Abstract
The development of airway hyperreactivity (AHR), a cardinal feature of asthma, requires the presence of invariant NKT (iNKT) cells. In a mouse model of asthma, we demonstrated that the induction of AHR required ICOS costimulation of iNKT cells. ICOS was highly expressed on both naive and activated iNKT cells, and expression of ICOS was greater on the CD4+ iNKT than on CD4− iNKT cells. Furthermore, the number of CD4+ iNKT cells was significantly lower in spleens and livers of ICOS−/− and ICOSL−/− mice, and the remaining iNKT cells in ICOS−/− mice were dysfunctional and failed to reconstitute AHR when adoptively transferred into iNKT cell-deficient Jα18−/− mice. In addition, direct activation of iNKT cells with α-GalCer, which induced AHR in wild-type mice, failed to induce AHR in ICOS−/− mice. The failure of ICOS−/− iNKT cells to induce AHR was due in part to an inability of the ICOS−/− iNKT cells to produce IL-4 and IL-13 on activation. Moreover, survival of wild-type iNKT cells transferred into ICOSL−/− mice was greatly reduced due to the induction of apoptosis. These results indicate that ICOS costimulation plays a major role in induction of AHR by iNKT cells and is required for CD4+ iNKT cell function, homeostasis, and survival in the periphery.
Bronchial asthma is an immunological disease resulting from Th2-driven inflammation in the airways. It is characterized by inflammation in the peribronchial space, with increased production of airway mucus, and by airway hyper-reactivity (AHR),3 a cardinal feature of asthma. Although conventional CD4+ T cells play a major role in asthma by recognizing exogenous Ags and initiating allergic inflammation in the lungs, we and others showed that invariant NK T (iNKT) cells are important effector cells in asthma. Thus, iNKT cell-deficient mice failed to develop AHR and have substantially reduced eosinophilia after sensitization and challenge with allergen, although Th2 responses developed normally (1, 2). The requirement for iNKT cells was specific, since adoptive transfer of iNKT cells from wild-type (WT) mice reconstituted the development of AHR in iNKT cell-deficient Jα18−/− mice. These studies of iNKT cells in mice and nonhuman primates (1–8) strongly suggest that iNKT cells play an important role in human asthma. Indeed, a significant number of CD4+ iNKT cells are present in the lungs of patients with asthma and not in the lungs of normal individuals (9, 10), although the degree of infiltration of iNKT cells in the lungs has been questioned (11).
NK T cells comprise a unique and relatively rare subset of lymphocytes that express markers of both αβTCR+ T cells and NK cells. Type I (or classical) NKT cells constitute a distinct subset of T cells expressing a highly restricted or conserved/invariant TCR repertoire consisting of Vα14-Jα18 (in mice) or Vα24-Jα18 (in humans). These NKT cells are often referred to as iNKT (12). iNKT cells are CD4+ or CD4−/CD8− (double negative; DN). Through their invariant TCRs, iNKT cells recognize exogenous and endogenous glycolipid Ags presented by the non-polymorphic MHC class I-like protein, CD1d (13). CD1d is widely expressed by intestinal and airway epithelial cells, T cells, hepatocytes, B cells, macrophages, and dendritic cells (DCs). Activation of iNKT cells results in the rapid production of large quantities of cytokines such as IL-4, IL-13, IL-10, and IFN-γ (14, 15). This capacity to produce cytokines rapidly is a manifestation of an innate-like immunity that endows the iNKT cell with the capacity to critically amplify adaptive immunity and regulate the development of polarized T cells.
Activation and function of conventional class II MHC-restricted CD4+ T cells is regulated by signals delivered through the TCR and costimulatory molecules. One important costimulatory molecule that plays a critical role in CD4+ T cell activation is the ICOS molecule, a member of the CD28 superfamily, expressed following activation of naive T cells and expressed preferentially on Th2 cells and regulatory T cells (16–20). ICOS has been shown to regulate production of Th2 cytokines (21) and to have a critical role in lung mucosal inflammatory responses (22). The specific costimulatory signals however, that are required for the activation and function of iNKT cells in asthma are not fully understood.
