Abstract
Background
Methamphetamine (MA)-dependent individuals prefer smaller immediate over larger delayed rewards in delay discounting (DD) tasks. Human and animal data implicate ventral (amygdala, ventral striatum, ventrolateral prefrontal cortex insula) and dorsal (dorsolateral prefrontal cortex, dorsal anterior cingulate cortex and posterior parietal cortex) systems in DD decisions. The ventral system is hypothesized to respond to the salience and immediacy of rewards while the dorsal system is implicated in the process of comparison and choice.
Methods
We used functional Magnetic Resonance Imaging to probe the neural correlates of DD in 19 recently abstinent MA-dependent patients and 17 age- and gender-matched controls.
Results
Hard DD choices were associated with greatest activation in bilateral middle cingulate, posterior parietal cortex (PPC), and the right rostral insula. Control subjects showed more activation than MA patients bilaterally in the precuneus and in the right caudate nucleus, anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC). Magnitude of discounting was correlated with activity in the amygdala, DLPFC, posterior cingulate cortex and PPC.
Conclusions
Our findings were consistent with a model wherein dorsal cognitive systems modulate the neural response of ventral regions. Patients addicted to MA, who strongly prefer smaller immediate over larger delayed rewards, activate the dorsal cognitive control system in order to overcome their preference. Activation of the amygdala during choice of delayed rewards was associated with a greater degree of discounting, suggesting that heavily discounting MA-dependent individuals may be more responsive to the negative salience of delayed rewards than controls.
Keywords: Methamphetamine, Delay discounting, Brain imaging
Introduction
Delay discounting (DD) is a measure of impulsivity that has been used extensively in drug dependent populations (Monterosso and Ainslie 1999). DD characterizes impulsivity as the relative preference for smaller immediate rewards over larger but delayed rewards (Ainslie 1974; Rachlin and Green 1972). Numerous studies have shown that people addicted to drugs devalue or discount future rewards more than non-users; that is, drug addicted individuals are willing to settle for relatively smaller immediate gratification rather than wait for a larger amount. Relative to controls, heightened DD has been demonstrated in users of tobacco (Baker et al. 2003; Bickel et al. 1999; Mitchell 1999), alcohol (Petry et al. 2002), heroin (Kirby et al. 1999; Odum et al. 2000), cocaine (Coffey et al. 2003; Monterosso et al. 2001), and methamphetamine [MA] (Hoffman et al. 2006; Monterosso et al. 2007), also see Reynolds (2006), and Bickel and Marsch (2001) for reviews.
The neuroanatomical substrates of DD can be understood in terms of a model proposed by Ernst and Paulus (2005) in which two separate neural systems process different aspects of the decision and its outcome. Anatomical regions associated with the “cognitive” circuit include the dorsal anterior cingulate cortex (dACC), dorsolateral prefrontal cortex (DLPFC), posterior parietal cortex (PPC) and the superior temporal gyrus (STG), while the “affective” circuit includes the amygdala, ventral striatum, ventrolateral prefrontal cortex (VLPFC), the ventral ACC and the anterior insula. The affective system is hypothesized to be particularly sensitive to the intrinsic salience of a stimulus, while the cognitive system, partly through a working memory mechanism, is necessary for the comparison of alternatives and assessment of (sometimes hypothetical) outcomes. Impulsivity in patients suffering from addiction has been hypothesized to be related to both overvaluation of immediate rewards and difficulty reflecting on the future consequences of a decision (Bechara 2005; Ernst and Paulus 2005).
Two studies in normal controls reported findings consistent with more involvement of the cognitive system with the choice of delayed rewards. Wittmann et al. (2007) reported greater activation associated with choice of delayed than immediate rewards in posterior insula bilaterally and left sided regions in the posterior cingulate cortex (PCC), STG and PPC (angular gyrus [AG] and inferior parietal lobule [IPL]). McClure et al. investigated a DD task variant and reported that trials including the possibility of immediate reward produced relatively greater activation of ventral striatum and orbitofrontal cortex (OFC), while trials that offered a choice between two delayed rewards activated PCC and DLPFC.
Monterosso et al. (2007) studied DD in non-treatment-seeking MA-dependent individuals and non-dependent controls. They did not detect activation in limbic regions, but found evidence of activation in both cognitive and affective systems. A contrast of DD trials vs. ‘no choice’ trials demonstrated bilateral activation in PPC, ACC/supplementary motor area (SMA) and both ventrolateral prefrontal cortex (VLPFC), DLPFC, and left rostral insula. When they compared the MA and control groups, they found significant clusters in the left DLPFC but only in a hard choice (near the indifference curve, where subjects have equal preference for immediate and delayed rewards) versus easy choice (far from the indifference curve) contrast. Interestingly, Boettiger et al. (2007), in a study that indirectly compared alcohol-dependent patients and controls, found that activity in DLPFC, PPC, and rostral parahippocampal gyrus correlated with preference for immediate rewards and a region in the ventrolateral OFC correlated with a preference for delayed rewards.
These data are consistent with the idea that substance-abusing individuals discount more heavily (prefer smaller immediate over larger later rewards) due to increased activity in the affective circuit and decreased activity in the cognitive circuit. The current study tested this model by examining the cortical and subcortical activity associated with DD in patients recently (2 to 8 weeks) abstinent from MA and in a matched group of normal controls. These subjects should be free from the acute effects of MA intoxication, but not yet recovered from the consequences of longer-term dependence (Volkow et al. 2001). We hypothesized that, in the combined groups, DD choices would activate DLPFC, OFC, and posterior parietal cortex (PPC) more than control choices. In addition, we expected that choices of immediate vs. delayed rewards would activate ventral striatum and sub-genual ACC. Finally, we hypothesized that increased discounting in the MA group relative to the control group would be reflected in increased activity in the affective circuit due to overvaluation of immediate rewards and decreased activity in the cognitive circuit due to difficulty making comparisons.
Materials and methods
Participants
Control subjects (n=22) were recruited from the Portland Veterans Affairs Medical Center and the general public. Subjects in the MA group (n=20) were recruited from treatment programs in Portland, OR and had been abstinent from all drugs for at least 2 weeks and not longer than 8 weeks. Six subjects (five controls and one MA) were excluded because of unusable MRI data for a final count of 17 controls and 19 MA patients. Subjects were excluded due to excessive motion [>1 mm or >1° in any direction] (three), technical defects (two) or because subjects could not complete the scan (one). MA-dependent subjects reported use of at least 0.5 g of MA per day, at least 5 days a week for the past year. All participants provided informed consent (approved by the Portland Veterans Affairs Medical Center (PVAMC) Institutional Review Board) at the time of enrollment and were given a copy of the consent form, which outlined all procedures involved in participation. Exclusion criteria for either group included current drug use, incompatibility with MRI (due to metal implants of any kind, pregnancy, welding history, etc.), physical health issues (active hepatitis B or C, diabetes, heart disease, HIV, asthma, tuberculosis, epilepsy/seizures, renal dysfunction), or significant psychiatric problems (present/past DSM-IVTR [American Psychiatric Association, 2000] diagnoses of schizophrenia, bipolar affective disorder, attention deficit hyperactivity disorder, major depression, obsessive compulsive disorder). Each MA participant was interviewed and diagnosed using the Structured Clinical Interview for DSM-IV (SCID) [Multi Health Systems, North Tonawanda, NY, 1998] in order to establish the diagnosis of MA dependence and confirm the absence of disqualifying psychiatric disorders. Controls were interviewed with the SCID-NP. A urine drug screen was performed on every participant at the time of evaluation. No subject tested positive for any drug.
