Abstract
Objectives
Evaluate the effects of pressure and duration of intracoronary (IC) infusion of mesenchymal stem cells (MSCs) on delivery efficiency and safety after myocardial infarction (MI).
Background
Standard IC delivery of MSCs can lead to intravascular plugging and reduced coronary blood flow. The optimal delivery pressure and duration is unknown.
Methods
Immediately after MI pigs were randomized to 1 of 3 delivery protocols of 5 × 107 iron-fluorescent microspheres labeled MSCs, control received 2 ml infusions at 1 ml/min (five times), very high flow rate (VHFR) a single 10 ml infusion at 60 ml/min and the high flow rate (HFR) group a single 10 ml infusion at 20 ml/min. TIMI grade flow was assessed throughout the procedure and at sacrifice (day 14). MSCs distribution was analyzed in isolated hearts by 4.7T MRI. Delivery efficiency was quantified via fluorescent microsphere recovery using a magnetic separation technique and by light microscopy.
Results
TIMI grade flow did not change following MI (all groups TIMI 3). However, following MSCs delivery only 18% (2/11) of control animals had TIMI 3 blood flow vs. 56% (5/9) in VHFR and 67% (4/6) in HFR (P = 0.03). As a consequence, 63% of control animals died within 24 hr, 33% in VHFR and none in HFR (P = 0.02). MSCs delivery in the infarct tissue did not differ between the groups (P = 0.06).
Conclusions
A single MSCs infusion at 20 ml/min resulted in improved coronary blood flow and decreased mortality, without sacrificing delivery efficiency.
Keywords: angioplasty balloon, microvascular plugging, allogeneic transplantation, cellular delivery, cardiomyoplasty, TIMI grade blood flow
INTRODUCTION
Irreversible cell loss due to myocardial infarction (MI) is a major cause of ventricular dysfunction, congestive heart failure, and death. One strategy being studied to potentially improve long-term outcomes of an MI is to reduce ventricular remodeling by cardiac transplantation of bone marrow-derived mesenchymal stem cells (MSCs) [1–3]. These cells represent 0.001–0.01% of the population of nucleated cells in the bone marrow and have the ability to differentiate into cardiomyocytes [4,5]. MSCs offer several advantages: they can modulate the immune response by altering the cytokine secretion profile and inhibiting T cell proliferation [4,6,7], and given their allogenicity, they can be isolated and prepared in advance thereby being immediately available for use after an MI.
We have previously shown that intracoronary (IC) infusion of MSCs in pigs resulted in a higher delivery efficiency and retention within myocardial tissue compared to intravenous and endomyocardial delivery [8]. However, IC delivery was associated with an increased incidence of impaired distal blood flow after delivery into an infarcted area secondary to clustering of MSCs in the microvasculature. To improve the delivery efficiency and reduce untoward events after IC delivery new approaches to IC MSCs delivery may be necessary. One such approach is delivering cells during a single infusion at higher flow rates, thereby reducing arterial no-reflow and improving subsequent cell delivery.
METHODS
MSCs Preparation
Allogeneic MSCs were derived from porcine bone marrow aspirates using a standardized protocol (Cambrex BioSciences Walkersville, Walkersville, MD). Briefly, the mononuclear cell fraction was isolated from the bone marrow aspirate using density-gradient centrifugation and then plated in culture flasks. These cells were then expanded out to third passage. Fluorescent iron particles (Bangs Laboratories, Fishers, IN) were added to cell culture media in the last 24 hr of culture. Pilot studies confirmed that the manufacturer's specification of 50 particles per cell was accurate and that the assay used to measure the cell number was sensitive enough to detect as few as 12,500 cells, or less than 0.03% of the target dose. Each cell batch was qualified for total cell number, viability, sterility, mycoplasma, and endotoxin. The cells were then frozen at –80°C until the delivery procedure. Before administration, 5 × 107 cells were thawed, counted, diluted to 10 ml and loaded into a sterile 10 ml syringe. To decrease aggregation of the cells, MSCs were kept at 37°C and the syringe was gently rocked until infusion.
