Abstract
Pandemic (H1N1) 2009 influenza is affecting countries in all five continents, with most cases so far having been reported in North and South America and Europe, and children and young adults being the most susceptible age groups. To date, the clinical course of disease is typically mild, with low hospitalization and mortality rates. Pandemic (H1N1) 2009 is susceptible to oseltamivir and, although few clinical data are yet available, current information suggests that treatment with oseltamivir appears to be beneficial. Only isolated cases of resistance to the drug have been reported to date, in keeping with the low frequency observed in clinical studies involving patients infected with seasonal influenza viruses. Current health authority guidelines recommend the use of oseltamivir in infected adults and children who have or are at elevated risk for severe disease, including pregnant women; use during the pandemic in infants <1 year has also been authorized in Europe and a number of other countries, including the USA and Canada. Before the onset of the current pandemic, F. Hoffmann-La Roche Ltd expanded annual production capacity for oseltamivir to 400 million treatment courses per year to meet anticipated demand. However, during an influenza pandemic, and despite increased production capabilities, resources are nonetheless likely to be initially in short supply. For this reason, Roche, in line with WHO recommendations, has advocated advance stockpiling of antivirals by governments as a pandemic preparedness measure. Between 2004 and December 2009, 350 million treatment courses were supplied to governments worldwide. Support for developing countries has been a priority. Roche has established a cluster of initiatives aimed at increasing access to Tamiflu for the world's developing economies, including, making donations to the WHO, establishing the Tamiflu Reserves Program (TRP) and sub-licensing and manufacturing contracts with local companies in Asia and Africa. Furthermore, Roche has published a document outlining how it would allocate limited supplies of Tamiflu during a pandemic, which are in line with WHO recommendations stating that ‘resources should be used to provide the maximum possible health benefit’. Roche is also offering support such as reprocessing of expiring capsule stocks (in development) and shelf-life extension to support governments in the management of their stockpiles. Clinical studies, either sponsored by or supported by Roche, are in progress. These trials are designed to investigate the effectiveness of oseltamivir in patients infected with the pandemic virus in greater depth, and include high-dose studies, assessment of natural and drug-induced resistance, and response to treatment in high-risk populations such as young infants, immunocompromised patients and the severely ill.
Keywords: antiviral, stockpiling, roles, responsibilities
Epidemiology and prevalence of pandemic influenza A (H1N1) 2009
As had been widely predicted by medical and public health experts, the current influenza pandemic reached Phase 6 on 11 June 2009, indicated by sustained community level outbreaks in more than one WHO region. Pandemic influenza A (H1N1) 2009 is a triple-reassortant virus containing genes from influenza viruses of avian, human and swine origin,1 and the first human infections caused by this virus were reported in Mexico in April 2009. By 22 November 2009, laboratory-confirmed cases of infection had been reported in 207 countries across five continents, affecting a total of >622 482 people, the great majority of which (84%) were reported to three WHO Regional Offices, namely the Americas (190 765 cases), Europe (>154 000) and the Western Pacific (176 796).2 These incidence figures underestimate the actual number of cases, because countries where community-wide transmission has occurred are no longer required to identify all individual cases.3 Indeed, by mid-July 2009, >1 million people were estimated to have been infected in the USA.4
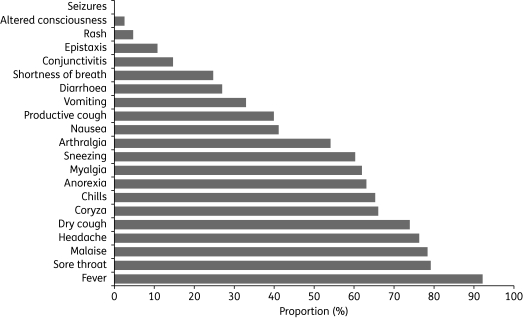
Children and young adults seem to be more susceptible to the disease than older people,5–7 although transmission to older adults may occur later in the epidemic. The incubation period for pandemic influenza A (H1N1) 2009 is not yet certain, but appears to range from 2 to 7 days.1 In most cases, clinical signs are mild and similar to those seen with seasonal influenza infections, typically including fever, cough and sore throat (Figure 1).5–7 So far, the numbers of patients hospitalized have been small and, given the large number of mild unreported cases, the hospitalization rate is probably <1%.4 Primary viral pneumonia is the most common complication, with bacterial pneumonia being less frequent.4 Severe disease appears to be most common in children <5 years old, pregnant women and those with underlying medical conditions such as lung or heart disease, or immunosuppression.8,9 Danger signs that signal progression to more severe disease include shortness of breath, difficulty breathing, turning blue, chest pain, altered mental status, persistent high fever and low blood pressure.9 The current mortality rate for pandemic (H1N1) 2009 is low—as of 22 November 2009, 7826 of the >622 482 cases (1.3%) reported to the WHO had a fatal outcome.2
Figure 1.