The groups did not differ in age (controls, 36.7±9.9 years, MA, 34.8±10.0 years; t(34)=0.65, NS(gender) (controls, 29% female), MA, 32% female) handedness (controls, 12% left, MA, 11% left), or race (controls, 100% Caucasian, MA, 85% Caucasian). The MA subjects had used (mean±SD) 1.2±1.1 g per day for 5.1±5.0 years and had been abstinent 48±17 days at the time of evaluation. More MA subjects than controls were smokers (75% vs. 25%). Subjects were not required to abstain from smoking for the study.
DD task
Participants performed a forced-choice block design task in the scanner comprised of eight DD blocks alternated with eight magnitude estimation (ME) control blocks. Each block contained ten trials for a total of 80 ME and 80 DD trials per run for two runs. The blocks were separated by a 16-s fixation interval. DD trials presented the subject with two choices: $100 after a delay between 0 and 365 days and an immediate reward from $1 to $99 (e.g., $100 in 12 days vs. $80 now). The set of choice pairs was generated so that the response space was evenly sampled. One hundred sixty immediate and delayed reward pairs were presented to each subject in pseudorandom sequence. Immediate and delayed rewards were randomly presented on the left or right. The ME trials consisted of two choices in which either the delay or reward was held constant (e.g., $50 in 12 days vs. $50 in 65 days, $50 in 12 days vs. $75 in 12 days). The ME task served as a control condition requiring similar scanning movement of the eyes, digital manipulation of the button box and similar lexical demand as the DD trials. Participants had 4 s to respond on each trial before the next was presented. Subjects indicated their choice by pressing buttons with the index and middle fingers of the right hand on an MR-compatible button box (Harvard Medical Systems, Holliston, MA). Stimuli were projected onto a screen located at the back of the bore of the magnet. Subjects read the choices by looking into a mirror affixed to the top of the head coil.
The responses on the DD task are characterized by a function that divides the stimulus space into choices of immediate versus delayed reward. Respondents prefer the immediate reward when its value is high or the delay is long but prefer the delayed reward when the immediate value is low or the delay short. For a given delay, the indifference point is defined as the value at which the preference switches between the immediate and delayed reward. The function that best fits these points is termed the indifference curve. This function is reasonably well represented by a hyperbolic equation (Green et al. 1997; Johnson and Bickel 2002):
| (1) |
where v(x) is the value of the immediate reward at the indifference point, M is the maximum dollar amount (in this case, $100), x is the delay time and Kn and n (both >0) are adjustable parameters. The most common implementation of Eq. 1 is with n=1. In this single parameter case, K1 indexes the rate at which the value of M is discounted as a function of delay. A participant is said to be more impulsive the steeper the gradient (larger K1) of the discounting function (Rachlin et al. 1991).
MRI
Imaging data were acquired on a 3 tesla (T) Siemens Trio MRI scanner. A localizer scan was acquired in order to guide slice alignment during the functional scans. Two T2*-weighted echo-planar imaging (EPI) functional runs were acquired (24 slices, 4 mm thick, gap width=1 mm, TR/TE/α = 2,000 ms/35 ms/80°, matrix = 128 × 128, FOV = 240 × 240 mm, 420 volumes per run) with an in-plane pixel size of 1.875 mm2. Finally, a high-resolution T1-weighted anatomical Magnetization-Prepared Rapid Gradient Echo [MPRAGE] (144 slices 1 mm thick, TR/TE/TI/α = 2,300 ms/4.38 ms/1,200 ms/12°, FOV = 208 × 256 mm) was acquired for co-registration with functional images and for statistical overlays.
Data analysis
All statistical calculations not performed by image analysis programs were performed with SYSTAT 11 (Point Richmond, CA; http://www.systat.com).
Analysis of the discount function
Choices made near the indifference curve (Eq. 1) demand more consideration (i.e., have longer reaction times) and are considered “hard” choices, whereas choices far from the indifference curve represent “easy” choices. The “best” indifference curve was determined by choice of the parame-ters, Kn and n, that maximized ϕ, the point biserial correlation between the actual and predicted choices. The choice of Kn and n was determined by a seeded iterative procedure, where n varied from 0 to ~2 in increments of ~0.003, and Kn was varied from 0 to ~0.4 in increments of ~0.0001. For each iteration, a 2 × 2 table was constructed between the actual choice (immediate or delayed) and the decision predicted by Eq. 1. The pair of parameters that gave the overall highest value of ϕ was used to determine hard vs. easy choices as described below. In general, the version of Eq. 1 with n ≠ 1 provided the maximum ϕ. The best value of K for the special case of n = 1, K1, was used for correlation with brain activity and comparison with past results.
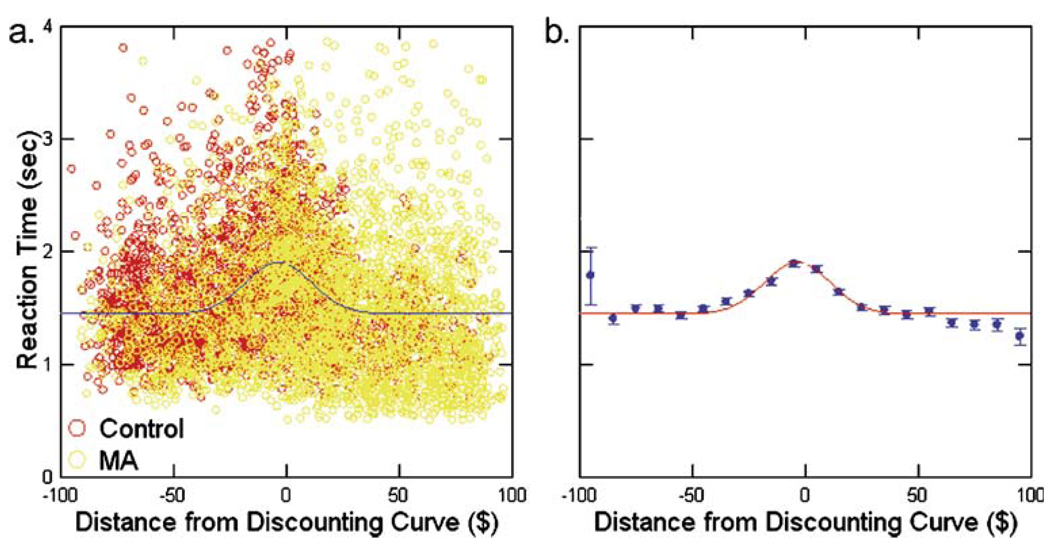
Examination of reaction time as a function of distance from the calculated indifference curve revealed that, although there was considerable variability in reaction time at every distance, there was a peak near the indifference curve. The raw data are shown in Fig. 1a and the means (±SEM) of bins $10 wide are plotted in Fig. 1b. The data were fit to a Gaussian function of the form,
| (2) |
where RT is the reaction time, A0 is the peak height of the function above baseline, d is the distance from the discounting curve, μ is the location of the peak and S is a measure of the width of the function. The non-linear regression yielded values of A0=0.461±0.021, μ=−3.093±0.767, C = 1.439±0.012, and S = 15.01±0.94. We used these results to choose ±$15 as the region near the indifference curve where the decisions were ‘hard’. All decisions outside this region were classified as ‘easy’. This choice represents choices that fall in the central region of the function that accounts for 66% of the area under the curve above baseline. This procedure classified 31% of the control responses and 26% of the MA responses as hard choices.
