Abstract
Background
Lean and Six Sigma are business management strategies commonly used in production industries to improve process efficiency and quality. During the past decade, these process improvement techniques increasingly have been applied outside of the manufacturing sector, for example, in health care and in software development. This article concerns the potential use of Lean and Six Sigma to improve the processes involved in clinical and translational research. Improving quality, avoiding delays and errors, and speeding up the time to implementation of biomedical discoveries are prime objectives of the NIH Roadmap for Biomedical Research and the NIH Clinical and Translational Science Award (CTSA) program.
Methods
This article presents a description of the main principles, practices, and methodologies used in Lean and Six Sigma. Available literature involving applications of Lean and Six Sigma to health care, laboratory science, and clinical and translational research is reviewed. Specific issues concerning the use of these techniques in different phases of translational research are identified.
Results
Examples are provided of Lean and Six Sigma applications that are being planned at a current CTSA site, which could potentially be replicated elsewhere. We describe how different process improvement approaches are best adapted for particularly translational research phases.
Conclusions
Lean and Six Sigma process improvement methodologies are well suited to help achieve NIH’s goal of making clinical and translational research more efficient and cost-effective, enhancing the quality of the research, and facilitating the successful adoption of biomedical research findings into practice.
Keywords: Lean, Six Sigma, process improvement, translational research, quality, TQM, CQI
Introduction
Various business management strategies have been developed to improve the performance of organizations by improving the processes by which they carry out their work. These strategies, which include Lean and Six Sigma, aim to implement process improvements through a coordinated set of principles and practices that promote greater efficiency and effectiveness, with fewer wasteful practices or errors. Evolving from their original application in manufacturing industries, these process improvement strategies have been extended to other settings including construction, software development, financial services, health care delivery, and laboratory sciences.
The creation of the Clinical and Translational Science Awards (CTSA) initiative as part of the National Institutes of Health (NIH) Roadmap for Medical Research is aimed at creating a clinical and translational research enterprise that assures maximal value is obtained from biomedical research investments. While the definition of clinical and translational research is still being debated, there is broad consensus that formal and sustained processes are needed to improve the timeliness and efficiency of research along the biomedical continuum. Reducing the time between biomedical research discoveries and their adoption into clinical practice requires increased coordination, systematic planning, and new types of connections within biomedical research organizations. This article suggests that better coordination, timeliness, efficiency and value of clinical and translational research can be achieved by applying the set of principles, practices and methods represented by Lean and Six Sigma.
What is Lean?
Lean (also known as Lean Production, Lean Enterprise, and Lean Thinking) involves a set of principles, practices and methods for designing, improving and managing processes. The development of Lean is attributed to Taiichi Ohno’s articulation of the Toyota Production System.1 Ohno aimed to improve efficiency by eliminating particular kinds of waste (called muda, in Japanese) which absorb time and resources but do not add value. Examples include mistakes which need rectification, unneeded process steps, movement of materials or people without a purpose, unnecessary waiting because upstream activity was not delivered on time, and the creation of goods or services that are not really needed by end users.2
A Lean process reflects the goal of continually reducing waste and improving work flow to efficiently produce a product or service that is perceived to be of high value to those who use it. Implementation of Lean involves systematic process assessment and analysis. The preliminary stages of Lean assessment include “value stream mapping” in which key people, resources, activities and information flows required to deliver a product or service are made explicit and depicted graphically. The value stream map is a key tool for identifying opportunities to reduce waste and more tightly integrate process steps, thus improving process efficiency.
Improvement approaches such as Lean and Six Sigma grow out of a long tradition of quality and process improvement efforts in manufacturing. For example, Frederick Winslow Taylor’s scientific management and Frank Gilbreth’s “time and motion” studies were among the earliest prescriptions for improving the quality and efficiency of production processes. Current thinking about process improvement draws heavily on the ideas of W. Edwards Deming, Joseph Juran and other statisticians whose data analysis tools and management philosophies were initially adopted by Japanese manufacturers, and have come to be known as Total Quality Management (TQM) or Continuous Quality Improvement (CQI).3,4
What is Six Sigma?
Six Sigma, like Lean, is a business management strategy used to improve the quality and efficiency of operational processes. While Lean focuses on identifying ways to streamline processes and reduce waste, Six Sigma aims predominantly to make processes more uniform and precise through the application of statistical methods.5 Six Sigma was originally developed by Bill Smith of Motorola in 1986 as a way of eliminating defects in manufacturing, where a defect is understood to be a product or process that fails to meet customers’ expectations and requirements. The name Six Sigma refers to a quality level defined as the near-perfect defect rate of 3.4 defects per million opportunities. As a process improvement strategy, Six Sigma gained much attention through its association with General Electric and its former CEO Jack Welsh.
A variety of systematic methodologies for identifying, assessing and improving processes have been developed as part of the Six Sigma approach. The Six Sigma improvement model, Define, Measure, Analyze, Improve, and Control (DMAIC) specifies the following sequence of steps for understanding and improving a process: 1) defining the project goals and customer (internal and external) requirements; 2) measuring the process to determine current performance; 3) analyzing and determining the root cause(s) of relevant defects; 4) improving the process by eliminating defect root causes, and 5) controlling future process performance. Another Six Sigma methodology, Design for Six Sigma (DFSS), is used to systematically design new products and services that meet customer expectations and can be produced at Six Sigma quality levels.6
Six Sigma also involves the training and certification of designated process specialists (called black belts, green belts, or other similar titles) within organizations to help guide Six Sigma improvement efforts. Other distinctive Six Sigma features include the expectation that process quality improvements be translated into financial metrics to assess value and the active involvement of top management in all Six Sigma initiatives.
