Abstract
MUC4, a high–molecular weight transmembrane glycoprotein, is overexpressed in pancreatic cancer and is implicated in its pathogenesis. It is a heterodimeric protein containing a large extracellular, heavily glycosylated subunit, MUC4α, and a transmembrane growth factor–like subunit, MUC4β. In the present study, we have shown the interaction of human MUC4 with the receptor tyrosine kinase HER2 in pancreatic adenocarcinoma cells by reciprocal coimmunoprecipitation and cocapping studies. MUC4 colocalized with HER2 at the cell surface and in the cytoplasm. Silencing of MUC4 by transient or stable expression of MUC4-targeted short-interfering RNA led to the down-regulation of HER2 with a concomitant decrease in its phosphorylated form (pY1248-HER2). Further analyses revealed that the MUC4-knockdown–mediated decrease in HER2 expression occurred due to the drop in the stability of the receptor. In MUC4-knockdown pancreatic cancer cells, we also observed a reduced phosphorylation of the focal adhesion kinase and p42/44 mitogen-activated protein kinase, which are downstream effector proteins in HER2 signaling. Our findings add a new dimension to MUC4 function as a modulator of cell signaling and provide mechanistic evidence for its role in pancreatic cancer progression.
Introduction
Human epidermal growth factor receptor-2 (HER2) belongs to the ErbB family of receptor tyrosine kinases and is implicated in cancer pathogenesis (1). The other members of the ErbB family are ErbB1 (also known as epidermal growth factor receptor or HER1), ErbB3/HER3, and ErbB4/HER4. These receptors have been implicated in multiple cellular functions, including cell proliferation, differentiation, cell survival, tumorigenesis, and metastasis (2, 3). Activation of these receptors occurs upon ligand binding, leading to the initiation of cell signaling. These receptors can be activated by multiple ligands that show either specific binding to the receptor or multiple specificities (2, 3). No ligand, however, has clearly been ascribed to HER2/ErbB2 and its activation is hypothesized to occur through overexpression via homodimerization or through heterodimerization with ligand-activated HER1, HER3, or HER4. In previous studies, sialomucin complex (SMC), which is a rat orthologue of MUC4 mucin (rMuc4), has been shown to act as an intramembrane ligand for ErbB2 inducing its tyrosine phosphorylation (4, 5). MUC4, a transmembrane mucin, shares significant structural similarity with SMC/rMuc4 and has been shown to be of pathogenic significance in pancreatic cancer (6, 7). Like rMuc4, human MUC4 also consists of two subunits: a large mucin-like subunit, MUC4α, containing a highly O-glycosylated tandem repeat (TR) region, and a transmembrane subunit, MUC4β, containing three epidermal growth factor–like domains and a short cytoplasmic tail (8). In the present study, we attempt to examine if human MUC4 mucin could interact with HER2. We provide experimental evidence for the interaction of MUC4 and HER2 in pancreatic cancer cells. Furthermore, we show that the short-interfering RNA (siRNA)–induced silencing of MUC4 leads to the down-regulation of HER2 and diminished downstream signaling. Our findings also suggest that MUC4 regulates the expression of HER2, not at the transcript level, but at the protein level by enhancing the stability of the receptor.
Materials and Methods
Cell culture
The human pancreatic cancer cell lines—CD18/HPAF, CaPan1, and Colo357 were cultured in DMEM supplemented with 10% fetal bovine serum and antibiotics (100 μg/mL penicillin and streptomycin). Cells were grown at 37°C with 5% CO2 in a humidified atmosphere. The medium was changed at alternate days and cells were split before they reached confluency.
