Abstract
Avian influenza A viruses continue to cause disease outbreaks in humans, and extrapulmonary infection is characteristic. In vitro studies demonstrate the activity of oseltamivir against avian viruses of the H5, H7 and H9 subtypes. In animal models of lethal infection, oseltamivir treatment and prophylaxis limit viral replication and improve survival. Outcomes are influenced by the virulence of the viral strain, dosage regimen and treatment delay; it is also critical for the compound to act systemically. Observational data on oseltamivir treatment in the early stages of disease suggest it is useful for improving survival in patients infected with H5 viruses, and drug-selected resistance has only rarely been reported. The WHO strongly recommends oseltamivir for the treatment of confirmed or suspected cases of human H5 infection and prophylaxis of those at high risk of infection. In addition to oral dosing, nasogastric administration appears to be a viable option for the management of severely ill patients, as is the use of higher doses and prolonged schedules. F. Hoffmann-La Roche Ltd, the manufacturer of oseltamivir, is developing a mathematical model to allow rapid prediction of appropriate dosage regimens for any future pandemic. Roche is also funding the Avian Influenza Registry, an online database that aims to collect information from clinicians worldwide on the course of avian influenza in humans.
Keywords: H5N1, treatment, prophylaxis, clinical, pre-clinical
Introduction
Influenza A viruses cause recurrent epidemics with substantial human morbidity and mortality, and are also associated with pandemics.1 As pandemic influenza A has its origins in avian influenza viruses, the highly pathogenic H5N1 viruses that have become panzootic in domestic and wild fowl have to be considered a serious threat.1–3 H5N1 is principally a zoonosis, with transmission in the first instance typically from fowl, or fowl product, to humans, although the primary source of exposure cannot always be determined.4 Recent evidence supports the occurrence of limited human to human transmission,4–9 while evidence from Pakistan supports human to human to human (third-generation) transmission.8
The typical clinical manifestation of H5 infection is influenza-like illness, with the development of dyspnoea and progression to severe illness, usually typified by fulminant pneumonia complicated by acute respiratory distress syndrome (ARDS); multiorgan failure and death may follow.2,10,11 Gastrointestinal disturbances may also be evident, and the illness may present as a gastroenteritis-like condition.12 Neurological signs and symptoms have also been reported.13,14 Cases of mild illness and asymptomatic infection are evident,4,15–18 but the extent of these less dramatic infections remains largely unknown.
Common laboratory findings of abnormality include leucopenia, lymphopenia and thrombocytopenia.2,11 Mortality is high; up to 31 August 2009, of 440 laboratory-confirmed cases of H5N1 infection reported to the WHO since 2003, 262 (60%) were fatal.19 Although most cases have been reported in South-East Asia,19 Egypt accounts for the third greatest number of cases, and a significant outbreak was reported from Eastern Turkey.18 Wild birds in a number of EU countries have tested positive for H5, and 14 outbreaks in domestic poultry had been reported in six member states by early December 2007.20
Avian viruses of the H7 subtype also cause human disease outbreaks, and appear to have a predilection for the conjunctiva, as well as causing respiratory illness. In an outbreak in the Netherlands, a H7N3 virus of avian origin was detected in 89 persons, of whom 85 presented with conjunctivitis and/or influenza-like illness.21 Symptoms were generally mild, but a fatal case of pneumonia in combination with ARDS was reported. In the UK, three outbreaks of H7N2 disease with influenza-like illness and/or conjunctivitis have been reported;22–25 in one of these outbreaks, three of the four cases documented were hospitalized.22 Cases of H7 disease have also been reported in the USA (H7N2),26 Canada (H7N3)27 and Italy (H7N3).28 In some of these outbreaks, seroconversion was not detectable in all patients with symptomatic disease. The reason for this is unclear, but it seem likely that some H7 infections go undetected.29 Symptomatic infections with H9N2 viruses were reported in Hong Kong, but the illness appears to be mild.30 Seropositivity for H9 has also been reported from China.31
In the face of these developments, the antiviral medication oseltamivir (Tamiflu®; F. Hoffmann-La Roche Ltd) is recommended on the basis of observational data and systemic activity by the WHO as the primary treatment for H5N1 infection, with particular potential for reducing mortality if used in the early stages of disease.32,33 Oseltamivir also appears active in the attempts to control disease outbreaks caused by H7 avian viruses.34,35
Treatment experience
Pre-clinical studies
H5N1 viruses isolated from infected humans have been shown to be susceptible to oseltamivir in pre-clinical studies in vitro and in animal models.36–38 Two studies focused on the influenza A/HK/156/97 (H5N1) strain that was transmitted to humans in Hong Kong in 1997 and killed 6 of 18 people infected. Leneva et al.38 showed that oseltamivir carboxylate inhibited replication of the virus in Madin–Darby canine kidney (MDCK) cells with an EC50 (50% effective concentration) of 7.5 ± 2.5 µmol/L. In this study, mice given 1 or 10 mg/kg oseltamivir after lethal challenge with A/HK/156/97 (H5N1) did not die and lost less weight than controls; mice treated with 0.1 mg/kg showed improved survival. In a similar study of the same viral strain, 90% of infected animals survived when given 10 mg/kg/day oseltamivir for 5 days starting 24 h after lethal infection.36 When treatment was started 60 h after infection, 65% survived. Systemic efficacy was demonstrated by the prevention of spread of virus to the brain by daily dosages of 1 and 10 mg/kg. Oseltamivir was also active against the viral subtype A/quail/HK/G1/97 (H9N2) in both of these studies.36,38
The importance of early treatment has been shown by effects on more recent clinical isolates successfully treated with oseltamivir in animals. Ferrets were protected from lethal infection with influenza A/Vietnam/1203/04 (H5N1) by 5 mg/kg oseltamivir daily when started 4 h after infection, but 25 mg/kg daily was needed when treatment was started after 24 h.37 A dosage of 10 mg/kg daily attenuated viral symptoms and blocked viral spread to internal organs when animals were challenged with the less pathogenic A/Turkey/15/06 strain.