Various combinations of Lean and Six Sigma techniques have been developed, which frequently are described as Lean Six Sigma approaches. The blended approach points to the common process-centered and data-driven foundations of both Lean and Six-Sigma. Proponents of a combined approach assert that organizations can benefit from utilizing both the customer-orientation and focus on eliminating waste inherent in Lean along with the statistical tools and systematic defect reduction strategies featured in Six Sigma.7,8
Lean and Six Sigma are just two of numerous approaches that are in use for systematically analyzing and improving process flow and efficiency within industries. Other similar approaches include Business Process Modeling (BPM), Business Process Reengineering (BPR), and Workflow Mapping (WM), as well as a variety of TQM and CQI-oriented techniques such as management accounting systems, Kaizen, and Shewhart cycles (PDCA). The selection of a particular process improvement approach will depend upon the specific circumstances and needs existing in a working environment, including the type of processes, the improvement objectives, and the skills, knowledge, and resources available in that setting. For example, some approaches may b better suited to statistical analysis of defects (e.g., Six Sigma), some to layout planning and product flow (e.g., BPM and WM), and some to optimizing transitions between process steps (e.g., Lean). We chose to focus primarily on Lean and Six Sigma in this article because of literature suggesting their applicability to biomedical and research settings (reviewed below).
Application of Lean and Six Sigma to Health Care
Health care organizations, especially large health systems, began studying and adopting industrial quality management methods in the late 1980’s including TQM and CQI approaches9-11 Early applications focused primarily on establishing programs and infrastructure to measure quality and enhancing organizational culture surrounding quality issues.12 Some hospitals used TQM methods to implement process improvements and redesign both non-clinical and clinical work flows.13 Examples of specific TQM interventions included the formation of cross-disciplinary teams to examine and improve work processes, training employees to identify quality improvement opportunities, and the use and application of statistical methods for process improvement.14
Under the banner of TQM and CQI (hereafter we will use “TQM” as short-hand for both TQM and CQI) health care institutions began to evaluate and make changes to a variety of care practices. For example, selected service functions such as basic laboratory, pharmacy, admitting and discharge, medical records, housekeeping, and material support services were relocated to patient care areas to improve organizational efficiency.15 Applying TQM principles, hospitals restructured processes to make care more patient focused. In one TQM application, the turnaround of radiology reports was improved by revising work flow to feature electronic signature by radiologists, elimination of a trainee signature requirement, accelerated transcription, structured reports, faster film delivery to reading desks, and training about the importance of radiology reports for clinical decision making.16 Many health care organizations, inspired by TQM, established broader and more customer-focused quality measurement systems including patient questionnaires, quality and appropriateness reviews, performance appraisals, patient monitoring reports, infection rate surveillance, and other quality-oriented metrics.17
Although TQM approaches became quite common in health care during the 1990s, many authorities expressed skepticism and reservations about the effectiveness of TQM and its ultimate effect on improving health care delivery and patient outcomes. Several critics characterized TQM as a vague and indistinct fad, with little tangible content.18,19 Shortell et al. (2000) found that whether or not a hospital adopted TQM had little effect on multiple outcomes of care for patients receiving coronary artery bypass graft surgery. 20 Blumenthal and Kilo (1998) have summarized the shortcomings of early applications of TQM to health care quality improvement.21
As described by Black and Revere (2006), Lean and Six Sigma “emerged from the fertile environment” created by TQM.22 Recent applications of Lean and Six Sigma in health care attempt to improve on previous experiences with TQM by making project deliverables more discrete and measurable, retaining a strong customer (rather than organizational) focus, quantifying results, and attempting to deliver specific quality improvements within a designated time frame.
