Abstract
Background
Transgenic (TG) Ca/calmodulin-dependent protein kinase II (CaMKII)δC mice have heart failure and isoproterenol (ISO)-inducible arrhythmias. We hypothesized that CaMKII contributes to arrhythmias and underlying cellular events and that inhibition of CaMKII reduces cardiac arrhythmogenesis in vitro and in vivo.
Methods and Results
Under baseline conditions, isolated cardiac myocytes from TG mice showed an increased incidence of early afterdepolarizations compared with wild-type myocytes (P<0.05). CaMKII inhibition (AIP) completely abolished these afterdepolarizations in TG cells (P<0.05). Increasing intracellular Ca stores using ISO (10−8 M) induced a larger amount of delayed afterdepolarizations and spontaneous action potentials in TG compared with wild-type cells (P<0.05). This seems to be due to an increased sarcoplasmic reticulum (SR) Ca leak because diastolic [Ca]i rose clearly on ISO in TG but not in wild-type cells (+20±5% versus +3±4% at 10−6 M ISO, P<0.05). In parallel, SR Ca leak assessed by spontaneous SR Ca release events showed an increased Ca spark frequency (3.9±0.5 versus 2.0±0.4 sparks per 100 μm−1·s−1, P<0.05). However, CaMKII inhibition (either pharmacologically using KN-93 or genetically using an isoform-specific CaMKIIδ-knockout mouse model) significantly reduced SR Ca spark frequency, although this rather increased SR Ca content. In parallel, ISO increased the incidence of early (54% versus 4%, P<0.05) and late (86% versus 43%, P<0.05) nonstimulated events in TG versus wild-type myocytes, but CaMKII inhibition (KN-93 and KO) reduced these proarrhythmogenic events (P<0.05). In addition, CaMKII inhibition in TG mice (KN-93) clearly reduced ISO-induced arrhythmias in vivo (P<0.05).
Conclusions
We conclude that CaMKII contributes to cardiac arrhythmogenesis in TG CaMKIIδC mice having heart failure and suggest the increased SR Ca leak as an important mechanism. Moreover, CaMKII inhibition reduces cardiac arrhythmias in vitro and in vivo and may therefore indicate a potential role for future antiarrhythmic therapies warranting further studies.
Keywords: arrhythmia, calcium, excitation-contraction coupling, heart failure, sarcoplasmic reticulum, calcium-calmodulin-dependent protein kinase type 2
Calcium/calmodulin-dependent protein kinase II (CaMKII) is a multifunctional serin/threonine protein kinase. Its expression is increased in human heart failure in both ischemic and dilated cardiomyopathy.1,2 CaMKII in the heart is predominantly present in its δ isoform with the splice variant δC being localized to the cytosol.3 CaMKII regulates numerous intracellular proteins, including sarcolemmal ion channels (eg, L-type Ca and Na channels) and sarcoplasmic reticulum (SR) Ca release channels (RyR) and phospholamban and therefore is important in regulating SR Ca release and Ca reuptake. Thus, CaMKII is critical in the fine tuning of cardiac excitation-contraction coupling.3-7
We previously showed that transgenic (TG) CaMKIIδC overexpression in mouse hearts induces severe heart failure,7 which is accompanied by an increased incidence in electrically and pharmacologically inducible arrhythmias.4 Interestingly, chronic CaMKII inhibition was shown to protect against maladaptive cardiac remodelling after excessive β-adrenergic stimulation with isoproterenol (ISO) and myocardial infarction.8 Moreover, CaMKII inhibition contributed to a reduction in arrhythmias in a TG CaMKIV and a calcineurin mouse model9,10 and after acidosis in rat heart.11 Several intracellular potentially CaMKII-dependent mechanisms have been shown to be involved in this arrhythmogenesis with a main focus on sarcolemmal L-type Ca channel-dependent early afterdepolarizations (EADs).12 However, it is not clear whether the increased incidence of cardiac arrhythmias in TG CaMKIIδC mice is due to a higher CaMKII activity itself or to the mice's heart failure phenotype including altered expression of Ca-handling proteins, as described previously7 and what the mode of action for the arrhythmogenesis might be, ie, whether other channels in addition to L-type Ca channels such as RyRs may be involved.
Therefore, we hypothesized that CaMKII activity contributes to cardiac arrhythmogenesis in TG CaMKIIδC mice with heart failure4 and that CaMKII inhibition would reduce proarrhythmogenic events despite the heart failure phenotype. To test this, we isolated cardiac myocytes from TG CaMKIIδC mice having heart failure and wild-type (WT) littermates.4,13 Myocytes were current clamped and investigated using epifluorescence and confocal microscopy. Cellular proarrhythmogenic events in vitro were defined as (i) afterdepolarizations of the membrane potential; (ii) spontaneous elementary SR Ca release events (Ca sparks); and (iii) nonstimulated (spontaneous) Ca transients and myocyte contractions (nonstimulated events [NSEs]). Experiments were performed under baseline and Ca-loaded conditions (using ISO). All experiments were performed with and without CaMKII inhibition (AIP or KN-93).4,7,13 Moreover, cells from mice specifically lacking CaMKIIδ (CaMKIIδ-KO) were used for comparison.14 In addition, we also tested whether pharmacological CaMKII inhibition could reduce ISO-dependent arrhythmias in TG CaMKIIδC mice in vivo.4
We found a dramatically increased incidence of proarrhythmogenic events in vitro in TG myocytes under basal and ISO-stimulated conditions presumably due to both L-type Ca channel (EADs) and RyR-malfunctions (delayed afterdepolarizations [DADs] and SR Ca sparks). Furthermore, we could show that pharmacological CaMKII inhibition clearly reduced arrhythmogenic events in vitro and cardiac arrhythmias in vivo in TG CaMKIIδC mice having heart failure. Moreover, CaMKIIδ-inhibition using a CaMKIIδ-KO mouse model14 also reduced arrhythmogenic events despite β-adrenergic stimulation. We conclude that CaMKII activity can contribute to cardiac arrhythmogenesis in TG CaMKIIδC mice and suggest the SR Ca leak as an important factor in arrhythmogenesis. Therefore, reducing CaMKII activity may provide novel antiarrhythmic therapies in heart failure, especially because CaMKII inhibition seems to act antiarrhythmic with respect to several intracellular arrhythmogenic triggers.