Confocal immunofluorescence microscopy
Cells were grown at low density on sterilized coverslips for 20 h. For immunohistofluorescence analysis of formalin-fixed paraffin-embedded pancreatic tumor tissues, serial sections of 4 μm thickness were prepared, deparaffinized with xylene, and rehydrated in an ethanol series. For antigen retrieval, microwave treatment [slides immersed in a 10 mmol/L citrated buffer (pH 6.0), at 95°C for 15 min total] was carried out. Cells/tissue sections were washed with Hanks buffer containing 25 mmol/L of HEPES, and fixed in ice-cold methanol at −20°C for 2 min and processed for the immunofluorescence procedure as described earlier (6). Mouse monoclonal anti-MUC4 antibody (9) and anti-HER2 rabbit polyclonal antibodies (Santa Cruz Biotechnology) were used for the analysis.
Immunoprecipitation and immunoblot analysis
SDS-PAGE, immunoprecipitation, and immunoblotting analyses were done as previously described (6, 9). Antibodies, mouse anti-MUC4 monoclonal antibody (9), anti-HER2 rabbit polyclonal antibody (Santa Cruz Biotechnology), pY1248-HER2 (Upstate Biotech), extracellular signal-regulated kinase (ERK), phosphorylated ERK, focal adhesion kinase (FAK), and phosphorylated FAK (Cell Signaling Tech., Inc.) were used for the analyses.
Capping of MUC4
Cells were detached by using a nonenzymatic cell dissociation solution (Sigma), suspended in PBS and incubated with FITC-conjugated anti-MUC4 antibody (25 μg/mL) for 1 h at 4°C. After washing, the cells were plated on coverslips and incubated for 75 min at 37°C for adherence. Cells were fixed with 3.7% formaldehyde solution, washed, blocked with serum and incubated with anti-HER2 and anti–α2-integrin rabbit polyclonal antibodies (Santa Cruz Biotechnology) for 60 min and processed for confocal microscopy as described earlier (6).
siRNA-mediated silencing of MUC4 expression
CD18/HPAF cells (1.0 × 106 cells) were plated in a six-well plate in 2 mL of antibiotic-free media and allowed to grow until they acquired ∼50% confluency. Cells were transfected with MUC4-targeted siRNA duplex for different time periods (72, 98, and 120 h) at 37°C and protein lysates were prepared for subsequent examination of MUC4 and HER2 expression.
RNA isolation and reverse transcription-PCR analysis
Total RNA was isolated from cell lines as previously described (6). cDNA was synthesized using 2 μg of total RNA, oligo(dT)18 primer, and Superscript reverse transcriptase (Invitrogen Corp.). Quantitative real-time PCR was performed using 2 μL of a 1:50 dilution of first-strand cDNA in FastStart DNA Master SYBR Green I (Roche Applied System) using primers specific to HER2 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in iCycler (Bio-Rad). The following forward and reverse primers were used: HER2, 5′-GGTCCTGGAAGCCACAAGG-3′ and 5′-GGTTTTCCCACCACATCCTCT-3′; GAPDH, 5′-AGCCTCGTCCCGTAGACAAAA-3′ and 5′-GATGACAAGCTTCCCATTCTCG-3′. The efficiency of the PCR reaction and the specificity of primers were determined using serial dilutions of template cDNA and analyzing the melt curve data. Each cDNA sample was used in duplicate, and a corresponding no-cDNA sample was included as a negative control. HER2 mRNA levels were then normalized to the GAPDH mRNA levels, and results were graphically represented as a fold difference in mRNA level in MUC4-overexpressing and knockdown cell lines.
Biotinylation assay
MUC4-overexpressing (CD18/HPAF-SCR) and MUC4-knockdown (CD18/HPAF-siMUC4) cells were surface-biotinylated with cell-impermeable EZ-link Sulfo-NHS-SS-Biotin (1.5 mg/mL; Pierce Biotechnology, Inc.) for 20 min at 0°C. The free biotin was quenched with 50 mmol/L of glycine solution in phosphate buffer saline. After quenching the free biotin, the cells were incubated with medium at 37°C for different time intervals (0, 30, 60, 120, 240, and 480 min). The total cell lysate was made in radioimmunoprecipitation assay lysis buffer and the biotinylated proteins were recovered by incubation with neutravidin beads (Pierce Biotechnology, Inc.).