Since 2000, there have been a variety of projects applying Lean and Six Sigma strategies to health care quality improvement. For example, pilot programs utilizing Lean approaches at Intermountain Healthcare resulted in substantially reduced turnaround time for pathologist reports from an anatomical pathology lab.23 Other Lean-facilitated improvements at Intermountain Healthcare included reducing IV backlog in the pharmacy, reducing the time needed to perform glucose checks on patients, decreasing time to enter new medication orders and complete chart entries, and streamlining electronic payment for large vendor accounts.23
De Koning et al. (2006) describe several applications of an integrated Lean Six Sigma approach instituted at a Dutch hospital that led to reducing the complexity of hiring part-time clinical staff, optimizing operating room scheduling by designing a new pre-surgical admissions process, and developing a new work planning system to expedited completion of equipment maintenance requests.24 The U.K.’s National Health System adopted a variety of Lean strategies, including redesigning the number of steps, and hence the time, needed for collection and processing of blood samples at Bolton Hospital.25 Successful applications of Lean and Six Sigma have been reported at numerous other health care settings.26-31
Application of Lean and Six Sigma to Laboratory Science
Lean and Six Sigma methodologies are well suited for application to laboratory settings because of the inherent need for statistical precision and quality control in laboratory testing and measurement activities, as well as the highly repetitive nature of laboratory work. Most laboratory applications of Lean and Six Sigma have occurred in clinical environments. A recent review article by Gras and Phillippe (2007) describes many of these applications.32 Nevalainen et al. have advocated using a Six Sigma scale (based on six standard deviations in variance representing a defect rate of 3.4 per 1,000,000 opportunities) as a way of tracking on laboratory quality, establishing benchmarks, and measuring changes in laboratory performance over time.33 Applications of Lean and Six Sigma in clinical laboratories have included efforts to reduce auto-verification errors in a laboratory information system,34 ensure sufficient volume of blood samples for use in a clinical microbiology laboratory,35 assure the repeatability and reproducibility of warfarin anticoagulation testing among different laboratories within a community,36 and establish continuous and efficient work flow within a hospital-based histology lab.37
There is substantially less research literature describing Lean and Six Sigma applications in basic science laboratories as compared to clinical laboratory settings. This difference may reflect the greater access to process improvement expertise available to clinical laboratories, as these facilities are generally part of larger health care delivery systems. Nonetheless, Lean and Six Sigma approaches are potentially applicable in both clinical and non-clinical laboratory settings. For example, Six Sigma techniques have been recommended as a means to avoid cross contamination of cell lines.38 Hollensead et al. (2004) outline potential uses of Lean, Six Sigma, and other quality assurance practices to reduce laboratory errors in a host of disciplines including molecular biology, cytology, microbiology, and pathology.39 Lean and Six Sigma have also been directed towards quality assurance in pharmaceutical laboratories and production facilities.40
Quality and Process Improvement in Clinical & Translational Research
The NIH’s Roadmap for Medical Research calls for “re-engineering the clinical research enterprise.” This initiative aims to develop new partnerships among organized patient communities, community-based health care providers, and academic researchers. The NIH envisions a clinical research process that becomes more efficient and effective by improving linkages between system components and better integrating the continuum spanning basic science, clinical studies, and the uptake of new practices by medical practitioners and their patients. The NIH calls for “new and more efficient approaches to discovery and clinical validation of research results . . .[that will] . . . contribute to accelerating and strengthening clinical research by adopting a systematic infrastructure that will better serve the evolving field of scientific discovery.”41
To accomplish its vision, the NIH in 2006 initiated a program of Clinical and Translational Science Awards (CTSA) for major medical research institutions throughout the United States.42 As of early 2009, 38 sites have been awarded CTSA funding. The NIH has charged the CTSA sites with four primary goals:1) to improve the way biomedical research is conducted across the country, 2) to reduce the time it takes for laboratory discoveries to become treatments for patients, 3) to engage communities in clinical research efforts, and 4) to train the next generation of clinical and translational researchers.
Underlying the NIH’s Roadmap is the belief that the clinical research enterprise is not currently as efficient or coherent as it ought to be. The NIH has identified a variety of impediments plaguing the current research environment, particularly the lengthy timeframe needed for conducting research, testing approaches in patient populations, and getting effective approaches accepted into clinical practice. The NIH hopes that establishment of the CTSA sites will address important problems, such as poor coordination between existing research networks and lack of data sharing among researchers. The CTSA awards contain funds for training of new researchers who will be expected to work collaboratively in a transdisciplinary environment that fosters new ideas and creates more efficient processes for moving novel practices and technologies into the health care delivery setting.
The NIH’s vision for the CTSA sites is clearly aligned with the objectives represented by Lean and Six Sigma approaches. These management strategies for process improvement, quality measurement, and reduction of errors and waste hold the potential for facilitating the transformation of the clinical and translational research enterprise envisioned by NIH. The remainder of this article will describe the specific components of clinical and translational research, as currently understood, and provide examples of ways in which Lean and Six Sigma methodologies can be applied to help achieve the specific goals of NIH’s clinical and translational research program.
What is Clinical and Translational Research?
The NIH has defined clinical and translational science as follows: “’Clinical Research’ comprises studies and trials in human subjects. Translational research includes two areas of translation. One is the process of applying discoveries generated during research in the laboratory, and in preclinical studies, to the development of trials and studies in humans. The second area of translation concerns research aimed at enhancing the adoption of best practices in the community. Cost-effectiveness of prevention and treatment strategies is also an important part of translational science.”43
Several scholars have proposed conceptual models of clinical and translational research as consisting of multiple linked phases. The Institute of Medicine’s Clinical Research Roundtable, which met from 2000 to 2003, distinguished two types of translational research domains, designated as T1 and T2. The “bench to bedside” T1 enterprise is concerned with transferring the discoveries and advances of basic laboratory science to clinical testing in human subjects. The T2 sphere extends the results of clinical studies into everyday clinical practice and health decision making.44 Woolf (2008) has commented on the inherent ambiguity in calling both types of activity “translational research,” and he has advocated a stronger governmental commitment to supporting T2 studies examining the uptake and use of new clinical care practices in community-based settings.45 Other researchers have recommended alternative nomenclature for describing these two domains including preclinical research and discovery research for T1 studies and applied clinical research and knowledge translation for T2 studies.46,47
Owing to the complexity of translational research and its continuum over a wide scope of activities bridging laboratory experiments, preclinical testing, clinical trials, knowledge transfer, adoption into accepted clinical practice, and ultimately assessing the effects on individuals and communities, some authorities have recommended more finely detailed conceptual models of translational research. Several theorists have developed translational research models with three or more stages.