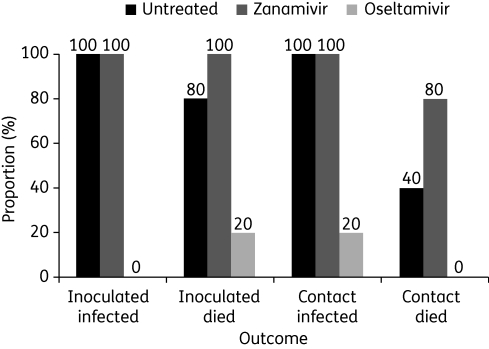
The significance of systemic action has been highlighted by results in chickens infected with influenza A/Chicken/Pennsylvania/1370/83 (H5N2). Chickens infected with virus were caged with contact chickens; treated birds received either intratracheal 1 mg/kg zanamivir or oral 120 mg/kg oseltamivir twice daily from 1 day before infection to 7 days afterwards. Monitoring for infection was carried out daily to day 8 and then on day 14. As indicated in Figure 1, zanamivir (which is distributed to the lungs only) had no effect on rates of infection and mortality while oseltamivir showed beneficial effects.39
Figure 1.
Infection and mortality in chickens inoculated with and in contact with influenza A/Chicken/Pennsylvania/1370/83 (H5N2); n = 5 chickens per group. ‘Infection’ refers to systemic infection indicated by virus isolation from the cloaca (with or without tracheal infection). Figure derived from data in Meijer et al.39
Clinical studies
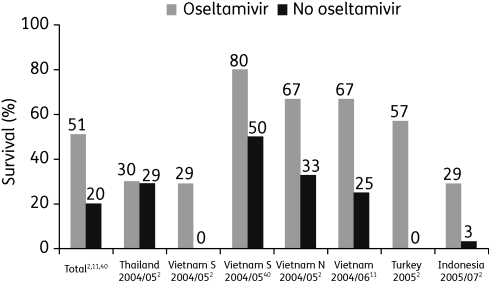
Evidence pointing to the activity of oseltamivir against avian influenza in pre-clinical studies is supported by data in humans who have contracted H5N1. A summary of results from clinical case series in patients who contracted clade 1 or 2 influenza A (H5N1) viruses and who recovered after treatment with oseltamivir is shown in Figure 2.2,11,40 Overall, results from uncontrolled studies suggest that survival is improved ∼2-fold by the use of oseltamivir, with early therapy being recommended.2,11,40 However, the WHO recommends that late presentation should not preclude the initiation of oseltamivir therapy.32
Figure 2.
Survival among H5N1-infected patients treated with oseltamivir. Figure derived from data in WHO Writing Committee,2 Liem et al.11 and Hien et al.40
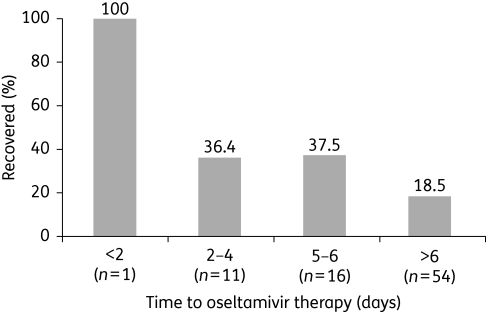
Mortality remains high despite use of oseltamivir, but delayed initiation of treatment appears to be a key factor, as exemplified by the most recent analysis of data from Indonesia,41 experience in Egyptian patients42 and pooling of results for the years 2004–06 from Vietnam, Thailand, Indonesia and Turkey.43 Indonesia has put in place surveillance and referral systems for human H5N1 infections since February 2004, and data are available for 127 patients with confirmed onset from June 2005 to February 2008 (this includes 54 included in a previous data set with a case fatality rate of 76%).44 Of the 127 patients, 103 (81%) died; 88 patients were treated with oseltamivir (median time to treatment of 7 days). Patients with early oseltamivir treatment showed significantly increased survival (P = 0.03 for trend; Figure 3).41 Pooled data from Vietnam, Thailand, Indonesia and Turkey show similar survival in patients treated with oseltamivir (13/34; 38%) and those not treated (4/16; 25%).43 However, when time to treatment was accounted for, patients receiving oseltamivir within 5 days of onset showed 53% survival (8/15) compared with 26% of those who received oseltamivir ≥6 days after onset of disease (5/19).
Figure 3.
Rates of recovery according to time to oseltamivir treatment in patients infected with influenza A/H5N1 in Indonesia from June 2005 to February 2008. Initiation of treatment within 2 days was associated with significantly lower mortality than initiation after 5–6 days or later (P < 0.0001). Figure derived from data in Kandun et al.41
To further investigate the clinical features, disease course and impact of antiviral treatment in human H5N1 infections, Roche is funding the AVEX Avian Influenza Registry, an online database that aims to collect information from clinicians worldwide.45 This will be one of the largest sources of such information available. Data from 177 patients in eight countries were available for initial analysis as of August 2009.45 The median age of patients was 20 years (range: 1.25–67 years) and 49% were male. Treatment with oseltamivir significantly improved crude survival rates for patients who received at least one dose [45/78 (58%) versus 10/87 (11%) for patients who received no antiviral; P < 0.0001]. A significant survival benefit over no antiviral treatment remained even when oseltamivir was not initiated until days 6–8 of illness [12/23 (52%) patients given oseltamivir surviving versus 20/101 (20%) for no antiviral; P = 0.003], but early treatment provided the greatest impact on mortality [survival on days 1–2; 6/8 (75%) among patients treated with oseltamivir versus 45/153 (29%) untreated patients; P = 0.014].45
Prophylaxis experience
Pre-clinical studies
Oseltamivir was effective as prevention in the murine studies of the 1997 Hong Kong virus discussed earlier when 5 day courses were started 4 h before potentially lethal infection with A/HK/156/97 (H5N1); 100% survival was reported with daily dosages ≥0.1 mg/kg (Table 1).36,38
Table 1.