Fig. 1.
Distribution of reaction times to DD choices as a function of distance from the discounting curve. a Shows the distribution of the raw reaction times and b that of the means in bins $10 wide. The non-linear regression fit of Eq. 2 to the mean values was good (corrected R2=0.661)
Image analysis
Analysis of functional and anatomical MRI data was performed using Analysis of Functional NeuroImages [AFNI; Cox and Hyde 1997]. Functional scans were motion corrected, corrected for slice scan time, spatially smoothed with a Gaussian kernel (full width at half maximum [FWHM] = 4 mm), detrended by fitting a linear model and temporally smoothed with a high-pass filter (three cycles per time course). The functional data were then co-registered to the MPRAGE and transformed into a standard space (Talairach and Tournoux 1988). Within-subjects regressions were performed using five conditions: ME, DD-immediate-choice—hard (DDih), DD-immediate-choice—easy (DDie), DD-delayed-choice—hard (DDdh), and DD-delayedchoice—easy (DDde). Within-subjects predictors were constructed by convolving a linear combination of two gamma functions, which approximates the hemodynamic response in the brain to a stimulus, with a binary variable that was 0 for an acquisition without that trial type and a 1 for an acquisition with that trial type (Glover 1999). Time series were corrected for autocorrelation before regression.
Within-subject maps were generated with 3dDeconvolve, which associates a regression coefficient with each condition. Contrasts on the within-subject variables (i.e., conditions) were calculated as differences between the mean regression coefficients for the effects of interest. For the combined groups random effects analysis 3dANOVA2 was used, with five within-subject levels (conditions) and 36 between-subject levels (subjects). This map was corrected for multiple comparisons using 3dFDR [false discovery rate; Benjamini and Hochberg 1995] and a Monte Carlo cluster threshold estimation (7.1 voxel minimum cluster size). Because the easy and hard trials are orthogonal, they have unique predictor time courses and therefore yield unique parameter estimates for each of these conditions.
For between-group and covariate [ln(K1)] analyses, the contrast of individual subject regression coefficients (DDdh+DDih−2ME, calculated as described above), were entered as dependent variables into 3dRegAna, which allows for random effects analyses of unbalanced models. The user specifies a full multiple linear regression model, which includes all of the variables of interest, and a reduced model with fewer variables. A statistical test is performed to determine if the full model accounts for statistically significantly more variance than the reduced model. Group membership was coded by a binary variable (±1) and Z-scores were calculated for ln(K1). The full regression model is,
| (3) |
where CDDh−ME is the balanced contrast between DDhard and ME (from the within-subjects general linear model [GLM]), and the γn are the estimated parameters in the multiple linear regression. Statistical maps were calculated in a hierarchical manner. Separate maps were calculated for each of the single variable equations containing only ln(K1) or Group. Maps were then calculated for the full model using each of the single variable reduced models. Minimum cluster sizes were generated using the Monte Carlo simulation method (Forman et al. 1995), which arrived at minimum cluster sizes of 6.9 voxels (ln(K1)) and 7.0 voxels (Group). The maps were then thresholded to p<0.001 (ln(K1) and Group, t=3.58, 3.539, respectively), and displayed on the high-resolution anatomical image.
Volumes of interest (VOIs) determined from the second level regression were subjected to further analysis to better characterize the responses of the regions identified by statistical maps of the significance of the associated second level regression coefficients. Within each VOI, the within-subject regression coefficients for each first level predictor were averaged over all voxels. These averaged coefficients were combined to form the contrast of interest, DDdh+DDih−2(ME), and then regressed against the between-subjects variables, ln(K1) and Group.
Results
Delay discounting
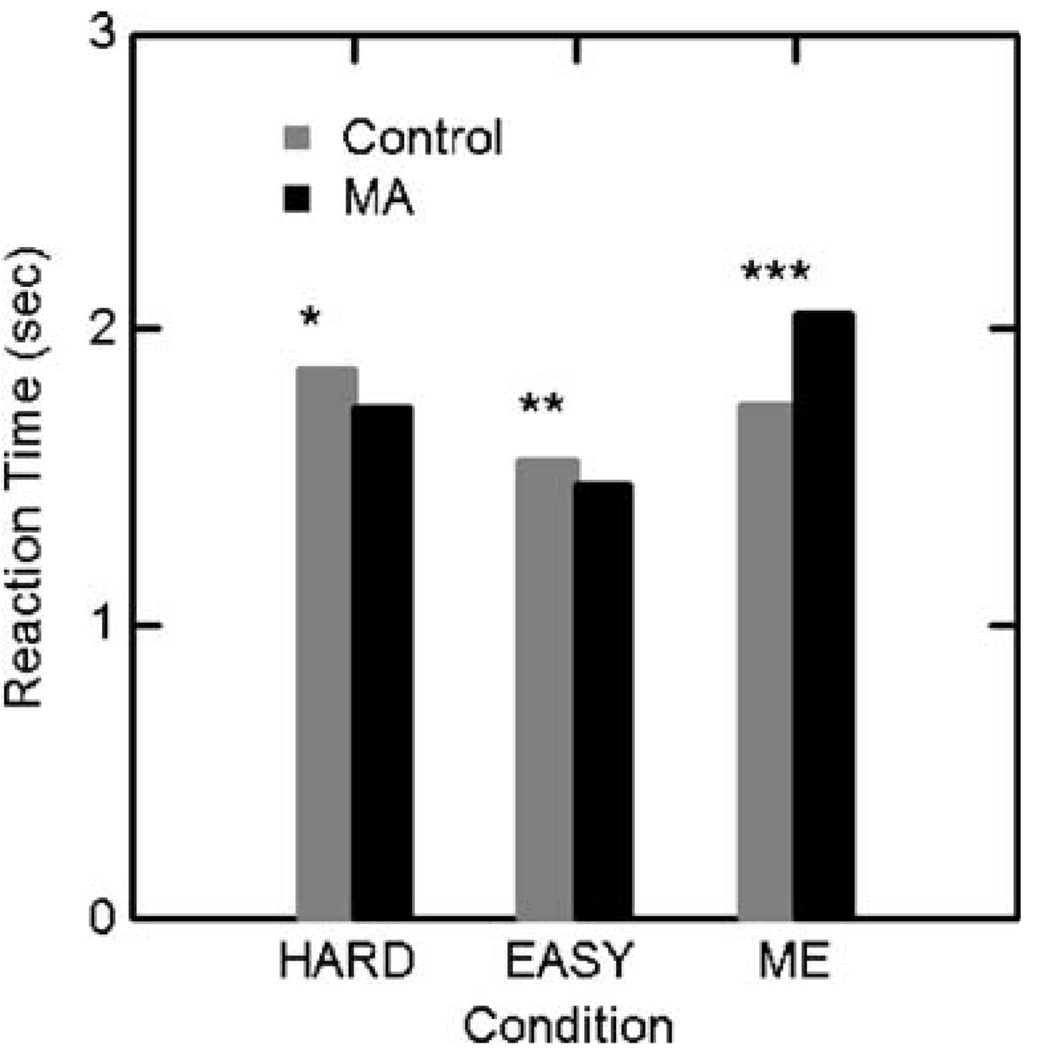
Reaction times for hard and easy choices and the ME control task followed the same pattern in both MA and control groups (Fig. 1 and Fig. 2). Hard choices took longer than easy choices in both groups and the groups did not differ in reaction times on the DD trials. MA subjects took 15% longer to respond to ME trials than controls. The proportion of hard to easy choices was similar in the two groups (31% [range 28% to 35%] in the control group and 26% [range 22% to 32%] in the MA group). The MA subjects, however, chose the immediate rewards substantially more often than the controls (65% vs. 40%, χ2=350, p<0.0001).