Westfall et al. (2007), for example, have distinguished three domains of translational research: T1, in which preclinical and animal testing is shifted to human subjects; T2, in which the results of initial testing in human subjects migrates to patients, and T3, involving implementation and dissemination of research discoveries into accepted clinical practice.48 Dougherty and Conway’s (2008) model shares Westfall’s conception of a linear process bridging the boundaries of discovery to broad-scale implementation, with T1 representing bench to bedside research, T2 designating clinical trials to test safety and efficacy, and T3 involving transfer to practices settings and populations.49 A four-phase model has been proposed by Khoury et al. (2007) in which the T1 phase concerns transfer for laboratory to potential health application, the T2 phase from health application to evidence-based guidelines, the T3 phase from guidelines to health care practice, and T4 from health care practice to effects in individuals and populations.50
The picture emerging of clinical and translational research is that of a complicated multi-phase process involving numerous participants including laboratory scientists, researchers, clinicians, patients, academic institutions, external funding sources, health care organizations, manufacturers and suppliers of health care technologies, communities, and others. The end goal of clinical and translational science initiatives sponsored by NIH is to make this process more rationale, coordinated, efficient, cost-effective, and timely, with fewer impediments and less wasted effort. NIH’s goal is to support integrated research efforts across the broad spectrum of phases in order to accelerate the entire process and increase the likelihood that research will identify effective clinical treatments and practices.
Application of Lean and Six Sigma to Clinical and Translational Research
There is a clear correspondence between NIH’s vision of a more integrated and efficient clinical and translational science enterprise and the process-focused strategies embodied by Lean and Six Sigma. These management strategies, imported from the industrial environment, can be applied to help systemically analyze and improve the array of process steps involved in most clinical and translational research projects. The CTSA structure that NIH has adopted facilitates the selection and introduction of process management techniques that can be applied to clinical and translation research programs. We are not aware of any published articles to date describing Lean or Six Sigma approaches to redesign the clinical and translation research enterprise at any of the CTSA sites. This is an opportunity that is waiting to be tested.
A few applications of Lean and Six Sigma techniques at other clinical and translational research sites have been reported. Ablowitz et al. (2008) describe a complex systems engineering analysis of the translational research process at the University of Virginia. In that analysis, the investigators developed and utilized a Translational Research Performance Index to quantify performance measures of translational research, such as the number of researchers in various cross-functional teams and the number of existing research partnerships.51 Based on their analysis, various “solution strategies” for enhancing the translational research process were proposed, including incentives to stimulate trans-departmental collaboration, design recommendations for facility infrastructure, and the recruitment of a specialist in Lean/Six Sigma to undertake studies of additional process changes.
Liu (2006) describes an application of Six Sigma methods to achieve a reduction of 70% in cycle time for entry of case record forms in a phase III clinical trial, while maintaining a statistically acceptable error rate requirement.52 The process redesign involved such steps as implementing an optical mark technique to convert study data into optically recognizable binary characters for processing data directly into data management systems without human intervention. Marti (2005) reported on an application of Lean Six Sigma in which the time needed to complete a phase 1 clinical trial was improved by redesigning standardized case record forms, setting up a dashboard system for monitoring key performance indicators, and acquiring new hardware and software systems for reducing cycle time for data analysis.53 Lean techniques have been applied to streamline the drug discovery process in the preclinical phase of research. For example, Lean techniques were used by a contract research organization to improve assay turnaround times and reduce assay result variance.54 In another preclinical pharmacologic research setting, Lean and Six Sigma were used for redesigning laboratory layout to align better with workflows, grouping work by assay type, and repositioning equipment and instrumentation to be in closer proximity to their eventual point of use.55
The Center for Clinical and Translation Science at The Ohio State University, a CTSA site, is planning to pursue various process improvement projects using Lean and Six Sigma methods. Some of the projects that are now being designed and initiated include:
A process improvement study using Lean and Six Sigma techniques to review, assess, and improve the process for establishing clinical research contracts between a sponsor (typically a pharmaceutical company) and the university’s clinical research center. This process is often prolonged and burdensome owing to the need to develop appropriate disclosure agreements, arrange and conduct sponsor qualification visits, and develop the language and attain legal review for the clinical research contract.
Studying the complex issues involved in transforming NIH’s former model of a “General Clinical Research Center” (GCRC) as a nexus for organizing and conducting clinical trials to the new paradigm of clinical and translational research units. There are questions about whether the GCRC should be retained as is, modified, or merged into the new “Center for Clinical and Translational Research” that was established at the university. A Lean analysis is being considered to examine these issues.
A related process study is being designed to expand a charge-back process for the clinical trials unit by which costs for different services will be compared (e.g., overnight stays, multiple blood draws), with charges being applied and routed back to appropriate cost units. Similar “charge-back” processes are being considered for other services offered by the Center for Clinical and Translational Science including biostatistical support and services being offered through biomedical informatics and their data warehouse.
Six Sigma and Lean methods are being used to investigate the process steps and issues involved in establishing reciprocal IRB agreements between affiliated academic and non-academic research institutions. The goal is to enact a new “fast-track” process to expedite the time needed to obtain final IRB approval.
The review process for soliciting, evaluating, and awarding of pilot project awards, clinical research traineeships, and test-bed projects for novel technologies will be examined using Six Sigma and Lean techniques, with the goal of making the process quicker, more efficient, and fair.