Methods
Mouse Cardiac Myocyte Isolation
TG CaMKIIδC mice (n=43 mice, 13.4±0.5 mg/g) had a ≈1.7-fold increased heart weight to body weight ratio compared with their WT littermates (n=26 mice, 8.0±0.5 mg/g, P<0.05), which is consistent with their heart failure phenotype reported previously.4,7,13 CaMKIIδ-KO mice had a heart to body weight ratio of 7.2±0.4 mg/g (n=8 mice) and were generated as published previously by Backs et al14 Cardiac myocyte isolation from TG, WT, and KO mouse hearts was performed as described previously.4,13 In brief, mice were anesthetized in a gas chamber with isoflurane, hearts were excised and mounted on a Langendorff perfusion apparatus and were retrogradely perfused with a nominally Ca-free Tyrodes solution containing (in mM) NaCl 113, KCl 4.7, KH2PO4 0.6, Na2HPO4×2H2O 0.6, MgSO4×7H2O 1.2, NaHCO3 12, KHCO3 10, HEPES 10, Taurine 30, 2,3-butanedione 10, glucose 5.5, phenol red 0.032 for 4 minutes at 37°C (pH 7.4). Then, 7.5 mg/mL liberase 1 (Roche Diagnostics, Mannheim, Germany), trypsin 0.6%, and 0.125 mmol/L CaCl2 were added to the perfusion solution. Perfusion was continued for ≈3 minutes until the heart became flaccid. Ventricular tissue was removed, cut into small pieces, and dispersed until no solid cardiac tissue was left. Ca reintroduction was performed carefully through stepwise increasing [Ca] from 0.1 to 0.8 mmol/L. Shortly after, cells were plated onto superfusion chambers, with the glass bottoms treated with laminin to allow cell adhesion and then used for immediate measurements. All investigations conform to the “Guide for the Care and Use of Laboratory Animals” published by the US National Institutes of Health (publication No. 85-23, revised 1996).
Current-Clamp Measurements
Current-clamp measurements were performed at 2 stimulation frequencies (0.5 and 1 Hz) to elicit action potentials (APs) on an inverted Nikon T 300 microscope using a HEKA EPC 10 patch-clamp-setup (Heka Electronics, Inc, Lambrecht, Germany). In brief, low-resistance pipettes (usually ≈3 MΩ) were pulled and filled with an internal solution containing (in mM) K-aspartic acid 120, KCl 8, NaCl 7, MgCl2 1, Mg-ATP 5, and HEPES 10. Cells were superfused with an external solution containing (in mM) KCl 5.4, NaCl 135, MgCl2 1, HEPES 10, and glucose 10 at physiological temperature (36±1°C) and [Ca] (1 mmol/L). Resting cell membrane potentials were similar in WT (−74.4±0.8 mV, n=25) and TG (±75.8±0.8 mV, n=31) ventricular myocytes. When appropriate, CaMKII was blocked by adding 0.1 μmol/L AIP to the pipette solution. EADs were observed as spontaneous oscillations during the plateau phase of the AP and verified by 3 different investigators. In some experiments, ISO (at 10−8 M) was superfused for 5 minutes and cells were investigated for DADs and spontaneous APs. All measurements were performed by a blinded investigator.
Shortening and Ca Measurements Using Epifluorescence Microscopy
Shortening and [Ca]i measurements were performed simultaneously and performed as reported previously6,13 using a Nikon Eclipse TE2000-U inverted microscope provided with an IonOptix fluorescence detection system. Myocytes were loaded with either fluo-3 or fura-2 by incubation with 10 μmol/L of the acetoxymethyl ester form of the dyes (Molecular Probes, Eugene, Ore) and incubated for 15 minutes at room temperature in darkness. Emitted fluorescence was collected using a photomultiplier at ≈535±20 nm for fluo-3 and ≈510 nm for fura-2, respectively. Ca transient amplitudes were calculated by means of peak fluorescence divided through baseline fluorescence (F/F0, fluo-3) after subtracting background fluorescence.13 The amount of Ca that forms a transient could be semiquantified using .7,15 For fura-2, Ca transient amplitude was assessed as the 340/380-nm fluorescence ratio (F340 nm/F380 nm). Myocytes were field stimulated (voltage ≈25% above threshold) at 1 Hz and were exposed to increasing concentrations of ISO (0, 10−9 to 10−6 M ISO) in an experimental solution containing (in mM) NaCl 140, KCl 4, MgSO4 7H2O 1, glucose 10, HEPES 5, and MgCl2 1 at 37°C and pH 7.4. To avoid interference with fluorescence measurements, cells were transilluminated by red light (>650 nm), and shortening was measured using a sarcomere length detection system (IonOptix Corp, Milton, Mass). In addition, we measured ISO-dependent changes in diastolic [Ca]i. CaMKII inhibition was ensured by superfusing isolated cells for 15 minutes using the organic CaMKII-inhibitor KN-93 (1 μmol/L)13 compared with its inactive analogue KN-92 (1 μmol/L)5,16-18 for comparison. However, some experiments were performed using cells from CaMKIIδ-KO mice specifically lacking CaMKIIδ.14 Cellular arrhythmias, which are detectable with our epifluorescence system, were defined as NSE that describe spontaneous Ca-transient increases with subsequent contractions of myocyte. Thereby early NSE (ENSE) as approximate measures for systolic arrhythmogenic events—occurring before the turning point of the Ca transient decay—were separated from late NSE (LNSE). LNSE were observed after the turning point of the Ca transient decay and considered as diastolic arrhythmogenic events. LNSE were categorized as sustained events if they outlast >3 seconds at an intrinsic unstimulated frequency above 3 Hz. All measurements were performed by blinded investigators.
Confocal Microscopy
Ca sparks measurements were performed in fluo-4-loaded (10 μmol/L, 20 minutes incubation) TG or KO cardiac myocytes on a laser scanning confocal microscope (Zeiss LSM 5 PASCAL, Göttingen, Germany) as reported previously.6,7,19 Fluo-4 AM-loaded cells were excited through an argon laser (at 488 nm), and emitted fluorescence was collected at 505 nm through a long-pass emission filter.19 Cells were superfused with normal Tyrode solution (see above) or the same solution containing 10−7 M ISO. Ca sparks were analyzed with using Zeiss software.
Arrhythmia Induction and CaMKII Inhibition In Vivo
TG mice were anesthetized using 2.5% Avertin as reported previously,4 and an equivalent of lead I ECG (ECG) recording was performed. After stabilization of the preparation, a control ECG was recorded for 5 minutes. This was followed by an intraperitoneal injection of ISO (2 mg/kg body weight) and a subsequent recording period of ≈10 minutes. During this period, ECGs were analyzed for ISO-induced arrhythmias. CaMKII inhibition was performed in the same mouse on different days. In brief, 20 μmol/L/kg KN-93 or KN-92, respectively, was injected intraperitoneal ≈12 minutes before ISO application under continuous ECG recording. Then, ISO was applied as described above, and ECGs were recorded for 10 minutes and analyzed for arrhythmias.
Statistics
Data are presented as mean±SEM. Two-way analysis of variance (ANOVA) for repeated measurement tests combined with Student-Newman-Keuls post hoc test was performed for Figure 2B through 2D, Student t tests for Figures 3C, 3D, 4B, 4C, and 6C, and Fisher 2-sided exact tests for Figures 1B, 1D, 5B through 5D, 5F, and 6D. Values of P<0.05 were considered statistically significant.
Figure 2.
