Abstract
σ-1 Receptors are endoplasmic reticulum (ER) chaperones that are implicated in the neuroplasticity associated with psychostimulant abuse. We immunocytochemically examined the distribution of σ-1 receptors in the brain of drug-naive rats and then examined the dynamics of σ-1 receptors and other ER chaperones in specific brain subregions of rats that self-administered methamphetamine, received methamphetamine passively, or received only saline injections. σ-1 Receptors were found to be expressed in moderate to high levels in the olfactory bulb, striatum, nucleus accumbens shell, olfactory tubercle, amygdala, hippocampus, red nucleus, ventral tegmental area, substantia nigra, and locus ceruleus. Methamphetamine, whether self-administered or passively received, significantly elevated ER chaperones including the σ-1 receptor, BiP, and calreticulin in the ventral tegmental area and substantia nigra. In the olfactory bulb, however, only the σ-1 receptor chaperone was increased, and this increase occurred only in rats that actively self-administered methamphetamine. Consistent with an increase in σ-1 receptors, extracellular signal-regulated kinase was found to be activated and protein kinase A attenuated in the olfactory bulb of methamphetamine self-administering rats. σ-1 Receptors in the olfactory bulb were found to be colocalized with dopamine D1 receptors. These results indicate that methamphetamine induces ER stress in the ventral tegmental area and substantia nigra in rats whether the drug is received actively or passively. However, the changes seen only in rats that actively self-administered methamphetamine suggest that D1 and σ-1 receptors in the olfactory bulb might play an important role in the motivational conditioning/learning aspects of methamphetamine self-administration in the rat.
Methamphetamine is a drug that is widely abused by humans and readily self-administered by laboratory animals such as rats. It is recognized that the underlying neurochemical mechanisms of methamphetamine abuse involve the mesolimbic dopamine system, where methamphetamine causes presynaptic vesicular release of dopamine that in turn activates postsynaptic dopamine receptors (Tidey and Bergman, 1998; Segal et al., 2005; Fleckenstein et al., 2009). However, nondopaminergic receptors have also been implicated in methamphetamine's abuse-related effects. The σ-1 receptor is one of those receptors (Ujike et al., 1992; Itzhak, 1993; Takahashi et al., 2000; Matsumoto et al., 2008).
The σ-1 receptor was once mistakenly termed as an opioid subtype of receptors (Su, 1982) but was later recognized as a nonopioid receptor (Su et al., 1988; Hellewell et al., 1994; Hanner et al., 1996; Herrera et al., 2008). It is now known as an endoplasmic reticulum (ER) resident protein that can regulate Ca2+ signaling from the ER into mitochondria by chaperoning and stabilizing type 3 IP3 receptors at the ER-mitochondrial interface in Chinese hamster ovary (CHO) cells (Hayashi and Su, 2001, 2007; Wu and Bowen, 2008). σ-1 Receptor ligands, including neurosteroids, regulate this receptor-chaperone activity in an agonist/antagonist fashion (Hayashi and Su, 2007; Hayashi et al., 2009). In addition, σ-1 receptors were recently found to bind the psychedelic drug N,N-dimethyltryptamine and eventually produce a blockade of sodium channels, possibly through translocation of σ-1 receptors from the ER to the plasma membrane region (Fontanilla et al., 2009; Johannessen et al., 2009; Su et al., 2009).
Methamphetamine binds to σ-1 receptors with micromolar affinity and has been described as exerting its locomotor stimulatory effect, at least in part, through σ-1 receptors (Nguyen et al., 2005). Repeated methamphetamine treatment causes an increase of σ-1 receptors in the mouse brain (Ujike et al., 1992; Itzhak, 1993), and the behavioral sensitization associated with repeated methamphetamine treatment can be blocked by a σ-1 receptor antagonist (Takahashi et al., 2000). High doses of methamphetamine cause cytotoxicity that is mediated via σ-1 receptors (Matsumoto et al., 2008). Methamphetamine, in a dose sufficient to produce apoptosis and neuronal degeneration, can increase the expression of the ER chaperone BiP, presumably by causing ER stress in the brain (Jayanthi et al., 2004).
We reported previously that σ-1 receptors were elevated in the midbrain of rats that self-administered methamphetamine, but not in control rats that passively received injections of the same dose of methamphetamine, or in rats that only received saline injections (Stefanski et al., 2004). In the present study, using the same behavioral paradigm, we systematically defined the σ-1 receptor-enriched areas in the rat brain by immunocytochemistry, used this information to micropunch relevant areas, and then performed Western blot analyses to compare region-specific alterations of σ-1 receptors and other ER chaperones in the three experimental groups of rats (Stefanski et al., 2004).
Materials and Methods
Animals.
Male Sprague-Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing approximately 345 to 385 g at the start of experiment were individually housed in a temperature- and humidity-controlled environment under a 12-h light/dark cycle (lights on at 7:00 AM). The rats were allowed 7 days of free feeding after being delivered to the animal facility. Before the start of the study, rats were diet-restricted for 10 days as follows: rats for intravenous self-administration were fed 6 to 7 NIH07 biscuits (Harlan Laboratories, Indianapolis, IN) per day. Under this feeding regimen, body weights of rats for intravenous self-administration were maintained close to their original values throughout the course of experiments. The diet restriction was maintained throughout the study. Water was available ad libitum in the home cage. Rats were tested in the light phase. They were experimentally and drug-naive at the start of this study.
Animals used in this study were maintained in facilities fully accredited by the American Association for the Accreditation of Laboratory Animals Care, and all the experimentation was conducted in accordance with the guidelines of the Institutional Care and Use Committee of the Intramural Research Program, National Institute on Drug Abuse, National Institutes of Health, and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Drugs.
S-(+)-Methylamphetamine hydrochloride (methamphetamine) was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in sterile 0.9% saline. Doses refer to the salt form of the drug.
Methamphetamine Self-Administration.