Faculty and doctoral students from the university’s systems engineering department are conducting work flow assessments in the clinical trials unit in which acuity factors are calculated estimating the time required to perform specific functions and procedures. This will result in time simulations for optimal process flow. Some changes have already been made as the result of preliminary investigation, such as revising patient scheduling procedures.
Because the nature of clinical and translational research activities varies considerably between the different research phases described previously (T1, T2, T3, T4), the application of process improvement strategies utilizing Lean and Six Sigma can be expected to also differ among the research phases. Adopting a four-phase model of translational research based loosely on Khoury et al. (2007), it is possible to distinguish the general type of Lean and Six Sigma approaches that may be most relevant and applicable to each of the four research phases. Table 1 details some of the specific practices associated with Lean and Six Sigma, and illustrates how applications of these two approaches could be relevant to each translational research phase.
Table 1.
Examples of Lean and Six Sigma Strategies Applied to the Phases of Translational Research
| Management Strategies | T1 Basic Laboratory Research Animal Testing Pre-Clinical Testing |
T2 Initial Application to Human Subjects Clinical Trials |
T3 Implementation Adoption in Clinical Practice Practice Guidelines |
T4 Outcomes Assessment Dissemination Impact on Populations |
|---|---|---|---|---|
| Lean | ||||
| Determine “value” as defined by CTR customers |
Novel research ideas Qualified researchers |
Research grant funding Protocol approval |
Payer reimbursement Evidence-based treatment |
Positive efficacy results Findings published |
| Assess customer pull: demand for products and services |
Appropriate instrumentation Assistance with IRB |
Biostatistical consultation Subject recruitment |
Treatment protocols EMR and IS adaptability |
Outcomes measurement Health policy implications |
| Identify and understand process steps |
Testing protocols Investigational new drug appl |
Availability of pilot data Clinical trial protocol accepted |
Post-marketing testing Inclusion in guideline |
Follow up with patients Social network analysis |
| Conduct value stream mapping to evaluate work flow |
Transfer of samples in lab Regulatory requests |
Advertising for patients Informed consent |
Distribution of guidelines Specific clinical training |
Data acquisition methods Methods to de-identify data |
| Eliminate waste (steps that do not add value) |
In vitro testing when possible Assure compounds available |
Minimize protocol amendment Are placebos really needed |
Unexplained variation in care Misuse of drugs & treatments |
Appropriate sampling strategy Is pilot project necessary |
| Integrate steps and test results on efficiency and goals |
Fast turnaround of tox. results Validation of assays |
Strict patient monitoring Consider database recruitment |
Quality assurance program MD performance incentives |
Research committee oversight Experienced PI |
| Six Sigma | ||||
| Define project goals and customer requirements |
Use appropriate animal model Select best target agents |
Select best endpoints Have qualified research team |
Increase physician awareness Standardizing care |
Assess health status Improve patient compliance |
| Designate resource specialists (“black belts”) |
Pharmacological expertise Bioinformatics capabilities |
Bioethics/informed consent Clinical trial office |
Clinical leaders in system Specialists with care approach |
Health economists Health services researchers |
| Measure the process to determine current performance |
Monitor side effects Analyze scheduling system |
Treatment scheduling Statistical analysis of defects |
EMR chart review Communication with MCOs |
Research team formation Patient self-reporting |
| Analyze and determine the root causes of relevant defects |
Identify reasons for attrition Inadequate number of samples |
Inadequate enrollment patient safety jeopardized |
Cooperation of nursing staff Incompatible IS systems |
Low response rates Inability to contact patients |
| Improving the process by eliminating defect root causes |
Set new safety standards Replace defective instruments |
Reasons for protocol deviation Early phase design errors |
Improve patient education Strengthen clinical leadership |
Use updated accurate data Ensure adequate sample sizes |
| Control future process performance |
Improve lab recording system Establish biological endpoints |
Address conflict of interest Enhanced patient self-reports |
Guideline review periodically Peer-review committee |
Validated measurement tools Community participation |
Case Study
The following case study provides an example of how Lean and Six Sigma principles were used in a recent process improvement project involving redesign of the scheduling system at the Clinical Trials unit of the Ohio State University. Historically, scheduling of patients within the unit was done using a conventional paper-based calendar system. This led to inefficiencies in matching staff and room availability with protocol requirements and patient needs, as measured by utilization of staff and rooms as well as patients waiting for their services to be completed. A process improvement project was undertaken to develop a more coherent and data-driven scheduling system based on multiple factors and assisted by specialized computer software. The project began with efforts to clearly understand each step of the existing scheduling process. Next, the process improvement team began to consider different scheduling approaches that incorporated the salient factors identified as important for an efficient schedule. Using repeated improvement cycles, scheduling algorithms were tested in the field and evaluated. A key result of this effort was development and validation of an “acuity table” that assigns an acuity estimate (in minutes per activity) for each of 89 specific activities. For example, the activity of “arterial line set-up” was assigned an acuity score of 15 (minutes) and the activity of “simple specimen collection” was given an acuity score of 5. A scheduling algorithm matched the acuity scores with other factors (such as the number of available nurses per shift, room availability, number of protocols underway, visit sequence in the protocol, protocol requirements, etc.) to optimize both patient and staff scheduling on any particular day. By developing a computerized model based on this scheduling approach and then training staff to use the model, the project team helped assure that the process improvement would be adopted as the new way of scheduling patients. A diagrammatic depiction of the process steps analyzed and relevant factors considered are shown in Figure 1.