Effects of ISO on intracellular Ca transients, relaxation time 80% (RT80%), and diastolic Ca. A, Original Ca tracings showing the clear positive inotropic effects of ISO in WT and the diminished effects in TG. Average values for intracellular Ca transient amplitudes (B), relaxation (C), and diastolic Ca (D). *Significant different values versus baseline using Student-Newman-Keuls post hoc test. #Significant difference versus corresponding value of WT using ANOVA.
Figure 3.
ISO increases SR Ca leak. A, Original line-scan images showing increased Ca spark frequency and decreased Ca spark occurrence in the presence of CaMKII inhibition (1 μmol/L KN-93) and in a myocyte specifically lacking CaMKIIδ isoform (left column). B, Original Ca spark tracings (middle column). C, Average values of Ca spark frequency. D, Average Ca spark amplitude. E, Calculated SR Ca leak (Ca spark frequency×amplitude×duration). Student t test was performed for C and D.
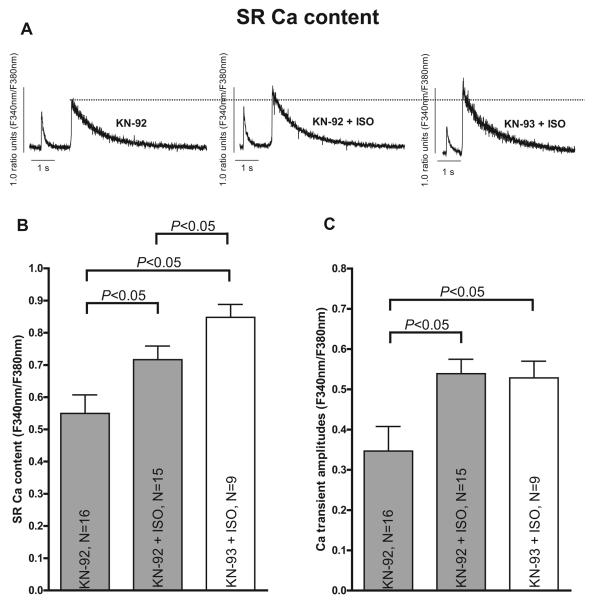
Figure 4.
ISO increases SR Ca content in TG CaMKII cells (1 μmol/L KN-92 and KN-93). A, Original SR Ca content tracings measured by caffeine contractures (10 mmol/L). Average values for SR Ca content (B) and Ca transient amplitude (C). P<0.05 indicates significance using Student t test.
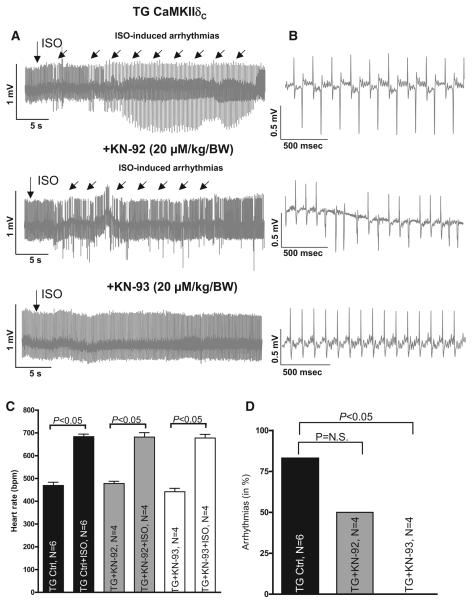
Figure 6.
A, Original ECG recordings in one representative mouse showing ISO-induced arrhythmias and inhibition of these using (20 μmol/L/kg body weight KN-93). B, Detailed tracings for respective arrhythmic events. C, Mean data showing similar effects of ISO on heart rate in all mice as calculated using Student t test. D, Mean data showing decreased arrhythmias in vivo by inhibition of CaMKII using Fisher 2-sided exact test.
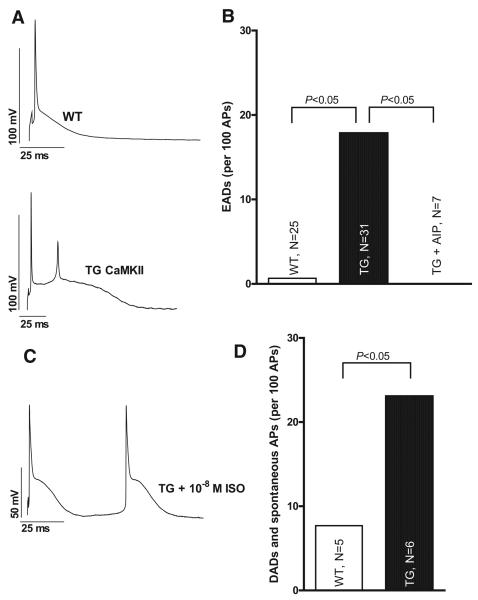
Figure 1.
CaMKII overexpression increases incidence of afterdepolarizations. A, Original patch-clamp recordings showing increased AP duration and EADs in a myocyte from a TG CaMKII mouse. B, Mean data for EAD occurrence at 0.5 Hz. CaMKII inhibition (0.1 μmol/L AIP) decreases EAD occurrence. C, ISO treatment (10−8 M ISO) induces DADs and spontaneous APs in TG myocytes. D, Average data for DAD and spontaneous AP occurrence. Statistical analysis was performed using Fisher 2-sided exact test.
Figure 5.
NSE of Ca transients and contractions. A, Original recordings showing ENSE and LNSE for Ca transients and shortening. B, Mean values for ENSE and LNSE showing increased incidence for TG. Decreased incidence of ENSE (C) and LNSE (D) after KN-93 treatment (1 μmol/L) and in cells from a mouse model specifically lacking CaMKIIδ isoform. E, Original recording showing sustained NSE and inhibition of these (F) using KN-93. No SNSEs were seen in KO cells. Statistical analysis was performed using Fisher 2-sided exact tests.