Fig. 2.
Reaction times (±SEM). Within groups (paired t test): *Control: Hard>Easy, p<0.01, MA: Hard>Easy, p<0.01; **Control: Easy<ME, p<0.01, MA: Easy<ME, p<0.001. Between groups (two sample t test): ***MA>Control (t(34)=2.591, p=0.015). The groups did not differ in reaction times on the Hard or Easy choices
Subjects in the MA group discounted more steeply than those in the control group; ln(K1) was significantly less negative in the MA patients (control: −5.95±1.10; MA: −4.01±1.92; t(34)=3.6, p<0.01). These values correspond to K1 of 0.0026 and 0.018 in the control and MA groups, respectively.
Functional MRI
Delay discounting vs. magnitude estimation: combined groups
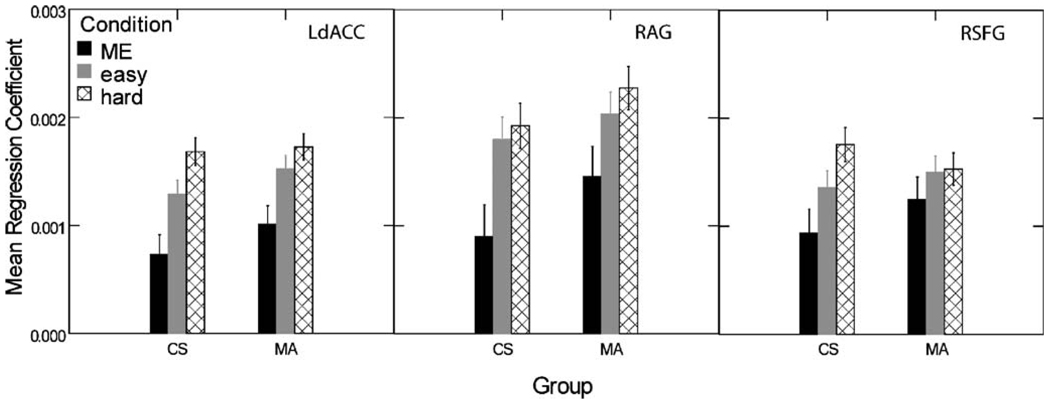
Examination of contrasts between DD trials categorized by difficulty (easy vs. hard) and ME control trials found a consistently greater contrast for hard vs. control than easy vs. control (Fig. 3). This is consistent with the reaction time data shown inFig. 2. Therefore, easy choice trials were omitted from the subsequent analyses and all contrasts between DD and the ME control condition used only responses on hard choices.
Fig. 3.
Mean regression coefficients calculated for three characteristic volumes of interest within the MA and control groups. The contrast between hard choices and ME is greater than that between easy choices and ME. Note also that the contrasts between hard choices and ME are smaller in the MA group than the control group. LdACC Left dorsal anterior cingulate cortex, RAG right angular gyrus, RSFG right superior frontal gyrus, ME magnitude estimation, MA methamphetamine group, CS control subject group
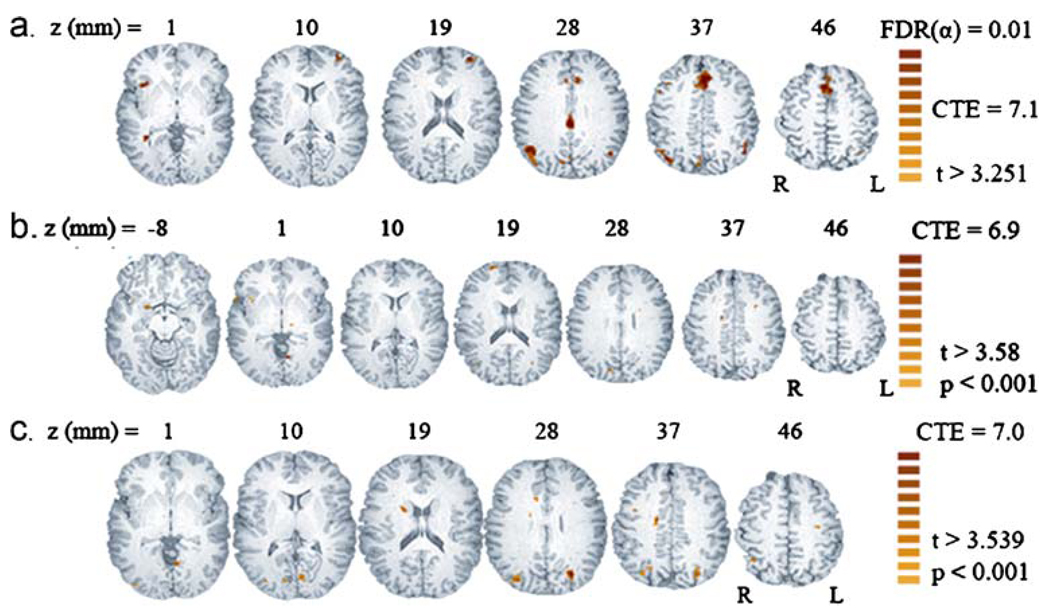
Contrast of DDhard vs. ME found 11 clusters with significantly greater signal during the decision-making than control trials (Table 1, Fig. 4a). The single unilateral volume was the right insular cortex. The largest bilateral area of activation appeared in the dorsal anterior cingulate cortex (dACC) and extended dorsally into the medial superior frontal gyrus (SFG). A second, smaller and more caudal, area of activation occurred in the posterior cingulate cortex (PCC). There were additional bilateral volumes of activation in V/DLPFC. In the parietal lobe, areas of activation appeared bilaterally: medially in the cunei and on the lateral surface in the angular gyri (AG). The latter cluster extended dorsally into the supramarginal gyrus (SMG) and the intraparietal sulcus (IPS).