Myocardial Infarction
An MI was induced in female Yorkshire pigs (25–40 kg) by prolonged balloon inflation in the mid left anterior descending coronary artery (LAD). Heparin was administered to maintain an activated clotting time (ACT) >300 sec. Using a 6 French guide catheter, a standard angioplasty balloon catheter was placed into the mid LAD, distal from the first diagonal artery. The balloon was then inflated for 1 hr at 1.1:1 ratio resulting in an infarct comprising ~22–26% of the left ventricular mass [3,8]. Following 60 min of occlusion, the balloon was deflated and the area reperfused for 20 min. The animals were then randomly assigned to one of the three groups for MSCs delivery. Blood pressure, arterial O2 saturation and electrocardiographic parameters were continuously monitored throughout the procedure. The study was approved by the University of Pennsylvania Institutional Animal Care and Use Committee (IACUC).
MSCs Delivery
Following 20 min of reperfusion, animals were randomized to 1 of 3 groups: control, very high flow rate (VHFR) and high flow rate (HFR). All three groups received a total number of 5 × 107 MSCs. Originally, we planned to enroll 18 animals (6 in each group), with percent of retained cells at 14 days as the primary efficacy endpoint. However, due to the early mortality, we enriched the sample size. Hence, 11 animals were enrolled in the control group, 9 in the VHFR group and the original 6 in the HFR group for a total of 26 enrolled animals.
Control delivery
A standard angioplasty balloon was placed at the infarct location in the LAD and 5 infusions were performed. The balloon was inflated to a 1:1 diameter ratio, the guide wire was removed and 2 ml of the MSCs suspension were infused at 1 ml/min. The balloon was then deflated and the vessel reperfused for 2 min. The balloon was then re-inflated and the process repeated until the entire 10 ml dose was delivered.
Experimental deliveries
Cells were delivered in a single 10 ml bolus using a prototype balloon catheter possessing a large inner lumen diameter which allows delivery at higher flow rates than can be achieved with standard angioplasty catheters. The catheter was placed in the mid LAD and the balloon inflated to a 1:1 ratio. In the VHFR group, MSCs were delivered over 10 sec (60 ml/min) while in the HFR cells were delivered over 30 sec (20 ml/min). Pilot studies in infarcted animals (n = 2) demonstrated that the distal coronary perfusion pressures achieved with this catheter exceeded physiologic pressure, with a mean distal pressure of 209 ± 5.7 mm Hg in the VHFR and 191 ± 28.3 mm Hg in the HFR group as determined by ComboWire® XT pressure and flow wires (Volcano Therapeutics, Rancho Cordova, CA).
During the reperfusion time and following MSCs delivery, angiograms were performed to estimate TIMI grade flow. In the instances of decreased TIMI flow, 100 μg of nitroglycerin was administered in a single bolus directly into the LAD. Left ventriculography was performed after cell injection and 14 days later, prior to sacrifice to assess ejection fraction.
MR Imaging of Delivered MSCs
Animals were sacrificed 14 days following MI and MSCs delivery. The hearts were rapidly removed, the left ventricles isolated and blood and thrombus removed. A rubber balloon was attached to a luer valve and placed into the left ventricular cavity with the valve sutured to the aortic opening. The entire ventricle with the balloon was immersed in a cylindrical glass vessel filled with perfluoropolyether (Fomblin®, manufactured by Solvay Solexis, Thorofare, NJ), which provides a homogenous media for the tissue sample but is not detectable by MRI because all hydrogen atoms in this compound are replaced by fluorine. A capsule was placed over the septum region for orientation. The valve was sealed and the vessel capped. The glass vessel was fit into a 1H volume transceiver coil of 6 cm inner diameter (Varian, Palo Alto, CA). MR imaging was performed on 4.7T horizontal bore INOVA spectrometer (Varian, Palo Alto, CA). T2-weighted 3D gradient echo images were acquired with the following parameters: repetition time (TR) of 0.1 sec, time of echo (TE) of 0.02 sec, flip angle of 10, field of view (FOV) of 5.5 × 5.5 × 5.5, matrix of 128 × 128 × 128 (leading to an isotropic resolution of 0.43 mm).