Engagement of CD40 or CD28 has been shown to provide important costimulatory signals to iNKT cells and to modulate production of IL-4 and IFN-γ in vitro and in vivo (23–25). In addition, we and others have shown that iNKT cells play a pathogenic role in asthma, but little is known about the costimulatory molecules that regulate the development and effector function of iNKT cells in asthma. Therefore, we hypothesized that ICOS might play an important role in iNKT cell activation and function. In this study, we found that in the absence of ICOS, the function of iNKT cells was greatly impaired and that ICOS−/− iNKT cells were unable to induce AHR. In addition, we found that ICOS is required for CD4+ iNKT cell survival and homeostasis in the periphery, as the number of CD4+ iNKT cells was significantly lower in spleen and liver of both ICOS−/− and ICOSL−/− mice. Moreover, the survival of WT iNKT cells transferred into ICOSL−/− mice was greatly decreased due to the induction of apoptosis, compared with those transferred into WT recipients. These studies demonstrate the importance of ICOS on iNKT cell function in development of AHR and in the homeostasis and survival of CD4+ iNKT cells.
Materials and Methods
Mice
Female BALB/cByJ mice (6–8-wk-old), CD28−/− mice, and an ICOSL−/− breeding pair were purchased from The Jackson Laboratory. Jα18−/− mice (backcrossed to BALB/c) were a gift from M. Taniguchi/T. Nakayama (Chiba University, Chiba, Japan) and S. Balk (Brigham and Women’s Hospital, Boston, Massachusetts). ICOS−/− mice were obtained from Arlene Sharpe (Harvard Medical School, Boston, Massachusetts) and backcrossed 11 times to BALB/cByJ. CD45.1 mice were obtained from J. Campbell (Brigham and Women’s Hospital, Boston, Massachusetts). Mice were maintained and used according to institutional and National Institutes of Health guidelines in a pathogen-free facility. The Animal Care and Use Committee, Children’s Hospital Boston approved all animal protocols.
In vivo Ab treatments
Mice were injected i.p. with 1 mg anti-ICOSL mAb 16F.7E5 (20) or rat isotype control mAb. Thymus, liver, and spleen were analyzed for the presence of NK T cells after 48 h.
Flow cytometry analysis
Cells were preincubated with anti-Fc blocking mAb (2.4G2) as well as normal rat serum, and washed before staining. iNKT cells were identified using various Ab combinations that included PE-conjugated CD1d:PBS-57 loaded tetramer (National Institutes of Health, National Institute of Allergy and Infectious Diseases MHC tetramer core facility), TCRb-PECy5 or allophycocyanin (clone H57–597; eBioscience), and CD4 allophycocyanin or allophycocyanin-Alexa750 (clone RM4–5) (eBioscience). FITC-Anti ICOS mAb 7E.17G9 was obtained from BD Biosciences. Cells were acquired on the FACS Calibur or FACS Canto flow cytometers (BD Biosciences), and 2500 events within the iNKT cell gate were collected. The data were analyzed with FlowJo 6.2 software (Tree Star).
