Abstract
Influenza is a transmissible viral pathogen that continues to cause substantial morbidity and mortality. Oseltamivir is an orally administered antiviral medication that selectively inhibits the influenza neuraminidase enzymes that are essential for viral replication. Treatment of infected children ≥1 year and adults of all ages may decrease the severity and duration of the symptoms of infection, while prophylactic dosing can prevent their onset. Oseltamivir is ingested in the form of a prodrug (oseltamivir phosphate) that is rapidly converted by hepatic esterases into the active metabolite, oseltamivir carboxylate. Oseltamivir carboxylate has high bioavailability and penetrates sites of infection at concentrations that are sufficient to inhibit viral replication. The pharmacokinetics of oseltamivir and oseltamivir carboxylate are dose proportional after repeated doses of up to 500 mg twice daily. This predictable profile means that oseltamivir is suitable for use in diverse patient populations, which may include young children and elderly patients, various ethnic groups and those with renal or hepatic impairment. As the potential for drug interactions is low, oseltamivir is also suitable for use in patients with co-morbid conditions who are likely to be receiving concomitant medications.
Keywords: mechanism of action, dosing, drug interactions
Introduction
Influenza is an infectious viral agent that continues to cause considerable morbidity and mortality.1 The pathogenicity of the influenza virus is now well understood, and this has led to the development of a range of antiviral agents for the treatment and prevention of its debilitating symptoms.2 Oseltamivir (Tamiflu®; F. Hoffmann-La Roche Ltd) is an orally administered antiviral for the management of influenza A and B infections in children ≥1 year and adults of all ages.3 It is a widely used medication, with global experience now exceeding 65 million treatment courses. For therapeutic use, oseltamivir is taken as a twice-daily regimen for 5 consecutive days, while for prophylaxis it is taken on a once-daily schedule for up to 42 days.3 Adults take oseltamivir at a standard dose of 75 mg, while children receive unit doses that are selected on the basis of body weight.3 Oral capsule (35, 40 and 75 mg) and suspension formulations are now readily available.3
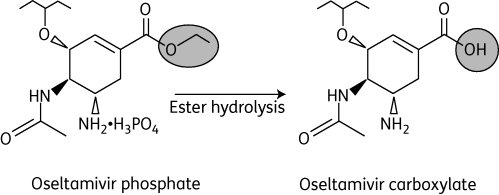
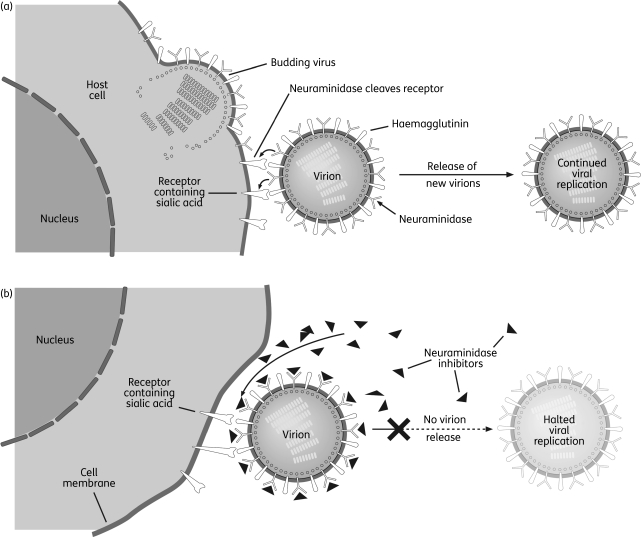
Oseltamivir is ingested in the form of an oral prodrug (oseltamivir phosphate; OP) that is rapidly metabolized to the active form, oseltamivir carboxylate (OC) (Figure 1).4 In infected patients, OC binds to and inhibits the active site of the neuraminidase enzymes that are present on all influenza viruses and are essential for the release of progeny virions from infected host cells (Figure 2).2 In this way, OC can reduce viral replication, which in turn can limit the viral load and course of infection in the host. When started within 48 h of the onset of illness, this action can limit the severity and duration of the symptoms of influenza and the risk of associated complications, such as bronchitis, pneumonia and otitis media.2 Symptomatic illness can also be prevented with prophylactic administrations.2
Figure 1.