Standard operant-conditioning chambers equipped with two holes containing nose-poke operanda were used (Coulbourn Instruments, Whitehall, PA). Self-administration procedures (including the catheter implantation and head-mount creation) were the same as described previously (Stefanski et al., 2004). In brief, 30 naive rats were divided into three groups and tested simultaneously. One group (n = 10) served as the methamphetamine self-administering group, which received response-contingent injections of methamphetamine (0.1 mg/kg/injection) during 2-h sessions. The second group (n = 10) served as yoked controls and passively received an injection of methamphetamine (0.1 mg/kg/injection) each time a response-contingent injection of 0.1 mg/kg methamphetamine was actively self-administered by the first group of rats. The third group (n = 10) received an injection of saline each time a response-contingent injection of 0.1 mg/kg methamphetamine was actively self-administered by the first group of rats. After responding by rats actively self-administering methamphetamine was initiated, the number of nose-poke responses required to produce each injection was gradually increased over a period of 9 to 12 sessions from one to a final value of five (a 5-response fixed-ratio schedule). Nose-poke responses by the yoked control rats were recorded but had no programmed consequences. One rat each from the yoked methamphetamine and yoked saline groups died before the end of the experiment and were not included in the analysis. After 5 weeks (25 sessions), brains from 28 animals were harvested 24 h after the last session ended.
Western Blotting.
Rat brains were freshly frozen (methamphetamine self-administering rats, n = 6; yoked methamphetamine rats, n = 6; yoked saline rats, n = 6) and then cut into 500-μm-thick coronal slices with a cryostat (HM505E; Thermo Fisher Scientific, Waltham, MA). Specific brain regions of interest were punched out from two immediately adjacent slices bilaterally from each rat brain with a needle (diameter, 0.2–1 mm). Tissues from the region of a rat thus obtained were combined and homogenized with a microsonicator in SDS sample buffer. After centrifugation (12,000g, for 20 min), protein concentrations in supernatants were measured with a bicinchoninic acid assay kit (Thermo Fisher Scientific). Proteins (50 μg/lane) were separated by 13% SDS-polyacrylamide gel electrophoresis and transblotted to a polyvinylidene difluoride membrane in methanol-free Towbin buffer. After blocking with 10% nonfat dry milk in Tris-buffered saline/Tween 20 (1 h), membranes were incubated with primary antibodies in Tris-buffered saline/Tween 20 at 4°C overnight. Specific protein bands were visualized by using horseradish peroxidase-conjugated secondary antibodies and the SuperSignal West Femto detection system (Thermo Fisher Scientific). σ-1 Receptor antibodies were raised as described previously (Hayashi and Su, 2003a,b). Anti-BiP and anti-calreticulin are from BD Biosciences Pharmingen (San Diego, CA); anti-cyclophilin B is from Abcam Inc. (Cambridge, MA); anti-extracellular signal-regulated kinase (ERK) is from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-pERK is from Cell Signaling Technology Inc. (Danvers, MA); anti-protein kinase A (PKA) RII and anti-PKA NT are from Millipore (Billerica, MA); and anti-β-tubulin is from Sigma-Aldrich (St. Louis, MO). Western blotting images were analyzed by Kodak 1D Image Analysis software (Eastman Kodak, Rochester, NY) to yield mean intensities on regions of interest (mean intensity = sum of all the pixel intensities in regions of interest ÷ area).
Immunofluorescence and Confocal Microscopy for Rat Brains.
After deep anesthesia with pentobarbital, animals (methamphetamine self-administering rats, n = 4; yoked methamphetamine rats, n = 3; yoked saline rats, n = 3) were perfused with phosphate-buffered saline (PBS; pH 7.4) through the aorta. Next, tissues were fixed by perfusing 4% paraformaldehyde (500 ml, pH 7.4) at room temperature. The brains were dissected, and postfixation and cryoprotection were performed by immersing brains successively in 4% paraformaldehyde-containing PBS (4°C, 1.5 h), 10% sucrose-containing PBS (4°C for 3 h), and 30% sucrose-containing PBS (4°C, 48 h). Brains were then cut coronally with a cryostat (HM505E; Microm) at a thickness of 40 μM.
After washing with PBS for 20 min, brain slices were incubated in PBS containing 10% fetal calf serum and 0.5% Triton X-100 for 30 min at room temperature. After a wash with PBS (10 min), brain slices were incubated with either σ-1 receptor antiserum (1:50; 23, 24) or preimmune serum in PBS containing 10% fetal calf serum, 4% bovine serum albumin, and 0.5% Triton X-100 at 4°C overnight with gentle shaking. In some experiments, slices were labeled with monoclonal anti-dopamine D1 receptor (D1R), dopamine D2 receptor, tyrosine hydroxylase, or dopamine β-hydroxylase antibody (Millipore Bioscience Research Reagents, Temecula, CA). After extensive washings with 0.5% Triton X100-containing PBS (10 min, once) and PBS (20 min, three times), sections were incubated with secondary antibodies (Alexa Fluor 488 goat anti-rabbit IgG or Alexa Fluor 594 goat anti-mouse IgG at 1:200 dilution; Invitrogen, Carlsbad, CA) in PBS containing 4% bovine serum albumin for 2 h at room temperature. After washing with PBS (20 min, three times), sections were mounted in antifade mount solution (Invitrogen). All the steps of the aforementioned immunohistochemical procedure were performed on the same days. Care was taken to digitize sections at identical confocal microscope settings (laser power output, 100%; pinhole, 25; attenuation, 1/3; exposure time, 1 s; contrast, 3000; brightness, 3200). A series of five adjacent sections in each brain region were microscopically observed in both right and left hemispheres. Images were captured using Zeiss confocal system software (Carl Zeiss GmbH, Jena, Germany).
Reverse Transcription-Polymerase Chain Reaction and Cloning of σ-1 Receptor cDNA.
Total RNAs from rat hippocampus and red nucleus were extracted by NucreoSpin kit (Clontech, Mountain View, CA). σ-1 Receptor cDNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR) (one-step RT-PCR kit; Clontech) with the forward primer SR-S1 (5′-CCAGGCTGCCCGCT-3′ corresponding to rat σ-1 receptor RNA 22–35) and the reverse primer SR-A2 (5′-GTATGTATGTCCTTCCTC-3′ corresponding to rat σ-1 receptor RNA 857-840). PCR products were cloned in pcDNA3.1 vectors with the TOPO cloning kit (Invitrogen, Carlsbad, CA), and the vectors were transfected to CHO cells with Lipofectamine 2000 (Invitrogen). The cloned cDNA was verified by gene sequencing.
Statistics.