Figure 1.
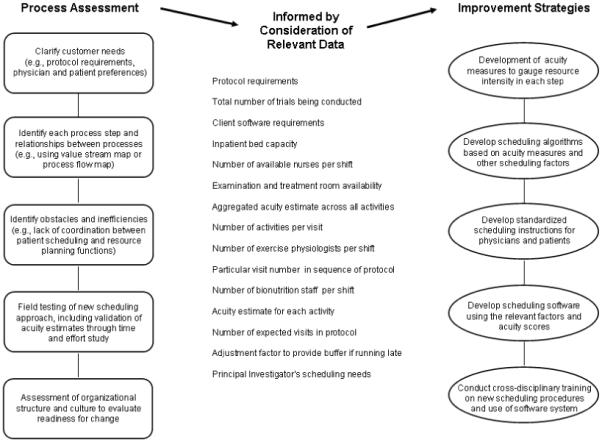
Example of a process improvement project at a clinical trial unit using Lean techniques
This process improvement project illustrates many of the steps typically involved in Lean and Six Sigma analyses. The analysis started with determining customer needs, systematically evaluating each process step in detail, and then identifying sources of inefficiency and waste, while also assessing organizational structure, culture, and management. The analysis was informed by on-site observation and acquisition of process data, for example, relating to patient load, nurse staffing needs, and protocol requirements. This led to the development of proposed strategies to optimize the process. After repeated improvement cycles and field testing, the improved scheduling strategy was incorporated into an integrated computer assisted scheduling system. Training and related support procedures were developed to assure staff understood how to use the new system and to address any concerns they might have with the revised process.
Conclusion
The traditional biomedical research model often features individual research projects that are only loosely linked by discipline and performed in distinctly separate work settings by specialized staff. Traditional research practices at clinical research centers too often suffer from poor coordination, inefficient use of resources, and burdensome administrative requirements. In that traditional model, there is considerable potential for process improvement. In manufacturing and business settings, the transformation of similarly disjointed and disconnected production processes has been enhanced significantly by the introduction of Lean and Six Sigma management strategies.
The NIH Roadmap envisions a new era of clinical and translational research characterized by expanded interconnection between preclinical discovery, clinical trials, and adoption of novel and effective treatments into practice. This new model demands that investigators work outside their organizational boundaries in transdisciplinary teams with greater awareness of the complex intertwined relationships among basic laboratory science, preclinical testing, clinical trials, adoption into practice, and the ultimate effects on individuals and communities.
It is naïve to believe that such a comprehensive view of biomedical research can be achieved without systematic tools and conceptual models for planning, understanding, analyzing, and implementing the diverse processes required for effective clinical and translational research. That is exactly the potential role that Lean and Six Sigma are intended to serve. Those methodologies have been developed and honed in the equally complex environment of manufacturing and systems engineering, where quality, precision, and customer uptake are as critical to overall project success as they are in biomedical research. The rational for the establishment of CTSA sites is rooted in the same underlying philosophies of lean production, customer orientation, cost effectiveness, and process efficiency.
It is important to note that mere application of Lean or Six Sigma techniques is generally not, in itself, sufficient to ensure a successful process improvement project. Achieving better efficiency and process flow also requires a receptive organizational climate, active management support and engagement, sufficient financial and other resources, and clear communications channels within the organization about the process change. As pointed out by Deming and other management theorists, implementing a truly transformative change takes time and shifts in organizational behavior that establish a foundation for process improvements to be accepted and fully integrated into the organization’s routine operations and expectations.55,56 Recognizing the importance of the “softer side” of organizational behavior in process change is a critical component for making a Lean or Six Sigma project succeed.
In this article, we have attempted to illustrate how high-level Lean and Six Sigma principles can be applied to clinical and translational research. The value of these approaches, ultimately, will be measured by whether new and effective treatments become widely used and population health is improved as a result. We expect that within the next two years, the first results of initial applications of these techniques in clinical and translational research will be attained. The results will be of great importance, not only to the NIH, but also to the long-term sustainability of America’s biomedical research enterprise.
Acknowledgments
The project described was supported by Award Number UL1-RR025755 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health..