Structures of oseltamivir phosphate (prodrug) and oseltamivir carboxylate (active metabolite).
Figure 2.
Influenza virus replication in (a) the absence and (b) the presence of neuraminidase inhibitors. Reproduced from Moscona2 with permission.
Since the active site of the neuraminidase enzyme is highly conserved, oseltamivir has activity against all of the neuraminidase subtypes so far tested in vitro.5 These include the neuraminidases of human seasonal viruses, avian viruses and pandemic viruses,5 including the newly emergent pandemic (H1N1) 2009 virus.6 A variety of laboratory strains and clinical isolates have shown susceptibility to OC, with 50% inhibitory concentrations (IC50s) ranging from 0.01 to 69.2 nM, depending on the influenza strain (Table 1).5,7–9 To place this in context, the average minimum plasma concentration of OC achieved with the twice-daily treatment regimen at the standard 75 mg dose is ∼330 nM.4 Importantly, OC is highly selective for influenza neuraminidase, and shows little or no activity against neuraminidases of other viruses, bacteria or human liver microsomes.10
Table 1.
Inhibitory concentrations (IC50s) of oseltamivir carboxylate against different neuraminidase subtypes in vitro5,7–9
| Virus subtype | IC50 (nM) | Virus subtype | IC50 (nM) |
|---|---|---|---|
| H1N1 | 0.78–2.28 | H6N5 | 33.29 |
| H1N9 | 0.35 | H7N2 | 2.79 |
| H2N2 | 0.35–9.07 | H7N7 | 4.49 |
| H2N3 | 0.018–3.39 | H8N4 | 0.45–47.09 |
| H3N2 | 0.28–0.68 | H9N2 | 1.99–15.07 |
| H3N8 | 0.85 | H10N7 | 0.85–8.29 |
| H4N6 | 0.45–3.29 | H10N8 | 29.79 |
| H4N8 | 69.29 | H11N9 | 9.59–17.79 |
| H5N1 | 7.07 | H12N5 | 1.55 |
| H5N3 | 2.99 | H13N6 | 4.99 |
| H6N1 | 36.19 | H14N5 | 36.89 |
| H6N2 | 0.848 | B | 1.75–24.38 |
Pharmacokinetics of oseltamivir and OC
To effectively limit viral replication and the viral load, therapeutic concentrations of OC must be achieved at all sites of infection and maintained for the duration of the dosing interval. For this reason, the pharmacokinetic profiles of oseltamivir and OC have been extensively studied in healthy volunteers and infected patients. Inter- and intra-subject variability across different demographic populations has also been widely investigated.
Absorption, distribution, metabolism and elimination
Following oral administration of OP, oseltamivir is rapidly absorbed from the gastrointestinal tract and converted by hepatic esterases into the active metabolite OC, giving an absolute bioavailability of ∼80%.4,11 OC is detectable in plasma within 30 min of dosing, and concentrations reach near-maximal levels after 3–4 h, exceeding oseltamivir concentrations by >20-fold.4,11 OC plasma concentrations display minimal inter- and intra-subject variability, and concomitant food intake has little effect on bioavailability.4,11 The absorption rate of oseltamivir is unaffected under conditions of altered gastric pH, such as that induced by cimetidine and antacids.4,11
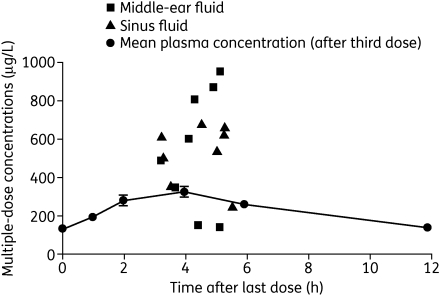
The volume of distribution of OC after intravenous administration in man is 23–26 L.4,11 This value is similar to the extracellular volume of body water in humans, suggesting that the metabolite may penetrate infection sites at concentrations similar to those in plasma. Indeed, oseltamivir and OC are systemically distributed, with therapeutic concentrations attained in the lung, trachea and nasal mucosa,4 as well as the sinuses and middle ear (Figure 3).12 Eisenberg et al.13 also confirmed that OC reached the lung following oral dosing in rats, and was detected in the bronchoalveolar lining fluid with equal or greater exposure to that in plasma and a slower clearance.