Table 1.
Significant clusters for random effects analysis of DDh vs. ME (all subjects)
| Region | Brodmann area | X | Y | Z | Volume (mm3) |
|---|---|---|---|---|---|
| L dACC | 24 | 0.6 | −21 | 40.7 | 4,819 |
| R AG | 39 | −44.5 | −61.2 | 32.6 | 2,413 |
| R dACC | 24 | −0.5 | 29.2 | 29.8 | 1,173 |
| L SFG | 9 | −32.6 | 48.4 | 14.1 | 962 |
| L AG | 39 | 44.5 | −58 | 35.2 | 956 |
| R insula | 13 | −38.1 | −17.3 | 1.3 | 442 |
| R cuneus | 7 | −4.3 | 69.5 | 33.5 | 389 |
| R MFG | 9 | −42.7 | −14 | 38.8 | 297 |
| R SFG | 9 | −29.4 | −50.9 | 15.4 | 270 |
| R MTG | 22 | −35 | 44.2 | 3 | 211 |
| R MFG | 9 | −40.8 | −45.1 | 10.8 | 198 |
Cartesian coordinates refer to Talairach and Tournoux space using the RAI convention.
L Left, R right, AG angular gyrus, dACC dorsal anterior cingulate cortex, MFG middle frontal gyrus, MTG middle temporal gyrus, SFG superior frontal gyrus
Fig. 4.
Statistical parametric maps displayed on a high-resolution anatomical image (MPRAGE) for within- and between-subjects contrasts. FDR false detection rate. CTE cluster threshold estimate (voxels). a DD vs. ME random effects analysis for combined groups. The active regions are identified and described in Table 1. b and c show maps related to a between-subjects multiple regression of the form shown in Eq. 3. b Linear-regression-map of t-statistic for the regression coefficient of ln(K1). Active regions are identified and described in Table 2. Positive t scores are related to positive correlation between the contrast, DDhard vs. ME and ln(K1). c. Linear-regression-map of t-statistic for regression coefficient of the effect of Group membership. Active regions are identified and described in Table 3. Contrast between DDhard and ME is greater for controls vs. MA subjects in all regions
As there were four left (L)-handed subjects (two controls and two MA subjects), their individual maps were inspected visually and did not qualitatively differ from the right (R)-handed subjects (furthermore, a fixed effects GLM with two left handed and two right handed controls, and two left and two right handed MA was run; no main effect was found for left vs. right in any voxel for overall F or for any contrast).
MA group vs. control group
The effect of diagnostic group was examined with the between-subjects linear regression model given in Eq. 3. The statistical map for the effect (t-statistic for the regression coefficient in Eq. 3) of ln(K1) on the contrast between DDhard and ME identified primarily right-sided regions: right amygdala, SFG, MCC, putamen, PCC, and temporal pole (TP; Table 2, Fig. 4b). The map for the effect of group membership identified bilateral regions at the parieto-occipital junction, the right caudate head, right ACC and smaller, primarily right hemisphere volumes (Table 3, Fig. 4c). Examination of mean regression coefficients for each trial type within the two groups (Fig. 3) revealed that the MA users’ decreased contrast between DDhard and ME occurred partly because the patient group exhibited more activation during ME and DDeasy trials than control subjects. Group did not account for more variance than ln(K1) in any voxel, but ln(K1) accounted for more variance than Group in the areas of activation shown in Fig. 4b. Moreover, adding an interaction term (Group x ln(K1)) to the model did not increase the percent of variance explained, suggesting that the regression slopes are parallel for the MA-dependent and control groups.
Table 2.
Significant clusters for second level linear regression (Eq. 3): effect of ln(K1)
| Region | Brodmann area | X | Y | Z | Volume (mm3) |
|---|---|---|---|---|---|
| R AN | −21.4 | −1.3 | −7.3 | 158 | |
| R PPC | 22/39 | −41 | 67.2 | 24 | 152 |
| R SFG | 9 | −18.7 | −53.7 | 20.5 | 132 |
| L LG | 18 | 7.5 | 59.1 | 0.7 | 119 |
| R putamen | −23.9 | 6.1 | 5.4 | 86 | |
| L HC | 14.7 | 40.2 | 8 | 79 | |
| R dACC | 24 | −14.9 | 7.7 | 35.1 | 73 |
| R cuneus | 29 | −12.8 | 73.2 | 27.9 | 59 |
| R TP | 38 | −55.5 | −12.4 | −0.1 | 53 |
Cartesian coordinates refer to Talairach and Tournoux space using the RAI convention.
L Left, R right, AN amygdaloid nucleus, HC hippocampal formation (CA), LG lingual gyrus, dACC dorsal anterior cingulate cortex, PPC posterior parietal cortex, SFG superior frontal gyrus, TP temporal pole
Table 3.
Significant clusters for second level linear regression (Eq. 2): effect of group
| Region | Brodmann area | X | Y | Z | Volume (mm3) |
|---|---|---|---|---|---|
| R precuneus | 7 | −32 | 67.7 | 33.6 | 1,582 |
| L precuneus | 7 | 26.3 | 66.4 | 31 | 989 |
| R CN | −19.6 | 0.6 | 23 | 376 | |
| R ACC | 24 | −11 | −14.8 | 33.3 | 303 |
| L cuneus | 18 | 6.5 | 74.4 | 7.2 | 290 |
| R cuneus | 18 | −11.1 | 75.6 | 8.8 | 283 |
| R MOG | 19 | −33.2 | 74.8 | 13.5 | 224 |
| R MCC | 24 | −18.2 | 12 | 36.3 | 204 |
| R precuneus | 7 | −9.2 | 71.8 | 41.1 | 204 |
| R MOG | 19 | −33.8 | 83.7 | 3.5 | 171 |
| R MFG | 9/6 | −25.3 | 7.9 | 57.9 | 132 |
| R LG | 18 | −24.8 | 75.8 | −3.9 | 112 |
| L PCG | 6 | 31.8 | 14 | 44.8 | 105 |
| L MOG | 19 | 44.7 | 71.6 | −7.3 | 99 |
| R PCG | 6 | −38.8 | 0.4 | 35.1 | 86 |
| R LG | 18 | −22.2 | 90.6 | −4.1 | 79 |
| R PCG | 6 | −46.6 | 4.4 | 26.2 | 73 |
Cartesian coordinates refer to Talairach and Tournoux space using the RAI convention.