Results
CaMKIIδC Overexpression Increases the Incidence of CaMKII-Dependent Afterdepolarizations In Vitro
Because Wagner et al4 found a higher incidence of inducible arrhythmias in vivo in TG CaMKIIδC mice, we tested whether isolated myocytes from TG hearts exhibited proarrhythmogenic afterdepolarizations on a cellular level. As depicted in an original trace in Figure 1A, TG CaMKIIδC overexpression resulted not only in increased AP duration as previously reported7 but also in a higher incidence of EADs, which may arise from the >2-fold increased AP duration. Average values showed significantly more EADs in TG compared with WT hearts (Figure 1B; 0.5 Hz; P<0.05, using Fisher 2-sided exact test). A clearly increased EAD incidence was also found at 1-Hz stimulation in TG versus WT myocytes (55 EADs during 661 APs in 30 myocytes of 15 TG hearts versus 13 EADs during 456 APs in 24 cells from 14 WT hearts; P<0.05, using Fisher 2-sided exact test). Importantly, CaMKII inhibition (using 0.1 μmol/L AIP) significantly reduced the incidence of EADs in TG CaMKIIδC at 0.5 Hz as shown in Figure 1B and similarly at 1-Hz stimulation (down to 0 EADs during 140 APs in 7 cells from 7 TG hearts; P<0.05 versus untreated TG myocytes, using Fisher 2-sided exact test). These data show that CaMKII-overexpressing cells from mice having heart failure have an increased incidence of proarrhythmogenic EADs on a cellular level. Most importantly, CaMKII inhibition reduced those events pointing to the fact that CaMKII activity directly may contribute to cardiac arrhythmogenesis under basal conditions in vitro. However, we failed to detect a significantly increased incidence of DADs under basal conditions (0.5- or 1-Hz stimulation rate; 1 mmol/L [Ca]o), despite a previously reported increased Ca spark frequency.6,7 This could be due to the well-known reduced SR Ca content of TG cells6,7,13 leading to small amplitude Ca sparks7 that were probably insufficient to produce distinct detectable oscillations of the membrane potential through the transient inward current (Iti).20 Interestingly, Fujiwara et al21 also found that low-K–perfused hearts exhibited Ca waves sporadically between Ca transients but without discernible membrane depolarization. Moreover, Ca waves induced membrane potential oscillations and triggered activity when they emerged synchronously and intensively in the heart. This gives nice evidence to the fact that Ca oscillations and hence sole Ca sparks do not necessarily result in detectable DADs if they do not reach a certain threshold. However, ISO treatment (10−8 M for 5 minutes) showed an increased incidence of DADs and spontaneous APs in TG cardiac myocytes (P<0.05 versus WT using Fisher 2-sided exact test; Figure 1C and 1D) This seems to be important because under physiological conditions (ie, at high stimulation rates or in the presence of β-adrenergic stimulation), SR Ca load and [Ca]i increases substantially and may induce further arrhythmogenic triggers that could be of a higher relevance for arrhythmias in vivo (and even under pathophysiological conditions such as in heart failure, catecholamine levels are known to be increased). Therefore, we decided to challenge the cells using ISO (up to 10−6 M) to load the SR Ca stores of TG cells and to further unmask their potential diastolic proarrhythmogenic events.
CaMKIIδC Overexpression Impairs the ISO-Dependent Inotropic Increase in Isolated Myocytes but Increases Diastolic [Ca]
We first examined isolated TG and WT myocytes using epifluorescence microscopy under basal and ISO-stimulated conditions (10−9 to 10−6 M ISO). Figure 2A shows that increasing the ISO concentrations increased the Ca transient amplitudes in TG cardiac myocytes (solid line) and in WT control cells (dashed line). However, average values shown in Figure 2B show an impaired increase in Ca transient amplitude (Δ[Ca]i in nanomolar) in TG cells (n=31 cells) versus WT (n=29 cells, P<0.05 using ANOVA). Similarly, ISO-dependent effects on fractional shortening (in % resting cell length, % resting cell length [RCL]) were significantly impaired in TG compared with WT myocytes (6.29±0.54% RCL at 10−9 M ISO versus 10.93±0.62% RCL at 10−7 M ISO in TG versus 7.41±0.56% RCL at 10−9 M ISO versus 12.2±0.73% RCL at 10−7 M ISO in WT, P<0.05 using ANOVA).
Whether this difference in the inotropic response was due to impaired SR Ca reuptake (and hence SR Ca release) can be assessed by twitch relaxation parameters. From Figure 2C, it can be appreciated that the extent of the ISO response (≈30% to 35%) was similar in CaMKIIδC-overexpressing cells and WT control myocytes, although TG myocytes had a ≈10% to 20% prolonged relaxation time throughout all ISO concentration steps (ANOVA P<0.05, Figure 2C), most likely due to the decreased sarcoplasmic reticulum Ca-ATPase expression.7 This behavior indicates an only slightly affected SR Ca uptake on ISO stimulation in TG myocytes but points to an increased SR Ca loss in TG cells. Indeed, a significant increase in diastolic [Ca]i could be detected in TG in the presence of ISO by 15±4% (at 10−7 M ISO) but without any changes at all in WT myocytes (+2±2% at 10−7 M ISO, ANOVA P<0.05, Figure 2D). Even when stimulating myocytes in the presence of 10−7 M ISO from 0.1 to 4 Hz, diastolic [Ca] increased clearly in TG by 29±6% but only modestly in WT (+14±2%, P<0.05 using ANOVA).
To test whether the effect of CaMKII on diastolic [Ca]i also occurs without heart failure phenotype (and without protein expression changes of intracellular Ca-handling proteins), we overexpressed CaMKII by adenoviral transfection for 24 hours in rabbit cardiac myocytes as reported previously.6,13 We saw a similar difference in the increase of diastolic [Ca]i in CaMKII-overexpressing rabbit myocytes with +37.5±8.6% versus LacZ control with +18.5±5.4% at 10−7 M ISO (P<0.05 using ANOVA) in the presence of unchanged protein expression, pointing to the importance of an CaMKII-dependent increased SR Ca leak even under these conditions.
Because elementary SR Ca release events are the main mechanism for SR Ca loss, we assessed SR Ca sparks in TG and WT mouse myocytes in a next step.
CaMKIIδC Overexpression Increases Diastolic SR Ca Leak Under Ca-Loaded Conditions In Vitro
Figure 3A shows representative SR Ca sparks in TG myocytes under basal conditions in the presence of KN-92 (first panel). ISO stimulation (10−7 M) increased Ca spark frequency (second panel) but not after preincubation with the CaMKII inhibitor KN-93 (third panel) or in myocytes specifically lacking CaMKIIδ isoform (KO, fourth panel). Mean values (Figure 3C) demonstrate a significantly lower SR Ca spark frequency in KN-93 versus KN-92 myocytes at 10−7 M ISO (2.0±0.4 versus 3.9±0.5 sparks per 100 μm−1·s−1, P<0.05 versus KN-92 using Student t test, Figure 3C). Moreover, CaMKIIδ-KO cells showed a Ca spark frequency of only 1.7±0.2 sparks per 100 μm−1·s−1 on ISO (P<0.05 versus KN-92+ISO using Student t test). The lowest values were found for KN-92 without ISO (2.0±0.4 sparks per 100 μm−1·s−1) and even lower for KN-93 without ISO (1.1±0.3 sparks per 100 μm·s, n=3) where almost no sparks occur at all (ie, only 7% sparking myocytes in the KN-93 without ISO group versus 22% in the KN-92 without ISO group). Although Ca spark characteristics were similar between all groups (Figure 3B and 3D), calculated SR Ca leak (Ca spark frequency×amplitude×duration) was almost doubled in KN-92+ISO group compared with control, and KN-93 was able to reduce this diastolic SR Ca leak almost to control levels (Figure 3E). Of note, diastolic Ca leak was found to be even further decreased to a third in cells from CaMKIIδ-KO mice on ISO-stimulated conditions.
Because Ca sparks and SR Ca leak, in general, depend largely on SR Ca load,22 we wanted to know whether the decreased SR Ca leak in the presence of KN-93 mainly is due to a reduced SR Ca content or whether it is a specific inhibiting effect on SR Ca release.