Behavioral results are expressed as total number of nose-poke responses in the active and the inactive hole per 2-h session, with each value representing a group mean (±S.E.M.). Data were analyzed using two-way analysis of variance (ANOVA) for repeated measures, followed by a pairwise multiple comparison procedure (Tukey's test) to locate differences between the groups. The significance level was set at p < 0.05. Biochemical data were analyzed, wherever appropriate, by one-way ANOVA followed by Tukey's multiple comparison test. The significance level was set at p < 0.05.
Results
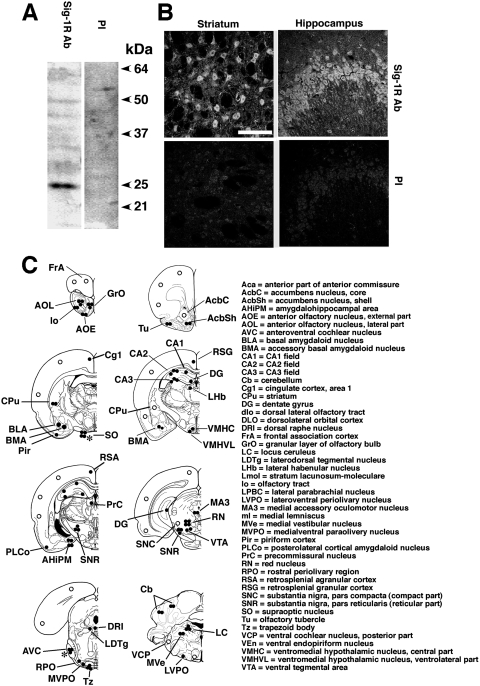
The immunospecificity of σ-1 receptor antibodies (Hayashi and Su, 2003a,b) used in this study was verified. The preimmune serum contained no antibody against σ-1 receptors as no signals were detected in Western blotting (Fig. 1A) or immunocytochemistry (Fig. 1B). The rostrocaudal regions in the rat brain containing immunodetectable σ-1 receptors are shown in Fig. 1C. The results are essentially similar to those described in other reports (Gundlach et al., 1986; Alonso et al., 2000; Kitaichi et al., 2000). In brief, moderate to highest immunoreactive densities (i.e., two to three dark spots in Fig. 1C) of σ-1 receptors were found rostrocaudally in the following regions in an overall estimation: olfactory bulb, shell of nucleus accumbens, olfactory tubercle, striatum, CA2 and CA3 of hippocampus, amygdala basal nucleus, amygdalohippocampal area, substantia nigra reticularis, ventral tegmental area, red nucleus, oculomotor nucleus, trapezoid body, medial vestibular nucleus, cerebellum, and locus ceruleus. Three neurobiological systems stood out as having significant levels of σ-1 receptors: 1) the nigrostriatal motor system; 2) the reward pathway involving the ventral tegmental area and nucleus accumbens; and 3) the limbic system consisting of the olfactory bulb, hippocampus, and amygdala.
Fig. 1.
Immunospecificity of the anti-σ-1 receptor (Sig-1R) antibody. A, Western blotting of rat brain protein lysates. Twenty micrograms of protein lysates from rat cortex was separated by 13% SDS-polyacrylamide gel electrophoresis and transblotted to a polyvinylidene difluoride membrane. Proteins were probed by either anti-Sig-1R antibody (Sig-1R Ab) or preimmune serum (PI) at a 1:750 dilution. The specific band with the 25-kDa molecular mass was detected. B, Sig-1R immunoreactivity in the rat striatum (left) or the hippocampal CA3 region (right). Top shows immunoreactivity derived from an anti-Sig-1R Ab (1:50). Bottom is from preimmune serum (1:50). Scale = 100 μm. C, schematic representation of the distribution of Sig-1R immunoreactivity in coronal sections of the rat brain. Intensities of immunoreactivities are ranked by the number of closed circles. The open circles indicate moderate expression. ∗, expression levels in pregnant rats.
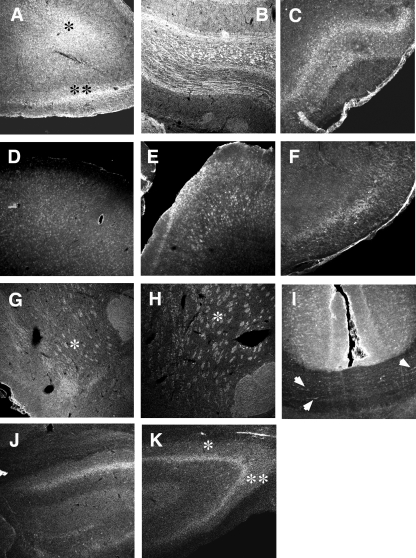
Detailed confocal microscopic examinations on immunoreactive σ-1 receptors in some of the above regions are shown in Figs. 2 and 3 in rostrocaudal order. Note that all the images in Fig. 2 (forebrain and hippocampus) are the same scale in size and were obtained under the same intensity of light source in the confocal setting (see Materials and Methods). Figure 3 shows σ-1 receptor immunoreactivities in midbrain, pons, and cerebellum. The red nucleus has higher σ-1 receptor immunoreactivity than the ventral tegmental area (Fig. 3D). Several large-sized neurons containing σ-1 receptors in the red nucleus are seen in Fig. 3E. The locus ceruleus also contains high levels of σ-1 receptors. σ-1 Receptors are seen as punctuate, globular, cytoplasmic structures inside a locus ceruleus neuron when observed at high magnification (Fig. 3L). The same distribution pattern is observed in several lines of cultured cells and is indicative of σ-1 receptors localizing at the mitochondria-associated ER membrane (Hayashi and Su, 2007). Thus, 10 distinct regions of rat brain high in σ-1 receptors were chosen for the following experiments. Those regions are the whole olfactory bulb as one region, striatum, nucleus accumbens, olfactory tubercle, amygdala, hippocampus, red nucleus, ventral tegmental area, substantia nigra compacta and reticulata, and locus ceruleus. Regions were bilaterally punched out with 0.2- to 1-mm needles (see Materials and Methods).
Fig. 2.
σ-1 Receptor (Sig-1R) immunoreactivity in forebrain and hippocampus. A, AOL (∗) and anterior olfactory nucleus, external part (∗∗) of olfactory bulb. B, granular layer of olfactory bulb. C, olfactory tubercle. D, frontal cortex. E, layers III through IV of the parietal cortex. F, postisometric relaxation of the dorsotemporal cortex. G, nucleus accumbens shell (∗). H, dorsofrontal striatum (∗). I, cingulate cortex and corpus callosum. Note immunoreactivities along the axonal tracts (arrows). J, dentate gyrus of hippocampus. K, hippocampus CA2 (∗) and CA3 (∗∗) regions. All images are in the same scale (bar = 300 μm).