Effects on survival of oseltamivir as prevention started 4 h before infection with avian influenza A viruses in animal models
| Reference (animal type) | Virus | Treatment | Dosage (mg/kg) | Survivors/total | Mean survival (days) |
|---|---|---|---|---|---|
| Govorkova et al.36 (mice) | A/HK/156/97 (H5N1) | oseltamivir ×5 days | 0.01 | 2/6 | 11.3 |
| 0.1 | 6/6 | 16.0 | |||
| 1.0 | 5/5 | 16.0 | |||
| control | 0/11 | 6.3 | |||
| A/quail/HK/G1/97 (H9N2) | oseltamivir ×5 days | 0.01 | 5/11 | 12.2 | |
| 0.1 | 4/11 | 11.9 | |||
| 1.0 | 11/11 | 16.0 | |||
| 10.0 | 12/12 | 16.0 | |||
| control | 0/11 | 8.0 | |||
| Leneva et al.38 (mice) | A/HK/156/97 (H5N1) | oseltamivir ×5 days | 0.1 | 4/5 | 14.0 |
| 1.0 | 5/5 | >16.0 | |||
| 10.0 | 5/5 | >16.0 | |||
| control | 0/4 | 7.5 | |||
| A/quail/HK/G1/97 (H9N2) | oseltamivir ×5 days | 0.1 | 5/10 | 9.8 | |
| 1.0 | 9/9 | >16.0 | |||
| 10.0 | 10/10 | >16.0 | |||
| 100.0 | 10/10 | >16.0 | |||
| control | 0/10 | 4.0 | |||
| A/HK/1074/99 (H9N2) | oseltamivir ×5 days | 0.1 | 3/5 | 11.2 | |
| 1.0 | 5/5 | >16.0 | |||
| 10.0 | 5/5 | >16.0 | |||
| control | 2/5 | 8.8 | |||
| Boltz et al.46 (ferrets) | A/Vietnam/1203/04 (H5N1) | oseltamivir ×10 days | 5 | 3/3 | |
| 10 | 3/3 | ||||
| control | 0/3 | ||||
| Yen et al.47 (mice) | A/Vietnam/1203/04 (H5N1) | oseltamivir ×5 days | 0.1 | 0/10 | 10.7 |
| 1.0 | 0/10 | 12.2 | |||
| 10.0 | 5/10 | 20.1 | |||
| control | 0/10 | 9.6 | |||
| oseltamivir ×8 days | 0.1 | 1/10 | 11.6 | ||
| 1.0 | 6/10 | 12.1 | |||
| 10.0 | 8/10 | 13.0 | |||
| control | 0/10 | 11.0 |
Dose-dependent effects have also been reported in prophylactic studies focusing on the more recent A/Vietnam/1203/04 (H5N1) virus, originally isolated from a throat swab from a fatally infected person.46,47 A 5 day regimen of 10 mg/kg/day protected 50% of mice and increased duration of survival (Table 1); an increase in duration of therapy to 8 days improved efficacy and conferred survival rates of up to 80%. This viral variant is more virulent in mice than the earlier influenza subtype A/HK/156/97 (H5N1), which points to the potential need for more prolonged therapy at higher dosages.47 In a more recent study, 100% survival was reported in ferrets protected with 10 day oseltamivir started before infection with influenza A/Vietnam/1203/04 (H5N1) (Table 1).46 When oseltamivir was given twice daily to these animals, clinical signs, systemic virus distribution and organ pathology were also prevented.
Clinical studies
There is currently only limited information available regarding the efficacy of oseltamivir for the prevention of human infection with avian influenza virus. No clinical trial has evaluated oseltamivir for the prevention of H5N1.33 However, modelling of an emerging outbreak in rural Asia has predicted the benefit of mass targeted antiviral prevention in terms of delaying or stopping spread of disease, and the WHO already has a stockpile of oseltamivir for this purpose.2
Kang et al.48 detailed an analysis of 2512 people involved in the management of influenza A/Chicken/Korea/ES/03 (H5N1) that caused 19 outbreaks in seven provinces in Korea in 2003–04. All those directly involved in the culling operations were equipped with personal protective equipment, were vaccinated with commercial seasonal influenza vaccine and received oseltamivir as prophylaxis. None of the persons studied developed any influenza-like symptoms or fever, although nine individuals seroconverted (0.36%), showing that they had been directly exposed to the virus but that infection was not associated with clinical signs (see later for discussion of serconversion data from animals and patients given oseltamivir).
An updated review by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) published in December 2007 of antiviral medicinal products for potential use during a pandemic also noted that ‘during the 2003 avian influenza H7N7 outbreak in the Netherlands, oseltamivir with the recommended prophylactic dose of 75 mg once daily seemed to be effective to protect poultry workers and their close contacts’.34 The use of oseltamivir as prophylaxis was also linked to a significant reduction in the risk of conjunctivitis (a common extrapulmonary symptom of H7N7 infection).35 These data also support a rationale for the potential utility of oseltamivir in avian influenza A infections of subtypes other than H5N1. Lim et al.49 presented a case study of an outbreak of highly pathogenic avian influenza A (H5N1) on a farm in the UK in 2007. Information was available for 482 people considered at risk of exposure to H5N1 during the outbreak. Oseltamivir was provided as post-exposure prevention to 11 farm workers exposed at the time of the outbreak, none of whom was subsequently hospitalized or reported to have developed clinical symptoms of H5N1 infection.
Seroconversion data
Information about the production of antibodies to avian influenza viruses during oseltamivir treatment is limited, but findings to date suggest that oseltamivir does not prevent the development of humoral immune responses to influenza A (H5N1) that are adequate to prevent re-infection.
Antibody production has been reported in ferrets inoculated with H5N1 virus and subsequently treated with oseltamivir, which supports the contention that the drug does not interfere with serum antibody production.37 Treated animals surviving lethal challenge with influenza A/Vietnam/1203/04 (H5N1) showed levels of HI antibody to influenza A/Vietnam/1203/04 (H5N1) that were low but nevertheless sufficient to provide complete protection against lethal rechallenge. Similar results were reported in animals initially challenged with influenza A/Turkey/15/06 (H5N1), although homologous HI titres were considerably higher (Table 2). Boltz et al.46 subsequently also reported low but sufficient homologous HI titres in treated ferrets surviving lethal challenge with A/Vietnam/1203/04 (H5N1), with higher titres of the heterologous virus A/HK/213/03 (H5N1). No clinical signs of infection were seen in any ferrets undergoing lethal rechallenge with influenza A/Vietnam/1203/04 (H5N1). Overall, these findings are compatible with the premise that, rather than preventing infection, prophylaxis limits the course of influenza infection within its early stages and prior to the onset of symptomatic disease.