Isoproterenol-Induced Changes in SR Ca Content in the Presence of CaMKII Inhibition
The increase in SR Ca content in the presence of ISO is expected and a well-known effect (Figure 4A and 4B). Thus, KN-92–treated TG cells showed a significant increase in SR Ca content by ≈30% on ISO stimulation (from 0.55±0.06, n=16 up to 0.72±0.04 at 10−7 M ISO, P<0.05 using Student t test, Figure 4B). Similarly, SR Ca content was found to be significantly increased up to 0.85±0.04 (n=9) in CaMKII-inhibited TG cells compared with basal conditions (≈+54% versus KN-92 without ISO, P<0.05 using Student t test) and compared with KN-93 (n=10) without ISO with 0.64±0.08 (≈+16%). Furthermore, CaMKII inhibition (KN-93) also induced a further increase in SR Ca loading compared with KN-92–treated cells on ISO stimulation (≈+18% versus KN-92 on 10−7 M ISO, P<0.05 using Student t test, Figure 4B). This seems to be in line with a ≈10% lower diastolic [Ca]i in KN-93– versus KN-92–treated cells on ISO stimulation (0.50±0.02, n=9, versus 0.55±0.03, n=15), which may point to the fact that CaMKII-inhibited cells indeed have a decreased diastolic Ca loss from the SR on ISO. However, this finding fits nicely to the decreased Ca spark frequency on ISO in KN-93–treated TG cells as shown in Figure 3C. In addition, data for intracellular Ca transients (Figure 4C) show ISO-dependent increases in parallel to SR Ca content in TG cells. It is well accepted that SR Ca spark frequency and amplitude usually correlate well with SR Ca load.22 Therefore, the decreased SR Ca leak, as shown in Figure 3E, is most likely due to a specific (inhibiting or stabilizing) effect on SR Ca release because we even found an increased SR Ca load in the presence of KN-93. A specific and inhibiting effect on SR Ca release is underlined by an additional finding that SR Ca fractional release was reduced during systolic activation in KN-93 myocytes compared with KN-92 (64±7%, n=9, versus 75±3%, n=15) on ISO stimulation.
Moreover, in contrast to the differences with respect to SR Ca release, SR Ca reuptake as assessed by Ca transient decay (to 50% of Ca transient amplitude) showed very similar ISO effects in the presence of KN-92 (to 106±5 ms versus 174±15 ms for baseline without ISO, P<0.05 using Student t test) compared with KN-93 (106±5 ms, P<0.05 versus KN-92 control using Student t test), suggesting contributions of SR Ca reuptake to be unlikely involved in decreased SR Ca leak. However, do these proarrhythmogenic effects on intracellular Ca cycling really translate into cellular arrhythmias?
CaMKIIδC Overexpression Increases the Incidence of CaMKII-Dependent NSEs on ISO Stimulation
Figure 5A shows representative nonstimulated Ca transients (above) and nonstimulated myocytes contractions (below) that have been frequently observed in TG myocytes. On the left panel, an ENSE is depicted with a nonstimulated Ca increase before the turning point of the Ca transient decay, which was accompanied by a minor cell contraction. In contrast, LNSEs, shown on the right panel (Figure 5A), occur after the turning point of the Ca transient decay and appear with major contractions of the myocytes. However, average data in Figure 5B show a significantly increased incidence of early (left, 54.4% versus 4.3%, P<0.05 using Fisher 2-sided exact test) and late (right, 86.4% versus 42.9%, P<0.05 using Fisher 2-sided exact test) NSEs in TG versus WT cells on increasing ISO concentrations. An arrhythmia-inducing protocol (100-second recording period at 10−7 M ISO, Figure 5C and 5D) shows the averaged incidence of ENSEs (Figure 5C) and LNSE (Figure 5D) with and without CaMKII inhibition. Pharmacological CaMKII inhibition (using KN-93) as well as CaMKIIδ-KO significantly decreased the incidence of ENSEs and LNSEs (using Fisher exact test, P<0.05), whereas KN-92 exhibited only minor, nonsignificant effects with respect to the NSE incidence, arguing against effects mediated by L-type Ca channel inhibition.
Interestingly, severe sustained NSEs (>3 seconds, unstimulated frequency >3 Hz, Figure 5E) were predominant in TG myocytes and rarely seen in WT (42.4% of 33 cells versus 3.2% of 31 cells, P<0.05 using Fisher exact test, Figure 5F). Moreover, these events could be completely abolished using CaMKII inhibition (down to 0% of 8 cells, P<0.05 using Fisher exact test) and were not seen in KO cells (n=52, P<0.05 using Fisher exact test).
In summary, CaMKII-overexpression not only increases the incidence of spontaneous proarrhythmogenic events such as afterdepolarizations (see above) and ENSEs but also leads to a dramatically enhanced frequency of LNSEs (and SNSEs) in vitro. Importantly, CaMKII inhibition was sufficient to reduce all types of proarrhythmogenic cellular events pointing to its potential antiarrhythmic relevance in failing cardiac myocytes with increased CaMKII activity.
However, because it was still unclear whether these cellular events could also be inhibited in vivo, we performed experiments in anesthetized mice.
CaMKII Inhibition Decreases the Incidence of Cardiac Arrhythmias In vivo in TG CaMKIIδC Mice Having Heart Failure
Because we had found several antiarrhythmic effects of CaMKII inhibition on cellular levels, we tested, in a last step, whether CaMKII inhibition is sufficient to reduce cardiac arrhythmias in vivo in TG CaMKIIδC mice having heart failure. Three representative ECG records are shown in Figure 6A, whereas Figure 6B depicts detailed tracings for respective arrhythmic events resembling most likely the previously described bidirectional tachycardias. All experiments were performed on the same mouse on different days. The TG mouse (upper panel) exhibited ISO-dependent arrhythmias even after KN-92 treatment (20 μmol/L/kg body weight, 12 minutes before ISO application, middle panel). However, KN-93 (20 μmol/L/kg body weight) was able to prevent cardiac arrhythmias in TG CaMKII mice (lower panel). Average values with respect to heart rate (in beats per minute) demonstrate that ISO-dependent increases in heart rate were similar for all groups (Figure 6C, P<0.05 using Student t test), making different ISO effects on heart rate after KN-93 and KN-92 treatment for arrhythmogenesis unlikely. Average data shown in Figure 6D showed that KN-93 significantly reduced cardiac arrhythmias in TG CaMKII mice in vivo (0 of 4 mice in KN-93–treated group versus 5 of 6 mice in untreated group exhibited arrhythmias in the first 10 minutes after ISO application, P<0.05 using Fisher exact test). However, KN-92 also had a slight effect on the incidence of cardiac arrhythmias in TG mice but this small effect failed to be significant. Therefore, CaMKII inhibition itself reduced cardiac arrhythmias in vivo, confirming its beneficial effects with respect to arrhythmogenesis on the cellular level and extending its relevance to the in vivo level.