Purification of iNKT cells
For isolation of splenic iNKT cells, cells were labeled with PE-conjugated CD1d-tetramer followed by anti-PE microbeads and then sorted by Auto MACS according to manufacturer’s instruction. Purity of iNKT cells was >80%. For isolation of CD4+ and CD4− iNKT cell subsets, cells were purified from spleen using FITC-PBS-57 (α-GalCer analog) loaded CD1d tetramers (National Institutes of Health, National Institute of Allergy and Infectious Diseases MHC tetramer core facility) and using MACS anti-FITC MultiSort kit (Miltenyi Biotech). Anti-FITC microbeads bound to tetramer+ cells were detached using MACS MultiSort release reagent and MACS MultiSort stop reagent according to the manufacturer’s instructions. The resultant tetramer+ cells were further enriched for CD4+ and CD4− cells by positive selection on a MACS column after incubating with anti-CD4 microbeads. In some experiments, selected cells were further enriched using a MoFlo high-speed cell sorter (DakoCytomation). CD4+ Tetramer+ and CD4− tetramer+ NK T cell preparations were confirmed to contain an average of >94 and >91% pure population, respectively. For detection of NK T cells in liver, liver lymphocytes were isolated with 35% Percoll, as described before (26). For detection of NK T cells in lung, single-cell suspensions from lung parenchyma were prepared as follows: lungs were flushed in situ with 20-ml cold PBS via cannulation of the heart to remove the intravascular blood pool. Minced lung tissue was incubated for 90 min at 37°C on a rocker in DMEM supplemented with 10% FCS, 50 U/ml DNase I (Boehringer Mannheim), and 250 U/ml collagenase I, Type 4197 (Worthington Biochemical). Passing the digested lung tissue through a nylon mesh and subsequent discontinuous Percoll gradient (Pharmacia) centrifugation (35–55%) yielded purified live lung parenchymal cells. In some experiments, the iNKT cells were negatively selected from splenocytes, using a mixture of biotinylated mAbs against B220, Gr1, CD62L, CD11c, followed by incubation with anti-biotin microbeads. The samples were subsequently enriched using magnetic cell sorting. The enriched iNKT cell splenocytes were confirmed to contain an average of >10% pure iNKT cell population.
In vitro culture of NKT cells
CD4+ and DN NKT cells were magnetically enriched from spleen of BALB/c or CD28−/− mice as described above. iNKT cells were cultured in round-bottom 96-well plates with 103 bone marrow-derived DCs from WT mice, prepared as previously described (27, 28) with some modifications (29, 30). As indicated, in some wells, WT CD4+ and DN iNKT cells were treated with 20 μg/ml anti ICOS-L blocking Abs. Supernatants were collected after 48 h, and measured for cytokines by ELISA or CTLL-2 bioassay.
Adoptive transfers
Purified CD4+ or DN NK T cells (3 × 106) were adoptively transferred into Jα18−/− mice that were sensitized to OVA (50 μg OVA in alum i.p., 1 wk previously) and recipients were challenged with three consecutive doses of OVA i.n. (50 μg). In some experiments, iNKT cells positively selected from spleen of BALB/c mice using CD1d tetramer were adoptively transferred (3 × 106 cells) into naive ICOS−/− recipient mice that were challenged with α-GalCer i.n.
Cytokine ELISAs
Cytokine secretion following in vitro challenge was determined by ELISA as previously described (1). The mAb pairs used were as follows (capture-detection): IFN-γ, R4–6A2-XMG1.2; IL-4, BVD4-BVD6; IL-5, TRFK-5-TRFK-4; and 2A5-SXC.1-biotin for IL-10. Reagents for mouse IL-13 were purchased from R&D systems. For the measurement of IL-2 production, aliquots of the supernatants were removed after 48 h of culture and tested for IL-2 content, with the IL-2-dependent cell line CTLL-2.
Induction of AHR and measurement of airway responsiveness
To induce AHR, mice were sensitized with 100 μg of OVA Ag (ICN Biomedical) in alum administered i.p. After 8 days, mice were exposed to intranasal OVA (50 μg i.n.) or PBS on three consecutive days. AHR responses were assessed by methacholine-induced airflow obstruction in conscious mice placed in a whole-body plethysmograph (Buxco Electronics), as described before (1, 31). In some experiments, we assessed AHR by invasive measurement of airway resistance, in which anesthetized and tracheostomized mice were mechanically ventilated using a modified version of a described method (32). Aerosolized methacholine was administered in increasing concentrations of methacholine and we continuously computed RL and Cdyn by fitting flow, volume, and pressure to an equation of motion. For acute induction of AHR, α-GalCer (Axxora LLC) or vehicle control was administered i.n. (2.0 μg) to mice anesthetized with ketamine and xylazine or isofluorane. AHR was measured at 22–24 h following terminal i.n. challenge.