Table 2.
Results of rechallenge of ferrets with a lethal dose of homologous H5N1 after earlier oseltamivir treatment; all three rechallenged ferrets survived with no clinical signs of disease; reproduced from Govorkova et al.37 with permission
| Range of pre-challenge HI titres (3 weeks after initial inoculation, expressed as reciprocal values) |
|||||
|---|---|---|---|---|---|
| Virus | Oseltamivir regimen used to treat initial infection | Rechallenge virus dose (EID50/ferret) | A/Vietnam/1203/04 | A/HK/213/03 | A/Turkey/15/06 |
| A/Vietnam/1203/04 | 25 mg/kg/day (24 h delay) | 102 | 20–40 | 80–160 | not tested |
| A/Turkey/15/06 | 10 mg/kg/day (24 h delay) | 107 | 20–40 | 320–640 | 160–320 |
EID50, 50% egg infectious dose; HI, haemagglutinin inhibition.
Seroconversion has also been noted in humans treated with oseltamivir for exposure to avian influenza, notably the nine individuals (see earlier) who seroconverted after exposure to influenza A/Chicken/Korea/ES/03 (H5N1).48 In a retrospective study of avian influenza in 705 persons involved in the 2003 outbreak of H7N7 virus in the Netherlands, a modified HI assay showed the presence of anti-H7 antibodies in 49% of 508 persons exposed to poultry and 64% of 63 persons exposed to infected humans.35 These findings are consistent with those observed in seasonal influenza.
Dose optimization
Whereas the majority of cases of H5N1 reported to date have been managed with the approved oseltamivir regimen of 75 mg, current dosages approved for seasonal influenza may need to be modified to combat effectively a potential pandemic virus based on the highly pathogenic influenza A/H5N1 strain. A higher dosage (e.g. 150 mg twice daily in adults) and increased duration of therapy may be reasonable in light of the high levels of replication of influenza A/H5N1, observations of progressive disease despite early treatment with standard dosages in some patients, and the apparent safety of higher dosages of oseltamivir in patients with seasonal influenza.2
The safety of a dosage of 150 mg twice daily given for 5 days has been demonstrated in two randomized and controlled studies in which 454 adult patients received this regimen for the treatment of acute seasonal influenza infection.50,51 In these studies, the 150 mg regimen was shown to be as effective as the 75 mg regimen, with no apparent effect of the increased dosage on the incidence or severity of adverse events, most of which were transient and gastrointestinal in nature. Favourable tolerability at the 150 mg dose level was also reported in a study of experimental influenza B infection,52 a dose-ranging study53 and three pharmacokinetic studies.54–56
Higher doses have also been well tolerated. One study exposed 391 healthy volunteers to dosages of oseltamivir up to 450 mg twice daily for 5 days.57 In this trial, in which dosages of 75 and 225 mg twice daily were also given, nausea, vomiting and headache were most commonly reported, and there was no apparent effect of oseltamivir at any dosage on QTc intervals or other electrocardiogram (ECG) parameters. No serious adverse events were reported. There was a dosage-related increase in gastrointestinal adverse events, with the majority of instances of nausea and vomiting being seen in the two highest dosage groups. Most were of mild to moderate severity and were transient. In other studies, oseltamivir was well tolerated at single doses of up to 675 mg58 and 1000 mg,59 and twice daily doses of up to 500 mg for 7 days.59
Given that individuals infected with influenza A/H5N1 are usually seriously ill (often with pneumonia) and need mechanical ventilation, the efficacy of nasogastric administration of oseltamivir is of considerable interest. Taylor et al.60 investigated the pharmacokinetics of nasogastrically administered oseltamivir at a dose of 150 mg twice daily for 10 days in three severely ill patients, two with H5N1 (including one pregnant female) and one with influenza A/H3N2. Treatment commenced on day 6 of illness or later in all patients. Virus was cleared from two patients (one male infected with H5N1 and one elderly female with H3N2) after 5 days of treatment although the female subsequently died of respiratory failure. The pregnant female also died of respiratory failure. Oseltamivir was well absorbed and converted extensively into oseltamivir carboxylate, with trough concentrations exceeding the IC50 (50% inhibitory concentration) for the H5N1 virus isolated from the pregnant female by 545- to 3956-fold. Good absorption and therapeutic concentrations were also detected after nasogastric administration of oseltamivir to a child with influenza B-associated encephalitis.61
Animal data show that benefit might be obtained through the use of longer courses of therapy,47 and this approach has been investigated in several human studies in seasonal influenza. Influenza prophylaxis with oseltamivir in the community has been given for up to 6 weeks in a study in children aged 1–12 years,62 in two large studies in 1559 adults63 and in >500 vaccinated frail elderly persons in the residential care setting.64 Prophylactic dosing has been used successfully and safely for 8 weeks in a study in 32 immunocompromised children and young people65 and, more recently, for 12 weeks in a placebo-controlled study involving 477 solid organ and stem cell transplant patients aged ≥1 year.66 Oseltamivir prophylaxis has also been given for up to 13 weeks in at-risk healthcare workers.67 In all reported studies, oseltamivir has been well tolerated and has reduced the incidence of infection substantially. An extended duration of prophylactic dosing is of particular relevance to healthcare workers in a pandemic situation, as these personnel play a key role in managing and containing an outbreak while being exposed to the virus over extended periods.