Discussion
This study shows that CaMKII contributes to cardiac arrhythmogenesis in TG CaMKIIδC mice having heart failure. We identified the increased SR Ca leak as an important proarrhythmogenic mechanism in failing TG CaMKII cells under Ca-loaded conditions. Most importantly, we demonstrate that CaMKII inhibition reduces these proarrhythmogenic events pointing to its potential antiarrhythmic relevance in heart failure. Moreover, we suggest a novel, genetic approach of isoform-specific CaMKIIδ inhibition to result in only few arrhythmogenic events despite β-adrenergic stimulation, which could be of importance in future antiarrhythmic therapies. In line with our cellular findings, we show that CaMKII inhibition also decreases arrhythmias in vivo in TG CaMKIIδC mice. Therefore, we conclude that targeting increased CaMKII activity may provide novel antiarrhythmic therapies in heart failure.
TG CaMKIIδC Mice as a Model of Increased CaMKII Activity and Heart Failure
TG CaMKIIδC overexpression was shown to be associated with heart failure7 and with arrhythmias in vivo.4 CaMKII activity is increased by ≈3-fold in TG CaMKIIδC mice,23 which is similar to its increased CaMKII activity observed in failing human hearts.1,2 Therefore, the TG CaMKIIδC mouse can be regarded not only as a model of increased CaMKII activity but also as a pathophysiological relevant model of heart failure.
However, no data investigating the role of CaMKII in the arrhythmogenesis in TG CaMKIIδC mouse and what potential mechanism may lead to proarrhythmogenic effects had been gathered yet. Moreover, it is not clear whether CaMKII activity itself had contributed to cardiac arrhythmias observed by Wagner et al,4 or whether they are simply due to the mice's heart failure phenotype, including altered expression of Ca-handling proteins.4,7
TG CaMKIIδC Overexpression Increases the Incidence of Systolic, CaMKII-Dependent EADS
The results of this study demonstrate that TG CaMKIIδC overexpression increases the incidence of cellular proarrhythmogenic events. First, we could identify an increased incidence of EADs under basal conditions (ie, without ISO), which is in line with reports from Mark Andersons Laboratory, showing that CaMKII activity is associated with the generation of these systolic proarrhythmogenic events.5,9 CaMKII activity can contribute to L-type Ca current facilitation7,8 and action potential duration prolongation,7,24 as shown previously, and therefore may favor EAD generation5,9 and probably torsade de pointes arrhythmias,25 which could provide one explanation for arrhythmias observed in vivo by Wagner et al4 However, we found that CaMKII inhibition clearly reduced the incidence of EADs in TG CaMKIIδC cells, thereby possibly inhibiting CaMKII-dependent effects on L-type Ca channels6,7 and maybe also late INa,4 pointing to the fact that CaMKII inhibition may contribute to arrhythmia reduction in vitro and therefore probably also in vivo.
ISO Fails to Increase Ca Transients but Unmasks an Increased Incidence of Diastolic, CaMKII-Dependent Cellular Arrhythmias in TG CaMKII Myocytes
Patch-clamp experiments under basal conditions showed systolic proarrhythmogenic events in TG cells (ie, EADs) rather than DADs, despite their well-documented diastolic SR Ca leak.6,7,26 Therefore, we decided to fill SR Ca stores of TG cells using isoproterenol (up to 10−6 M ISO) to unmask potential diastolic cellular arrhythmias. Thus, ISO (at 10−8 M) induced an increased incidence of DADs and spontaneous APs, which points to possible diastolic events on ISO stimulation probably generated by Iti as previously reported by Wu et al20 These authors could convincingly show that the Na/Ca-exchanger was responsible for Iti because the Na/Ca-exchanger–inhibiting peptide XIP (Na/Ca exchanger inhibitory peptide) clearly reduced this arrhythmogenic current. In the same study, it was shown that SR Ca release contributed to the arrhythmogenesis because SR storage–interfering drugs (eg, ryanodine or thapsigargin) as well as CaMKII inhibition decreased Iti.20 This is in line with our findings in CaMKIIδC-overexpressing myocytes showing increased SR Ca leak (ie, increased Ca spark frequency),6,7 which can be dramatically reduced by CaMKII inhibition using KN-93 or AIP6,7 or by direct inhibition of SR Ca leak using tetracaine.6
However, to our surprise, ISO-dependent increase in Ca transient amplitudes was greatly impaired in TG cells, despite an almost similar acceleration of relaxation on ISO stimulation. In parallel, diastolic [Ca]i rose dramatically in TG compared with WT control cells, most likely due to the increase in SR Ca spark frequency as a measure for an increased SR Ca leak. Indeed, when calculating this leak, it was increased 2-fold on ISO addition but could be decreased back to control levels in the presence of CaMKII inhibition. To assess that the decrease in Ca sparks in the presence of CaMKII inhibition does not depend on a decreased SR Ca content, we also measured SR Ca load by caffeine contractures and did not find a decreased, but a rather increased, SR Ca content confirming previous results27 and the CaMKII dependency of the arrhythmogenic SR Ca leak (rather than simply decreased SR Ca leak due to decreased SR Ca content). Interestingly, CaMKIIδ-KO14 resulted not only in a clearly decreased Ca spark frequency, which resulted in low diastolic Ca leak despite β-adrenergic stimulation, but also in a significantly lowered incidence of all types of proarrhythmogenic NSEs. Therefore, this specific, genetic approach may indicate a future therapeutic alternative to pharmacological CaMKII inhibition, which unspecifically affects all CaMKII isoforms. In summary, these data give further evidence to the relevance of the SR Ca leak in TG CaMKII mice because it dramatically disturbs myocytes' SR Ca handling not only under basal conditions7 but also during β-adrenergic stimulation.
In fact, ISO unmasked a dramatically increased incidence of LNSEs in TG cells compared with WT, which is in line with the increased SR Ca leak and increased diastolic [Ca] (as shown in Figures 2 and 3). It should be noted that ISO may also activate CaMKII directly through exchange protein directly activated by cAMP-dependent pathways and indirectly through increasing [Ca]i,28 thus resulting in more dramatic CaMKII-dependent cellular arrhythmias in vitro. Moreover, these results could have an important clinical impact because ISO concentrations (eg, 10−7 M) used in this study reflect pathophysiological ranges found in human heart failure,29 and of note, ≈40% of this patients die because of arrhythmias.30
However, LNSEs occur after the turning point of the Ca transient during diastole and therefore represent an additional class of arrhythmogenic events in case of TG CaMKII overexpression. Again, and most importantly, pharmacological and genetic CaMKII inhibition dramatically reduced their appearance. Especially, sustained self-preserving NSEs seemed to be of great CaMKII dependency, as Figure 5E and 5F show that sustained NSE could be abolished by pharmacological CaMKII inhibition and were not seen in CaMKIIδ-KO cells. Regarding these self-preserving NSEs, we suggest CaMKII autophosphorylation of Thr287 as an important mechanism.31 The enzyme's preserved activation augments further diastolic [Ca]i increase and increases CaMKII activity from beat to beat.32 One possible consequence of this positive feedback is a decreasing threshold for NSE in vitro and accordingly for cardiac arrhythmias in vivo.