Figure 3.
Concentration–time curves for oseltamivir carboxylate in plasma, middle ear and sinuses after five twice-daily doses of 75 mg of oseltamivir (n = 24). Reproduced from Kurowski et al.12 with permission.
At least 75% of an oral dose of the prodrug is converted into OC by first-pass metabolism, and <5% is recovered in urine as OP.4,11 Neither oseltamivir nor OC interacts with human cytochrome P450 mixed-function oxidases or glucuronyl transferases in vitro. OP and OC are eliminated primarily by renal excretion, but small amounts (<20% of the oral dose) of both compounds are also eliminated in faeces.4 Following oral dosing, plasma concentrations of oseltamivir decline rapidly (apparent elimination half-life of 1–3 h), while OC concentrations persist for longer (apparent elimination half-life of 6–10 h), permitting twice-daily dosing. Renal clearance of both compounds exceeds the glomerular filtration rate, indicating that renal tubular secretion contributes to elimination; for OC, this has been shown to proceed via the anionic transport process.4,11
Oseltamivir has shown a high safety margin in acute, subacute and chronic toxicity studies.4 The pharmacokinetics of oseltamivir are linear and dose proportional at doses of up to 500 mg twice daily.4,11 Only modest accumulation (<2-fold) of OC is noted prior to attainment of steady state. The pharmacokinetics of multiple dose administration can be predicted from those of single dosing and provide no indication of a temporal change in the disposition of either oseltamivir or OC. Steady-state plasma concentrations of OC are achieved within 2–3 days of twice-daily administration.4,11 Recently, the pharmacokinetics of high doses of oseltamivir have been examined in healthy Thai volunteers.14 In a dose-escalation study, 21 individuals received single doses of oseltamivir at four increasing dose levels, giving a total of 125 individual series. Doses of up to 675 mg were well tolerated. Pharmacokinetics were dose-linear, with rapid absorption and conversion (median = 93%) into OC. Median [95% confidence interval (CI)] elimination half-lives were 1.0 (0.9–1.1) h for oseltamivir and 5.1 (4.7–5.7) h for OC. One subject repeatedly showed markedly reduced conversion of oseltamivir into OC due to constitutionally impaired carboxylesterase activity.14
Influence of patient demographics and ethnicity
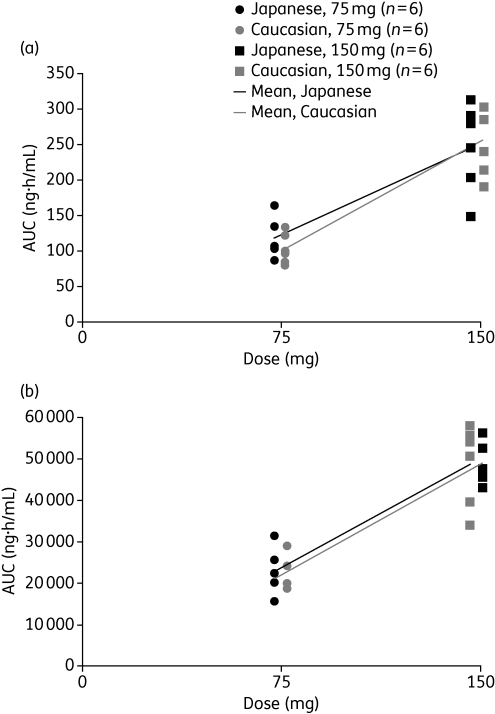
Overall, there are no clinically relevant differences in the pharmacokinetics of oseltamivir or OC between healthy volunteers and infected patients; this applies in adults of different sexes, ages and weights.11 Equally, ethnicity does not appear to affect the pharmacokinetics of the prodrug or active metabolite. In a study in which healthy Japanese and Caucasian subjects were randomized to 75 or 150 mg of oseltamivir or placebo twice daily for 6 days, with a single dose on day 7, individual and mean pharmacokinetics of oseltamivir and OC were similar between the two groups (Figure 4).15 Ethnic differences produced no observable effect on individual exposures at steady state. Although a prospective comparison has not been performed in children, data from four separate studies involving 141 Caucasian and 18 Japanese children have been pooled to compare plasma concentrations.16 After normalization to a 2 mg/kg dose, interquartile ranges for oseltamivir concentration were 3.63–26.75 ng/mL (Caucasian) and 3.95–22.05 ng/mL (Japanese). Results for OC were very similar.