Cellular Delivery
Tissue sectioning and particle quantification
Following the MRI, the heart was cleaned of residual oil. Transverse slices at 1 cm intervals from apex to base were obtained and further divided into sections. The sections were classified into infarct, border (1 cm to each side of the infarct) and normal zones. Each section was weighed and a representative sample was removed for histolopathology from infarct, border and normal tissues areas.
The remaining tissues were placed into individually labeled plastic centrifuge tubes filled with 7 ml of 2 M Potassium hydroxide (KOH) and heated in a water bath at 60°C for 3 hr. Following removal from the water bath, a series of magnetic separation steps to isolate the magnetic particles from the degraded tissue slurry were performed. Each tube was placed on a rare-earth magnet (Dynal Biotech ASA, Oslo, Norway) tube holder for 10 min. The supernatant was then drawn off and replaced with distilled water, vortexed for 20 sec and the process was repeated three times to ensure removal of all residual alkaline solution or tissue. Following the final wash step, 1 ml of water was added to the tube and the suspension was placed in an ultrasound bath for 10 sec and vortexed for a minute.
Three samples, 200 μl each, were drawn from each tube and placed in a 96-well fluorometry plate, scanned at 480 nm excitation and recorded at 520 nm. On the basis of the calibration using a serial dilution of a known concentration of the seed particles, the number of particles per g of tissue was reported. The number of cells present in the assayed tissue was normalized to the mass of the tissue in each region (normal and infarct).
Histopathology
Samples obtained were divided into infarct, border, and normal tissues. After adequate processing, the segments were stained using H&E and Prussian blue staining. Light microscopy was used to identify iron-labeled cells in the tissue samples (blue staining in the cytoplasm). The concentration of cells was determined at a magnification of 400× in two different sections for each zone. The area of each section was also quantified.
Statistical Analysis
Data are presented as mean ± standard deviation unless noted otherwise. Analysis was performed using site-licensed SPSS® statistical software (SPSS, Chicago, IL). Initial comparisons were made among control, VHFR, and HFR groups using one-way ANOVA or Chi square tests. Comparison of post MSCs delivery TIMI flow rate and subsequent mortality used the Fisher's Exact Test. Individual comparisons between flow rate groups and control were made using Student's t-tests for independent variables, as well as paired analyses for within subject differences at normal, border and infarct zones. Schieffe correction was used to control for multiple comparisons. Statistical significance was set at the 0.05 level.
RESULTS
In vivo Blood Flow
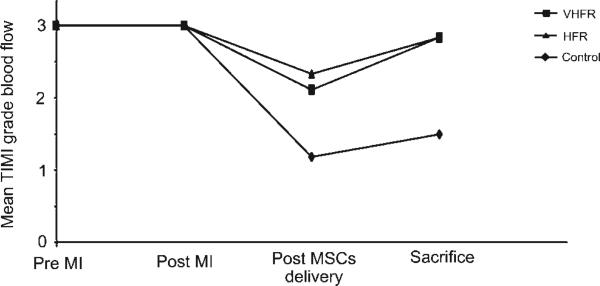
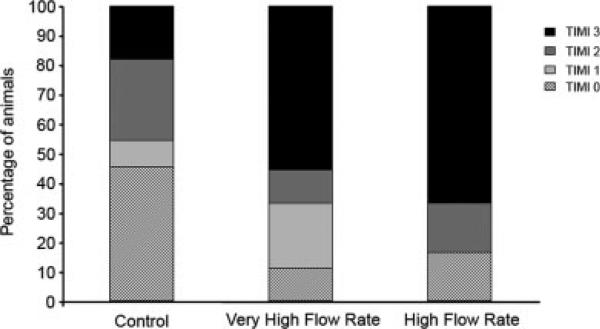
All the animals had TIMI 3 grade distal blood flow at baseline and after infarction (Fig. 1). Following MSCs delivery, the mean TIMI grade blood flow decreased in the three groups, although it was more pronounced in the control group (Mean TIMI grade blood flow: control: 1.18; VHFR: 2.11; HFR: 2.33). In the control group, only 2 of 11 animals (18%) exhibited TIMI 3 grade flow after MSCs delivery (Fig. 2). In the VHFR and HFR groups, 5 of 9 (56%) and 4 of 6 (67%) animals respectively exhibited a TIMI score of 3 (P = 0.03 vs. control). At 14 days, 2 of 4 animals of the control group (50%) exhibited TIMI 3 grade blood flow, while the other two animals had TIMI grade 0. In each experimental group, 5 of 6 animals showed TIMI 3 grade blood flow (83%) with the remaining animal exhibiting TIMI 2 grade flow.