Fig. 3.
σ-1 Receptor (Sig-1R) immunoreactivity in midbrain, pons, and cerebellum. A, amygdala (∗). B, substantia nigra reticulata. C, substantia nigra compacta (∗). D, red nucleus (∗), VTA (∗∗). E, large-sized neurons in the red nucleus positive to Sig-1R. F, cochlear nucleus of a pregnant rat. G and H, trapezoid nucleus (∗) and paraolivary nucleus. ∗∗, medialventral paraolivery nucleus; #, laterolventral periolivery nucleus. I, cerebellum. J–L, locus ceruleus. Arrows, highly clustered Sig-1R at cytoplasmic structures. Scale = 50 μm in E and K, 20 μm in L. Others are in the same scale to A (bar = 300 μm).
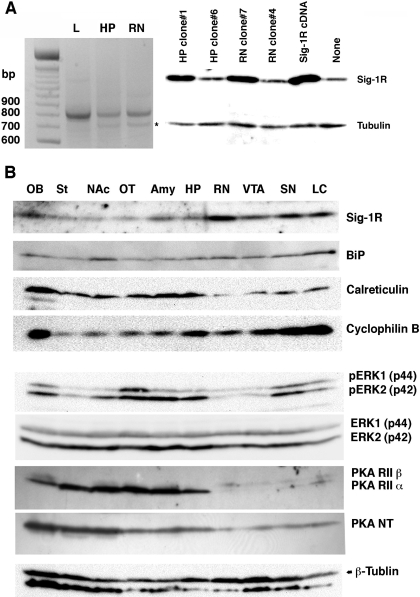
To verify the expression of σ-1 receptor transcripts in the rat brain, total RNAs from the hippocampus and red nucleus, together with those from the liver (where there is abundance of σ-1 receptors), were applied to RT-PCR amplified by the σ-1 receptor primers (Hayashi and Su, 2001). The PCR products were transfected into CHO cells. The sequence and functionality of the resultant PCR products were verified by sequencing and Western blotting on CHO cell lysates. The hippocampus and red nucleus express σ-1 receptors (Fig. 4A) with a higher expression level in the red nucleus, which is consistent with results from Western blotting and immunohistochemistry. Although a splice variant of σ-1 receptors is reported in lymphocytes (Ganapathy et al., 1999), we could not detect any variants in our samples from rat brains. Together with σ-1 receptors, three ER chaperones (BiP, calreticulin, and cyclophilin B) and two kinases (ERK and PKA) were also examined in this study because they are related to the action of σ-1 receptors (Stefanski et al., 2004; Cormaci et al., 2007) or methamphetamine (Jayanthi et al., 2004). Representative Western blotting of the chaperones and the kinases in the chosen brain region are shown in Fig. 4. For ERK, the total ERK and the phosphorylated active form of ERK were both examined (Fig. 4B). For PKA, both the regulatory subunit and the catalytic subunit were examined (Fig. 4B).
Fig. 4.
Brain expression and distribution of σ-1 receptors (Sig-1Rs), ER chaperones, PKA, and ERK. A, expression of Sig-1R transcripts in the rat brain. Left, the result of RT-PCR using total RNAs from the liver (L), hippocampus (HP), and red nucleus (RN). One microgram of rat liver total RNA or 2 μg of rat brain total RNA was applied to RT-PCR. ∗, the 5-methyltetrahydrofolate-homocysteine methyltransferase gene nonspecifically amplified by the Sig-1R primers. All the PCR products were identified by gene sequencing. Western blotting of Sig-1Rs (right) verified the cloned HP or RN cDNA expressing Sig-1Rs in the mammalian cells. Total cell lysates (15 μg/lane) were prepared from CHO cells transfected with PCR products cloned in the pcDNA3.1 vector. HP clone 6 and RN clone 4 serve as negative controls containing the nonfunctional gene. Sig-1R cDNA is a positive vector containing the Sig-1R cDNA previously cloned (Hayashi and Su, 2001). B, brain distribution of ER chaperones and kinases. See Materials and Methods for details.
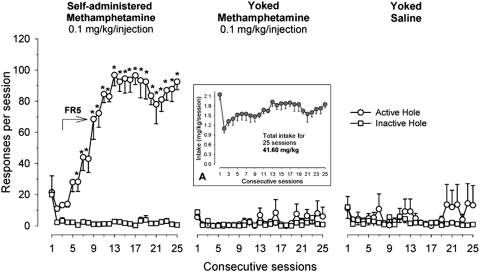
In the methamphetamine self-administration experiment, a two-way repeated-measures ANOVA of the nose-poke responding data revealed a significant interaction between nose-poke hole (active versus inactive hole) and sessions in the methamphetamine self-administering group (Fig. 5, left) [F(24,216) = 26.19, p < 0.001]; post hoc analysis revealed that a significant preference for the active hole occurred during sessions 5 to 25 (p < 0.01). In the yoked methamphetamine (Fig. 5, middle) and yoked saline groups (Fig. 5, right), in which nose-poke responding had no programmed effect, the effects of nose poke, session, and their interaction were not significant. The total intake of methamphetamine in the first two groups of rats over 25 consecutive sessions was 41.77 mg/kg (Fig. 5, inset A).
Fig. 5.
Yoked methamphetamine self-administration. The mean number (±S.E.M.) of responses in the active and inactive holes for rats that were allowed to acquire self-administration of methamphetamine at a dose of 0.1 mg/kg/injection (n = 10) and their yoked controls that received yoked infusions of methamphetamine (n = 9) or saline (n = 9) during each of the daily 2-h sessions. The arrow indicates the period when methamphetamine self-administration was maintained under the final five-response fixed-ratio schedule of reinforcement. Asterisks (∗) denote significant differences p < 0.01 between active and inactive nose pokes. Inset A, the values (mean ± S.E.M.) represent the total amount of actively self-administered (first group) or passively delivered (yoked methamphetamine group) methamphetamine during each of 25 experimental sessions.