Frequency of symptoms at presentation in confirmed cases of pandemic (H1N1) 2009 infection in the UK as of 31 May 2009 (n = 175). Reproduced from HPA, Health Protection Scotland, National Public Health Service for Wales, HPA Northern Ireland Swine influenza investigation teams7 with permission.
Activity of oseltamivir against pandemic influenza A (H1N1) 2009
Oseltamivir (Tamiflu®; F. Hoffmann-La Roche Ltd) is currently approved for use in adults and children aged ≥1 year of age, and has also recently been approved in Europe for children <1 year of age infected with pandemic influenza.10 Emergency authorization has recently been granted in the USA, Canada and several other countries for use in infants <1 year old with pandemic (H1N1) 2009.11,12 In addition to patients with severe disease caused by the pandemic virus, guidance from the WHO, the CDC and the European Centre for Disease Prevention and Control (ECDC)11,13,14 recommends that pregnant women should be added to the other recognized groups of high-risk patients (children aged <5 years, the over-65s, immunosuppressed patients and those with concomitant diseases) for whom oseltamivir treatment should be used. Early treatment initiation within 48 h of symptoms is also recommended, although initiation after 48 h is considered beneficial.11,13,14 The WHO and the CDC have confirmed that the pandemic (H1N1) 2009 virus is susceptible to oseltamivir,2,11 with susceptibility being very similar to that of drug-susceptible seasonal viruses (Table 1).1
Table 1.
Susceptibility of 37 isolates of swine-origin influenza A (H1N1) virus to neuraminidase inhibitors compared with control values from seasonal influenza viruses; adapted from Novel S-OIV Investigation Team 20091
| Oseltamivir IC50 (nmol/L) |
Zanamivir IC50 (nmol/L) |
|||
|---|---|---|---|---|
| mean | median | mean | median | |
| All 37 isolates | 0.57 | 0.54 | 0.59 | 0.59 |
| Reference seasonal viruses | ||||
| oseltamivir susceptible | 0.63 | — | 0.60 | — |
| oseltamivir resistant | 265.27 | — | 1.27 | — |
IC50, concentration producing inhibition in 50% of the sample.