Fig. 1.
Delivery of mesenchymal stem cells (MSCs) at 60 ml/min (VHFR) and 20 ml/min (HFR) was associated with improved TIMI flow rates after infusion. VHFR, very high flow rate; HFR, high flow rate; MI, myocardial infarction.
Fig. 2.
TIMI grade flow following mesenchymal stem cells (MSCs) infusion after infarction. Delivery at either 60 ml/min (n = 9) or 20 ml/min (n = 6) resulted in a higher percentage of animals exhibiting TIMI 3 grade flow following MSCs infusion compared to the control group (n = 11) (P = 0.03).
Mortality and Morbidity of IC Delivery
There were no significant differences in the left ventricular ejection fraction either following infarction/MSCs infusion or at 14 days: Post MI/MSCs infusion: Control: 42.6 ± 15.4%, VHFR: 44.0 ± 11.4%, HFR: 46.4 ± 2.4% (P = 0.91). At 14 days: Control: 36.9 ± 17.5%, VHFR: 42.6 ± 12.3%, HFR: 44.7 ± 12.5% (P = 0.79).
Every animal enrolled in the HFR group completed the study without major complications. In the VHFR group, three animals (33%) died within 24 hr of MSCs infusion. Of the 11 animals in the control group, 7 (63%) died within 24 hr of the study (P = 0.02 vs. experimental groups, Table I). Early death was associated with persistent decreased TIMI 0–2 grade distal blood flow following MSCs infusion. Nine of 15 animals exhibiting TIMI 0–2 grade flow after MSCs infusion died compared to 1 of 11 with TIMI 3 grade flow (P = 0.01). The actual cause of death may have been due to ventricular arrhythmias, leading to sudden death, in the setting of persistently decreased distal arterial blood flow. Clinically, angiographic no-reflow phenomenon is associated with increased short- and long-term mortality following percutaneous transluminal coronary angioplasty [9].
TABLE I.
Mortality Rates
| Groups | Immediate post MSCs delivery deaths | Cause of death | Post procedural deaths (≤24 hs) | Cause of death | Completed study |
|---|---|---|---|---|---|
| Control (n = 11) | 2 | Ventricular fibrillation | 5 | Sudden death | 4 (36%) |
| VHFR (n = 9) | 2 | Ventricular fibrillation | 1 | Sudden death | 6 (67%) |
| HFR (n = 6) | 0 | 0 | 6 (100%) |
MSCs, mesenchymal stem cells. Sudden death thought secondary to ventricular arrhythmias.
Cellular Delivery
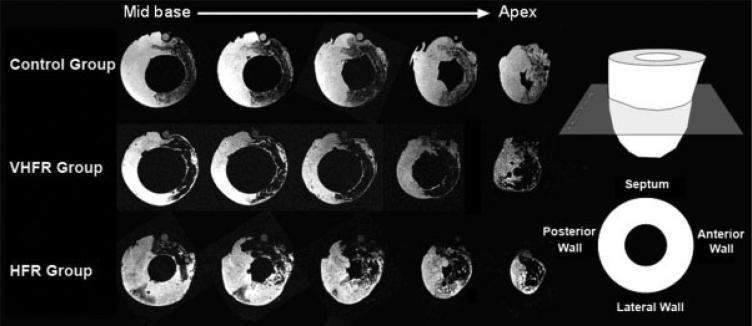
The total infarct tissue collected for each ventricle averaged 19.7 ± 4.1 g. Representative cardiac MRI slices from base to apex of the heart are displayed in Fig. 3. In all animals the hypointense signal induced by super paramagnetic iron oxide labeled MSCs spread was localized over the anterior-septal wall in the LAD distribution. The relatively uniform hypointense signal on MR images also suggests that MSCs were effectively delivered to the LAD territory.
Fig. 3.
Representative MRI examples showing the presence of iron-labeled cells (dark areas) 14 days following delivery in the three groups. Transversal slice imaging shows cells located in the approximate infarct region fed by the mid and distal LAD (anterior and septal walls).