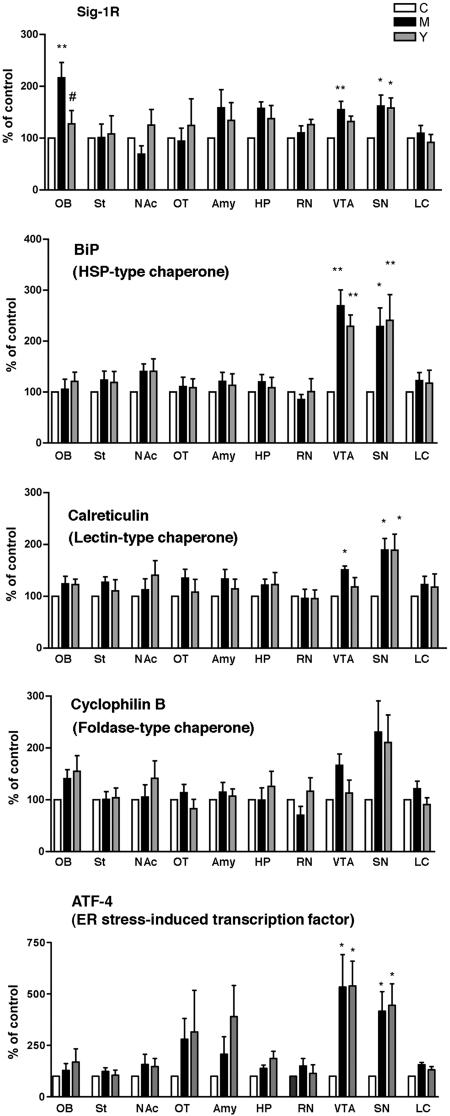
Twenty-four hours after the last self-administration session, brains were removed and processed for Western blotting (see Materials and Methods). Of the 10 regions examined in the three groups of rats, only the olfactory bulb, ventral tegmental area, and substantia nigra showed a difference in the level of ER chaperones. In both groups of rats that received methamphetamine, the ER chaperones σ-1 receptor, BiP, and calreticulin were all up-regulated in the ventral tegmental area and substantia nigra, whereas another ER chaperone, cyclophilin B, was not affected in any brain region (Fig. 6). Furthermore, in the two regions where the three ER chaperones were up-regulated, no differences in their densities were observed between the methamphetamine self-administering group and the yoked methamphetamine group. σ-1 Receptors were slightly elevated in the ventral tegmental area in the methamphetamine self-administering group compared with the yoked methamphetamine group, but this difference was not significant (Fig. 6, top). However, the olfactory bulb stood out as a distinct region where only σ-1 receptors were up-regulated compared with other ER chaperones; furthermore, σ-1 receptors were significantly up-regulated only in rats that actively self-administered methamphetamine. Yoked methamphetamine rats exhibited the same level of σ-1 receptors as did the yoked saline group (Fig. 6). We also examined the level of activating transcription factor 4 (ATF4) in the 10 brain regions. ATF4 is an ER stress marker downstream of the protein kinase-like ER kinase and is activated by unfolded proteins as a result of ER stress (Schröder and Kaufman, 2005). ATF4 was significantly and specifically up-regulated in the ventral tegmental area and substantia nigra in rats that received methamphetamine either actively or passively (Fig. 6, bottom).
Fig. 6.
Effect of methamphetamine self-administration on expression levels of ER chaperones. Protein levels of each molecular chaperone were measured by Western blotting. Data are shown as percentage of animals receiving saline (control). ∗∗, p < 0.01, ∗, p < 0.05 compared with control (n = 6/group). Data analyzed by one-way ANOVA followed by Tukey's multiple comparison test. See Materials and Methods for details in the sample preparation. C, yoked saline control; M, methamphetamine self-administration; Y, yoked methamphetamine control. OB, olfactory bulb; St, striatum; NAc, nucleus accumbens; OT, olfactory tubercle; Amy, basolateral amygdala; HP, dorsolateral hippocampus; RN, red nucleus; SN, substantia nigra (compacta and reticulata); LC, locus ceruleus.
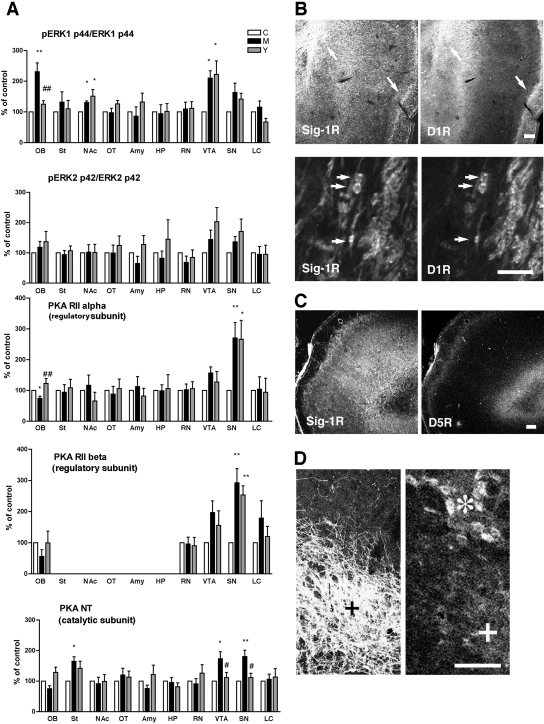
In this study, we also examined the densities of ERK and PKA in the three groups of rats from the self-administration experiment because the activation of ERK or PKA has been previously shown by us to increase or decrease, respectively, the level of σ-1 receptors in B-104 cells (Cormaci et al., 2007). Regarding ERK, the degree of phosphorylation (i.e., the activation) of two types of ERK, ERK1 (p44) and ERK2 (p42), was examined. Phosphorylation of ERK1 was increased in the nucleus accumbens and ventral tegmental area of rats that received methamphetamine either in an active or passive fashion compared with the yoked saline group (Fig. 7A, top). In the olfactory bulb, the degree of ERK1 phosphorylation was significantly increased only in the rats that actively self-administered methamphetamine (Fig. 7A, top). No differences in ERK2 phosphorylation were observed between the three groups of rats in any region (Fig. 7A, second panel).
Fig. 7.
