Abstract
Resting metabolic rates at thermoneutral (RMRts) are unexpectedly variable. One explanation is that high RMRts intrinsically potentiate a greater total daily energy expenditure (DEE), but recent work has suggested that DEE is extrinsically defined by the environment, which independently affects RMRt. This extrinsic effect could occur because expenditure is forced upwards in poor habitats or enabled to rise in good habitats. We provide here an intraspecific test for an association between RMRt and DEE that separates intrinsic from extrinsic effects and forcing from enabling effects. We measured the DEE and RMRt of 75 free-living short-tailed field voles at two time points in late winter. Across all sites, there was a positive link between individual variation in RMRt and DEE. This correlation, however, emerged only because of an effect across sites, rather than because of an intrinsic association within sites. We defined site quality from the survivorship of voles at the sites and the time at which they commenced breeding in spring. The associations between DEE/RMRt and site quality suggested that in February voles in poorer sites had higher energy demands, indicating that DEE was forced upwards, but in March the opposite was true, with higher demands in good sites, indicating that high expenditure was enabled. These data show that daily energy demands are extrinsically defined, with a link to RMRt that is secondary or independent. Both forcing and enabling effects of the environment may pertain at different times of year.
The basal metabolic rate (BMR) is defined as the metabolic rate of a quiescent animal, in the thermoneutral zone, that is neither digesting food nor engaged in reproduction or growth (1). A slightly less rigorously defined measurement, resting metabolic rate at thermoneutral (RMRt), incorporates all of these requirements except that the animal need not be postabsorptive (2). BMR and RMRt are highly variable. This variability is most manifest at the interspecific level, where species that have the same body mass may differ in their RMRts by almost an order of magnitude (3–5). However, intraspecific variation in these traits is also substantial, particularly in small mammals, where individuals of the same body mass may differ by 100% in their BMR or RMRt (6–10).
Understanding the nature of these differences is important because RMRt (and BMR) are major components of total energy budgets. Typically in free-living animals, RMRt accounts for ≈30–40% of total daily energy demands (11–14). Because animals must spend time feeding to sustain their daily energy demands, including the component comprising their RMRts, there is presumably selection on animals to minimize this component of their daily energy budgets [to reduce foraging times and exposure to predation or adverse environmental conditions (15) or to allocate the saved energy to the processes of growth and reproduction (16)]. Attempts at understanding why some individual animals have much greater RMRts than others have therefore focused on defining advantages that might accrue to individuals that maintain high RMRts.
One hypothesis is that high RMRts reflect the capacity of an individual to sustain a high total daily energy expenditure (DEE) (11, 17, 18), because sustaining high DEE requires large and costly internal organs and RMRt reflects the costs of maintaining these organs in the resting state. Consequently, a high RMRt may be advantageous because it confers on the individual greater capacity for DEE (the potentiation hypothesis). The advantage of having a high DEE may be because the total capacity to expend energy may be an important limit on the ability of the animals to reproduce or survive. This idea is supported by observations that at the interspecific level sustained maximum DEE appears to be a fixed ratio of RMRt (11, 17–19). Given this prior observation that RMRt and DEE do appear to be linked at the intraspecific level, debate around this issue has generally focused on whether the link between high RMRt and high DEE is reflective of a central processing limit [i.e., the costs of sustaining the alimentary system where energy is absorbed (14, 20–22)] or a peripheral limit [i.e., the costs of maintaining the organs where energy is expended (14)]. Both of these ideas, however, are fundamentally similar in that they posit that the level of DEE is intrinsically defined by aspects of the animal's morphology or physiology that also define the RMRt (and BMR) and subsequently lead to an association between these two traits. This intrinsic setting of morphology and physiology that then determines the RMRt and DEE might involve genetic variation between individuals or epigenetic phenomena such as fetal and perinatal programming. The important point is that the locus of control over the association between the traits stems from variation that is intrinsic to the animals themselves.