Determination of the half-life of HER2
The band intensities of biotinylated HER2 following different durations of chase experiments were measured by densitometry. The half-lives were estimated by plotting the curve for the dependent variable of HER2 levels (band intensity) for MUC4-overexpressing (CD18/HPAF-SCR) and MUC4-knockdown (CD18/HPAF-siMUC4) cell lines, and time (30, 60, 120, 240 and 480 min) as an independent variable. We considered the following models:
For each model, the fit was examined using scatter diagrams, the adjusted R-squared statistic, and residual plots. The adjusted R-squared measures how much of the variation in the outcome variable (protein) is accounted for in the model, with 0 indicating that none of the variance was accounted for and 1.00 indicating that 100% of the variance was accounted for.
Results and Discussion
MUC4 colocalizes and interacts with the HER2 oncoprotein in pancreatic cancer cells
Normal epithelial cells have a well-defined morphology with apical, basal, and lateral organization and many cellular proteins exhibit restricted localization to these regions including MUC4, which is present at the apical borders. However, during the course of transformation, the cells lose its polarity, which facilitates novel protein-protein interactions (8). In fact, it has been shown that in nonpolarized cells, MUC1, another member of the transmembrane mucin family, interacts with growth factor receptors such as ErbB family members, which are otherwise restricted to the lateral and basal cell borders (10–12). In previous studies, the rat homologue of MUC4 (rMuc4/SMC) was also shown to interact with ErbB2/neu in multiple cell types (4, 5). Here, we examined the expression and subcellular localization of human MUC4 and HER2 in two MUC4-expressing pancreatic cancer cell lines (CD18/HPAF and CaPan1) using confocal microscopy. In both cell lines, a diffused localization of MUC4 (cell membrane and in cytoplasm) was observed, although HER2 was predominantly observed at the membrane and moderately in the cytoplasmic compartment (Fig. 1A). Digital merging of confocal microscopic images of MUC4 (green) and HER2 (red) depicted a considerable colocalization (yellow) of these proteins in CD18/HPAF (Fig. 1A, a) and Capan1 (Fig. 1A, b) cells. The colocalization of MUC4 and HER2 was observed in both membrane and cytoplasmic regions. Similar observations were also made in pancreatic tumor tissue samples, suggesting their clinical significance (Fig. 1B). We next studied whether MUC4 and HER2 were capable of interacting with each other. For this, we performed reciprocal coimmunoprecipitation analyses for MUC4 and HER2. Our data showed that HER2 was pulled down in MUC4 immunoprecipitates and vice versa; however, the efficiency of direct and cross–pull down was limited (Fig. 2A). This could be either due to an inherent limitation of the antibodies or it may be a masking effect by the interacting protein(s) under immunoprecipitation conditions or due to some other technical regions. Therefore, to further support this interaction at the cell surface on live cells, we carried out MUC4-HER2 cocapping experiments. Live cells in suspension were incubated with FITC-conjugated anti-MUC4 antibody (8G7) generated against the tandem repeat peptide (9). Due to the repeated nature of the epitope, this antibody efficiently clustered the cell surface MUC4 in the form of caps (Fig. 2B). Interestingly, and supporting our coimmunoprecipitation data, when these cells were immunostained with anti-HER2 antibody to study its localization, we observed the coclustering of HER2 protein in MUC4 caps (Fig. 2B, top). This event was specific for HER2 as another surface protein, α2-integrin, did not cocluster with MUC4 (Fig. 2B, bottom). These two observations (coimmunoprecipitation and cocapping) clearly indicate that MUC4 and HER2 exist in a complex in pancreatic cancer cells and thus may be of functional significance in pancreatic cancer progression. What yet remains to be investigated is the structural basis of this interaction and whether MUC4 and HER2 interact directly or require an intermediate adapter molecule to facilitate their interaction. Based on the previous findings with rat Muc4, we may speculate a direct interaction involving one of the three epidermal growth factor domains present in the transmembrane MUC4-β subunit of MUC4 (4, 5). It is also likely that the cytoplasmic tail of MUC4 or glycans present on MUC4 may indirectly interact with HER2. In fact, in a recent report, the interaction of MUC1 with epidermal growth factor receptors has been shown to be bridged by galectin-3, which interacts with the glycosylated MUC1 extracellular domain (13). This finding is important because, in our recent studies, we have also observed that MUC4 interacts with galectin-3.4
Figure 1.