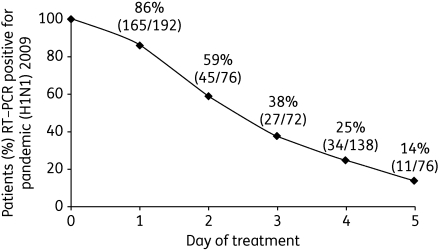
Although information on clinical effectiveness of oseltamivir in pandemic (H1N1) 2009 is currently limited, early reports from Japan and Vietnam suggest that treatment may be beneficial. All except 1 of 49 mostly adolescent patients hospitalized in Kobe City, Japan with pandemic (H1N1) 2009 received either oseltamivir (22) or zanamivir (26).15 Both medications were well tolerated, and earlier administration seemed to be associated with a reduced duration of fever [median duration of fever of 1.5 days (range 1–4 days) with oseltamivir and 1 day (range 1–5 days) with zanamivir when antiviral treatment began on the day of fever onset, compared with a median of 3 days (range 2–5 days) when either drug was initiated the day after fever onset]. Most patients were discharged shortly after antiviral administration and all recovered without complication.15 In the USA, data were collected on 272 patients with influenza-like illness who tested positive for the pandemic virus and were admitted to hospital for at least 24 h.16 In a multivariable analysis of outcomes, the only variable that was significantly associated with a positive outcome was the receipt of antiviral drugs within 2 days of illness onset.16 Between 29 May 2009 and 28 July 2009, the Hospital for Tropical Diseases in Ho Chi Minh City, Vietnam also collected data on antiviral efficacy in 292 hospitalized patients (average age: 26.4 years; range: 1–69 years) who tested positive for the pandemic (H1N1) 2009 virus by RT–PCR.17 All patients received the standard treatment dose of oseltamivir (75 mg twice daily) until RT–PCR negative. By day 3 of treatment, 62% of patients were RT–PCR negative, and by day 5, 86% were negative (Figure 2).17 After 24 h, 228/292 patients (78%) were afebrile and all patients had a mild illness course. Two patients remained RT–PCR positive for an extended period of time (one up to day 8 and one up to day 14) despite oseltamivir treatment, but no specimens collected after day 5 cultured positive for the virus.17
Figure 2.
Proportion of patients who were RT–PCR positive for pandemic (H1N1) 2009 by the day following initiation of oseltamivir treatment (n = 292). Figure derived from data in Hien et al.17
Of over 19 000 pandemic H1N1 viruses that had been evaluated by laboratories in the Global Influenza Surveillance Network for antiviral drug resistance up to 4 December 2009, almost all were susceptible to both oseltamivir and zanamivir.18 At this time, 96 cases of oseltamivir-resistant infections had been reported by the WHO, all of which carried the H275Y mutation located in the neuraminidase gene.18 Many of these cases were associated with post-exposure prophylaxis or long-term oseltamivir treatment in immunocompromised patients. The WHO has not changed its clinical treatment guidance,19 and antiviral drugs remain a key component of the public health response when used as recommended.14,19
Roles and responsibilities of Roche during influenza pandemics
In the interests of transparency, Roche issued a document in May 2008 setting out its role and responsibilities in a pandemic, and how the company plans to work with other stakeholders during pre-pandemic and pandemic stages.20 This was developed in consultation with the Internal Public Policy Advisory Council on Pandemic Preparedness (PAC), a body that includes experts from governments, medicine and academia.
One of Roche's key roles is to ensure a sustainable supply of oseltamivir. Although manufacturing capacity at the end of 2004 was sufficient to produce 28 million treatment courses annually, the company has since invested in the development of a global manufacturing network, involving 19 external partner companies that can produce 400 million treatment courses annually if required. Roche has also appointed sub-licensees in China and India, and has undertaken a knowledge transfer agreement with a company in Africa, allowing these companies to supply generic forms of oseltamivir for local use and for use in selected developing nations. These initiatives are described in more detail in the next section. Before the current pandemic was declared, global oseltamivir stockpiles were sufficient to treat <5% of the world's population, so the initial demand from health authorities responding to the new pandemic is expected to exceed production capacity. To address this challenge, Roche has been allocating supplies of oseltamivir according to protocols consistent with the WHO publication, ‘Ethical Considerations in Developing a Public Health Response to Pandemic Influenza’,21 which states that ‘resources should be used to provide the maximum possible health benefit’. The principles of the policy are to support pandemic response efforts of both the WHO and national governments, while balancing the needs of Roche's shareholders and employees and society as a whole, and maintaining a dialogue with the WHO and other international agencies regarding antiviral allocation needs.20 These principles will apply to the extent permitted under local emergency legislation affecting the company's manufacturing and supply capabilities.