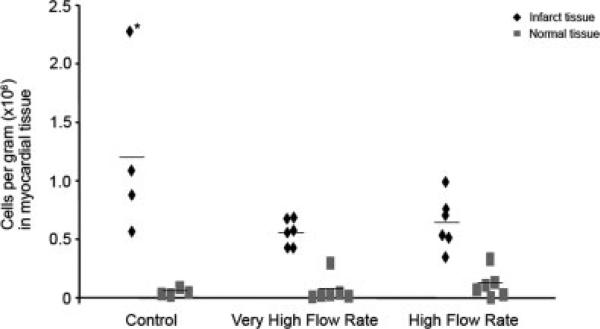
Figure 4 shows the concentration of delivered cells within the myocardium for individual animals. The control group had an increased concentration of cells within the infarct tissue compared to the other two groups, although this difference did not achieve statistical significance (control: 11.9 × 105 ± 7.49 × 105 cells per g, VHFR: 5.48 × 105 ± 1.15 × 105 cells per g, HFR: 6.32 × 105 ± 2.25 × 105 cells per g. P = 0.06). However, the increased delivery in the control group reflects an increased concentration noted in an animal with arterial occlusion and persistence of cells in the mid and distal LAD. The number of delivered cells in the infarct region was greater than the number present in the normal tissue for the same animal in the VHFR and HFR groups (P < 0.0001 in VHFR; P = 0.007 in HFR), while approaching significance in the control group (P = 0.06).
Fig. 4.
Intracoronary delivery of mesenchymal stem cells (MSCs) in infarct tissue was similar across the groups (control vs. experimental P = 0.06). Lines represent mean values for each group. *Shows animal with persistent occlusion of LAD following MSCs delivery, associated with the highest retention of cells.
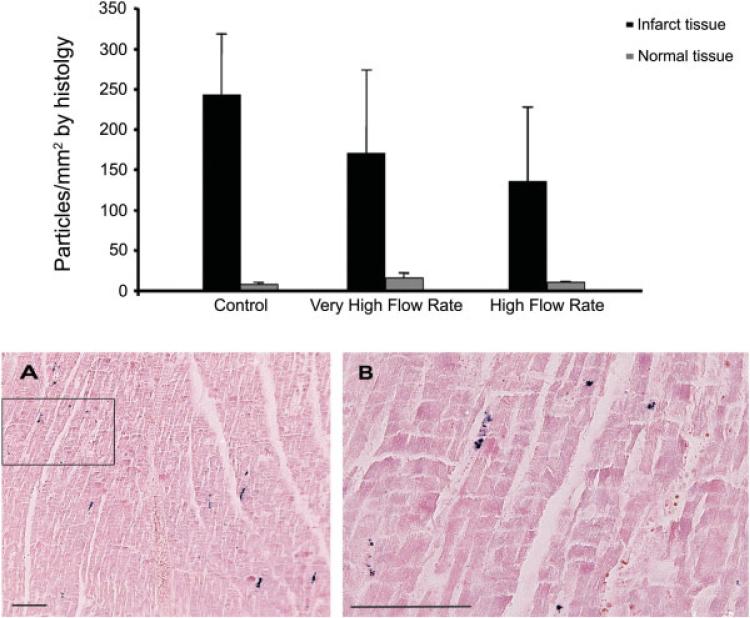
Histologic analysis demonstrated that cells were generally noted in the myocardium and not the vasculature. There was a higher concentration of Prussian blue-positive cells in the control group (Fig. 5). However, there was no significant difference in particle concentration within the infarct zones among the three groups (Results expressed as mean ± SEM. Control: 241 ± 76 particles/mm2, VHFR: 169 ± 104 particles/mm2, HFR: 134 ± 93 particles/mm2. P = 0.71).
Fig. 5.