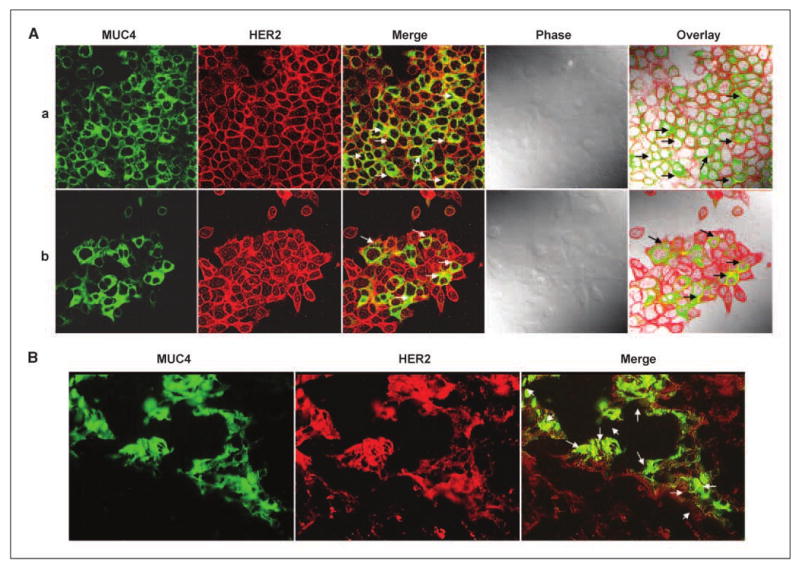
Colocalization of MUC4 and HER2 in pancreatic cancer cells. A, CD18/HPAF (a) and CaPan1 (b) cells were grown at low density on sterilized coverslips, washed, and fixed with methanol, and permeabilized before incubation with antibodies against MUC4 and HER2. Fluorescence signals from each scan were acquired sequentially. MUC4 (green) was detected by using a fluorescein-conjugated secondary antibody; HER2 was identified with CY3-conjugated secondary antibody (red). Dual-merge of MUC4/HER2 (green and red) showed colocalization (white arrows). B, immunohistofluorescence analysis was carried out on formalin-fixed and paraffin-embedded pancreatic tumor tissue sections after rehydration and antigen-retrieval. H&E staining was also performed on the serial sections to study the cellular morphology. Digital merging of the immunostained tissue section clearly showed the colocalization of MUC4 (green) and HER2 (red) in an adenocarcinoma lesion.
Figure 2.
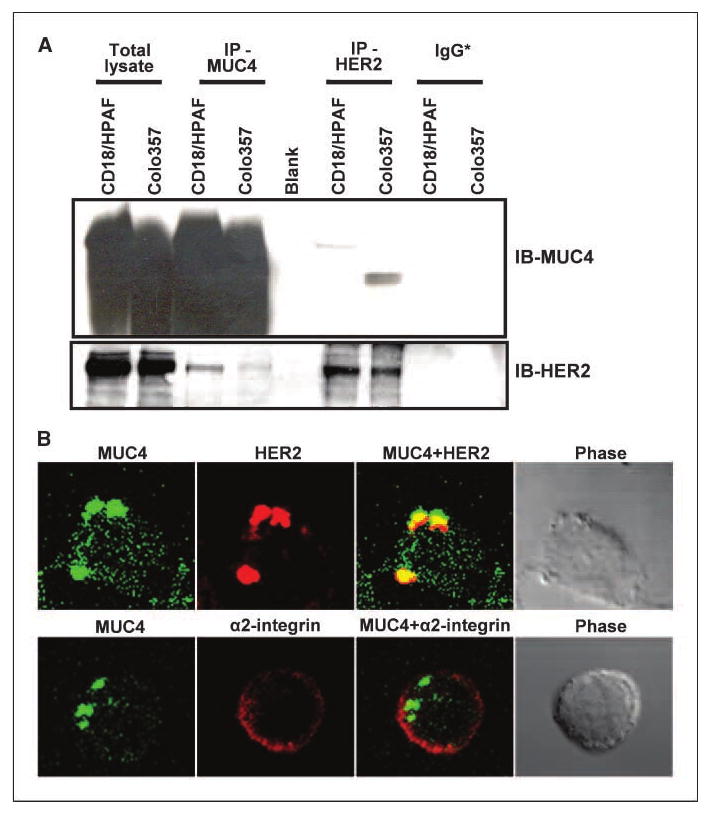
MUC4 and HER2 interact in vivo. A, coimmunoprecipitation assay. Lysates from MUC4-overexpressing cell lines (CD18/HPAF and Colo357) were used for immunoprecipitation with mouse anti-MUC4 and rabbit anti-HER2 antibodies, separately. All cell lines express HER2 at significant levels. The immunoprecipitates were electrophoretically resolved on a 2% agarose (for MUC4) and 10% polyacrylamide gel (for HER2), and immunoblotted with anti-MUC4 or anti-HER2 antibodies. The respective nonspecific IgG* (mouse or rabbit IgGs) was used for immunoprecipitation controls. B, cocapping. CD18/HPAF cells in suspension were incubated with FITC-conjugated anti-MUC4 antibodies for 1 h at 4°C. After washing, the cells were seeded on coverslips and incubated for 75 min at 37°C for adherence. Cells were then fixed, washed, and incubated with anti-HER2 or anti–α2-integrin rabbit polyclonal antibodies for 60 min. After washing, the cells were incubated with CY3-conjugated anti-rabbit secondary antibody and mounted on glass slides in antifade mounting medium. Immunofluorescence staining was detected by a laser-scanning confocal microscope (original magnification, ×100). The digital merging shows that HER2 colocalizes with MUC4 in the clustered area, whereas α2-integrin remains distributed all over the cell surface.
MUC4 regulates the expression of HER2 via enhancing its stability
In our previous study, we observed a correlative decrease in HER2 expression in antisense-RNA–induced stable MUC4-knockdown cells (6). To further validate this finding and examine if this was not an adaptive response in stable cultures, we silenced the expression of MUC4 in CD18/HPAF cells by transient transfection of MUC4-specific siRNA oligos. Significant knockdown of MUC4 expression was observed in siRNA-transfected CD18/HPAF cells at 72, 96, and 