Recent work, however, has brought into question the fundamental notion that limits on DEE and RMRt might be associated with intrinsic factors. Despite early hope (20, 21, 23), studies of lactating mice have failed to define the intrinsic limits during lactation (9, 24–27). Attempts to link reproductive capacity (7–9, 28) to individual variation in RMRt have proved negative, although a weak link to overwinter survival has been shown (10). The only previous attempt to find associations between intraindividual variation in RMRt and DEE in free-living animals also failed to find a significant association (29). Moreover, in a comprehensive reappraisal of the links between DEE and RMRt at the interspecific level in small mammals, Speakman (14) came to the conclusion that DEE was most likely to be set by extrinsic factors, such as the supply of energy from the environment, rather than intrinsic factors, such as components of physiology. A correlation with RMRt may then emerge either because RMRt is pulled along by DEE or because it is independently determined by similar environmental factors. Similar conclusions were reached by Tinbergen and Verhulst (30) in their study of breeding blue tits, and the notion of extrinsic influences on DEE has formed a fundamental component of recent studies that explain links between changes in global climate and animal distributions (31). Interspecific comparisons of RMRt in the genus Peromyscus have also produced similar conclusions (32). This viewpoint is fundamentally different from the intrinsic hypothesis because it postulates that the source of the association between RMRt and DEE arises because of extrinsic rather than intrinsic factors.
The nature of extrinsic limitation on DEE could reflect two alternative processes. First, high DEE (and the consequent high RMRt to support it) could reflect high levels of environmental resources, with animals being enabled to elevate their DEE (and RMRt) by the resource availability. A key aspect of this idea is that animals could potentially choose in this environment to have lower expenditures and still survive. They are selected, however, to have elevated expenditures because of the fitness benefits this brings. However, an alternative view is that poor environments may lead to greater levels of both DEE and RMRt, because metabolism is forced upwards by the harsh conditions (31). A critical difference between the forcing and enabling processes is that under environmental forcing the animals could not survive if they chose to expend energy at lower levels. Both of these “extrinsic limitation hypotheses” (14) are actually rooted in ideas prevalent in the 1970s concerning animal energy budgeting (see, for examples, refs. 16 and 33) but force a radical rethinking of the whole nature of the association between RMRt and DEE. To our knowledge, however, no objective intraspecific tests of these hypotheses, to separate intrinsic from extrinsic limits or to separate enabling from forcing effects, have been performed. We present such a test here.
Materials and Methods
The study was carried out in Kielder Forest (55° 13′ N, 2° 33′ W), a large spruce plantation straddling the border between Scotland and England. The forest covers an area of ≈600 km2 and is dominated by spruce, which are managed on an average rotation time of 40–60 years. Harvesting of timber provides well defined islands of successional habitat that progress from clear-cuts, to grassland dominated by Deschampsia cespitosa, Agrostis tenuis, Juncus effusus, and bryophytes, to prethicket forest, and, finally, to a thicket stage after 12–15 years. The forest therefore consists of a mosaic of dense conifer stands with little ground vegetation and ephemeral grassland patches. The grassland patches range in size from 5 hectare (ha) to >100 ha, with the smallest occupying the valley bottoms. About 16–17% of the forest is occupied by the patches suitable for field voles. The field vole is common in these ephemeral habitats but completely absent from forested areas, which lack grass cover. Long-term monitoring has shown that field vole populations in the Kielder forest have cyclic dynamics with a 3- to 4-year period (34). Habitat patches are very similar in terms of vegetation and differ primarily in aspect. Vole populations fluctuate out of phase over relatively small spatial scale in this area (35). At a given time, the main difference and potential selection pressure is the prevailing population density, which depends on the phase of the cycle in any particular patch.
This study took place during late winter 1998 in four separate patches 1–20 km apart and followed a transplant experiment in which overwintering field voles had been transplanted between sites before the breeding season (36). By transferring the animals between sites before our measurements, we eliminated the possibility that differences between sites might reflect locally adapted genotypes in the subpopulations. Hence, any site differences would reflect the extrinsic impact of the sites, rather than intrinsic properties due to local adaptation. This procedure overcomes a primary difficulty with the study of Mueller and Diamond (32), where differences between habitats are completely confounded by differences in species occupying those habitats.