Effect of methamphetamine self-administration on expression levels of PKA or phosphorylation of ERK. Protein levels or phosphorylation were measured by Western blotting. Data are shown as percentage of animals receiving saline (control). ∗∗, p < 0.01, p < 0.05 compared with control (n = 6/group). Data analyzed by one-way ANOVA followed by Tukey's multiple comparison test. See Materials and Methods for details of the sample preparation. C, yoked saline control; M, methamphetamine self-administration; Y, yoked methamphetamine control. OB, olfactory bulb; St, striatum; NAc, nucleus accumbens; OT, olfactory tubercle; Amy, basolateral amygdala; HP, dorsolateral hippocampus; RN, red nucleus; SN, substantia nigra (compacta and reticulata); LC, locus ceruleus. B, localization of σ-1 receptors (Sig-1Rs) with D1R in the olfactory bulb. Arrows indicate the potential colocalization between Sig-1Rs and D1R seen at lower magnification of AOL (left) and anterior olfactory nucleus, external part (right) regions. Bottom two panels are at the higher magnification of the AOL region. Note the clear localization of Sig-1Rs in cell bodies of D1R-postive neurons. C, no colocalization of Sig-1Rs with dopamine D5 receptors (D5R) in the olfactory bulb. D, expression of Sig-1Rs in tyrosine hydroxylase-positive VTA neurons (+). ∗, large neurons in the red nucleus positive to Sig-1R immunoreactivity. Scale = 300 μm (B, top, and C), and 100 μm (B, bottom, and D).
The densities of two regulatory subunits of PKA, PKA RIIα and PKA RIIα, and the catalytic subunit PKA NT were also examined. The two regulatory subunits were increased in the substantia nigra of the two groups of rats that actively or passively received methamphetamine (Fig. 7A, third and fourth panels). In the olfactory bulb, the level of regulatory subunit PKA RIIα was decreased in the methamphetamine self-administering group but not the yoked methamphetamine control group (Fig. 7A, third panel). Although PKA RIIα and the catalytic subunit PKA NT showed the same tendency as PKA RIIα in the olfactory bulb, no statistically significant differences were found between the groups (Fig. 7A, fourth and fifth panels). However, in the ventral tegmental area and substantia nigra, PKA NT was significantly elevated in the methamphetamine self-administering group and not in the yoked methamphetamine control group (Fig. 7A, fifth panel).
As the olfactory bulb is innervated by dopaminergic neurons (Davila et al., 2003), the localization of σ-1 receptors, which is mainly postsynaptic, was compared with postsynaptic D1 and D5 dopamine receptors. Rats from the yoked saline group were used for this analysis. σ-1 Receptors and D1 receptors were both present in the lateral and external parts of the olfactory bulb (Fig. 7B, arrows in top). Higher magnifications of the neurons in the lateral part of the olfactory bulb show a striking pattern of colocalization of σ-1 receptors and D1 receptors at the soma of the same neuron (Fig. 7B, arrows in bottom). However, σ-1 receptors were not colocalized with D5 receptors in the olfactory bulb nor, apparently, with tyrosine hydroxylase in the VTA (Fig. 7, C and D).
Discussion
We previously reported an increase of σ-1 receptors in the midbrain of rats that actively self-administered methamphetamine (Stefanski et al., 2004). However, in the present experiment, we found that expression of σ-1 receptors in rats that self-administered methamphetamine was only increased in the olfactory bulb. A possible reason for this discrepancy is that in the first study the midbrain region was dissected out as a whole, whereas in the present study, which focuses mainly on the reward system, only the ventral tegmental area, substantia nigra, and red nucleus were micropunched and examined. The σ-1 receptors in the ventral tegmental area showed only a nonsignificant increase in the actively self-administering rats compared with the yoked methamphetamine group. It is currently unknown whether σ-1 receptors in other areas of the midbrain might have been quantitatively altered and thus implicated in the self-administration of methamphetamine.
It is interesting to note that three of four ER chaperones were up-regulated in the ventral tegmental area and substantia nigra of rats that received methamphetamine either actively or passively. As ER chaperones are up-regulated by ER stress, the results suggest that methamphetamine causes ER stress in those two areas of the brain. Attested to the notion of ER stress incurring in those two areas of the brain is the increase of the transcription factor AFT4 downstream of the protein kinase-like ER kinase signaling. Thus, ER stress did occur in those two areas of the brain. However, whether apoptosis or cell death occurred in those two areas of the brain was not examined in this study. High doses of methamphetamine have been known to cause specific pattern of stereotypy (Kitanaka et al., 2009) in mice and induce apoptosis or cell death in the brain (Jayanthi et al., 2004; Zhu et al., 2006). However, because the amount of methamphetamine received by the rats in the present study is low (41.60 mg/kg over 25, 2-h sessions), it would not be likely to cause apoptosis or cell death but might cause some milder form of ER stress. It is interesting to note that chronic cocaine administration at moderate doses (20 mg/kg, 7–9 days) also causes ER stress (e.g., up-regulation of BiP and caspase-12) in the rat brain, and that this can be blocked by N-methyl-d-aspartate receptor antagonists or a dopamine D1 antagonist (e.g., Shin et al., 2007). Because up-regulation of ER chaperones alter the capacity of the ER in folding and secretion of proteins (including those important for neuronal excitation and synaptogenesis), the region-specific ER stress caused by low to moderate doses of psychostimulants might contribute to the establishment of neuronal adaptation that lead to psychostimulant addiction. Thus, ER stress might also participate in neuronal adaptation that underlies addictive processes.
The olfactory bulb in this study turns out to be the only area where σ-1 receptors were significantly up-regulated in the methamphetamine self-administering rats compared with the yoked methamphetamine rats. Because the yoke methamphetamine group did not differ from saline-exposed controls, this up-regulation cannot simply be attributed to methamphetamine exposure. Therefore, this finding suggests the possibility that σ-1 receptors in the olfactory bulb play an important role in the motivational conditioning/learning aspects of methamphetamine self-administration. The olfactory bulb is recognized as a constituent of the cortical-hippocampal-amygdala limbic region, which contributes to the emotional and memory-related components of behavior (Cecchi et al., 2001; Rolls, 2005; Song and Leonard, 2005). Many reports show that σ-1 receptors are important for learning and memory (Maurice et al., 2001) and play an important role in neuritogenesis (Takebayashi et al., 2004). Thus, the present results reveal the olfactory bulb to be a potentially important brain region for studying the role of σ-1 receptors in methamphetamine self-administration and perhaps the brain reward system in general. Our results with ERK (specifically ERK1) being activated while PKA (specifically PKA RIIα) is down-regulated in the olfactory bulb are consistent with our previous report showing that in B-104 cells ERK activation or PKA inactivation causes an increase in σ-1 receptors (Cormaci et al., 2007). However, it is not presently known how the interplay between the ERK and PKA might affect levels of σ-1 receptors in the ventral tegmental area or substantia nigra.