Figure 4.
Distribution of the mean and individual values of the area under the concentration–time curves from time 0 to 12 h post-dose (AUC0–12) for (a) oseltamivir and (b) oseltamivir carboxylate following 7 days of treatment with oseltamivir in healthy Caucasian and Japanese adult volunteers. Reproduced from Schentag et al.15 with permission.
Influence of renal or hepatic impairment
Exposure to OC is increased as a result of decreased renal function, and severe renal insufficiency (creatinine clearance rates of <30 mL/min) is associated with a marked increase in exposure.4,17 In this patient group, a reduced treatment dose of 75 mg once daily is recommended.3 Oseltamivir is not recommended for patients with end-stage renal disease (creatinine clearance <10 mL/min).3 In vitro experiments indicate that the metabolic pathway of oseltamivir conversion into OC in the liver is unlikely to be appreciably altered in subjects with moderate hepatic impairment.18 This has been confirmed in a clinical study, and no dose adjustment is recommended for patients with mild to moderate hepatic impairment.11
Influence of age
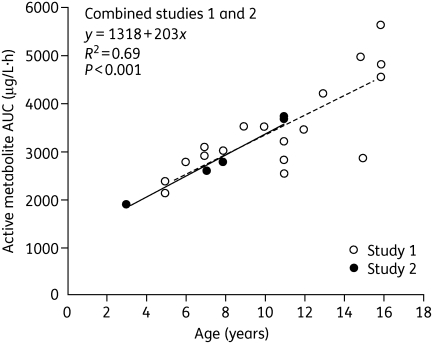
Exposure to oseltamivir in elderly patients increases in proportion to the usual age-related decline in renal function.4,11,19 However, as this increase is small relative to the known safety margin, no dose adjustment is necessary. There are no clinically relevant differences in the pharmacokinetics of oseltamivir or OC between healthy children and infected children.20 Clearance of OC increases with increasing body weight, and hence age, and this is particularly apparent when the OC clearance is expressed per kg of body weight. The OC rate of clearance per kg of body weight in children decreases with advancing age, such that exposure in children ≥13 years is similar to that in adults.20 Although the range of exposures in children and adolescents ≤16 years following a 2 mg/kg oseltamivir dose was within the range that was safe and effective in adults receiving 75 or 150 mg doses twice daily, there was a clear trend towards higher systemic clearance per kg of body weight in younger children. Administration of 2 mg/kg to children in the age range 3–16 years demonstrated a significant downward trend with decreasing age (Figure 5). Therefore, a simple age- and weight-based unit dosing algorithm was developed to ensure exposures in children comparable to those in adults.3 The algorithm is based on a target exposure window and a simple linear regression analysis of pharmacokinetic data from children and adolescents following a single 2 mg/kg dose of oseltamivir. The algorithm was shown to maintain drug exposure within the target efficacy/safety window, while also allowing for a convenient dose regimen.20
Figure 5.
Area under the plasma concentration–time curve (AUC) for oseltamivir carboxylate in children aged 3–16 years administered 2 mg/kg oseltamivir (two pooled studies). The broken line represents the regression line for the open circles (study 1) and the continuous line represents the regression line for the filled circles (study 2). Reproduced from Oo et al.20 with permission.
Drug interactions
Patients with influenza often take over-the-counter medications to ease their symptoms. In addition, many patients with co-morbidities receive concomitant drugs. Drug interactions could adversely affect the pharmacokinetics of the co-administered medications, and thus their efficacy and safety. With this in mind, the potential for clinically relevant interactions between oseltamivir and OC and a variety of commonly used medications has been widely studied.