The populations at the four sites were monitored by capture–mark–recapture methodology biweekly from February 1, following the procedure described in ref. 36. The trapping study revealed large variation in survival and the spring onset of reproduction between sites (Table 1). This variation between the quality of the four sites leads to an ability to distinguish both intrinsic from extrinsic limitation, and also to compare enabling with forcing interpretations of extrinsic limits. In brief, if voles were intrinsically limited, then one might anticipate that a link between RMRt and DEE would be apparent within the individual sites but that differences between sites would be less apparent. In contrast, if extrinsic factors were predominant, then one would expect a relationship between RMRt and DEE, but this would reflect only the impact of the site differences. Finally, if the environment were enabling high levels of DEE and RMRt, we would anticipate that both traits would be higher at high-quality sites. However, if voles were forced to cope with the harsher environments then the opposite trend would be revealed.
Table 1. Details of the sampling sites.
| Distance from, km
|
Average body mass in sampling period,* g ± SE
|
Date when 50% of females had mated†
|
Biweekly survival rate‡
|
||||
|---|---|---|---|---|---|---|---|
| Site | A | B | C | D | |||
| A | 4 | 20 | 20 | 19.6 ± 0.5 | April 9 | 0.74 | |
| B | 4 | 21 | 22 | 21.2 ± 0.5 | March 18 | 0.86 | |
| C | 20 | 21 | 1 | 20.1 ± 0.3 | March 30 | 0.85 | |
| D | 20 | 22 | 1 | 20.7 ± 0.4 | March 27 | 0.86 | |
There was no significant change in body mass over the sampling period.
Estimated from logistic regression on proportion of females with perforate vagina.
Geometric mean of biweekly estimates of survival probability. Survival probabilities are estimated from capture—recapture data collected at 2-week intervals between mid-March and mid-April by using the Cormack—Jolly—Seber model with time-specific survival and recapture probability in the program mark.
Field Rates and RMRts. Voles were captured for measuring field rates and RMRts over an 8-week period between the start of February and the end of March. We divided this capture period into early phase (February) and the late phase (March). Traps were set just before dusk and checked the next morning after dawn. On capture, female voles (identifiable by coded ear tags) were injected with 0.3 ml of doubly-labeled water [20 atom percent excess (APE) oxygen/10 APE deuterium], left in the trap for 60 min to allow the isotopes to reach equilibrium, and bled by tail-tipping into capillary tubes to obtain an initial blood sample for isotope analysis. The mass of the animal was recorded before injection and after tail bleeding. After injection and blood sampling, voles were released immediately at the exact site of capture. No attempt was made to recapture injected animals until the next morning, thus maximizing the amount of time the vole spent in natural field conditions. The next morning, 12 traps, centered on the site where the vole was initially captured and subsequently released, were set before dawn in an attempt to recapture individuals after ≈24 h in the field. Traps were then checked at 2- to 3-h intervals throughout the day. In the event that a vole had not been recaptured in the first 24 h, traps were left set overnight to maximize the chance of recapture within 48 h. Recaptured voles were tail bled for the second time, weighed, and transported to a field laboratory where measures of RMRts were made following the protocols previously established for this species (37). Briefly, animals were confined in a small cylindrical chamber (30 cm in length, 10 cm in diameter) that contained a perforated base to separate animals from their feces. The chamber was located inside a temperature-controlled incubator maintained at 25°C, which is inside the thermoneutral zone for this species (38). A dry airflow through the chamber was controlled by a mass flow controller (Sierra Instruments, Monterey, CA) and metered by using a high-precision rotameter (Alexander Wrights, Westminster, U.K.). Excurrent air was dried and then passed through an oxygen analyzer at 1100 hours (Sevomex, Crowborough, Sussex, U.K.). We did not absorb the CO2 in the excurrent stream to maximize the precision of the measurement (39) when RQ is unknown. We maintained voles in the chamber for 3 h. The output of the analyzer was continuously logged at ≈20 Hz via an A to D card in a standard PC (Viglen, Middlesex, U.K.), and the average oxygen consumption was calculated at 30-sec intervals. Data were processed by using customized software, and RMRt was defined as the lowest 10 consecutive readings equivalent to 5 min in the chamber. Direct observations of the voles and inspections of the traces revealed that most voles settled down within about an hour of entry to the chamber. Voles were released back to the field within 1 h of RMRt measurement. RMRt measurements of four voles that did not settle down to rest within an hour from initial entry to the respirometer were rejected.