An additional important finding in this study is that σ-1 receptors colocalize with D1Rs in the olfactory bulb. The olfactory bulb is known to be innervated by dopaminergic terminals (Davila et al., 2003; Jayanthi et al., 2004), and D1Rs appear to be involved in the reinforcing (e.g., Stefanski et al., 1999) and subjective (e.g., Tidey and Bergman, 1998) effects of methamphetamine in rats. Although the exact relation between σ-1 receptors and D1Rs in the olfactory bulb is not clear at present, an interesting link is suggested by results of the present and previous studies. Mizoguchi et al. (2004) found that activation of ERK1 via D1Rs was associated with methamphetamine-induced conditioned place preference. We reported previously that the activation of ERKs up-regulated σ-1 receptors (Cormaci et al., 2007). We found in the present study that ERK1 is activated in the olfactory bulb of methamphetamine self-administering rats but not in yoked methamphetamine rats that receive the drug passively. Although more experiments are needed to show a causal link, these findings suggest that motivational conditioning/learning aspects of methamphetamine self-administration involve activation of D1Rs in the olfactory bulb, which in turn activate ERKs, leading to an increase of σ-1 receptors that might affect neuronal structure and thus the persistence of addiction (Takebayashi et al., 2004; Martin-Fardon et al., 2007). Although high dose of methamphetamine has been found to cause apoptosis and cell death in the olfactory bulb (Deng et al., 2007), the dose of methamphetamine used in the present study might not cause apoptosis, cell death, or even ER stress in the olfactory bulb. Levels of ER chaperones and that of the ER stress sensor ATF4 were not significantly altered in the olfactory bulb of rats receiving either methamphetamine or saline (Fig. 6). Finally, σ-1 receptors have been implicated in increases of dopamine in hippocampal neurons (Meurs et al., 2007). However, whether σ-1 receptors might increase dopamine in the olfactory bulb and thus cause a “feed-forward” increase of themselves via the proposed D1R-ERK pathway is unknown at present.
Acknowledgments
We thank Eric Thorndike for excellent programming and technical assistance during the self-administration experiments and Dr. Leigh Panlilio for comments on the final version of the manuscript.
This research was supported by the Intramural Research Program of the National Institutes of Health National Institute on Drug Abuse.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.109.159244.
- ER
- endoplasmic reticulum
- CHO
- Chinese hamster ovary
- ERK
- extracellular signal-regulated kinase
- PKA
- protein kinase A
- PBS
- phosphate-buffered saline
- D1R
- dopamine D1 receptor
- RT-PCR
- reverse transcription-polymerase chain reaction
- ANOVA
- analysis of variance
- ATF4
- activating transcription factor 4
- AOL
- anterior olfactory nucleus, lateral part
- VTA
- ventral tegmental area.
References
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. (2000) Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience 97:155–170 [DOI] [PubMed] [Google Scholar]
- Cecchi GA, Petreanu LT, Alvarez-Buylla A, Magnasco MO. (2001) Unsupervised learning and adaptation in a model of adult neurogenesis. J Comput Neurosci 11:175–182 [DOI] [PubMed] [Google Scholar]
- Cormaci G, Mori T, Hayashi T, Su TP. (2007) Protein kinase A activation down-regulates, whereas extracellular signal-regulated kinase activation up-regulates sigma-1 receptors in B-104 cells: implication for neuroplasticity. J Pharmacol Exp Ther 320:202–210 [DOI] [PubMed] [Google Scholar]
- Davila NG, Blakemore LJ, Trombley PQ. (2003) Dopamine modulates synaptic transmission between rat olfactory bulb neurons in culture. J Neurophysiol 90:395–404 [DOI] [PubMed] [Google Scholar]
- Deng X, Ladenheim B, Jayanthi S, Cadet JL. (2007) Methamphetamine administration causes death of dopaminergic neurons in the mouse olfactory bulb. Biol Psychiatry 61:1235–1243 [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Hanson GR. (2009) Psychostimulant-induced alterations in vesicular monoamine transporter-2 function: neurotoxic and therapeutic implications. Neuropharmacology 56 (Suppl 1):133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanilla D, Johannessen M, Hajipour AR, Cozzi NV, Jackson MB, Ruoho AE. (2009) The hallucinogen N,N-dimethyltryptamine (DMT) is an endogenous sigma-1 receptor regulator. Science 323:934–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy ME, Prasad PD, Huang W, Seth P, Leibach FH, Ganapathy V. (1999) Molecular and ligand-binding characterization of the sigma-receptor in the Jurkat human T lymphocyte cell line. J Pharmacol Exp Ther 289:251–260 [PubMed] [Google Scholar]
- Gundlach AL, Largent BL, Snyder SH. (1986) Autoradiographic localization of sigma receptor binding sites in guinea pig and rat central nervous system with (+)3H-3-(3-hydroxyphenyl)-N-(1-propyl)piperidine. J Neurosci 6:1757–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig J, Kempner E, Glossmann H. (1996) Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A 93:8072–8077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. (2009) MAM: more than just a housekeeper. Trends Cell Biol 19:81–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2001) Regulating ankyrin dynamics: roles of sigma-1 receptors. Proc Natl Acad Sci U S A 98:491–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2003a) Intracellular dynamics of sigma-1 receptors (sigma(1) binding sites) in NG108–15 cells. J Pharmacol Exp Ther 306:726–733 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2003b) Sigma-1 receptors (sigma(1) binding sites) form raft-like microdomains and target lipid droplets on the endoplasmic reticulum: roles in endoplasmic reticulum lipid compartmentalization and export. J Pharmacol Exp Ther 306:718–725 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131:596–610 [DOI] [PubMed] [Google Scholar]
- Hellewell SB, Bruce A, Feinstein G, Orringer J, Williams W, Bowen WD. (1994) Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol 268:9–18 [DOI] [PubMed] [Google Scholar]