Concentration of Prussian blue-positive cells in the infarct and normal zones detected by histology. Upper panel: There was no significant difference in particle concentration in the infarct tissue among the 3 groups (P = 0.71). Results are expressed as mean ± SEM. Lower panel: (A) ×100 magnification of the infarct tissue showing Prussian Blue-positive mesenchymal stem cells following delivery at 60 ml/min (B) ×400 magnification of the black box in panel A. Bar indicates 100 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
The current study was designed to correlate increased distal arterial flow rate to safety and retention of MSCs delivered shortly after reperfusion of infarcted myocardium. As such, the study addressed an important aspect of MSCs delivery, namely injection pressure and whether a single infusion of cells results in increased retention compared to multiple smaller infusions obtained during short periods of transient ischemia. The results indicate delivery of MSCs in infarct zones following five injections of 2 ml each was associated with reduced distal blood flow and substantially increased early mortality. A single, continuous infusion at increased flow rates resulted in an increased incidence of preserved blood flow and decreased mortality with a similar degree of cell delivery.
We have previously demonstrated that IC delivery of MSCs after infarction results in decreased distal blood flow [8]. This observation may reflect of the use of relatively large cells, ~10–20 μm into the vasculature of newly infarcted myocardium, a milieu characterized by endothelial damage and increased interstitial pressure secondary to cellular edema. Multiple balloon inflations performed 20 min after the onset of reperfusion, as carried out in the control group, may have resulted in additional endothelial and/or intimal damage thereby triggering vasospasm, cellular aggregation, and subsequent microvascular plugging, as reflected by the impaired distal blood flow and increased mortality. Using a similar multiple inflation approach, Vulliet et al. observed that the delivery of mesenchymal stromal cells in healthy, noninfarcted dogs caused electrocardiographic ST changes, microvascular plugging and microinfarcts [10]. A beneficial effect of a delivery approach that avoided repetitive injury to the vascular wall was shown by Perin et al. in which IC delivery of MSCs 7 days after an MI in dogs, using multiple infusions without proximal occlusion of the vessel, resulted in only one death due to no-reflow and recurrent infarction out of seven animals [11]. Extensive microvascular plugging was observed on histology. Hence, comparison of the current results with those reported by Perin et al. would indicate that either eliminating sequential balloon inflations or increasing the time between reperfusion and delivery may be necessary to reduce no-reflow, although species differences may also play a role. Clinically, vessel dilatation during percutaneous coronary intervention increases the plasma cytokine levels, thereby triggering an inflammatory cascade [12].
VHFR was associated with higher mortality and impaired coronary blood flow compared to HFR, demonstrating a potential effect of infusion velocity (Table II). This observation supports data showing an association between increased velocity of IC infusion and incidence of micro-infarcts in noninfracted dogs [11]. We postulate that the higher infusion velocities may damage stem cells leading to increased aggregation of platelets, neutrophils and MSCs which impairs the distal coronary blood flow and increases mortality.
TABLE II.
Association of TIMI Grade Flow Following MSCs Delivery and Death Within <24 hr
| Groups | Deaths/TIMI 0 | Deaths/TIMI 1 | Deaths/TIMI 2 | Deaths/TIMI 3 |
|---|---|---|---|---|
| Control | 3/5 | 0/1 | 3/3 | 1/2 |
| VHFR | 1/1 | 1/2 | 1/1 | 0/5 |
| HFR | 0/1 | 0/0 | 0/1 | 0/4 |
The association of bone marrow derived 1 stem cells with subsequent decreased distal blood flow has not been reported in humans [13–17]. An important difference between these clinical studies and the current porcine study is the time of delivery in relationship to the infarct. In the human studies, cells were delivered between 1 and 8 days after the infarction, while we injected the cells 20 min following reperfusion. The delay in clinical studies reflects the processing and preparation of autologous stem cells, steps not necessary when using allogeneic MSCs. However, the time delay may be necessary to allow recovery of microvascular function. Currently optimal delivery timing for stem cell delivery including MSCs remains unknown [4,6,7,18] however, the current results suggest that a single infusion at 20 ml/min may be optimal from a safety standpoint without significantly decreasing cellular delivery and potential engraftment and should be studied further.