Blood samples were distilled by using the pipette method of Nagy (40). Mass spectrometric analysis of deuterium enrichment was performed by using H2 gas, produced from the distilled water after reaction with LiAlH4 (41, 42). For analysis of 18O enrichment, distilled water was equilibrated with CO2 gas by using the small sample equilibration technique (43). 2H:1H and 18O:16O ratios were measured by using dual-inlet gas source isotope ratio mass spectrometry (Optima IR MS, Micromass, Manchester, U.K.), with isotopically characterized gases of H2 and CO2 (chemically pure grade gasses from British Oxygen, Guildford, U.K.) in the reference channels. Reference gasses were characterized every 3 months relative to SMOW and SLAP supplied by the International Atomic Energy Agency. Each batch of samples was run with triplicates of three laboratory standards to correct for day-to-day variation in performance of the mass spectrometers. All isotope enrichments were measured in δ (‰) relative to the working standards and converted to ppm, using the established ratios for these reference materials. Measures of isotope enrichment were based on independent analysis of two subsamples of the water distilled from the blood samples. We estimated CO2 production by using the single pool deuterium equation from ref. 44. The error in individual estimates was determined by using the iterative procedures outlined in ref. 45. Conversion to DEE was made by using an assumed respiratory quotient of 0.8. All calculations were made by using natureware dlw software (available from the corresponding author).
Statistical Analysis. We tested for normality in the distributions of all variables included in the analysis by the Anderson–Darling test and transformed them accordingly when not normally distributed. Linear least-square models were fitted with the lm-function in s-plus (Mathsoft, Cambridge, MA). Model selection was based on the lowest AIC (Akaike's Information Criterion), which measures the tradeoff between simple models (high precision but also high bias in the predicted values) and complex models (low bias but also low precision). Among a set of candidate models, the model with the lowest AIC is most likely to give predictions that are closest to the truth. The model fits were assessed by diagnostics plots (normality and homogeneity of residuals), and we checked for influential data points by examining Cook's distances.
Results
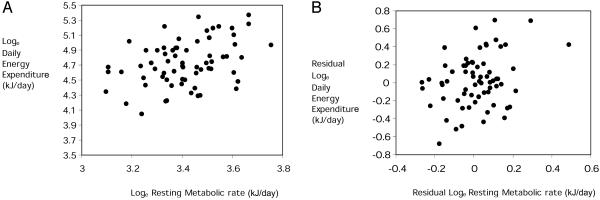
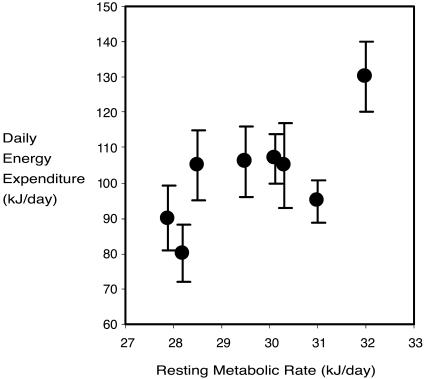
Measurements of RMRt and DEE were made on a total of 75 individual voles. To our knowledge, this is the largest sample of dlw measurements made on a single species at a restricted time of year (late winter, prebreeding) to date. Body mass, RMRts, and DEEs were all not normally distributed (Anderson–Darling tests) but had positive skew. We therefore normalized the variables by log-transformation. When pooling data across sites and sampling times there was a significant positive relationship between measurements of Loge RMRt and Loge DEE (Fig. 1A). The least-squares fit linear regression explained 14.7% of the variation in DEE (F1,74 = 11.22, P < 0.001). This positive association could reflect the fact that both traits were significantly related to body mass. We therefore removed the effect of body mass by calculating residuals to the fitted regressions of both Log-converted traits to Loge body mass. The relationship between residual Loge RMRt and residual Loge DEE remained significant when the effect of body mass on the two traits was removed (Fig. 1B). The least-squares fit linear regression explained 9.7% of the variation in DEE (F1,74 = 6.42, P = 0.026). However, when the influence of both sampling site and sampling date, in addition to the effect of body mass, were accounted for, the RMRt and DEE were no longer significantly related (Table 2; model = site × date + BM, where BM is body mass). Consequently, the correlation between measurements of mass-independent RMRt and DEE could be accounted for by correlated effects of site and sampling time on both RMRt and DEE, rather than individual differences within each site and sampling occasion. Confirming this fact, when we used the average values of the energetics traits measured at each site and sampling time, there was a significant association between mass-adjusted DEE and mass-adjusted RMRt (Fig. 2). The least-squares fit regression on the averaged values across sites and sampling times explained 56.5% of the variation in DEE (F1,7 = 7.69, P = 0.037).