References
- 1.Ohno T. Toyota Production System: Beyond Large-Scale Production. Productivity Press; New York: 1988. Original Japanese edition published in 1978 by Diamond Press. [Google Scholar]
- 2.Womack J, Jones D. Lean Thinking, Banish Waste and Create Wealth in Your Corporation. Simon & Schuster; New York: 1996. pp. 15–16. [Google Scholar]
- 3.Hackman JR, Wageman R. Total quality management: empirical, conceptual, and practical issues. Admin Sci Quart. 1995;40:309–342. [Google Scholar]
- 4.Powell A, Rushmer R, Davies H. Effective quality improvement: TQM and CQI approaches. Brit J Healthcare Manage. 2009;15(3):114–120. [Google Scholar]
- 5.Bendell T. A review and comparison of Six Sigma and the Lean organizations. TQM Magazine. 2006;18(3):255–262. [Google Scholar]
- 6.Kwak YH, Anbari FT. Benefits, obstacles, and future of six sigma approach. Technovation. 2006;26:708–715. [Google Scholar]
- 7.Arnheiter ED, Maleyeff J. The integration of lean management and Six Sigma. TQM Magazine. 2005;17(1):5–18. [Google Scholar]
- 8.George ML. Lean Six Sigma: Combining Six Sigma Quality with Lean Speed. McGraw-Hill Professional; New York, NY: 2002. [Google Scholar]
- 9.Berwick DM. Continuous improvement as an ideal in health care. N Engl J Med. 1989;320(1):53–56. doi: 10.1056/NEJM198901053200110. [DOI] [PubMed] [Google Scholar]
- 10.Laffel G, Blumenthal D. The case for using industrial quality management science in health care organizations. JAMA. 1989;262(20):2869–2873. [PubMed] [Google Scholar]
- 11.McLaughlin CP, Kaluzny AD. Total quality management in health: making it work. Health Care Manage Rev. 1990;15(3):7–14. doi: 10.1097/00004010-199001530-00002. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin CP, Kaluzny AD. Continuous Quality Improvement in Health Care: Theory, Implementation, and Applications. Aspen Publications; Gaithersburg, MD: 1994. [Google Scholar]
- 13.Young GY, Charns MP, Shortell SM. Top manager and network effects on the adoption of innovative management practices: a study of TQM in a public hospital system. Strat Mgmt J. 2001;22(10):935–951. [Google Scholar]
- 14.McLaughlin CP, Kaluzny AD. Defining quality improvement: past, present, and future. In: McLaughlin CP, Kaluzny AD, editors. Continuous Quality Improvement in Health Care: Theory, Implementation, and Applications. 2nd edition Jones and Bartlett Publishers, Inc.; Sudbury, MA: 1999. p. 10. [Google Scholar]
- 15.Wakefield DS, Cyphert ST, Murray JF, et al. Understanding patient-centered care in the context of total quality management and continuous quality improvement. Jt Comm J Qual Improv. 1994;20(3):152–61. doi: 10.1016/s1070-3241(16)30058-x. [DOI] [PubMed] [Google Scholar]
- 16.Seltzer SE, Kelly P, Adams DF, et al. Expediting the turnaround of radiology reports in a teaching hospital setting. Am J Roentgenol. 1997;168:889–893. doi: 10.2214/ajr.168.4.9124134. [DOI] [PubMed] [Google Scholar]
- 17.Lin B, Clousing J. Total quality management in health care: a survey of current practices. Total Quality Management. 1995;6(1):69–78. [Google Scholar]
- 18.Zbaracki MJ. The rhetoric and reality of total quality management. Admin Sci Quarterly. 1998;43(3):602–636. [Google Scholar]
- 19.Bigelow B, Arndt M. Total quality management: Field of dreams? Health Care Manage Rev. 1995;20(4):15–25. doi: 10.1097/00004010-199502040-00003. [DOI] [PubMed] [Google Scholar]
- 20.Shortell SM, Jones RH, Rademaker AW, et al. Assessing the impact of total quality management and organizational culture on multiple outcomes of care for coronary artery bypass graft surgery patients. Med Care. 2000;38(2):207–217. doi: 10.1097/00005650-200002000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Blumenthal D, Kilo CM. A report card on continuous quality improvement. Milbank Q. 1998;76(4):625–648. doi: 10.1111/1468-0009.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Black K, Revere L. Six Sigma arises from the ashes of TQM with a twist. Inter J Health Care Qual Assur. 2006;19(3):259–266. doi: 10.1108/09526860610661473. [DOI] [PubMed] [Google Scholar]
- 23.Jimmerson C, Weber D, Sobek DK., II Reducing waste and errors: piloting Lean principles at Intermountain Healthcare. J Qual Patient Care. 2005;31(5):249–257. doi: 10.1016/s1553-7250(05)31032-4. [DOI] [PubMed] [Google Scholar]
- 24.De Koning H, Verver JPS, den Heuvel J, et al. Lean Six Sigma in healthcare. J Healthcare Q. 2006;28(2):4–11. doi: 10.1111/j.1945-1474.2006.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 25.Jones D, Mitchell A. Lean Thinking for the NHS. NHS Confederation; London, U.K.: 2006. [Google Scholar]
- 26.King DL, Ben-Tovim DI, Bassham J. Redesigning emergency department patient flows: application of Lean Thinking to health care. Emerg Med Australasia. 2006;18(4):391–397. doi: 10.1111/j.1742-6723.2006.00872.x. 7. [DOI] [PubMed] [Google Scholar]
- 27.Chassin MR. Is health care ready for Six Sigma quality? Milbank Q. 1998;76(4):565–591. doi: 10.1111/1468-0009.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Womack JP, Byrne AP, Flume OJ, et al. Going Lean in Health Care. Institute for Healthcare Improvement; Cambridge, MA: 2005. [Google Scholar]