There are potential limitations in this study. The method used to calculate the delivery of cells was based on the detection of the iron particles placed within the injected MSCs. However, delivered MSCs may die thereby releasing the particles, where they undergo phagocytosis. Terrovitis et al. recently demonstrated that after injection of human MSCs into immunocompetent rats the iron content (assessed by MRI and Prussian blue staining) persisted in the myocardium 3 weeks after delivery, even after death of the injected cells [19]. Although data evaluating xenotransplantation of MSCs may not apply to allogeneic delivery, their observation suggests that MRI evaluation of the presence of iron-loaded cells may reflect delivery and not long-term engraftment, per se. The results of the current study should be assessed within that perspective. However, as this study was designed to correlate subsequent mortality and TIMI grade blood flow with cellular delivery approaches, the results remain valid.
In summary, the current investigational study shows a significant improvement in the TIMI grade flow in the target vessel when MSCs are delivered in a single bolus at 20 ml/min for 30 sec after an MI. This approach resulted in decreased early mortality rate, without a significant decrease in cellular delivery compared to the current standard delivery approach.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the assistance of Harrilla Profka in the care of the animals and the performance of the procedures.
Footnotes
Conflict of interest: R.L.W. is the recipient of research grants from Boston Scientific Corp.; S.E. and T.F. were former employees of Boston Scientific Corp.
REFERENCES
- 1.Boomsma RA, Swaminathan PD, Geenen DL. Intravenously injected mesenchymal stem cells home to viable myocardium after coronary occlusion and preserve systolic function without altering infarct size. Int J Cardiol. 2007;122:17–28. doi: 10.1016/j.ijcard.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 2.Amado LC, Saliaris AP, Schuleri KH, St. John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc Natl Acad Sci USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A placebo controlled, dose-ranging, safety study of allogenic mesenchymal stem cells injected by endomyocardial delivery after an acute myocardial infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 4.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 5.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 6.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cells responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 7.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- 8.Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 9.Morishima I, Sone T, Okumura K, Tsuboi H, Kondo J, Mukawa H, Matsui H, Toki Y, Ito T, Hayakawa T. Angiographic no-reflow phenomenon as a predictor of adverse long-term outcome in patients treated with percutaneous transluminal coronary angioplasty for first acute myocardial infarction. J Am Coll Cardiol. 2000;36:1202–1209. doi: 10.1016/s0735-1097(00)00865-2. [DOI] [PubMed] [Google Scholar]
- 10.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronary arterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 11.Perin EC, Silva GV, Assad JAR, Vela D, Buja LM, Sousa ALS, Litovsky S, Lin J, Vaughn WK, Coulter S, Fernandes MR, Willerson JT. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J Mol Cell Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 12.Paroli M, Mariani P, Accapezzato D, D'Alessandro M, Di Russo C, Bifolco M, Sirinian MI, Fedele F, Bruno G, Sardella G. Modulation of tachykinin and cytokine release in patients with coronary disease undergoing percutaneous revascularization. Clin Immunol. 2004;112:78–84. doi: 10.1016/j.clim.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: Double-blind, randomised controlled trial. Lancet. 2006;367:113–121. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 14.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 15.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey D, Hamm C, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher A. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 16.Strauer BE, Brehm M, Tobias Z, Köstering M, Hernandez A, Sorg RV, Kögler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch A, Nijveldt R, van der Vleuten PA, Tio RA, van der Giessen WJ, Marques KMJ, Doevendans PA, Waltenberger J, ten Berg JM, Aengevaeren WRM, Biemond BJ, Tijssen JGP, van Rossum AC, Piek JJ, Zijlstra F. Intracoronary infusion of autologous mononuclear bone marrow cells in patients with acute myocardial infarction treated with primary PCI: Pilot study of the multicenter HEBE trial. Catheter Cardiovasc Interv. 2008;71:273–281. doi: 10.1002/ccd.21337. [DOI] [PubMed] [Google Scholar]
- 18.Dimmeler S, Zeiher AM, Schneider MD. Unchain my heart: The scientific foundations of the cardiac repair. J Clin Invest. 2005;115:572–583. doi: 10.1172/JCI24283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terrovitis J, Stuber M, Youssef A, Preece S, Leppo M, Kizana E, Schär M, Gerstenblith G, Weiss RG, Marbán E, Abraham MR. Magnetic resonance imaging overestimates ferumoxide-labeled stem cell survival after transplantation in the heart. Circulation. 2008;117:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.732073. [DOI] [PubMed] [Google Scholar]