120 hours as compared with the parental/control cells with a concomitant decrease in HER2 expression (Fig. 3A). This confirmed that MUC4 could indeed alter HER2 expression. Next, we sought to determine whether the MUC4-mediated regulation of HER2 occurs at the transcript level or is solely a posttranscriptional phenomenon. For this, we used a MUC4-knockdown model that is comprised of scrambled short hairpin RNA–expressing pooled population (CD18/HPAF-SCR) and MUC4-targeted short hairpin RNA–expressing pooled population (CD18/HPAF-siMUC4) derived from CD18/HPAF pancreatic cancer cells (7). The expression of HER2 transcript was measured by quantitative real-time reverse-transcription assay in MUC4-expressing and knockdown cells. In reproducible experiments, we observed no down-regulation of HER2 at the transcript level (data not shown). These observations suggested that MUC4 does not affect the HER2 transcription or the stability of HER2 transcript. Therefore, we investigated the effect of MUC4 overexpression on HER2 turnover by performing the biotinylation-based pulse chase experiments. The cell surface proteins of CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells were biotinylated and incubated at 37°C for different durations to allow endocytosis and subsequent recycling or degradation prior to protein extraction. The biotinylated proteins were immunoprecipitated using neutravidin beads and subsequently immunoblotted for HER2. Band intensity was measured by densitometry to estimate the levels of biotinylated HER2 at different time points. The half-life of HER2 was determined by using growth and exponential models as described in Material and Methods. Figure 3B displays the scatter plots of the HER2 values in CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells. Both models provided similar estimates of the half-life of HER2, and had similar adjusted R-squared values (data not shown). Each model had a large adjusted R-squared value, indicating that each model provides an excellent fit to the observed data. Based on these models, the estimated half-life of HER2 was 198 minutes (3.3 hours) for CD18/HPAF-SCR and 95 minutes (∼1.6 hours) for CD18/HPAF-siMUC4. Altogether, our data clearly show that MUC4 regulates HER2 expression by enhancing its stability. Notably, our observations were not consistent with the previous findings in which rMuc4, although it complexed with ErbB2 and induced phosphorylation, did not affect ErbB2 expression (4, 5). This discrepancy indicates that human MUC4 may follow a distinct mechanism compared with its rat orthologue to deliver its biological action. In a recent study, MUC1 was shown to inhibit the degradation of ErbB1/HER1 by regulating receptor trafficking (12), and therefore, a similar mechanism may also exist for MUC4-mediated HER2 regulation, which needs to be investigated.