L left, R right, ACC anterior cingulate cortex, CN caudate nucleus, LG lingual gyrus, MCC middle cingulate cortex, MFG middlefrontal gyrus, MOG middle occipital gyrus, PCG precentral gyrus
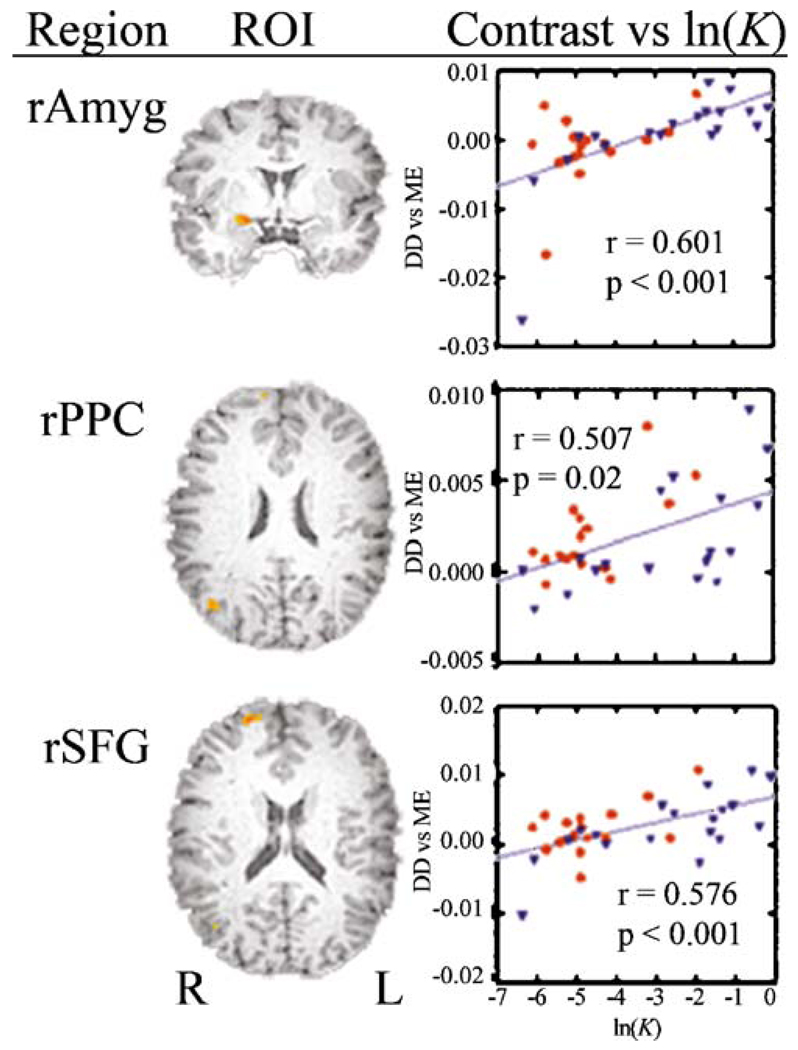
Examination of the relationship between the component values of the contrast between active and control tasks and ln(K1) yielded insight into the attribution of cerebral activation in Fig. 4b. The more heavily subjects discounted (less negative ln(K1)), the greater was the difference between hard choices and control choices (Fig. 5). A post hoc inspection of the contrast DDdh+DDih−2(ME) revealed that the regression coefficient of the DDih and ME trials did not correlate with ln(K1), while the coefficient of the DDdh trials was significantly positively correlated with ln(K1). For example, the Pearson correlations between DDdh and ln(K1) in amygdala, RPPC and RSFG were 0.673 (p<0.001), 0.403 (p<0.02), and 0.509 (p<0.002), respectively. Correlations with other terms in the contrast were not significant.
Fig. 5.
Mean value of contrast, DDhard-ME vs. ln(K1) in the regions of interest shown for the statistical map identified by the regression coefficient of ln(K1). red circles Controls, blue triangles MA, rAmyg right amygdala, rPPC right posterior parietal cortex, rSFG right superior frontal gyrus
Discussion
Behavioral observations
The values of K1 determined in the scanner for MA and control subjects (0.018 and 0.0026, respectively) are similar to those reported in our previous investigation (0.018, 0.004; Hoffman et al. 2006) and that of Monterosso et al. (2007), (0.045, 0.013). As expected, MA subjects discount more heavily than the non-addicted control subjects.
Functional MRI
Delay discounting task
Difficult choices between immediate and delayed rewards activated dACC (BA 24), V/DLPFC (BA 10/9), inferior parietal lobule (IPL; BA 39) the anterior insular cortex (BA 13) and the R MTG (BA 22). This pattern of regional activity is quite similar to that in the δ areas identified by McClure et al. (2004; 2007) and in the combined group analysis of Monterosso et al.. As expected, the regions identified in this analysis are associated with both the cognitive and affective systems hypothesized by Ernst and Paulus (2005). Although amygdala and ventral striatum, important components of the affective system, were not identified in the within-subjects analysis, focal activation of the amygdala was associated with heavier discounting in the between-subjects analysis (Fig. 4b). This broad pattern of cerebral activation likely reflects the complex nature of the decision-making process. The DD task requires evaluation of the immediate and delayed options, comparison of the choices, selection of the preferred option and a motor response. The task used in this study did not allow dissection of this process in a manner that would allow identification of anatomical correlates of subtasks (e.g., stimulus evaluation or stimulus comparison). We expect, however, that evaluation of the options would rely more heavily on the affective system, whereas comparison of the options and choice would engage the cognitive system (Bechara 2005; Ernst and Paulus 2005).
V/DLPFC, dACC, anterior insula, PPC, and more caudal regions of the MFG make up an executive network that exerts cognitive control over decisions (Cole and Schneider 2007). These executive regions integrate contextual and mnemonic information (e.g., current internal state, past experience, expectation of longer-term consequences) with raw salience and modulate the subsequent choice (Ernst and Paulus 2005). Verbal and spatial working memory are important subcomponents of executive function (Owen et al. 2005; Ricciardi et al. 2006). Working memory reflects a set of functions that involves both selective attention and mechanisms for the maintenance of cognitively manipulable representations in different sensory modalities (Baddeley et al. 2001; Goldman-Rakic 1996). In this context, the dorsal network may be related to calculation and comparison of the two alternatives (Simon et al. 2002). Capacity to evaluate multiple outcomes simultaneously is limited by the amount of information that one can hold online in the brain and manipulate. That is, expression of a preference between two possibilities relies on the brain’s capacity to simultaneously evaluate more than one potential outcome. The comparison may go awry if the network is inefficient or impaired. Interestingly, a recent study of DD and working memory found that the two functions show a strong inverse relationship, as increased working memory load was associated with more impulsive responses on the DD task (Hinson et al. 2003).