In summary, these data suggest that the CaMKII-mediated SR Ca leak, which has a well-documented relevance in the development of heart failure, contributes also to the development of cellular arrhythmias in failing cells during β-adrenergic stimulation, which is in line with previous findings by Zhang et al.8
CaMKII Activity Contributes to Cardiac Arrhythmogenesis In Vivo in TG CaMKIIδC Mice Having Heart Failure
As already mentioned above, CaMKIIδC-overexpressing mice were shown to have more arrhythmias in vivo.4 However, it was not clear whether these arrhythmias depend on CaMKII activity itself or whether they are simply due to the heart failure phenotype of TG mice.4,7,13 Therefore, we injected 20 μmol/L/kg body weight of the CaMKII-inhibiting drug KN-93 in TG CaMKIIδC mice having heart failure and found a pattern of arrhythmias resembling the previously described bidirectional tachycardias associated with increased SR Ca leak (Figure 6B). We then performed a pharmacological arrhythmia-induction protocol (2 mg/kg body weight ISO).4 In the presence of KN-93, we found a clear decrease in the incidence of resulting arrhythmias compared with the untreated control group, whereas the inactive analogue KN-92 only tended to decrease arrhythmias. However, and of great relevance, CaMKII inhibition clearly and significantly reduced the incidence of cardiac arrhythmias in vivo, thereby extending our in vitro findings to the in vivo level.
Pathophysiological Implications and Potential Limitations of CaMKII Inhibition
CaMKII overexpression contributes to the development of heart failure,7 is associated with arrhythmias,4,9-11 and has detrimental consequences in irreversible ischemia and perfusion injury.33 In line with this, CaMKII inhibition protects against maladaptive structural cardiac remodeling after excessive β-adrenergic stimulation and myocardial infarction.8 Moreover, targeting CaMKII activity reduced arrhythmias in several experimental studies.9-11 Furthermore, this study extends this body of knowledge, suggests the SR Ca leak as an important, diastolic CaMKII-dependent cellular trigger in failing TG CaMKII cells with respect to cardiac arrhythmogenesis, and gives further evidence to the potential antiarrhythmic role of CaMKII inhibition in heart failure. Moreover, we suggest a novel genetic approach of isoform-specific CaMKII inhibition (CaMKIIδ-KO) as a promising tool in future antiarrhythmic therapy. Therefore, growing evidence has been gathered that CaMKII inhibition (either pharmacologically or genetically) would provide new therapeutic options in a wide area of cardiovascular diseases.
In contrast, CaMKII seems to have some specific physiological roles, including a contribution to a preserved contractility during acidosis13,34,35 or during stunning.36 Furthermore, frequency-dependent acceleration of relaxation has been discussed to be CaMKII dependent, but the role of CaMKII for frequency-dependent acceleration of relaxation remains highly controversially.37,38 However, further studies are needed to evaluate the possible detrimental effects of long-term CaMKII inhibition with respect to its physiological functions.
In summary, we conclude that CaMKII contributes to cardiac arrhythmogenesis in TG CaMKIIδC mice in vitro and in vivo and that CaMKII inhibition may provide a novel antiarrhythmic approach in heart failure.
CLINICAL PERSPECTIVE.
Heart failure is a life-threatening disease, and its prevalence is still increasing in a population of rising life expectancy. An important cause of death for patients suffering from heart failure is cardiac arrhythmia. Therefore, investigating cardiac arrhythmogenesis in heart failure is of major clinical relevance. It has been reported that myocardium of patients with heart failure shows increased levels of Ca/calmodulin-dependent protein kinase II (CaMKII) expression and activity. CaMKII is a multifunctional protein kinase that phosphorylates several intracellular target proteins, including sarcolemmal L-type Ca channels and Na channels as well as ryanodine receptors (RyR), which regulate Ca release from intracellular Ca stores (ie, from the sarcoplasmic reticulum). Of note, CaMKII has already been suggested to be involved in cardiac arrhythmogenesis. However, early afterdepolarizations occurring during systole, mainly caused by increased L-type Ca channel activity, were thought to be the main cause. The actual study now provides a novel mechanism for CaMKII-dependent arrhythmogenesis in heart failure, which has been unmasked on isoproterenol stimulation (using isoproterenol concentrations found in human heart failure). We found that CaMKII activity contributed to a class of diastolic arrhythmogenic events, which were most likely due to higher sarcoplasmic reticulum Ca release, as measured by a higher incidence of elementary sarcoplasmic reticulum Ca release events (Ca sparks) and late nonstimulated events (eg, nonstimulated Ca transients and nonstimulated myocytes shortening). Most importantly, CaMKII inhibition was sufficient to reduce those arrhythmogenic events not only in vitro but also in an in vivo approach. Therefore, CaMKII inhibition may offer a future antiarrhythmic therapy for patients suffering from heart failure.
Acknowledgments
We thank the technical assistance of Sarah Weber, Timo Schulte, and Thomas Sowa.
Sources of Funding
Dr Lars Maier was funded by the Deutsche Forschungsgemeinschaft (DFG) through grants for a Clinical Research Group KFO155 (MA 1982/2-1 and 2-2) and a Heisenberg-Professorship (MA 1982/4-1), as well as by a Hengstberger grant through the Deutsche Gesellschaft für Kardiologie (DGK). Dr Backs was supported by the Emmy Noether-Program of the DFG. Dr Heller Brown was supported by NIH grant HL080101. Dr Sag was funded by a grant from the Faculty of Medicine (Anschubfinanzierung) of the Georg-August University Göttingen. Dr Olson was supported by grants from the NIH, the American Heart Association, the Donald W. Reynolds Foundation, the Leducq Foundation, and the Robert A. Welch Foundation. This work was supported in part by the Fondation Leducq Award to the Alliance for Calmodulin Kinas Signaling in Heart Disease.
Footnotes
Disclosures
None.