Fig. 1.
The relationships between Loge DEE and Loge RMRt (A) and residual Loge DEE and residual Loge RMRt (B) across all of the sampled individuals. In both cases, there was a significant positive correlation between the two traits, even though in B the shared variation due to body mass was removed.
Table 2. Correlations between residual RMRt and DEE from various models.
| Predictors in models* | No. of parameters | AIC response = log10 (RMRt) | AIC response = log10 (DEE) | Correlation between residuals (95% C.I.)† | t H0: zero correlation | P value |
|---|---|---|---|---|---|---|
| Intercept only | 1 | +25.4 | +7.4 | 0.34 [0.12, 0.59] | 3.06 | 0.0031 |
| BM‡ | 2 | +0.2 | +2.2 | 0.26 [0.03, 0.51] | 2.28 | 0.026 |
| Site | 4 | +30.6 | +9.9 | 0.37 [0.15, 0.62] | 3.31 | 0.0014 |
| Site + BM | 5 | +3.6 | +3.0 | 0.26 [0.03, 0.51] | 2.29 | 0.025 |
| Site × date§ | 8 | +28.1 | +7.1 | 0.28 [0.06, 0.53] | 2.48 | 0.015 |
| Site × date + BM¶ | 9 | 0.0 | 0.0 | 0.16 [-0.07, 0.40] | 1.37 | 0.18 |
Given are Pearson correlation coefficients between residual RMR and residual DEE from models with the same predictors. Also given are the models' AIC values for each of the two response variables. The AIC model selection criterion ranks the models according to their predictive ability (models with lower values give better predictions; see Materials and Methods). The values given are the differences from the model with the lowest value (i.e., site × date + BM for both response variables). A difference in AIC of less than one unit is considered unimportant. [We have added 95% confidence interval (C.I.) because this is relevant for the discussion on insufficient power to detect within-site correlations.]
All models include intercept.
Confidence intervals calculated by using Fisher's transformation (46).
BM is body mass.
Site-specific intercept and slope on date effect.
Interaction effect on loge(RMR): F3, 145 = 3.66, P = 0.01. Interaction effect on loge(DEE): F3,74 = 3.33, P = 0.02.
Fig. 2.
Mean DEE plotted against mean RMRt within sampling groups (site and times) standardized for body mass (adjusted to the marginal mean of 20.2 g). The linear correlation was significant at P = 0.037 (F1,6 = 7.15). Error bars represent standard deviations.
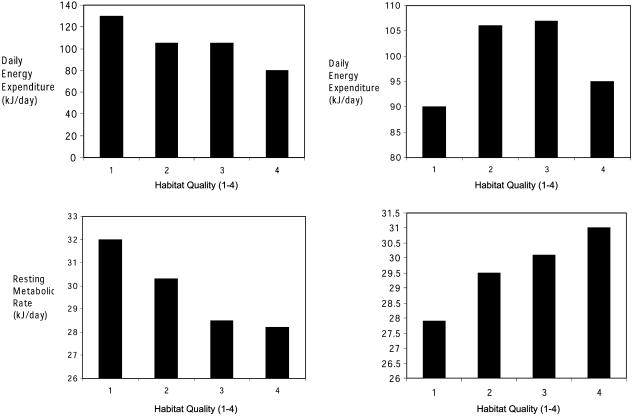
We plotted the average mass-adjusted DEE and RMRt at each site for the two sampling periods with the sites ranked in order of increasing quality defined from the site-specific survivorship and the time of onset of breeding (Table 1). At the earlier sampling time (February), both average RMRt and average DEE decreased as site quality increased (Fig. 3). The highest RMRt and DEE were observed at site A. Voles at this site also had lower body masses, poorer survival, and later onset of reproduction than voles at the other sites (Table 1). This suggests that the between-site variation in energy expenditure at this early sampling time was due to the fact that voles were forced to cope with the harsh environment rather than enabled by the good environment. In the second sampling period, this pattern changed (Fig. 3). Change in DEE between the two sampling periods within each site probably reflected both changes in ambient temperature and the fact that some animals late in the sampling period were preparing to breed, although none of the voles were palpably pregnant or lactating when measured (some females in sites B and D had perforate vagina at the last sampling occasion, although none of these had significantly increased body mass). During the second sampling period (March), the sites where breeding started earliest (site B, Table 1) had higher average energy expenditures (especially RMRts), whereas at site A, where voles started to breed ≈3 weeks later, the animals had lower average DEEs and RMRts. This pattern is more consistent with the environment enabling higher rates of metabolism.