Like Monterosso et al. (2007) and unlike McClure et al. (2004; 2007), we did not observe activation in the OFC or ventral striatum, critical nodes in the network for assigning representative value to stimuli and holding that representation on line during decision making (Bechara 2005; Ernst and Paulus 2005; McClure et al. 2004; Wallis 2007). Differences in task design or contrasts used to construct statistical maps likely account for some of these differences. The present study was designed so that both ME and DD trials require the subject to evaluate two stimuli and make an assessment regarding which is more desirable. Note, however, that the ME trials require an evaluation about which of two numbers is larger, a decision which has a correct answer, while the DD trials require a more complex assessment that involves temporal discounting. To the extent that the ME task is more purely cognitive, we might expect a diminution of contrast with DD in cognitive regions and enhancement in the affective circuit. We find, however, the highest contrast between the two tasks in the dorsal, cognitive network. In addition, blood oxygen level dependent (BOLD) response in the OFC is difficult to detect because of the artifact resulting from the magnetic susceptibility change at the interface between the brain and the air-filled frontal sinuses (Ojemann et al. 1997). In this study, the signal to noise ratio in the gyrus rectus was 10.55 while that in the SFG, for example, was 114.1.
Methamphetamine dependent patients and controls
Control subjects exhibited more robust cortical activation than MA patients in several regions. The precuneus (BA 7) is implicated in spatially directed attention, episodic memory retrieval and self awareness (Cavanna and Trimble 2006). Other medial occipital lobe activation in the cuneus and MOG may be related to visually directed attention. The ACC and dACC (BA 24) along with the R DLPFC (BA 9) and the head of the caudate nucleus (HCN) form the anterior part of a brain system that subtends working memory, spatially directed attention (Owen et al. 2005; Ricciardi et al. 2006) and cognitive control (Cole and Schneider 2007). MA-dependent individuals may have primary anatomical and metabolic deficits in the cingulate cortex. For example, Thompson et al. (2004) found decreased cortical thickness in several regions of the ACC in a group of MA-dependent patients while London et al. (2004), in a positron emission tomography (PET) study of MA users, found a correlation between mood disturbances and cerebral metabolic rate in the ACC. Monterosso et al. (2007) also found evidence of decreased activity in frontoparietal regions in MA users, but they identified parietal activation somewhat more laterally, in the PPC, while the frontal activation was more rostral. The activity we observed in the precuneus is quite near the junction with the PPC and likely represents the same observation. Examination of the average regression coefficients for DD and control stimuli indicated that subjects in both groups exhibited greater cortical activation during hard choices relative to easy choices (Fig. 3), although this contrast was not significant in whole brain analyses. The figure shows, however, that MA subjects, unlike controls, exhibited more activation on easy and ME questions and, as a result, showed less difference between DD and ME trials. A similar finding was reported previously by Monterosso et al. (2007). A possible interpretation of these findings is that MA users exhibited less efficient cognitive control, i.e., comparing alternative choices was generally more difficult. This executive control inefficiency may subsequently bias decisions to less complex, albeit smaller, immediate rewards. The longer reaction times of the MA group on ME trials further suggests that this group has a harder time in general making decisions than the controls. This interpretation is bolstered by the multiple regression results.
Multiple linear regression (Eq. 3) allowed identification of regions in which the discounting parameter, ln(K1), was associated with variance in addition to that accounted for by group membership. Greater BOLD response was correlated with magnitude of discounting in R amygdala, R SFG, R PPC and R PCC. It is important to recognize that these correlations resulted predominantly from the effect of trials in which the subjects picked the delayed reward in a hard choice pair. This right hemisphere circuit is more heavily recruited when subjects with a preference for immediate reward, instead, choose a delayed reward. This is consistent with the idea that subjects who prefer immediate rewards do so because of inefficient cognitive control and must robustly activate the cognitive circuit in order make a delayed choice.
In this sample, most of the heavy discounters were in the MA-dependent group but some controls also discounted heavily. The regression lines for MA-dependent and control participants appeared to have the same slopes and intercepts. Therefore, this association between choice of a delayed reward and amygdalar activation may be a general feature of delay discounting. Due to range restriction (i.e., limited variance in discounting) it might not be possible to detect amygdalar activation in a sample of controls nor in a sample of MA-dependent subjects. By combining control participants with MA-dependent subjects, however, the variance in discounting is expanded and it becomes possible to detect the associated amygdalar activation.
There are similarities between the regions identified with this regression and regions found in other fMRI studies of discounting and decision making.McClure et al. (2004; 2007) identified regions in the ventral striatum and anterior insula that were associated with choice of smaller immediate rewards, while Monterosso et al. (2007) found a region in the right VLPFC in which activity in a hard choice–no choice contrast was correlated negatively with log(K1). Boettiger et al. (2007) identified regions, similar to those we report (RPPC, LdPFC, R parahippocampal gyrus [PHG]), that were correlated with the subjects’ preference for immediate rewards. The authors suggest that activation of parietofrontal regions and PHG biases the individual toward choosing the immediate reward. Our data suggests an alternative interpretation; namely, that frontoparietal activation is necessary to exert cognitive control over amygdalar activation in order for steep discounters to select delayed rewards. This is consistent with the findings of Wittmann et al. (2007). In a sample of normal controls, they found choice of delayed reward associated with activation of posterior insular cortex, left STG, AG, IPL, and cuneus. They concluded that activation of the cognitive system was necessary for choices that delayed gratification.
The amygdala is generally felt to be part of the ventral, affective system and might be expected to be more sensitive to immediate rewards. For example, cue-induced craving for drugs is associated with activation of amygdala, ventral ACC, insula, and nucleus accumbens (Garavan et al. 2000; Grant et al. 1996; Kilts et al. 2001; Wexler et al. 2001). Note that subjects who discount heavily (primarily in the MA group) make many fewer choices of delayed than immediate rewards. It is possible that choosing a delayed reward is associated with amygdalar activation because the delayed reward represents an aversive choice or a loss. For example, Nitschke et al. (2006) reported activation of dorsal amygdala, anterior insula, dorsal ACC, right DLPFC, and right posterior OFC when subjects were shown aversive photos. These findings are consistent with a model in which MA-addicted patients make impulsive decisions (prefer the smaller immediate reward) due both to inefficient cognitive control (and consequent difficulty comparing the options) and to difficulty executing a choice of an aversive delayed reward. We did not find evidence in this study that MA users make impulsive decisions due to affective system hyperactivity leading to overvaluation of immediate rewards.
The greater number of smokers in the MA group could have influenced our results either through the effects of chronic smoking or due to withdrawal during the MRI. The latter possibility was minimized by allowing the subjects who smoked access to nicotine before the procedure and by limiting the time without a cigarette to less than 2 h. None of the subjects who smoked complained of nicotine craving during or after the procedure. The effects of chronic smoking on fMRI results are not well understood; however, a recent study (Friedman et al. 2008) found an increase in BOLD activation in chronic smokers (compared to non-smokers) on a simple visual activation task. As we did not find any areas of increased activation in the heavier smoking MA group, this effect is unlikely to have contributed artifactually to our results. Nevertheless, future studies should carefully match patients and controls for current and historical smoking.