Oseltamivir has limited potential for clinically relevant interactions with commonly co-administered drugs. When oseltamivir was taken with paracetamol (acetaminophen), plasma concentrations of OC and paracetamol remained unaffected.4 Equally, co-administration of aspirin21 or antacids22 with oseltamivir had no relevant effect on the pharmacokinetics of these compounds. Further studies indicated that neither cimetidine nor amoxicillin exhibited any interaction with oseltamivir.23 Furthermore, the latter investigation confirmed that oseltamivir is a weak competitor for the anionic renal tubular secretion processes and that its potential to compete effectively with other organic acids that undergo renal tubular secretion is minimal.23 Care should be taken with medications with a narrow therapeutic margin that are co-excreted, e.g. methotrexate. In renal transplant patients, co-administration of oseltamivir was shown not to change the pharmacokinetics of the immunosuppressant drugs cyclosporin, mycophenolate mofetil and tacrolimus, and the pharmacokinetics of oseltamivir itself in these patients were found to be similar to those in patients with native kidneys.24
Several groups have studied the co-administration of oseltamivir and probenecid.14,25,26 The ability of probenecid to modify the renal secretion of other drugs has previously been exploited, and its use as a ‘sparing agent’ to extend oseltamivir supplies in the event of an influenza pandemic has been suggested.27,28 In one study, co-administration of oseltamivir and probenecid resulted in an increase of ∼2.5-fold in exposure to OC, indicating that tubular secretion of the latter proceeds via the anionic pathway.23 Although this pathway is shared by some other drugs, clinically relevant interactions are unlikely. A more recent investigation found a mean decrease in the apparent volume of OC distribution of 40% (95% CI, 37%–44%) and mean reduction in renal elimination of 61% (58%–62%), resulting in an increase in the median AUC for OC of 154% (range, 71%–278%).14
Two groups have recently investigated the pharmacokinetics of oseltamivir/probenecid combinations in more detail.25,26 In the first of these, Holodniy et al.25 compared the pharmacokinetics of the combination (oseltamivir 75 mg once every 48 h plus probenecid 500 mg four times a day) with oseltamivir alone (75 mg once daily). Oral clearance of OC was found to be ∼25% lower with the combination, and 48 h plasma exposure results showed a lack of bioequivalence. However, the mean trough concentrations of OC at the end of a dosing interval in the two groups were not significantly different, suggesting that co-administration of probenecid might allow reduction of oseltamivir dose without compromising neuraminidase inhibition.25 In the second study, Rayner et al.26 used a population pharmacokinetic model to simulate combination regimens of oral oseltamivir (45 and 30 mg twice daily) and oral probenecid (500 mg/6 hourly). Probenecid plus 45 mg of oseltamivir achieved all the pharmacokinetic parameters expected of oseltamivir alone, but combination with 30 mg of oseltamivir and dose interval extension approaches did not.26 It is noteworthy, however, that unsuitable oseltamivir/probenecid regimens, or non-compliance with suitable regimens, could increase the risk of emergence of resistant virus via suboptimal dosing. Moreover, co-administration could compromise the systemic distribution of OC, which could be vital in cases of infection with highly pathogenic virus. Alongside the potential for multiple, clinically relevant drug interactions and adverse events with probenecid, the utility of the combination strategy appears to be limited.26
Oseltamivir has not previously been associated with haematological adverse reactions or interference with the coagulation process. However, following sporadic post-marketing reports of bleeding in patients on anticoagulant therapy, the pharmacokinetic interaction between the agents was re-evaluated in 20 patients receiving daily warfarin and with international normalized ratio (INR) values of 2.0–3.5 during the previous 2 weeks.29 These volunteers were randomized to twice-daily 75 mg of oseltamivir for 4 days with a single 75 mg dose on day 5, or warfarin alone in a two-way crossover design separated by a wash-out of 4–8 days. Anticoagulant effects were assessed by calculating overall change (AUEC0–96) and maximum absolute change from baseline (Emax) in INR and Factor VIIa, and change in vitamin K1 concentrations. For both treatments, changes from baseline in INR and Factor VIIa during treatment were small and there were also no statistically significant differences between treatments in mean Emax for INR and Factor VIIa, or in the change from baseline in vitamin K1 concentration. Oseltamivir did not alter warfarin pharmacokinetics. Oseltamivir was well tolerated in this population, with no adverse safety findings.29
Conclusions
Oseltamivir is an orally administered antiviral for the treatment and prophylaxis of influenza A and B infections in adults and children aged ≥1 year. After dosing, the prodrug (OP) is readily absorbed from the gastrointestinal tract and rapidly converted into the active metabolite, OC. In all patient groups, OC has high bioavailability and is systemically distributed to infection sites at concentrations sufficient to inhibit a range of influenza virus neuraminidases. Oseltamivir has a predictable linear pharmacokinetic profile and is suitable for a variety of patient populations and age groups. The potential for clinically relevant drug interactions is low. These characteristics underpin the use of oseltamivir in the diverse patient populations that are likely to be affected by seasonal and pandemic influenza viruses.