- 29.Sewail L, DeToung C. Six Sigma in health care. Leadership in Health Services. 2003;16(4):1–5. [Google Scholar]
- 30.Arnold C. Decreasing antibiotic overuse in neonatal intensive care units: quality improvement research. Proc(Bayl Univ Med Cent) 2005;18(3):280–282. doi: 10.1080/08998280.2005.11928083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Young T, Brailsford S, Connell C, et al. Using industrial processes to improve patient care. BMJ. 2004;328:162–164. doi: 10.1136/bmj.328.7432.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gras JM, Philippe M. Application of the Six Sigma concept in clinical laboratories: a review. Clin Chem Lab Med. 2007;45(6):789–796. doi: 10.1515/CCLM.2007.135. [DOI] [PubMed] [Google Scholar]
- 33.Nevalainen D, Berte L, Kraft C, et al. Evaluating laboratory performance on quality indicators with the Six Sigma scale. Arch Pathol Lab Med. 2000;124(4):516–519. doi: 10.5858/2000-124-0516-ELPOQI. [DOI] [PubMed] [Google Scholar]
- 34.Riebling N, Tria L. Six Sigma project reduces analytical errors in an automated lab. Med Lab Observer. 2005;37(6):20, 22–23. [PubMed] [Google Scholar]
- 35.Elder BL. Six Sigma in the microbiology laboratory. Clin Microbiol Newsletter. 2008;30(19):143–147. [Google Scholar]
- 36.Hurley B, Taylor T, Levett J. Implementation of Six Sigma and Lean methodology into the anticoagulation management process. J Thromb Thrombolysis. 2008;25:106. [Google Scholar]
- 37.Condel JL, Sharbaugh DT, Raab SS. Error-free pathology: applying Lean production methods to anatomic pathology. Clin Lab Med. 2004;24(4):865–899. doi: 10.1016/j.cll.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 38.Lindgren V. To err is human; to follow the SOP devine. Int J Cancer. 2008;123:979–980. doi: 10.1002/ijc.23567. [DOI] [PubMed] [Google Scholar]
- 39.Hollensead SC, Lockwood WB, Elin RJ. Errors in pathology and laboratory medicine: consequences and prevention. J Surg Onccol. 2004;88:161–181. doi: 10.1002/jso.20125. [DOI] [PubMed] [Google Scholar]
- 40.Carleysmith SW, Dufton AM, Altria KD. Implementing Lean Sigma in pharmaceutical research and development: a review by practitioners. R&D Management. 2009;39(1):95–106. [Google Scholar]
- 41.National Institutes of Health (NIH) [Accessed March 16, 2009];Re-engineering the Clinical Research Enterprise. [NIH Roadmap for Medical Research Web site]. Available at: http://nihroadmap.nih.gov/clinicalresearch/
- 42.Zerhouni EA. Translational research: moving discovery to practice. Clin Pharmacol Ther. 2007;81:126–128. doi: 10.1038/sj.clpt.6100029. [DOI] [PubMed] [Google Scholar]
- 43.National Institutes of Health (NIH) [Accessed March 16, 2009];Institutional Clinical and Translational Science Award. [NIH Request For Applications, Number: RFA-RM-09-004 Web site]. Available at: http://grants.nih.gov/grants/guide/rfa-files/RFA-RM-09-004.html.
- 44.Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 45.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]
- 46.Fiscella K, Bennett NM, Szilagyi PG. Nomenclature in translational research. JAMA. 2008;299(18):2148–2149. doi: 10.1001/jama.299.18.2148-b. [DOI] [PubMed] [Google Scholar]
- 47.Graham ID, Tetroe J. Nomenclature in translational research. JAMA. 2008;299(18):2149. doi: 10.1001/jama.299.18.2149-a. [DOI] [PubMed] [Google Scholar]
- 48.Westfall JM, Mold J, Fagnan L. Practice-based research - “Blue Highways” on the NIH roadmap. JAMA. 2007;297(4):403–406. doi: 10.1001/jama.297.4.403. [DOI] [PubMed] [Google Scholar]
- 49.Dougherty d, Conway PH. The “3t’s” road map to transform us health care. the “how” of high-quality care. JAMA. 2008;299(19):2319–2321. doi: 10.1001/jama.299.19.2319. [DOI] [PubMed] [Google Scholar]
- 50.Khoury MJ, Gwinn M, Yoon PW, et al. The continuum of translation research in genomic medicine: how can we accelerate the appropriate integration of human genome discoveries into health care and disease prevention? Genetics in Medicine. 2007;9(10):665–674. doi: 10.1097/GIM.0b013e31815699d0. [DOI] [PubMed] [Google Scholar]
- 51.Ablowitz JL, Calhoun TD, Farmer MR, et al. A systems approach to the promotion and implementation of medical translational research at the University of Virginia. Systems and Information Engineering Design Symposium. 2008 April 25;:210–215. [Google Scholar]
- 52.Lui EW. Clinical research: the Six Sigma way. J Assn Lab Automat. 2006;11(1):42–49. [Google Scholar]
- 53.Marti F. Lean Six Sigma method in phase 1 clinical trials: a practical example. Qual Assur J. 2005;9:35–39. [Google Scholar]
- 54.Ullman F, Boutellier R. A case study of Lean drug discovery: from project driven research to innovation studios and process factories. Drug Discovery Today. 2008;13(1112):543–550. doi: 10.1016/j.drudis.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 55.Sewing A, Winchester T, Carnell1 P, et al. Helping science to succeed: improving processes in R&D. Drug Discovery Today. 2008;13(56):227–231. doi: 10.1016/j.drudis.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Deming WE. Out of the Crisis. MIT Press; Cambridge MA: 2000. [Google Scholar]
- 57.Detert JR, Schroeder TG, Mauriel JJ. A framework for linking culture and improvement initiatives in organizations. Acad Mmgt Rev. 2000;25(4):850–863. [Google Scholar]