Bronchoalveolar lavage (BAL) fluid
Following measurement of AHR and euthanasia, the lungs were lavaged twice with 1 ml of PBS 2% FCS and the fluid pooled as described previously (1). The relative number of different types of leukocytes was determined from slide preparations of BAL fluid stained with H&E.
Results
Cytokine production by iNKT cells requires ICOS costimulation
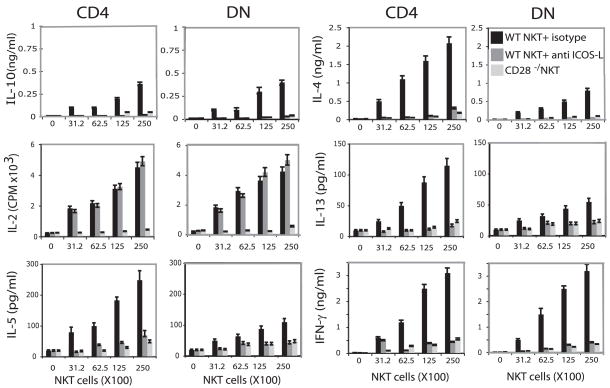
Thymocytes, hepatic mononuclear cells (MCs) and splenocytes from naive BALB/c mice were stained with α-GalCer-loaded CD1d tetramer, and analyzed for cell surface expression of ICOS on CD4+ and DN iNKT cells. In all three samples, CD4+ iNKT cells constitutively expressed high levels of ICOS, while DN iNKT cells expressed lower levels (Fig. 1). iNKT cells from lungs of WT mice that had been sensitized and challenged with OVA to induce AHR also expressed high levels of ICOS (Fig. 1). ICOSL was not expressed on CD4+ or DN iNKT cells, naive or activated (data not shown). To determine the role of ICOS costimulation in cytokine production by iNKT cells, CD4+ and DN NKT cells were magnetically enriched from spleens of BALB/c mice using α-GalCer-loaded CD1d tetramer. The positively selected iNKT cells produced high levels of cytokines without further addition of Ag when cultured with bone marrow derived-dendritic cells (BMDC) as APC. Blockade of the ICOS-ICOSL costimulatory pathway with anti-ICOSL mAb inhibited production of IL-4, IL-5, IFN-γ, IL-13, and IL-10 but had no effect on IL-2 production, indicating that IL-2 production by iNKT cells was not dependent on ICOS costimulation (Fig. 2). CD4+ iNKT cells produced substantially higher levels of IL-4, IL-5, and IL-13 than DN NKT cells but similar levels of IFN-γ, IL-2, and IL-10. iNKT cells from CD28−/− mice produced only barely detectable levels of cytokines (Fig. 2).
FIGURE 1.
NKT cells express high levels of ICOS. Thymocytes, hepatic MC, and spleen cells from naive BALB/c mice or lymphoid cells purified from lungs of BALB/c mice that had been immunized and challenged with OVA to induce AHR (bottom panel), were stained with α-Gal-Cer-loaded PE-conjugated CD1d tetramers, anti-TCR Vβ, and anti-CD4 mAb. ICOS expression was analyzed by gating on CD4+ (thick line) and DN iNKT (thin line) cells. Filled histograms represent the isotype control.
FIGURE 2.
Production of cytokines by iNKT cells requires ICOS-ICOSL pathways. CD4+ and DN NKT cells were magnetically enriched from spleen of BALB/c or CD28−/− mice as described in Materials and Methods. Titrating numbers of iNKT cells were cultured with 103 BMDCs from WT mice. As indicated, in some wells WT CD4+ and DN iNKT cells were treated with 20 μg/ml anti ICOSL blocking Abs or isotype control. Supernatants were collected after 48 h and measured for cytokines by ELISA or CTLL-2 bioassay. ELISA data are representative of three separate experiments and are shown as mean ± SD for triplicate samples.