Resistance to oseltamivir in H5N1 viruses
Most neuraminidases of H5N1 viruses appear to be susceptible to oseltamivir. Comparison of virus isolates from Asia from 1997 to 2005 found that viral neuraminidases from strains isolated in 2004 and 2005 were ∼10 times more susceptible to oseltamivir than H5N1 isolates from 1997 and 2003 and a H1N1 isolate from 2004.68 Fifty-three of 55 strains of H5N1 virus from Australia and South-East Asian countries analysed between 2004 and 2006 were shown to be susceptible to neuraminidase inhibitors, their IC50 values being similar to those for H1N1 strains circulating in this region between 2001 and 2006.69 The remaining two strains exhibited higher IC50 values of oseltamivir than wild-type viruses. However, these values represented only small decreases in susceptibility, and would not be expected to be associated with reduced clinical efficacy at recommended doses. A second example of such a natural variant was reported in Egypt, where the N294S mutation was found in H5N1 virus isolates taken before and after oseltamivir treatment from two infected patients who later died.70 This mutation reduced susceptibility to the drug by 12–15 times. Recent data confirm that the N294S mutation, and the H274Y mutation that confers high level resistance to oseltamivir, are rare in circulating H5N1 viruses; of 676 isolates, only 1 contained N294S and 4 contained H274Y.71
The available information suggests that the incidence of selection of clinically significant resistance during treatment of H5N1 infection with oseltamivir is low. In a study of Indonesian patients treated with oseltamivir for H5N1 infections, no H275Y mutants were found in samples taken from 75 patients on admission or taken from 25 patients during the first 3 days of treatment follow-up.72 Indeed, only three cases of infection with oseltamivir-resistant A/H5N1 viruses (all H275Y mutants) have been reported, all in Vietnamese patients. A patient who received oseltamivir treatment at the prevention dose (75 mg once daily) for 4 days despite being symptomatic recovered after a dose increase led to clearance of the virus.9 In two other patients, both of whom died, oseltamivir treatment was given at the correct dose, but was started late in one case (sixth day of illness).73 In all three cases, resistant virus was isolated after treatment had started.
Combination chemotherapy
For other viral infections, combination chemotherapy has been successfully used to improve efficacy and/or reduce the emergence of drug resistance. By combining oseltamivir with an antiviral that has an alternative mechanism of action, similar advantages might be obtained. Amantadine and rimantadine are established antiviral agents for influenza that have a mechanism of action different from that of oseltamivir, and work by inhibiting the M2 ion channel. Recent publications suggest that combination therapy with either agent and oseltamivir could be advantageous over monotherapy.74–79 Addition of ribavirin to this combination has also been investigated.80
Clinical pharmacokinetics
In a recent study, Morrison et al.74 investigated the pharmacokinetic profiles of amantadine and oseltamivir in healthy human volunteers. Seventeen subjects took part in the study, and each received amantadine and oseltamivir as monotherapy and as combination chemotherapy, with an appropriate wash-out between each regimen. Compared with monotherapy, neither drug had any clinically significant effect on the pharmacokinetics of the other when co-administered. There was also no increase in adverse events for the combination versus oseltamivir or amantadine monotherapy. The authors concluded that combination chemotherapy with oseltamivir and amantadine appeared safe and was without pharmacokinetic consequences.74
Pre-clinical efficacy
The activity of the oseltamivir/amantadine combination has been studied in vitro and in mice infected with seasonal and avian influenza viruses.75,77 In MDCK cells infected with influenza A/Hong Kong/1/68 (H3N2) or influenza A/PR/8/34 (H1N1), a combination of oseltamivir and amantadine reduced viral replication to a greater extent than that seen with either antiviral alone.75 In mice infected with lethal doses of the same mouse-adapted H1N1 or H3N2 strains, oseltamivir or amantadine monotherapy conferred 50%–60% survival, while complete protection was achieved with an oseltamivir/amantadine combination in which oseltamivir was administered at a lower dose than that used for monotherapy.75 The combination was also effective against amantadine-resistant H1N1. In a further study, mice were given preventative doses of oseltamivir and amantadine, either separately or in combination.77 A lethal dose of amantadine-susceptible or amantadine-resistant influenza A/Vietnam/1203/04 (H5N1) was then administered. Combination chemotherapy provided 60%–90% greater protection than monotherapy, depending on the amantadine dose. The efficacy of the combination against amantadine-resistant H5N1 was comparable to that with oseltamivir alone. Oseltamivir monotherapy produced dose-dependent protection against both amantadine-susceptible and amantadine-resistant viruses.
Combination therapy with oseltamivir and rimantadine has also been studied in vitro and in vivo. In MDCK cells infected with influenza A/New Caledonia/20/99 (H1N1) or A/Panama/2007/99 (H3N2), amantadine plus rimantadine effectively reduced virus yields and was more effective than either agent alone.78 Combination chemotherapy also provided greater protection against lethal infection and prolonged survival versus monotherapy in mice infected with influenza A/Aichi/2/68 (H3N2).79
Resistance
In addition to improved efficacy, combination chemotherapy with oseltamivir and amantadine or rimantadine could also decrease the emergence of resistant variants. Ilyushina et al.76 investigated this property in MDCK cells infected with human seasonal influenza A/Nanchang/1/99 (H1N1) and A/Panama/2007/99 (H3N2) or avian influenza A/Hong Kong/156/97 (H5N1). Yields of all three viruses were significantly reduced when the cells were treated with a combination of oseltamivir and amantadine. While mutations associated with reduced antiviral susceptibility were noted with oseltamivir and amantadine monotherapy after repeated passages in MDCK cells, no mutations of the haemagglutinin, neuraminidase or M2 proteins were detected when the medications were used in combination.
Ongoing studies
To address the issues surrounding dosing requirements in a future pandemic, Roche is developing a mathematical model based on viral kinetics in the presence and absence of oseltamivir therapy to facilitate rapid prediction of appropriate regimens.81
Roche is also supporting an open-label clinical study that will compare the efficacy and safety of oseltamivir monotherapy with oseltamivir/amantadine combination chemotherapy for 5 days in adults aged between 18 and 65 years with confirmed influenza A infections (clinicaltrials.gov reference NCT00830323). As published evidence on other combination chemotherapies is currently lacking, the study will also investigate the activity of oseltamivir when used in combination with zanamivir, a second neuraminidase inhibitor. The study, which is being conducted in France, aims to recruit 60 subjects. A further randomized, double-blind, placebo-controlled study in France (clinicaltrials.gov reference NCT00799760) will compare the efficacy and safety of combination therapy with oseltamivir and zanamivir versus oseltamivir and zanamivir monotherapy (plus placebo to maintain the blind) in patients aged ≥12 years. Each drug will be taken at the standard dose for 5 days, whether alone or in combination, and patients will be stratified by time to treatment start from symptom onset (<36 h and ≥36 h). The target recruitment is 300 patients per group. The results of these studies should further inform influenza pandemic planning strategies.