Fig. 3.
Fitted predictions of DEE (Upper) and RMRt (Lower) both adjusted for body mass in the first (Left, February) and second (Right, March) sampling periods. The predictions were obtained from the best statistical models selected by the lowest AIC (Table 2). Sites are ranked by their quality by using the criteria in Table 1, indicated by 1–4 on the abscissa of each plot, where 1 is the poorest and 4 is the best (1 = A, 2 = C, 3 = D, and 4 = B).
Discussion
Our study provides a demonstration in a free-living animal that mass-independent RMRt and DEE are positively correlated (Fig. 1B). One possibility is that the association between RMRt and DEE was an artifact that occurred because the voles were held in captivity for relatively short periods of time for measurements of their RMRt. One might imagine that during this period differences in stress might have affected the individual measurements of RMRt. An association between RMRt and DEE might therefore arise because the least stressed individuals settled more during their RMRt measurements and were also less affected by the dlw procedures. This interpretation seems unlikely, however, for three reasons. First, such an influence on both dlw and RMRt would be likely to mask any intersite differences and enhance within-site effects. The fact that withinsite effects were trivial compared with between-site differences strongly suggests that stress effects on individuals did not cause the association. Second, there was no direct indication that voles were stressed during the RMRt measurements. Almost all of the voles settled down to sleep in the respirometer within about an hour after initial entry and were not continuously active as might be anticipated for animals under capture stress. The four individuals that did not settle down and were active throughout the measurement period had their RMRt measurements rejected (see Materials and Methods). Finally, previous field studies have been unable to establish any negative effects of the dlw methodology on the behavior and activity patterns of subject animals, indicating that stress effects of the dlw method are relatively low (44). It seems probable, therefore, that the present result is not an artifact of the methods used.
We predicted that if the anticipated relationship between RMRt and DEE was a reflection of intrinsic limitations, then the association would be evident within sites but that large differences between sites would not be apparent. We found that the significant relationship occurred because both DEE and RMRt varied in the same manner across the sites and sampling times, rather than reflecting individual level associations within the sites and sampling occasions. Hence, our results support the notion that variation in RMRt and DEE is influenced more by extrinsic factors than by intrinsic aspects of the physiology of the individuals. The absence of significant relationships between DEE and RMRt within sites matches the observation by Meerlo et al. (29) in the same species that DEE and RMRt were not significantly associated within a single study site.
We also addressed the second issue: whether extrinsic factors operate on levels of DEE by high levels of resource availability enabling them or because poor environmental conditions force large expenditures of energy by animals attempting to cope with them. Defining the quality of an environment is notoriously difficult because resource availability may depend on a myriad of factors and the energy demands of living in a given location are similarly complex. Rather than attempting to quantify these traits directly, we instead used an empirical definition of habitat quality. The poor sites were those where voles had lower body masses, slower growth rates in spring, lower survival rates, and delayed onset of reproduction. If resource availability enables high levels of DEE, we would predict that voles in the good habitats would have high rates of expenditure, whereas voles in poor habitats would not be so enabled. In contrast, if animals were forced to cope with the poor habitats, the opposite trends would be apparent.