Figure 3.

MUC4 regulates the expression of HER2 by enhancing its stability. A, CD18/HPAF cells were transfected with either MUC4-specific siRNA oligos (lanes 3, 6, and 9), scrambled siRNA (lanes 2, 5, and 8), or TransIT-TKO transfection reagent only (lanes 1, 4, and 7) as a control. The cells were harvested at 72, 96, and 120 h, and the lysates were resolved on 2% SDS-agarose gel (for MUC4) and 10% SDS-polyacrylamide gel (for HER2 and β-actin) and immunoblotted with the respective antibodies. B, cells were biotinylated for 20 min at 0°C and the free biotin was quenched. Cells were then incubated with medium at 37°C for different time intervals (0 and 30 min, and 1, 2, 4, and 8 h). The total cell lysates were made in radioimmunoprecipitation assay lysis buffer, the biotinylated proteins pulled down by incubating with neutravidin beads and immunoblotted using anti-HER2 rabbit polyclonal antibodies. The HER2 band intensities for different durations were determined and displayed as scatter plots of the normalized HER2 values in scrambled siRNA-transfected CD18/HPAF-SCR and CD18/HPAF-siMUC4 cells, respectively. The values plotted in the scatter plot represent three independent experiments. Half-lives of HER2 in MUC4-expressing (CD18/HPAF-SCR) and knockdown (CD18/HPAF-siMUC4) cells were ∼3.3 and 1.6 h, respectively.
Effect of MUC4 down-regulation on HER2 downstream signaling
The tumorigenic and metastatic propensity of HER2-overexpressing cells has been contributed to its constitutive activation in tumor cells, which has also been associated with poor prognosis (2). HER2 regulates proliferation and metastasis by activating downstream mitogen-activated protein kinase (MAPK) and phosphoinositide-3-kinase/Akt pathways (14). Enhanced stabilization of HER2 may be linked to the augmentation of downstream signaling, and therefore, we attempted to determine the status of HER2 downstream signaling in our MUC4-knockdown model. Consistent with our previous observations, the data revealed a reduced expression of HER2 in the MUC4-knockdown cells (CD18/HPAF-siMUC4), which also correlated with a decrease in the level of the phosphorylated form of HER2 (pY1248-HER2; Fig. 4A). pY1248 is one of the major autophosphorylation sites in the cytoplasmic region of HER2, and studies with rat Muc4 have previously shown that Muc4 induces the limited phosphorylation of ErbB2 at this site, however, it does not alter the expression of HER2 (4, 5). We next determined the activation status of MAPKs, Akt and FAK, a c-src family kinase that also acts downstream of HER2. We observed a reduced phosphorylation of ERKs (p42/44 MAPK) in CD18/HPAF-siMUC4 cells as compared with CD18/HPAF-SCR cells (Fig. 4A); however, no changes were observed in the activation of JNK and p38 MAPKs (data not shown). The HER2-mediated activation of the ERK pathway plays a crucial role in mediating transformation and cell proliferation. The induction of this pathway is primarily dependent on the Tyr1248/1253 phosphorylation of HER2 and mutating this residue has been shown to substantially diminish the transforming potential of HER2 (15, 16). HER2-mediated transformation can be regulated by other pathways, but all these pathways, such as induction of cyclin D1, also primarily depend on ERK activity (17, 18).
