde VL, Hofseth LJ, Nagarkatti M, Nagarkatti P. (2007) Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72:1508–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh R, Singh S, Karls RK, Quinn FD, Taub DD, Lillard JW., Jr (2008a) CXCL10+ T cells and NK cells assist in the recruitment and activation of CXCR3+ and CXCL11+ leukocytes during Mycobacteria-enhanced colitis. BMC Immunol 9:25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh S, Singh R, Cong Y, Taub DD, Lillard JW., Jr (2008b) CXCL10-producing mucosal CD4+ T cells, NK cells, and NKT cells are associated with chronic colitis in IL-10(−/−) mice, which can be abrogated by anti-CXCL10 antibody inhibition. J Interferon Cytokine Res 28:31–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh UP, Singh S, Taub DD, Lillard JW., Jr (2003a) Inhibition of IFN-gamma-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol 171:1401–1406 [DOI] [PubMed] [Google Scholar]
- Singh UP, Singh S, Weaver CT, Iqbal N, McGhee JR, Lillard JW., Jr (2003b) IFN-gamma-inducible chemokines enhance adaptive immunity and colitis. J Interferon Cytokine Res 23:2000 [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA, Guarente L. (2001) Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature 410:227–230 [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]