References
- 1.Kirchhefer U, Schmitz W, Scholz H, Neumann J. Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res. 1999;42:254–261. doi: 10.1016/s0008-6363(98)00296-x. [DOI] [PubMed] [Google Scholar]
- 2.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 3.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–640. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+channels. J Clin Invest. 2006;116:3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson ME, Braun AP, Wu Y, Lu T, Wu Y, Schulman H, Sung RJ. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharmacol Exp Ther. 1998;287:996–1006. [PubMed] [Google Scholar]
- 6.Kohlhaas M, Zhang T, Seidler T, Zibrova D, Dybkova N, Steen A, Wagner S, Chen L, Brown JH, Bers DM, Maier LS. Increased sarcoplasmic reticulum calcium leak but unaltered contractility by acute CaMKII overexpression in isolated rabbit cardiac myocytes. Circ Res. 2006;98:235–244. doi: 10.1161/01.RES.0000200739.90811.9f. [DOI] [PubMed] [Google Scholar]
- 7.Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–911. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 8.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Temple J, Zhang R, Dzhura I, Zhang W, Trimble R, Roden DM, Passier R, Olson EN, Colbran RJ, Anderson ME. Calmodulin kinase II and arrhythmias in a mouse model of cardiac hypertrophy. Circulation. 2002;106:1288–1293. doi: 10.1161/01.cir.0000027583.73268.e7. [DOI] [PubMed] [Google Scholar]
- 10.Khoo MS, Li J, Singh MV, Yang Y, Kannankeril P, Wu Y, Grueter CE, Guan X, Oddis CV, Zhang R, Mendes L, Ni G, Madu EC, Yang J, Bass M, Gomez RJ, Wadzinski BE, Olson EN, Colbran RJ, Anderson ME. Death, cardiac dysfunction, and arrhythmias are increased by calmodulin kinase II in calcineurin cardiomyopathy. Circulation. 2006;114:1352–1359. doi: 10.1161/CIRCULATIONAHA.106.644583. [DOI] [PubMed] [Google Scholar]
- 11.Said M, Becerra R, Palomeque J, Rinaldi GJ, Kaetzel M, Díaz-Sylvester P, Copello JA, Dedman JR, Mundina-Weilenmann C, Vittone L, Mattiazzi A. Increased intracellular Ca2+ and SR Ca2+ load contribute to arrhythmias after acidosis in rat heart. Role of Ca2+-calmodulin dependent protein kinase II. Am J Physiol Heart Circ Physiol. 2008;295:H1669–H1683. doi: 10.1152/ajpheart.00010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res. 2007;73:657–666. doi: 10.1016/j.cardiores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Sag CM, Dybkova N, Neef S, Maier LS. Effects on recovery during acidosis in cardiac myocytes overexpressing CaMKII. J Mol Cell Cardiol. 2007;43:696–709. doi: 10.1016/j.yjmcc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson JA, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci USA. 2009;106:2342–2347. doi: 10.1073/pnas.0813013106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Blair LA, Marshall J. CaMKII-independent effects of KN93 and its inactive analog KN92: reversible inhibition of L-type calcium channels. Biochem Biophys Res Commun. 2006;345:1606–1610. doi: 10.1016/j.bbrc.2006.05.066. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca(2+)-calmodulin-dependent protein kinase II on cardiac excitation-contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501(Pt 1):17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris TA, DeLorenzo RJ, Tombes RM. CaMK-II inhibition reduces cyclin D1 levels and enhances the association of p27kip1 with Cdk2 to cause G1 arrest in NIH 3T3 cells. Exp Cell Res. 1998;240:218–227. doi: 10.1006/excr.1997.3925. [DOI] [PubMed] [Google Scholar]
- 19.Mauritz C, Schwanke K, Reppel M, Neef S, Katsirntaki K, Maier LS, Nguemo F, Menke S, Haustein M, Hescheler J, Hasenfuss G, Martin U. Generation of functional murine cardiac myocytes from induced pluri-potent stem cells. Circulation. 2008;118:507–517. doi: 10.1161/CIRCULATIONAHA.108.778795. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y, Roden DM, Anderson ME. Calmodulin kinase inhibition prevents development of the arrhythmogenic transient inward current. Circ Res. 1999;84:906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res. 2008;103:509–518. doi: 10.1161/CIRCRESAHA.108.176677. [DOI] [PubMed] [Google Scholar]
- 22.Shannon TR, Ginsburg KS, Bers DM. Quantitative assessment of the SR Ca2+ leak-load relationship. Circ Res. 2002;91:594–600. doi: 10.1161/01.res.0000036914.12686.28. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J, Jr, Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–919. doi: 10.1161/01.RES.0000069686.31472.C5. [DOI] [PubMed] [Google Scholar]
- 24.Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca/Calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–3847. doi: 10.1529/biophysj.107.114868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazur A, Roden DM, Anderson ME. Systemic administration of calmodulin antagonist W-7 or protein kinase A inhibitor H-8 prevents torsade de pointes in rabbits. Circulation. 1999;100:2437–2442. doi: 10.1161/01.cir.100.24.2437. [DOI] [PubMed] [Google Scholar]
- 26.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/Calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res. 2006;99:398–406. doi: 10.1161/01.RES.0000236756.06252.13. [DOI] [PubMed] [Google Scholar]
- 27.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 28.Pereira L, Métrich M, Fernández-Velasco M, Lucas A, Leroy J, Perrier R, Morel E, Fischmeister R, Richard S, Bénitah JP, Lezoualc'h F, Gómez AM. The cAMP binding protein Epac modulates Ca2+ sparks by a Ca2+/calmodulin kinase signalling pathway in rat cardiac myocytes. J Physiol. 2007;583:685–694. doi: 10.1113/jphysiol.2007.133066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klensch H. The basal noradrenaline level in human peripheral venous blood. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290:218–224. [PubMed] [Google Scholar]
- 30.Packer M. Sudden unexpected death in patients with congestive heart failure: a second frontier. Circulation. 1985;72:681–685. doi: 10.1161/01.cir.72.4.681. [DOI] [PubMed] [Google Scholar]
- 31.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annu Rev Physiol. 1995;57:417–445. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 32.Maier LS, Bers DM. Calcium, calmodulin, and calcium-calmodulin kinase II: heartbeat to heartbeat and beyond. J Mol Cell Cardiol. 2002;34:919–939. doi: 10.1006/jmcc.2002.2038. [DOI] [PubMed] [Google Scholar]
- 33.Vila-Petroff M, Salas MA, Said M, Valverde CA, Sapia L, Portiansky E, Hajjar RJ, Kranias EG, Mundiña-Weilenmann C, Mattiazzi A. CaMKII inhibition protects against necrosis and apoptosis in irreversible ischemia-reperfusion injury. Cardiovasc Res. 2007;73:689–698. doi: 10.1016/j.cardiores.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Mattiazzi A, Vittone L, Mundiña-Weilenmann C. Ca2+/calmodulin-dependent protein kinase: a key component in the contractile recovery from acidosis. Cardiovasc Res. 2007;73:648–656. doi: 10.1016/j.cardiores.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 35.DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca transients and SR Ca reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol. 2004;36:67–74. doi: 10.1016/j.yjmcc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Valverde CA, Mundiña-Weilenmann C, Reyes M, Kranias EG, Escobar AL, Mattiazzi A. Phospholamban phosphorylation sites enhance the recovery of intracellular Ca2+ after perfusion arrest in isolated, perfused mouse heart. Cardiovasc Res. 2006;70:335–345. doi: 10.1016/j.cardiores.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 37.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002;34:975–984. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- 38.Huke S, Bers DM. Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII. J Mol Cell Cardiol. 2007;42:590–599. doi: 10.1016/j.yjmcc.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]