Figure 4.
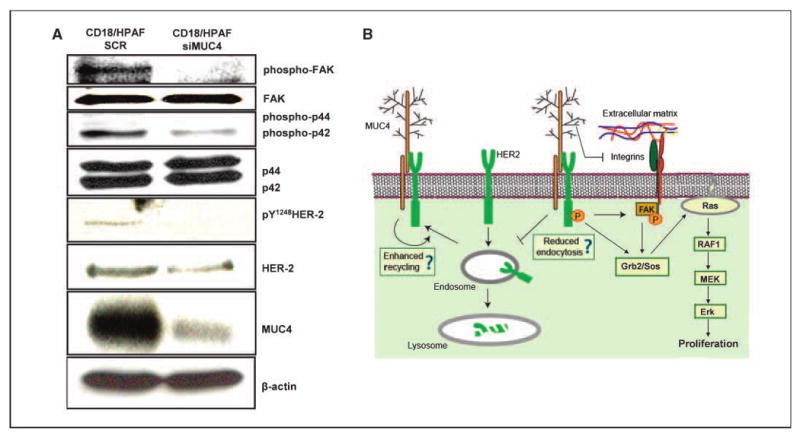
Effect of MUC4 silencing on HER2-downstream signaling. A, total cell lysates from CD18/HPAF-SCR (mixed population of scrambled siRNA-transfected cells) and CD18/HPAF-siMUC4 (pooled population of MUC4 siRNA-transfected cells) cells were used for immunoblotting with MUC4, HER2, pY1248 HER2, ERK, phosphorylated ERK, FAK, and phosphorylated FAK antibodies. For MUC4 detection, proteins were resolved on 2% SDS-agarose gel; other proteins were resolved on 10% SDS-polyacrylamide gel. β-Actin was used as an internal control. B, schematic representation of the proposed functional significance of MUC4-HER2 interaction. We propose that MUC4 enhances the stability of HER2 by either suppressing its endocytosis and/or by augmenting the recycling. Also, the negative effect of MUC4 on integrin downstream (FAK) signaling that may be caused by its interference with the integrin binding to extracellular matrix is neutralized by an alternate mechanism, i.e., induction by HER2.
Interestingly, FAK activation was also significantly reduced in CD18/HPAF-siMUC4 cells as compared with scrambled siRNA-transfected CD18/HPAF-SCR cells (Fig. 4A). FAK is a major protein of the focal adhesion complex that plays a key role in cell migration and matrix survival signals (19, 20). FAK acts downstream of the ErbB receptors and integrins, and its activation results in the induction of multiple signaling molecules, including Src family kinases, Ras-MAPKs and the adaptor protein Grb2. The finding that MUC4-overexpressing cells have heightened the phosphorylation of FAK is particularly important in the context of our previous observations indicating the role of MUC4 in interfering with integrin signaling (7). The present data suggests that the negative effect of MUC4 on integrin signaling is counterbalanced by its potentiation of HER2 signaling. A model has been proposed to depict the functional significance of MUC4 and HER2 interaction in pancreatic cancer (Fig. 4B).
Acknowledgments
Grant support: NIH RO1 grants CA78590, CA113903, CA133774, and LB506.
We thank Drs. Rakesh Singh and Anguraj Sadanandam for help with real-time quantitative PCR, Erik Moore for technical support, the Molecular Biology Core Facility (University of Nebraska Medical Center) for oligonucleotide synthesis and DNA sequencing, the Confocal facility for imaging, and Kristi L.W. Berger (Eppley Institute) for editorial assistance.
Footnotes
Unpublished data.