During the early stages of the current pandemic, orders for oseltamivir from the WHO and governments were given priority over new corporate orders and normal retail sales. Now that Phase 6 has been reached, Roche will continue dialogue with international agencies so that the objective of providing maximum possible health benefit in the allocation of oseltamivir is still achieved. Where priorities for allocation cannot be agreed on, Roche will allocate oseltamivir supplies for government orders according to the ‘first come, first served’ principle, whereby the date of the letter of intent or order will determine the date of delivery of the first consignment in a schedule of deliveries.
Responding to the pandemic: current Roche activities
Sustainable supply
Between 2004 and December 2009, Roche has fulfilled pandemic orders from governments worldwide, amounting to 350 million treatment courses of oseltamivir (3.5 billion doses). When pandemic alert Phase 5 was announced in April 2009, Roche immediately took steps to increase oseltamivir production and, during May to September 2009, was able to produce 110 million treatment courses (1.1 billion capsules). After September 2009, production output was accelerated, with the goal of reaching a maximum of 33 million treatment courses per month by early 2010, if this is required.
Support for developing countries
In 2005, Roche donated 3 million treatment courses of oseltamivir to the WHO for their exclusive use as a ‘rapid response stockpile’ to contain or slow the spread of infection at the site of a pandemic outbreak. A ‘regional stockpile’ consisting of a further 2 million treatment courses was also donated to serve the needs of developing countries. In May 2009, the rapid response stockpile was deployed by the WHO for use in Mexico and 72 of the world's lowest economy countries. In response, Roche has made further donations to replenish these stockpiles and to establish a ‘paediatric stockpile’ of 650 000 courses of small capsules for use by the WHO. Roche has also initiated the Tamiflu Reserves Program (TRP) for the world's lowest economy countries. This provides those countries with an insurance policy, by securing supply of oseltamivir for the management of a novel influenza strain defined by the WHO to have significant and current pandemic potential. Eligible countries will pay a substantially reduced price for oseltamivir stockpiles to be built and shipped at the time of an influenza pandemic or when required by the government. The countries covered by this are all 72 countries of GAVI (Global Alliance for Vaccines and Immunizations) except India, which has its own generic manufacturing capability.
As mentioned previously, sub-licences have been granted to Shanghai Pharmaceuticals and the HEC Group to produce a generic version of Tamiflu for China, and to Hetero for supply to India and some developing countries. Roche also signed a knowledge transfer agreement with the South African manufacturer Aspen Pharmaceuticals to support them in supplying a generic version of oseltamivir for African countries.
Increasing access to oseltamivir
Other activities Roche is pursuing will help governments and other provider organizations to gain the maximum benefit from their oseltamivir stockpiles during the pandemic. The first stockpiles were started in 2004, and the 5 year shelf-life for some of those stocks expired (technically) in 2009. As longer term stability data indicate that this stock is still viable, Roche has applied for shelf-life extension from 5 to 7 years. The shelf-life of stockpiled product has already been extended to 7 years in the USA and some European countries.
Roche will shortly seek regulatory approval for reprocessing expired capsules in government stockpiles to recover active pharmaceutical ingredient (API), so that oseltamivir base can be incorporated into new capsules and small capsules with a 5 or 7 year shelf-life and supplied at a lower cost than de novo manufacture. Smaller oseltamivir capsules (30 and 45 mg) have been developed by Roche specifically for use in younger patients, and offer governments the opportunity to build stockpiles that are appropriate for local demographics.
Clinical study programme
A number of clinical trials are under way that will generate information on the treatment of pandemic influenza (H1N1) 2009 with oseltamivir (Table 2). Patients infected with the pandemic virus and other seasonal influenza viruses are being recruited.
Table 2.