- Herrera Y, Katnik C, Rodriguez JD, Hall AA, Willing A, Pennypacker KR, Cuevas J. (2008) Sigma-1 receptor modulation of acid-sensing ion channels a (ASC1a) and ASC1a-induced Ca2+ influx in rat cortical neurons. J Pharmacol Exp Ther 327:491–502 [DOI] [PubMed] [Google Scholar]
- Itzhak Y. (1993) Repeated methamphetamine-treatment alters brain sigma receptors. Eur J Pharmacol 230:243–244 [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Noailles PA, Ladenheim B, Cadet JL. (2004) Methamphetamine induces neuronal apoptosis via cross-talks between endoplasmic reticulum and mitochondria-dependent death cascades. FASEB J 18:238–251 [DOI] [PubMed] [Google Scholar]
- Johannessen M, Ramachandran S, Riemer L, Ramos-Serrano A, Ruoho AE, Jackson MB. (2009) Voltage-gated sodium channel modulation by sigma-receptors in cardiac myocytes and heterologous systems. Am J Physiol Cell Physiol 296:C1049–C1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitaichi K, Chabot JG, Moebius FF, Flandorfer A, Glossmann H, Quirion R. (2000) Expression of the purported sigma(1) (sigma(1)) receptor in the mammalian brain and its possible relevance in deficits induced by antagonism of the NMDA receptor complex as revealed using an antisense strategy. J Chem Neuroanat 20:375–387 [DOI] [PubMed] [Google Scholar]
- Kitanaka J, Kitanaka N, Tatsuta T, Hall FS, Uhl G, Tanaka K, Nishiyama N, Morita Y, Takemura M. (2009) Sigma1 receptor antagonists determine the behavioral pattern of the methamphetamine-induced stereotypy in mice. Psychopharmacology (Berl) 203:781–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Maurice T, Aujla H, Bowen WD, Weiss F. (2007) Differential effects of sigma1 receptor blockade on self-administration and conditioned reinstatement by cocaine vs natural reward. Neuropsychopharmacology 32:1967–1973 [DOI] [PubMed] [Google Scholar]
- Matsumoto RR, Shaikh J, Wilson LL, Vedam S, Coop A. (2008) Attenuation of methamphetamine-induced effects through the antagonism of sigma (sigma) receptors: evidence from in vivo and in vitro studies. Eur Neuropsychopharmacol 18:871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice T, Urani A, Phan VL, Romieu P. (2001) The interaction between neuroactive steroids and the sigma1 receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev 37:116–132 [DOI] [PubMed] [Google Scholar]
- Meurs A, Clinckers R, Ebinger G, Michotte Y, Smolders I. (2007) Sigma 1 receptor-mediated increase in hippocampal extracellular dopamine contributes to the mechanism of the anticonvulsant action of neuropeptide Y. Eur J Neurosci 26:3079–3092 [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Yamada K, Mizuno M, Mizuno T, Nitta A, Noda Y, Nabeshima T. (2004) Regulations of methamphetamine reward by extracellular signal-regulated kinase 1/2/ets-like gene-1 signaling pathway via the activation of dopamine receptors. Mol Pharmacol 65:1293–1301 [DOI] [PubMed] [Google Scholar]
- Nguyen EC, McCracken KA, Liu Y, Pouw B, Matsumoto RR. (2005) Involvement of sigma (sigma) receptors in the acute actions of methamphetamine: receptor binding and behavioral studies. Neuropharmacology 49:638–645 [DOI] [PubMed] [Google Scholar]
- Rolls ET. (2005) Taste, olfactory, and food texture processing in the brain, and the control of food intake. Physiol Behav 85:45–56 [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ. (2005) The mammalian unfolded protein response. Annu Rev Biochem 74:739–789 [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, O'Neil ML, Melega WP, Cho AK. (2005) Prolonged exposure of rats to intravenous methamphetamine: behavioral and neurochemical characterization. Psychopharmacology 180:501–512 [DOI] [PubMed] [Google Scholar]
- Shin EH, Bian S, Shim YB, Rahman MA, Chung KT, Kim JY, Wang JQ, Choe ES. (2007) Cocaine increases endoplasmic reticulum stress protein expression in striatal neurons. Neuroscience 145:621–630 [DOI] [PubMed] [Google Scholar]
- Song C, Leonard BE. (2005) The olfactory bulbectomised rat as a model of depression. Neurosci Biobehav Rev 29:627–647 [DOI] [PubMed] [Google Scholar]
- Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. (2004) Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls. Psychopharmacology 175:68–75 [DOI] [PubMed] [Google Scholar]
- Stefanski R, Ladenheim B, Lee SH, Cadet JL, Goldberg SR. (1999) Neuroadaptations in the dopaminergic system after active self-administration but not after passive administration of methamphetamine. Eur J Pharmacol 371:123–135 [DOI] [PubMed] [Google Scholar]
- Su TP. (1982) Evidence for sigma opioid receptor: binding of [3H]SKF-10047 to etorphine-inaccessible sites in guinea-pig brain. J Pharmacol Exp Ther 223:284–290 [PubMed] [Google Scholar]
- Su TP, Hayashi T, Vaupel DB. (2009) When the endogenous hallucinogenic trace amine N,N-dimethyltryptamine meets the sigma-1 receptor. Sci Signal 2:pe12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, London ED, Jaffe JH. (1988) Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science 240:219–221 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Miwa T, Horikomi K. (2000) Involvement of sigma 1 receptors in methamphetamine-induced behavioral sensitization in rats. Neurosci Lett 289:21–24 [DOI] [PubMed] [Google Scholar]
- Takebayashi M, Hayashi T, Su TP. (2004) Sigma-1 receptors potentiate epidermal growth factor signaling towards neuritogenesis in PC12 cells: potential relation to lipid raft reconstitution. Synapse 53:90–103 [DOI] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. (1998) Drug discrimination in methamphetamine-trained monkeys: agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther 285:1163–1174 [PubMed] [Google Scholar]
- Ujike H, Okumura K, Zushi Y, Akiyama K, Otsuki S. (1992) Persistent supersensitivity of sigma receptors develops during repeated methamphetamine treatment. Eur J Pharmacol 211:323–328 [DOI] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. (2008) Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. J Biol Chem 283:28198–28215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JP, Xu W, Angulo JA. (2006) Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience 140:607–622 [DOI] [PMC free article] [PubMed] [Google Scholar]