Conclusions
Avian influenza A viruses continue to cause disease outbreaks in humans. Animal models of avian infection demonstrate that oseltamivir is active against H5, H7 and H9 viruses when given as treatment or prophylaxis; outcomes are influenced by the virulence of the viral strain, dosage regimen and treatment delay. Observational data on oseltamivir treatment in human disease suggest its usefulness for reducing the frequency of H5-associated mortality, while resistance has only rarely emerged during treatment. On this basis and the systemic action of the drug, the WHO recommend oseltamivir as the primary intervention for the treatment and prevention of human H5N1 disease. Modified regimens and antiviral combinations may further improve the control of viral replication in these patients.
Funding
Funding for this manuscript and the Supplement to which it belongs was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland, the manufacturer of oseltamivir.
Funding for the medical writing support was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Transparency declarations
This article is part of a Supplement sponsored by F. Hoffmann-La Roche Ltd.
The author is employed by F. Hoffmann-La Roche Ltd.
Medical writing support (drafting of text and editorial assistance) for this manuscript was provided by Scott Malkin and Stephen Purver of Gardiner-Caldwell Communications Ltd, Macclesfield, UK.
References
- 1.Peiris JS, de Jong MD, Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20:243–67. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus. N Engl J Med. 2008;358:261–73. doi: 10.1056/NEJMra0707279. Update on avian influenza A (H5N1) virus infection in humans. [DOI] [PubMed] [Google Scholar]
- 3.Lina B. [New pandemic influenza: what are the risks?] Rev Prat. 2008;58:1679–86. [PubMed] [Google Scholar]
- 4.Kandun IN, Wibisono H, Sedyaningsih ER, et al. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–94. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
- 5.Olsen SJ, Ungchusak K, Sovann L, et al. Family clustering of avian influenza A (H5N1) Emerg Infect Dis. 2005;11:1799–801. doi: 10.3201/eid1111.050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–40. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 7.Wang H, Feng Z, Shu Y, et al. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371:1427–34. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Human cases of avian influenza A (H5N1) in North-West Frontier Province, Pakistan, October-November 2007. Wkly Epidemiol Rec. 2008;83:359–64. [PubMed] [Google Scholar]
- 9.Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. doi: 10.1038/4371108a. [DOI] [PubMed] [Google Scholar]
- 10.Buchy P, Mardy S, Vong S, et al. Influenza A/H5N1 virus infection in humans in Cambodia. J Clin Virol. 2007;39:164–8. doi: 10.1016/j.jcv.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 11.Liem NT, Tung CV, Hien ND, et al. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis. 2009;48:1639–46. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambotto A, Barratt-Boyes SM, de J, et al. Human infection with highly pathogenic H5N1 influenza virus. Lancet. 2008;371:1464–75. doi: 10.1016/S0140-6736(08)60627-3. [DOI] [PubMed] [Google Scholar]
- 13.Apisarnthanarak A, Kitphati R, Thongphubeth K, et al. Atypical avian influenza (H5N1) Emerg Infect Dis. 2004;10:1321–4. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Jong MD, Bach VC, Phan TQ, et al. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352:686–91. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 15.Buxton BC, Katz JM, Seto WH, et al. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181:344–8. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 16.Bridges CB, Lim W, Hu-Primmer J, et al. Risk of influenza A (H5N1) infection among poultry workers, Hong Kong, 1997–1998. J Infect Dis. 2002;185:1005–10. doi: 10.1086/340044. [DOI] [PubMed] [Google Scholar]
- 17.Katz JM, Lim W, Bridges CB, et al. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–70. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 18.Oner AF, Bay A, Arslan S, et al. Avian influenza A (H5N1) infection in Eastern Turkey in 2006. N Engl J Med. 2006;355:2179–85. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- 19.WHO. Cumulative Number of Confirmed Human Cases of Avian Influenza A/(H5N1) Reported to WHO, 31 August 2009. http://www.who.int/csr/disease/avian_influenza/country/cases_table_2009_08_31/en/index.html. (10 September 2009, date last accessed) [Google Scholar]
- 20.Needham H. H5N1 in wild and domestic birds in Europe—remaining vigilant in response to an ongoing public health threat. Euro Surveill. 2007;12 doi: 10.2807/esw.12.49.03324-en. pii=3324. [DOI] [PubMed] [Google Scholar]
- 21.Koopmans M, Wilbrink B, Conyn M, et al. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363:587–93. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen-Van-Tam JS, Nair P, Acheson P, et al. Outbreak of low pathogenicity H7N3 avian influenza in UK, including associated case of human conjunctivitis. Euro Surveill. 2006;11 doi: 10.2807/esw.11.18.02952-en. pii=2952. [DOI] [PubMed] [Google Scholar]
- 23.Kurtz J, Manvell RJ, Banks J. Avian influenza virus isolated from a woman with conjunctivitis. Lancet. 1996;348:901–2. doi: 10.1016/S0140-6736(05)64783-6. [DOI] [PubMed] [Google Scholar]
- 24.Banks J, Speidel E, Alexander DJ. Characterisation of an avian influenza A virus isolated from a human—is an intermediate host necessary for the emergence of pandemic influenza viruses? Arch Virol. 1998;143:781–7. doi: 10.1007/s007050050329. [DOI] [PubMed] [Google Scholar]
- 25.Editorial team. Avian influenza A/(H7N2) outbreak in the United Kingdom. Euro Surveill. 2007;12 pii=3206. [PubMed] [Google Scholar]
- 26.CDC. Update: influenza activity—United States and worldwide, 2003–04 season, and composition of the 2004–05 influenza vaccine. MMWR Morb Mortal Wkly Rep. 2004;53:547–52. [PubMed] [Google Scholar]
- 27.Skowronski DM, Li Y, Tweed SA, et al. Protective measures and human antibody response during an avian influenza H7N3 outbreak in poultry in British Columbia, Canada. CMAJ. 2007;176:47–53. doi: 10.1503/cmaj.060204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puzelli S, Di TL, Fabiani C, et al. Serological analysis of serum samples from humans exposed to avian H7 influenza viruses in Italy between 1999 and 2003. J Infect Dis. 2005;192:1318–22. doi: 10.1086/444390. [DOI] [PubMed] [Google Scholar]