Clinical trials investigating the treatment of pandemic (H1N1) 2009 with oseltamivir
| Clinicaltrials.gov/Roche registration numbera | Patient group | Treatment regimen | Outcomes to be assessed |
|---|---|---|---|
| NCT00949533 | infected patients ≥5 years (Brazil) (n = 125) | 75 or 150 mg of oseltamivir twice daily for 5 days | clinical outcomes, virological assessment |
| NV22155 | infected patients ≥1 year (USA) (n = 400) | 75 or 150 mg of oseltamivir twice daily for 5 or 10 days | clinical outcomes, virological assessment |
| NV20237 | infected patients ≥1 year (northern and southern hemispheres) (n = 1200 per year) | determined by treating physician | clinical outcomes, resistance |
| MV22841 | infected patients ≥1 year (South Africa) (n = 125) | 75 mg of oseltamivir twice daily for 5 days | clinical outcomes, virological assessment |
| ML22879 | infected patients ≥1 year (UK) (n = 100) | 75 mg of oseltamivir twice daily for 5 days | clinical outcomes, virological assessment |
| NCT00545532 | immunocompromised transplant patients ≥1 year (USA and Europe) (n = 250) | 75 or 150 mg of oseltamivir twice daily for 10 days | efficacy and safety, resistance |
| NCT00988325 | infected infants <1 year (Europe) (n = 100) | 2–3 mg/kg oseltamivir twice daily for 5 days | clinical outcomes, pharmacokinetics, safety |
| NCT01010087 | infected patients with critical illness (Canada) (n = 240) | 75 or 225 mg of oseltamivir twice daily for 10 days | pharmacokinetics, safety |
| NCT00844155 | infected patients with respiratory failure (Canada) (n = 60) | 75 mg or 150 mg of oseltamivir, single dose | pharmacokinetics, efficacy and safety, resistance |
| NP22770 | healthy non-infected adult volunteers (n = 40) | 75 mg of oseltamivir or 100 mg of rimantadine twice daily for 5 days alone and in combination | pharmacokinetics, safety |
| NCT01002729 | obese non-infected adult volunteers (Canada) (n = 20) | 75 mg of oseltamivir twice daily for 7 days | pharmacokinetics, safety |
| NCT00873886 | pregnant female volunteers (USA) (n = 39) | 75 mg of oseltamivir, single dose | pharmacokinetics, safety |
| NCT00830323 | infected adults 18–65 years (France) (n = 60) | 75 mg of oseltamivir either alone or combined with 100 mg of amantadine or 5 mg of zanamivir twice daily for 5 days | clinical outcomes, virological assessment |
| NCT00799760 | infected adults 18–65 years (France) (n = 900) | 75 mg of oseltamivir or 10 mg of zanamivir or both combined twice daily for 5 days | clinical outcomes, virological assessment |
| NV25182 | children <2 years (Europe) (n ≤ 900) | determined by physician | safety |
| Not applicable (registry) | infected pregnant women (Europe and Japan) | determined by treating physician | pregnancy outcome |
aClinicaltrials.gov registration number (commencing NCT) supplied if known; otherwise, Roche internal clinical trial code provided.
The standard treatment regimens for adults and children are expected to be effective against the pandemic (H1N1) 2009 virus, but clinical evidence for this is still to be collected. It is possible that increasing the dosage could help to control viral shedding, particularly as anecdotal reports suggest this is prolonged in patients with pandemic (H1N1) 2009 disease. New studies have been initiated to evaluate the virological efficacy of doubling the dose (from 75 to 150 mg twice daily) or the duration (from 5 to 10 days), or both of these; these studies also monitor emergence of resistance. Further trials specifically assess the emergence and transmissibility of drug-selected resistant variants with the standard treatment regimen. One of these, the Influenza Resistance Information Study (IRIS; NV20237), aims to recruit 1200 patients per year (adults and children aged ≥1 year) from northern and southern hemisphere countries over three consecutive influenza seasons, finishing in 2011. The study does not have a fixed treatment regimen—physicians choose the most appropriate therapy for each patient, allowing comparison of a variety of treatments in patients infected with resistant and susceptible virus strains. Two smaller observational studies in the UK and South Africa look specifically at the incidence of drug resistance in patients treated with a standard course of oseltamivir. In the UK study, both the emergence of resistance and the transmission of resistant strains to household members are being measured.