In our data, we observed both trends across the sites at the two different sampling times. Early in the sampling period, both average RMRt and average DEE were negatively related to average body mass across sites, even though there was a positive relation within sites. The site with the smallest average body mass and the highest average RMRt and DEE during mid-winter (site A) also had poorer survival, lower growth rates, and later onset of spring reproduction than the other sites (Table 1 and ref. 36). This finding suggest that the environmental conditions at this site were harsher and energetically more costly, and that the increase in energy expenditure was forced by a poor environment rather than enabled by a good environment. Similar patterns were observed in blue tits, where birds breeding at times when resource availability was not yet elevated had high rates of energy expenditure and low survival (31). In contrast to the pattern early in our sampling period, the average RMRt and DEE at the end of the sampling period was positively correlated with average body mass across sites, as would be expected from the within-site correlation. At the sites with highest average body mass (sites B and D), spring reproduction started shortly after the last sampling occasion (Table 1). This pattern is more consistent with the hypothesis that a good environment enables a higher energy expenditure, in this case favoring faster growth and earlier reproduction. This situation is more similar to the differences in RMRt observed between species in the genus Peromyscus that were also positively linked to environmental productivity (32). It seems likely that the effect of the environment on energetics may encompass both modes of action under different circumstances. Because we used an empirical definition of habitat quality based on responses of the animals to that environment, it remains uncertain exactly what features of the environment stimulate these effects.
Acknowledgments
J.R.S. was supported by a Royal Society Leverhulme Senior Research Fellowship, and X.L. was supported by a Leverhulme Research Fellowship. This work was funded by the Natural Environmental Research Council (X.L. and J.R.S.) and the Norwegian Research Council (T.E.). We thank the Forestry Commission for permission to work on their land.
Abbreviations: AIC, Akaike's Information Criterion; BMR, basal metabolic rate; DEE, daily energy expenditure; RMRt, resting metabolic rate at thermoneutral.
References
- 1.Kleiber, M. (1961) The Fire of Life: An Introduction to Animal Energetics (Wiley, New York).
- 2.Brody, S. (1945) Bioenergetics and Growth (Reinhold, New York).
- 3.McNab, B. K. (2000) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 127, 309–329. [DOI] [PubMed] [Google Scholar]
- 4.McNab, B. K. (1988) Oecologia 77, 343–349. [DOI] [PubMed] [Google Scholar]
- 5.Harvey, P. H., Pagel, M. D. & Rees, J. A. (1991) Am. Nat. 137, 556–566. [Google Scholar]
- 6.Earle, M. & Lavigne, D. M. (1990) Can. J. Zool. 68, 381–388. [Google Scholar]
- 7.Hayes, J. P., Garland, T. & Dohm, G. L. (1992) Funct. Ecol. 6, 5–14. [Google Scholar]
- 8.Derting, T. L. & McClure, P. A. (1989) J. Mammal. 70, 520–531. [Google Scholar]
- 9.Johnson, M. S., Thomson, S. C. & Speakman, J. R. (2001) J. Exp. Biol. 204, 1937–1946. [DOI] [PubMed] [Google Scholar]
- 10.Jackson, D. M., Trayhurn, P. & Speakman, J. R. (2001) J. Anim. Ecol. 70, 633–640. [Google Scholar]
- 11.Drent, R. & Daan, S. (1980) Ardea 68, 225–252. [Google Scholar]
- 12.Nagy, K. A., Girard, I. A. & Brown, T. K. (1999) Annu. Rev. Nutr. 19, 247–277. [DOI] [PubMed] [Google Scholar]