- 29.Belser JA, Bridges CB, Katz JM, et al. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg Infect Dis. 2009;15:859–65. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peiris M, Yuen KY, Leung CW, et al. Human infection with influenza H9N2. Lancet. 1999;354:916–7. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 31.Jia N, de Vlas SJ, Liu YX, et al. Serological reports of human infections of H7 and H9 avian influenza viruses in northern China. J Clin Virol. 2009;44:225–9. doi: 10.1016/j.jcv.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Clinical Management of Human Infection With Avian Influenza A (H5N1) Virus. http://www.who.int/csr/disease/avian_influenza/guidelines/ClinicalManagement07.pdf. (10 September 2009, date last accessed) [Google Scholar]
- 33.WHO. WHO Rapid Advice Guidelines on Pharmacological Management of Humans Infected With Avian Influenza A (H5N1) Virus. doi: 10.1016/S1473-3099(06)70684-3. http://www.who.int/medicines/publications/WHO_PSM_PAR_2006.6.pdf. (10 September 2009, date last accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Medicines Agency (EMEA) Updated Review of Influenza Antiviral Medicinal Products for Potential Use During Pandemic by the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMEA) http://www.emea.europa.eu/pdfs/human/pandemicinfluenza/59210207en.pdf. (10 September 2009, date last accessed) [Google Scholar]
- 35.Meijer A, Bosman A, van de Kamp EE, et al. Measurement of antibodies to avian influenza virus A(H7N7) in humans by hemagglutination inhibition test. J Virol Methods. 2006;132:113–20. doi: 10.1016/j.jviromet.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Govorkova EA, Leneva IA, Goloubeva OG, et al. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother. 2001;45:2723–32. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Govorkova EA, Ilyushina NA, Boltz DA, et al. Efficacy of oseltamivir therapy in ferrets inoculated with different clades of H5N1 influenza virus. Antimicrob Agents Chemother. 2007;51:1414–24. doi: 10.1128/AAC.01312-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leneva IA, Roberts N, Govorkova EA, et al. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000;48:101–15. doi: 10.1016/s0166-3542(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 39.Meijer A, van der Goot JA, Koch G, et al. Oseltamivir reduces transmission, morbidity, and mortality of highly pathogenic avian influenza in chickens. International Congress Series. 2004;1263:495–8. [Google Scholar]
- 40.Hien ND, Ha NH, Van NT, et al. Human infection with highly pathogenic avian influenza virus (H5N1) in northern Vietnam, 2004–2005. Emerg Infect Dis. 2009;15:19–23. doi: 10.3201/eid1501.080073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kandun IN, Tresnaningsih E, Purba WH, et al. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372:744–9. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Ghafar AN. Working on the front line with H5N1. Perspectives in Interpandemic Influenza, Madrid, 2007. Oral presentation. [Google Scholar]
- 43.de Jong MD. Human H5N1 disease: insights in pathogenesis and treatment. Options for the Control of Influenza VI, Toronto, 2007. Oral presentation. [Google Scholar]
- 44.Sedyaningsih ER, Isfandari S, Setiawaty V, et al. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005-June 2006. J Infect Dis. 2007;196:522–47. doi: 10.1086/519692. [DOI] [PubMed] [Google Scholar]
- 45.Toovey S. First results from an avian influenza case registry. Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009; San Francisco. Washington, DC, USA: American Society for Microbiology; Abstract V-533. [Google Scholar]
- 46.Boltz DA, Rehg JE, McClaren J, et al. Oseltamivir prophylactic regimens prevent H5N1 influenza morbidity and mortality in a ferret model. J Infect Dis. 2008;197:1315–23. doi: 10.1086/586711. [DOI] [PubMed] [Google Scholar]
- 47.Yen HL, Monto AS, Webster RG, et al. Virulence may determine the necessary duration and dosage of oseltamivir treatment for highly pathogenic A/Vietnam/1203/04 influenza virus in mice. J Infect Dis. 2005;192:665–72. doi: 10.1086/432008. [DOI] [PubMed] [Google Scholar]
- 48.Kang C, Kwong D, Lee J-Y, et al. Asymptomatic infection with avian influenza A/H5N1 in Korea during 2003–2004 outbreaks. Abstracts of the Options for the Control of Influenza VI Conference; 2007; Toronto. Abstract P337. MediTech Media Conferencing, Inc., Atlanta, GA, USA. [Google Scholar]
- 49.Lim C, Sundqvist T, Nair P, et al. Public health response to an outbreak of highly pathogenic avian influenza (H5N1) on a poultry farm in Suffolk, UK. Abstracts of the Options for the Control of Influenza VI Conference; 2007; Toronto. Abstract 018. MediTech Media Conferencing, Inc., Atlanta, GA, USA. [Google Scholar]
- 50.Nicholson KG, Aoki FY, Osterhaus AD, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–450. doi: 10.1016/s0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 51.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24. doi: 10.1001/jama.283.8.1016. [DOI] [PubMed] [Google Scholar]
- 52.Hayden FG, Jennings L, Robson R, et al. Oral oseltamivir in human experimental influenza B infection. Antivir Ther. 2000;5:205–13. [PubMed] [Google Scholar]
- 53.Ward P, Small I, Smith J, et al. Oseltamivir (Tamiflu) and its potential for use in the event of an influenza pandemic. J Antimicrob Chemother. 2005;55(Suppl 1):i5–21. doi: 10.1093/jac/dki018. [DOI] [PubMed] [Google Scholar]
- 54.Snell P, Oo C, Dorr A, et al. Lack of pharmacokinetic interaction between the oral anti-influenza neuraminidase inhibitor prodrug oseltamivir and antacids. Br J Clin Pharmacol. 2002;54:372–7. doi: 10.1046/j.1365-2125.2002.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hill G, Cihlar T, Oo C, et al. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion—correlation of in vivo and in vitro studies. Drug Metab Dispos. 2002;30:13–9. doi: 10.1124/dmd.30.1.13. [DOI] [PubMed] [Google Scholar]