The effectiveness of oseltamivir in treating pandemic (H1N1) 2009 in some patient sub-populations known to be at higher risk of severe disease, such as young children and those with co-existing illnesses, has yet to be established. This is being addressed through a series of four studies; the first of these, which started in 2007, is comparing a standard-dose with a high-dose regimen in 250 immunocompromised solid organ or stem cell transplant recipients. A second study is examining the pharmacokinetics and safety of oseltamivir in young infants with pandemic (H1N1) 2009; this open-label study uses an investigational treatment regimen (2–3 mg/kg twice daily for 5 days) in 100 influenza-infected infants, stratified into those aged 3–<12 months and those aged <3 months. Two studies are being performed in intensive care unit (ICU) patients infected with influenza in Canada to evaluate administration of oseltamivir by nasogastric tube. A smaller initial study is assessing pharmacokinetics and safety, and will be followed by a nationwide multicentre study in ∼240 critically ill influenza patients that will also assess efficacy and the incidence of resistance with standard and high doses of oseltamivir. Pharmacokinetics will also be assessed in pregnant women.
Two further studies are also assessing the pharmacokinetics of oseltamivir; the first is investigating the utility of oseltamivir and rimantadine as a combination therapy in healthy volunteers and the second is investigating whether the pharmacokinetic profile of oseltamivir is the same in obese individuals as in non-obese individuals. Evidence from the Americas suggests that obese patients could be at higher risk of complications if infected with pandemic (H1N1) 2009 influenza.4 Two further studies in France are assessing the efficacy, safety and incidence of resistance of combinations of oseltamivir with amantadine or zanamivir compared with monotherapy.
Finally, Roche is working with various groups to measure the real-life utilization of Tamiflu during the pandemic, by analysing patient registries that will collect information from specified vulnerable populations infected with pandemic (H1N1) 2009 virus. By doing this, a better understanding will be gained of the clinical course of the disease and the safety of oseltamivir treatment in these populations. In addition, Roche will adapt its existing pharmacovigilance process to collect data on the use of Tamiflu in a pandemic situation.
Funding
Funding for this manuscript and the Supplement to which it belongs was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland, the manufacturer of oseltamivir.
Funding for the medical writing support was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Transparency declarations
This article is part of a Supplement sponsored by F. Hoffmann-La Roche Ltd.
The author is a paid employee of F. Hoffmann-La Roche Ltd and holds stock and stock options in the company.
Medical writing support (drafting of text and editorial assistance) for this manuscript was provided by Scott Malkin and Roger Nutter of Gardiner-Caldwell Communications Ltd, Macclesfield, UK.
References
- 1.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Pandemic (H1N1) 2009 Update 76. http://www.who.int/csr/don/2009_11_27a/en/index.html. (10 December 2009, date last accessed) [Google Scholar]
- 3.WHO. Changes in Reporting Requirements for Pandemic (H1N1) 2009 Virus Infection. http://www.who.int/csr/disease/swineflu/notes/ h1n1_surveillance_20090710/en/index.html. (22 October 2009, date last accessed) [Google Scholar]
- 4.WHO. Human infection with pandemic A (H1N1) 2009 influenza virus: clinical observations in hospitalized patients, Americas, July 2009—update. Wkly Epidemiol Rec. 2009;84:305–8. [PubMed] [Google Scholar]