Funding
Funding for this manuscript and the Supplement to which it belongs was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland, the manufacturer of oseltamivir.
Funding for the medical writing support was provided by F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Transparency declarations
This article is part of a Supplement sponsored by F. Hoffmann-La Roche Ltd.
The author is employed by F. Hoffmann-La Roche Ltd.
Medical writing support (drafting of text and editorial assistance) for this manuscript was provided by Scott Malkin and Stephen Purver of Gardiner-Caldwell Communications Ltd, Macclesfield, UK.
References
- 1.Nicholson KG. Human influenza. In: Nicholson KG, Webster RG, Hay AJ, editors. Textbook of Influenza. Oxford, UK: Blackwell Science; 1998. pp. 219–64. [Google Scholar]
- 2.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–73. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 3.Tamiflu SmPC. Tamiflu Summary of Product Characteristics. http://emc.medicines.org.uk/ (22 October 2009, date last accessed) [Google Scholar]
- 4.He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999;37:471–84. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 5.Roberts NA, Wiltshire HR, Mendel DB, et al. Oseltamivir carboxylate is effective against all subtypes of influenza neuraminidase. Abstracts of the ASM Biodefense Research Meeting; 2003; Baltimore. Poster. [Google Scholar]
- 6.WHO. Pandemic (H1N1) 2009 - Update 65. http://www.who.int/csr/don/2009_09_11/en/index.html. (23 October 2009, date last accessed) [Google Scholar]
- 7.Leneva IA, Roberts N, Govorkova EA, et al. The neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against A/Hong Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral Res. 2000;48:101–15. doi: 10.1016/s0166-3542(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 8.Bantia S, Parker CD, Ananth SL, et al. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45:1162–7. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Govorkova EA, Leneva IA, Goloubeva OG, et al. Comparison of efficacies of RWJ-270201, zanamivir, and oseltamivir against H5N1, H9N2, and other avian influenza viruses. Antimicrob Agents Chemother. 2001;45:2723–32. doi: 10.1128/AAC.45.10.2723-2732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendel DB, Tai CY, Escarpe PA, et al. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–6. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dutkowski R, Thakrar B, Froehlich E, et al. Safety and pharmacology of oseltamivir in clinical use. Drug Saf. 2003;26:787–801. doi: 10.2165/00002018-200326110-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kurowski M, Oo C, Wiltshire H, et al. Oseltamivir distributes to influenza virus replication sites in the middle ear and sinuses. Clin Drug Invest. 2004;24:49–53. doi: 10.2165/00044011-200424010-00006. [DOI] [PubMed] [Google Scholar]
- 13.Eisenberg EJ, Bidgood A, Cundy KC. Penetration of GS4071, a novel influenza neuraminidase inhibitor, into rat bronchoalveolar lining fluid following oral administration of the prodrug GS4104. Antimicrob Agents Chemother. 1997;41:1949–52. doi: 10.1128/aac.41.9.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wattanagoon Y, Stepniewska K, Lindegardh N, et al. Pharmacokinetics of high dose oseltamivir in healthy volunteers. Antimicrob Agents Chemother. 2009;53:945–52. doi: 10.1128/AAC.00588-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schentag JJ, Hill G, Chu T, et al. Similarity in pharmacokinetics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian subjects. J Clin Pharmacol. 2007;47:689–96. doi: 10.1177/0091270007299761. [DOI] [PubMed] [Google Scholar]