- 56.Schentag JJ, Hill G, Chu T, et al. Similarity in pharmacokinetics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian subjects. J Clin Pharmacol. 2007;47:689–96. doi: 10.1177/0091270007299761. [DOI] [PubMed] [Google Scholar]
- 57.Dutkowski R, Thakrar B, Froehlich E, et al. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 2003;26:787–801. doi: 10.2165/00002018-200326110-00004. [DOI] [PubMed] [Google Scholar]
- 58.Wattanagoon Y, Stepniewska K, Lindegardh N, et al. Pharmacokinetics of high dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother. 2009;53:945–52. doi: 10.1128/AAC.00588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Massarella JW, He GZ, Dorr A, et al. The pharmacokinetics and tolerability of the oral neuraminidase inhibitor oseltamivir (Ro 64-0796/GS4104) in healthy adult and elderly volunteers. J Clin Pharmacol. 2000;40:836–43. doi: 10.1177/00912700022009567. [DOI] [PubMed] [Google Scholar]
- 60.Taylor WR, Thinh BN, Anh GT, et al. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS ONE. 2008;3:e3410. doi: 10.1371/journal.pone.0003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Straumanis JP, Tapia MD, King JC. Influenza B infection associated with encephalitis: treatment with oseltamivir. Pediatr Infect Dis J. 2002;21:173–5. doi: 10.1097/00006454-200202000-00021. [DOI] [PubMed] [Google Scholar]
- 62.Reisinger K, Marcadis I, Cupelli LA. Oseltamivir for prevention of seasonal influenza in children. Abstracts of the Options for the Control of Influenza VI Conference; 2007; Toronto. Abstract P1309. MediTech Media Conferencing, Inc., Atlanta, GA, USA. [Google Scholar]
- 63.Hayden FG, Atmar RL, Schilling M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999;341:1336–43. doi: 10.1056/NEJM199910283411802. [DOI] [PubMed] [Google Scholar]
- 64.Peters PH, Jr, Gravenstein S, Norwood P, et al. Long-term use of oseltamivir for the prophylaxis of influenza in a vaccinated frail older population. J Am Geriatr Soc. 2001;49:1025–31. doi: 10.1046/j.1532-5415.2001.49204.x. [DOI] [PubMed] [Google Scholar]
- 65.Chik KW, Li CK, Chan PK, et al. Oseltamivir prophylaxis during the influenza season in a paediatric cancer centre: prospective observational study. Hong Kong Med J. 2004;10:103–6. [PubMed] [Google Scholar]
- 66.Ison MG, Szakaly P, Shapira MY, et al. Reduced incidence of seasonal influenza with oseltamivir prophylaxis in solid organ transplant recipients. Abstracts of the American Transplant Congress; 2009; Boston. Abstract 483. http://www.abstracts2view.com/atc/ (9 November 2009, date last accessed) [Google Scholar]
- 67.McGeer A, Borgundvaag B, Drews S. Experience with seasonal oseltamivir prophylaxis in Canadian health care workers. Abstracts of the Joint Forty-eighth Interscience Conference on Antimicrobial Agents and Chemotherapy and Infectious Diseases Society of America Forty-sixth Annual Meeting; 2008; Washington DC. Abstract K-4208, p. 581. American Society for Microbiology, Washington, DC, USA/Infectious Diseases Society of America, Arlington, VA, USA. [Google Scholar]
- 68.Rameix-Welti MA, Agou F, Buchy P, et al. Natural variation can significantly alter the sensitivity of influenza A (H5N1) viruses to oseltamivir. Antimicrob Agents Chemother. 2006;50:3809–15. doi: 10.1128/AAC.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurt AC, Selleck P, Komadina N, et al. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antiviral Res. 2007;73:228–31. doi: 10.1016/j.antiviral.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 70.Saad MD, Boynton BR, Earhart KC, et al. Detection of oseltamivir resistance mutation N294S in humans with influenza A H5N1. Abstracts of the Options for the Control of Influenza VI Conference; 2007; Toronto. Abstract P909. MediTech Media Conferencing, Inc., Atlanta, GA, USA. [Google Scholar]
- 71.Hill AW, Guralnick RP, Wilson MJ, et al. Evolution of drug resistance in multiple distinct lineages of H5N1 avian influenza. Infect Genet Evol. 2009;9:169–78. doi: 10.1016/j.meegid.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 72.Sedyaningsih ER. The indonesian experience. Abstracts of the Tenth International Symposium on Respiratory Viral Infections; 2008; Singapore. Plenary session. [Google Scholar]
- 73.de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353:2667–72. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 74.Morrison D, Roy S, Rayner C, et al. A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS ONE. 2007;2:e1305. doi: 10.1371/journal.pone.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masihi KN, Schweiger B, Finsterbusch T, et al. Low dose oral combination chemoprophylaxis with oseltamivir and amantadine for influenza A virus infections in mice. J Chemother. 2007;19:295–303. doi: 10.1179/joc.2007.19.3.295. [DOI] [PubMed] [Google Scholar]
- 76.Ilyushina NA, Bovin NV, Webster RG, et al. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antiviral Res. 2006;70:121–31. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Ilyushina NA, Hoffmann E, Salomon R, et al. Amantadine-oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12:363–70. [PubMed] [Google Scholar]
- 78.Govorkova EA, Fang HB, Tan M, et al. Neuraminidase inhibitor-rimantadine combinations exert additive and synergistic anti-influenza virus effects in MDCK cells. Antimicrob Agents Chemother. 2004;48:4855–63. doi: 10.1128/AAC.48.12.4855-4863.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Galabov AS, Simeonova L, Gegova G. Rimantadine and oseltamivir demonstrate synergistic combination effect in an experimental infection with type A (H3N2) influenza virus in mice. Antivir Chem Chemother. 2006;17:251–8. doi: 10.1177/095632020601700502. [DOI] [PubMed] [Google Scholar]
- 80.Nguyen JT, Hoopes JD, Smee DF, et al. Triple combination of oseltamivir, amantadine, and ribavirin displays synergistic activity against multiple influenza virus strains in vitro. Antimicrob Agents Chemother. 2009;53:4115–26. doi: 10.1128/AAC.00476-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gieschke R, Jonsson N, Boak L, et al. A dose prediction model for the neuraminidase inhibitor oseltamivir. Abstracts of the Tenth International Symposium on Respiratory Viral Infections; 2008; Singapore. Abstract. [Google Scholar]