Our results have clinical implications, as MA patients who discount more heavily may also be at greater risk for relapse. Although the association has not been demonstrated in the MA population, increased discounting has been associated with a greater probability of relapse in pregnant female smokers (Yoon et al. 2007). Therefore, heavy discounting may help identify individuals at risk for resumption of drug use and provide a target for pharmacological and psychosocial therapies aimed at changing the magnitude of preference for immediate rewards. For example, cognitive behavioral treatments aimed at increasing cognitive control could form the basis for increasing the subjective desirability of future rewards (Monterosso and Ainslie 2007). In summary, we have identified brain regions activated by temporal discounting in controls and in patients recovering from MA addiction. In particular, we have identified regions that are specifically activated when subjects with a preference for immediate rewards choose delayed rewards. The findings suggest that patients addicted to MA, who strongly prefer smaller immediate rather than larger delayed rewards, must activate the dorsal cognitive control system in order to overcome their preference. When these heavy discounters choose a delayed reward, increased amygdalar activation likely represents the aversive nature of picking the deferred option.
Acknowledgments
Supported by the Medical Research Foundation of Oregon, Department of Veterans Affairs Merit Review Program (WFH), Stanley Medical Research Institute (MSH), NIH grants P50 DA018165 (WFH, SHM, BHM), and DA015543 (SHM).
Footnotes
Financial Disclosures None of the authors reported any biomedical financial interests or potential conflicts of interest.
Contributor Information
William F. Hoffman, Email: hoffmanw@ohsu.edu, Mental Health and Clinical Neurosciences Division P35C, Veterans Affairs Medical Center, 3710 SW US Veterans Hospital Road, Portland, OR 97239, USA; Research Service, Veterans Affairs Medical Center, Portland, OR, USA; Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA; Methamphetamine Abuse Research Center, Oregon Health & Science University, Portland, OR, USA.
Daniel L. Schwartz, Research Service, Veterans Affairs Medical Center, Portland, OR, USA Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA.
Marilyn S. Huckans, Mental Health and Clinical Neurosciences Division P35C, Veterans Affairs Medical Center, 3710 SW US Veterans Hospital Road, Portland, OR 97239, USA Research Service, Veterans Affairs Medical Center, Portland, OR, USA; Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA.
Bentson H. McFarland, Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA Kaiser Permanente Center for Health Research, Portland, OR, USA.
Gal Meiri, Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA; Methamphetamine Abuse Research Center, Oregon Health & Science University, Portland, OR, USA; Ben Gurion University of the Negev, Beer-sheva, Israel.
Alexander A. Stevens, Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR, USA Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA.
Suzanne H. Mitchell, Department of Behavioral Neuroscience, Oregon Health & Science University, Portland, OR, USA Department of Psychiatry, Oregon Health & Science University, Portland, OR, USA; Methamphetamine Abuse Research Center, Oregon Health & Science University, Portland, OR, USA.
References
- Ainslie GW. Impulse control in pigeons. J Exp Anal Behav. 1974;21:485–489. doi: 10.1901/jeab.1974.21-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A, Chincotta D, Adlam A. Working memory and the control of action: evidence from task switching. J Exp Psychol Gen. 2001;130:641–657. [PubMed] [Google Scholar]
- Baker F, Johnson MW, Bickel WK. Delay discounting in current and never-before cigarette smokers: similarities and differences across commodity, sign, and magnitude. J Abnorm Psychol. 2003;112:382–392. doi: 10.1037/0021-843x.112.3.382. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decisionmaking, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J Roy Stat Soc B Met. 1995;57:289–300. [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–454. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Boettiger CA, Mitchell JM, Tavares VC, Robertson M, Joslyn G, D'Esposito M, Fields HL. Immediate reward bias in humans: fronto-parietal networks and a role for the catechol-O-methyltransferase 158(Val/Val) genotype. J Neurosci. 2007;27:14383–14391. doi: 10.1523/JNEUROSCI.2551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Cox RW, Hyde JS. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(sici)1099-1492(199706/08)10:4/5<171::aid-nbm453>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biol Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friedman L, Turner JA, Stern H, Mathalon DH, Trondsen LC, Potkin SG. Chronic smoking and the BOLD response to a visual activation task and a breath hold task in patients with schizophrenia and healthy controls. Neuroimage. 2008;40:1181–1194. doi: 10.1016/j.neuroimage.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pankiewicz J, Bloom A, Cho JK, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The prefrontal landscape: implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351:1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci U S A. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Mem Cognit. 1997;25:715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Hinson JM, Jameson TL, Whitney P. Impulsive decision making and working memory. J Exp Psychol LearnMem Cogn. 2003;29:298–306. doi: 10.1037/0278-7393.29.2.298. [DOI] [PubMed] [Google Scholar]
- Hoffman WF, Moore M, Templin R, McFarland B, Hitzemann RJ, Mitchell SH. Neuropsychological function and delay discounting in methamphetamine-dependent individuals. Psychopharmacology (Berl) 2006;188:162–170. doi: 10.1007/s00213-006-0494-0. [DOI] [PubMed] [Google Scholar]
- Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP. Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry. 2001;58:334–341. doi: 10.1001/archpsyc.58.4.334. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503, 507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27:5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berl) 1999;146:339–347. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- Monterosso J, Ainslie G. The behavioral economics of will in recovery from addiction. Drug Alcohol Depend. 2007;90 Suppl 1:S100–S111. doi: 10.1016/j.drugalcdep.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso J, Ehrman R, Napier KL, O'Brien CP, Childress AR. Three decision-making tasks in cocaine-dependent patients: do they measure the same construct? Addiction. 2001;96:1825–1837. doi: 10.1046/j.1360-0443.2001.9612182512.x. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28:383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. Neuroimage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend. 2000;60:259–266. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Kirby KN, Kranzler HR. Effects of gender and family history of alcohol dependence on a behavioral task of impulsivity in healthy subjects. J Stud Alcohol. 2002;63:83–90. [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Raineri V, Cross D. Subjective probability and delay. J Exp Anal Behav. 1991;55:233–244. doi: 10.1901/jeab.1991.55-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–667. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Ricciardi E, Bonino D, Gentili C, Sani L, Pietrini P, Vecchi T. Neural correlates of spatial working memory in humans: a functional magnetic resonance imaging study comparing visual and tactile processes. Neuroscience. 2006;139:339–349. doi: 10.1016/j.neuroscience.2005.08.045. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier CP, Sim T, Richardson K, Rawson RA, Ling W. Cognitive performance of current methamphetamine and cocaine abusers. J Addict Dis. 2002;21:61–74. doi: 10.1300/j069v21n01_06. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–6036. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JD. Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci. 2007;30:31–56. doi: 10.1146/annurev.neuro.30.051606.094334. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC. Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry. 2001;158:86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol. 2007;15:176–186. doi: 10.1037/1064-1297.15.2.186. [DOI] [PubMed] [Google Scholar]