- 5.CDC. 2009 pandemic influenza A (H1N1) virus infections—Chicago, Illinois, April-July 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 6.Gilsdorf A, Poggensee G. Influenza A(H1N1)v in Germany: the first 10,000 cases. Euro Surveill. 2009;14 doi: 10.2807/ese.14.34.19318-en. pii=19318. [DOI] [PubMed] [Google Scholar]
- 7.HPA, Health Protection Scotland, National Public Health Service for Wales, HPA Northern Ireland Swine influenza investigation teams. Epidemiology of new influenza A (H1N1) virus infection, United Kingdom, April–June 2009. Euro Surveill. 2009;14 pii=19232. [PubMed] [Google Scholar]
- 8.CDC. Hospitalized patients with novel influenza A (H1N1) virus infection—California, April–May, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:536–41. [PubMed] [Google Scholar]
- 9.WHO. Pandemic Influenza in Pregnant Women. http://www.who.int/csr/disease/swineflu/notes/ h1n1_pregnancy_20090731/en/index.html. (22 October 2009, date last accessed) [Google Scholar]
- 10.European Medicines Agency (EMEA) Tamiflu. http://www.emea.europa.eu/influenza/antivirals/tamiflu/tamiflu.html. (22 October 2009, date last accessed) [Google Scholar]
- 11.CDC. Updated Interim Recommendations for the Use of Antiviral Medications in the Treatment and Prevention of Influenza for the 2009–2010 Season. http://www.cdc.gov/h1n1flu/recommendations.htm. (22 October 2009, date last accessed) [Google Scholar]
- 12.Public Health Agency of Canada (PHAC) Guidance for Expanded Use of Oseltamivir (Tamiflu®) in Children Under One Year of Age in the Context of Pandemic (H1N1) 2009. http://www.phac-aspc.gc.ca/alert-alerte/h1n1/guidance-orientation-07-20-eng.php. (22 October 2009, date last accessed). [Google Scholar]
- 13.European Centre for Disease Prevention and Control (ECDC) On Public Health Use of Influenza Antivirals During Influenza Pandemics (With Particular Reference to the Pandemic (H1N1) 2009) http://www.ecdc.europa.eu/en/healthtopics/Documents/0908_Influenza_AH1N1 _On_Public_Health_Use_of_Influenza_Antivirals_during_Influenza_Pandemics.pdf. (22 October 2009, date last accessed) [Google Scholar]
- 14.WHO. WHO Guidelines for Parmacological Management of Pandemic (H1N1) 2009 Influenza and Other Influenza Viruses. http://www.who.int/entity/csr/resources/publications/swineflu/ h1n1_guidelines_pharmaceutical_mngt.pdf. (22 October 2009, date last accessed) [Google Scholar]
- 15.WHO. Human infection with new influenza A (H1N1) virus: clinical observations from a school-associated outbreak in Kobe, Japan, May 2009. Wkly Epidemiol Rec. 2009;84:237–44. [PubMed] [Google Scholar]
- 16.Jain S, Kamimoto L, Bramley AM, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–44. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 17.Hien TT, Bryant JE, Truong NT, et al. Influenza Pandemic (H1N1) 2009 (68): Viet Nam, Virus Clearance. http://www.promedmail.org/pls/otn/f?p=2400:1001:6983346141275563::NO:: F2400_P1001_BACK_PAGE,F2400_P1001_PUB_MAIL_ID:1000,79587. (22 October 2009, date last accessed) [Google Scholar]
- 18.WHO. Pandemic (H1N1) 2009 – Update 77 (Virological Surveillance) http://www.who.int/csr/disease/swineflu/laboratory04_12_2009/en/index.html. (10 December 2009, date last accessed) [Google Scholar]
- 19.WHO. Oseltamivir-resistant pandemic (H1N1) 2009 influenza virus, October 2009. Wkly Epidemiol Rec. 2009;84:453–68. [PubMed] [Google Scholar]
- 20.F Hoffmann-La Roche Ltd. Preparing for the Next Influenza Pandemic: Roles and Responsibilities of Roche and Other Stakeholders. http://www.roche.com/sus_csoc-acc_influenza.pdf. (3 August 2009, date last accessed) [Google Scholar]
- 21.WHO. Ethical Considerations in Developing a Public Health Response to Pandemic Influenza. http://www.who.int/csr/resources/publications/WHO_CDS_EPR_GIP_2007_2c.pdf. (22 October 2009, date last accessed) [Google Scholar]