- 16.Gieschke R, Dutkowski R, Smith J, et al. Similarity in the pharmacokinetics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian children. Abstracts of the Options for the Control of Influenza VI Conference; 2007; Toronto. Abstract P921. MediTech Media Conferencing, Inc., Atlanta, GA, USA. [Google Scholar]
- 17.Robson R, Buttimore A, Lynn K, et al. The pharmacokinetics and tolerability of oseltamivir suspension in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2006;21:2556–62. doi: 10.1093/ndt/gfl267. [DOI] [PubMed] [Google Scholar]
- 18.Snell P, Dave N, Wilson K, et al. Lack of effect of moderate hepatic impairment on the pharmacokinetics of oral oseltamivir and its metabolite oseltamivir carboxylate. Br J Clin Pharmacol. 2005;59:598–601. doi: 10.1111/j.1365-2125.2005.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abe M, Smith J, Urae A, et al. Pharmacokinetics of oseltamivir in young and very elderly subjects. Ann Pharmacother. 2006;40:1724–30. doi: 10.1345/aph.1H174. [DOI] [PubMed] [Google Scholar]
- 20.Oo C, Barrett J, Hill G, et al. Pharmacokinetics and dosage recommendations for an oseltamivir oral suspension for the treatment of influenza in children. Paediatr Drugs. 2001;3:229–36. doi: 10.2165/00128072-200103030-00005. [DOI] [PubMed] [Google Scholar]
- 21.Oo C, Barrett J, Dorr A, et al. Lack of pharmacokinetic interaction between the oral anti-influenza prodrug oseltamivir and aspirin. Antimicrob Agents Chemother. 2002;46:1993–5. doi: 10.1128/AAC.46.6.1993-1995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snell P, Oo C, Dorr A, et al. Lack of pharmacokinetic interaction between the oral anti-influenza neuraminidase inhibitor prodrug oseltamivir and antacids. Br J Clin Pharmacol. 2002;54:372–7. doi: 10.1046/j.1365-2125.2002.01678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hill G, Cihlar T, Oo C, et al. The anti-influenza drug oseltamivir exhibits low potential to induce pharmacokinetic drug interactions via renal secretion—correlation of in vivo and in vitro studies. Drug Metab Dispos. 2002;30:13–9. doi: 10.1124/dmd.30.1.13. [DOI] [PubMed] [Google Scholar]
- 24.Aoki F, Lam H, Jeffrey JR, et al. Oseltamivir does not interact pharmacokinetically with cyclosporine, mycophenolate or tacrolimus in renal transplant patients. Abstracts of the Annual Meeting of the Infectious Diseases Society of America; 2005; San Francisco. Abstract 522. Infectious Diseases Society of America, Arlington, VA, USA. [Google Scholar]
- 25.Holodniy M, Penzak SR, Straight TM, et al. Pharmacokinetics and tolerability of oseltamivir combined with probenecid. Antimicrob Agents Chemother. 2008;52:3013–21. doi: 10.1128/AAC.00047-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rayner CR, Chanu P, Gieschke R, et al. Population pharmacokinetics of oseltamivir when coadministered with probenecid. J Clin Pharmacol. 2008;48:935–47. doi: 10.1177/0091270008320317. [DOI] [PubMed] [Google Scholar]
- 27.Butler D. Wartime tactic doubles power of scarce bird-flu drug. Nature. 2005;438:6. doi: 10.1038/438006a. [DOI] [PubMed] [Google Scholar]
- 28.Howton JC. Probenecid with oseltamivir for human influenza A (H5N1) virus infection? N Engl J Med. 2006;354:879–80. doi: 10.1056/NEJMc052951. [DOI] [PubMed] [Google Scholar]
- 29.Davies BE, Aceves Baldo P, Brewster M. Effect of oseltamivir on anticoagulation: a crossover study in patients stabilized on warfarin. Abstracts of the Forty-ninth Interscience Conference on Antimicrobial Agents and Chemotherapy; 2009; San Francisco. Washington, DC, USA: American Society for Microbiology; Abstract A1-596. [Google Scholar]