- 13.Ricklefs, R. E., Konarzewiski, M. & Daan, S. (1996) Am. Nat. 147, 1047–1071. [Google Scholar]
- 14.Speakman, J. R. (2000) Adv. Ecol. Res. 30, 177–297. [Google Scholar]
- 15.Lagos, V. O., Bozinovic, F. & Contreras, L. C. (1995) J. Mammal. 76, 900–905. [Google Scholar]
- 16.Gadgil, M. & Bossert, W. H. (1970) Am. Nat. 104, 1–24. [Google Scholar]
- 17.Weiner, J. (1992) Trends Ecol. Evol. 7, 384–388. [DOI] [PubMed] [Google Scholar]
- 18.Hammond, K. A. & Diamond, J. (1997) Nature 386, 457–462. [DOI] [PubMed] [Google Scholar]
- 19.Peterson, C. C., Nagy, K. A. & Diamond, J. (1990) Proc. Natl. Acad. Sci. USA 87, 2324–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammond, K. A. & Diamond, J. (1994) Physiol. Zool. 67, 282–303. [Google Scholar]
- 21.Hammond, K. A. & Diamond, J. (1992) Physiol. Zool. 65, 952–977. [Google Scholar]
- 22.Hammond, K. A. & Kristan, D. M. (2000) Physiol. Biochem. Zool. 73, 547–556. [DOI] [PubMed] [Google Scholar]
- 23.Hammond, K. A. & Janes, D. N. (1998) J. Exp. Biol. 201, 2081–2090. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, M. S., Thomson, S. C. & Speakman, J. R. (2001) J. Exp. Biol. 204, 1925–1935. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, M. S., Thomson, S. C. & Speakman, J. R. (2001) J. Exp. Biol. 204, 1947–1956. [DOI] [PubMed] [Google Scholar]
- 26.Speakman, J. R., Gidney, A., Bett, J., Mitchell, I. P. & Johnson, M. S. (2001) J. Exp. Biol. 204, 1957–1965. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, M. S. & Speakman, J. R. (2001) J. Exp. Biol. 204, 1967–1977. [DOI] [PubMed] [Google Scholar]
- 28.Rogowitz, G. L. (1998) Physiol. Zool. 71, 312–320. [DOI] [PubMed] [Google Scholar]
- 29.Meerlo, P., Bolle, L., Visser, G. H., Masman, D. & Daan, S. (1997) Physiol. Zool. 70, 362–369. [DOI] [PubMed] [Google Scholar]
- 30.Tinbergen, J. M. & Verhulst, S. (2000) J. Anim. Ecol. 69, 323–334. [Google Scholar]
- 31.Thomas, D. W., Blondel, J., Perret, P., Lambrechts, M. M. & Speakman, J. R. (2001) Science 291, 2598–2600. [DOI] [PubMed] [Google Scholar]
- 32.Mueller, P. & Diamond, J. (2001) Proc. Natl. Acad. Sci. USA 98, 12550–12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoener, T. W. (1971) Annu. Rev. Ecol. Syst. 2, 369–404. [Google Scholar]
- 34.Lambin, X., Petty, S. J. & MacKinnon, J. L. (2000) J. Anim. Ecol. 69, 106–118. [Google Scholar]
- 35.Lambin, X., Elston, D. A., Petty, S. J. & MacKinnon, J. L. (1998) Proc. R. Soc. London Ser. B 265, 1491–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ergon, T., Lambin, X. & Stenseth, N. C. (2001) Nature 411, 1043–1045. [DOI] [PubMed] [Google Scholar]
- 37.Hayes, J. P., Speakman, J. R. & Racey, P. A. (1992) Physiol. Zool. 65, 604–619. [Google Scholar]
- 38.Hayes, J. P., Speakman, J. R. & Racey, P. A. (1992) Physiol. Zool. 65, 742–762. [Google Scholar]
- 39.Koteja, P. (1996) Funct. Ecol. 10, 675–677. [Google Scholar]
- 40.Nagy, K. A. (1983) The Doubly-Labelled Water (3HH18O) Method: A Guide to Its Use (Univ. of California, Los Angeles, publication 12-1417).
- 41.Ward, S., Scantlebury, M., Krol, E., Thomson, P. J., Sparling, C. & Speakman, J. R. (2000) Rapid Comm. Mass Spectrom. 14, 450–453. [DOI] [PubMed] [Google Scholar]
- 42.Krol, E. & Speakman, J. R. (1999) J. Exp. Biol. 202, 2839–2849. [DOI] [PubMed] [Google Scholar]
- 43.Speakman, J. R., Nagy, K. A., Masman, D., Mook, W. G., Poppitt, S. D., Strathearn, G. E. & Racey, P. A. (1990) Anal. Chem. 62, 703–708. [Google Scholar]
- 44.Speakman, J. R. (1997) Doubly-Labelled Water: Theory and Practice (Chapman & Hall, London).
- 45.Speakman, J. R. (1995) Obesity Res. 3, 31–39. [DOI] [PubMed] [Google Scholar]
- 46.Zar, J. H. (1984) Biostatistical Analysis (Prentice–Hall, Englewood Cliffs, NJ), 2nd Ed., p. 310.