References
- 1.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341–54. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 3.Warren CM, Landgraf R. Signaling through ERBB receptors: multiple layers of diversity and control. Cell Signal. 2006;18:923–33. doi: 10.1016/j.cellsig.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Carraway KL, III, Rossi EA, Komatsu M, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–6. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 5.Jepson S, Komatsu M, Haq B, et al. Muc4/sialomucin complex, the intramembrane ErbB2 ligand, induces specific phosphorylation of ErbB2 and enhances expression of p27(kip), but does not activate mitogen-activated kinase or protein kinase B/Akt pathways. Oncogene. 2002;21:7524–32. doi: 10.1038/sj.onc.1205970. [DOI] [PubMed] [Google Scholar]
- 6.Singh AP, Moniaux N, Chauhan SC, Meza JL, Batra SK. Inhibition of MUC4 expression suppresses pancreatic tumor cell growth and metastasis. Cancer Res. 2004;64:622–30. doi: 10.1158/0008-5472.can-03-2636. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi P, Singh AP, Moniaux N, et al. MUC4 mucin potentiates pancreatic tumor cell proliferation, survival, and invasive properties and interferes with its interaction to extracellular matrix proteins. Mol Cancer Res. 2007;5:309–20. doi: 10.1158/1541-7786.MCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 8.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–6. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 9.Moniaux N, Varshney GC, Chauhan SC, et al. Generation and characterization of anti-MUC4 monoclonal antibodies reactive with normal and cancer cells in humans. J Histochem Cytochem. 2004;52:253–61. doi: 10.1177/002215540405200213. [DOI] [PubMed] [Google Scholar]
- 10.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–76. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Ren J, Raina D, Chen W, Li G, Huang L, Kufe D. MUC1 oncoprotein functions in activation of fibroblast growth factor receptor signaling. Mol Cancer Res. 2006;4:873–83. doi: 10.1158/1541-7786.MCR-06-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pochampalli MR, el Bejjani RM, Schroeder JA. MUC1 is a novel regulator of ErbB1 receptor trafficking. Oncogene. 2007;26:1693–701. doi: 10.1038/sj.onc.1209976. [DOI] [PubMed] [Google Scholar]
- 13.Ramasamy S, Duraisamy S, Barbashov S, Kawano T, Kharbanda S, Kufe D. The MUC1 and galectin-3 oncoproteins function in a MicroRNA-dependent regulatory loop. Mol Cell. 2007;27:992–1004. doi: 10.1016/j.molcel.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holbro T, Hynes NE. ErbB receptors: directing key signaling networks throughout life. Annu Rev Pharmacol Toxicol. 2004;44:195–217. doi: 10.1146/annurev.pharmtox.44.101802.121440. [DOI] [PubMed] [Google Scholar]
- 15.Amundadottir LT, Leder P. Signal transduction pathways activated and required for mammary carcinogenesis in response to specific oncogenes. Oncogene. 1998;16:737–46. doi: 10.1038/sj.onc.1201829. [DOI] [PubMed] [Google Scholar]
- 16.Ben-Levy R, Paterson HF, Marshall CJ, Yarden Y. A single autophosphorylation site confers oncogenicity to the Neu/ErbB-2 receptor and enables coupling to the MAP kinase pathway. EMBO J. 1994;13:3302–11. doi: 10.1002/j.1460-2075.1994.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seton-Rogers SE, Lu Y, Hines LM, et al. Cooperation of the ErbB2 receptor and transforming growth factor β in induction of migration and invasion in mammary epithelial cells. Proc Natl Acad Sci U S A. 2004;101:1257–62. doi: 10.1073/pnas.0308090100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Timms JF, White SL, O'Hare MJ, Waterfield MD. Effects of ErbB-2 overexpression on mitogenic signalling and cell cycle progression in human breast luminal epithelial cells. Oncogene. 2002;21:6573–86. doi: 10.1038/sj.onc.1205847. [DOI] [PubMed] [Google Scholar]
- 19.Ilic D, Furuta Y, Kanazawa S, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–44. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 20.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]