To confirm the requirement for ICOS in costimulation of iNKT cells, we compared cytokine production by iNKT cells from WT and ICOS−/− BALB/c mice (Fig. 3A). iNKT cells from ICOS−/− mice produced substantially lower levels of IL-4, IL-5, IFN-γ, IL-13, and IL-10 than iNKT cells from WT BALB/c but similar levels of IL-2. Moreover, iNKT cells produced substantially lower levels of IL-4 and IFN-γ but similar levels of IL-2 when cultured with BMDC from ICOSL−/− mice as APC than with BMDC from WT mice (Fig. 3B), confirming the requirement for ICOS costimulation in production of IL-4 and IFN-γ and iNKT cells (Fig. 3B).
FIGURE 3.
NKT cells from ICOS−/− mice produce IL-2 but do not produce IL-4, IL-10, IL-13, and IFN-γ. (A) CD4+ and DN iNKT cells were magnetically enriched as described in Materials and Methods and titrating numbers of iNKT cells was cultured with 103 BMDCs. (B) iNKT cells were negatively selected as described in Materials and Methods and incubated for 48 h with α-GalCer-loaded BMDCs prepared from WT or ICOSL−/− mice. Supernatants were collected after 48 h and measured for cytokines by ELISA or CTLL-2 bioassay. ELISA data are representative of three separate experiments and are shown as mean ± SD for triplicate samples.
ICOS costimulation of iNKT cells is required for the induction of AHR
We have previously shown that iNKT cell-deficient Jα18−/− mice do not develop AHR, and that adoptive transfer of iNKT cells from WT mice restores the development of AHR. In these studies, recipient iNKT cell-deficient mice were sensitized with OVA to prime Th2 responses before adoptive transfer of iNKT cells. It should be noted that in this transfer model, the recipient Jα18−/− mice have normal expression of ICOS on conventional CD4+ T cells, which allows the normal development of OVA-specific Th2 cells. To determine whether ICOS costimulation of iNKT cells was required for induction of AHR, CD4+ and DN iNKT cells were positively selected from the spleen of WT or ICOS−/− mice and adoptively transferred into OVA-sensitized Jα18−/− mice, which were then challenged with OVA to induce AHR. Only the CD4+ and not the DN iNKT cells from WT BALB/c restored AHR, measured as airway resistance (RL) and dynamic compliance (Cdyn) in anesthetized, tracheostomized, and mechanically ventilated mice (Fig. 4A), or as Penh (Fig. 4B). These results are consistent with the lower levels of IL-4 and IL-13 produced by DN compared with CD4+ iNKT cells (Fig. 2). The Jα18−/− mice reconstituted with iNKT cells from ICOS−/− mice failed to develop AHR, while mice reconstituted with iNKT cells from WT BALB/c developed high levels of AHR (Fig 4), similar to that observed in WT BALB/c mice. Neither the CD4+ nor DN NK T cells from ICOS−/− mice restored AHR, demonstrating that ICOS costimulation of iNKT cells was required for the induction of AHR. Moreover, examination of the lungs of mice that received ICOS−/− NKT cells also showed a reduction in airway inflammation (Fig. 4C).
FIGURE 4.
ICOS−/− NKT cells cannot transfer AHR. A and B, CD4+ or DN iNKT cells were positively selected from the spleen of BALB/c or ICOS−/− mice and adoptively transferred (3 × 106 cells) into OVA-sensitized Jα18−/− mice which were then challenged with OVA i.n. on 3 consecutive days and assessed for AHR by measuring lung resistance (RL), dynamic compliance (Cydn) (A), and PenH (B). Nonrecipient mice were sensitized and challenged with OVA or PBS as control. Data are the mean ± SEM Penh, representative of three experiments (n = 5). C, Lung histopathology of Jα18−/− recipient mice from A. Left panel, Lung tissue from an OVA-challenged Jα18−/− mouse showing normal airway and surrounding parenchyma. Middle panel, Lung tissue from an OVA-treated Jα18−/− mouse that received 3× WT iNKT cells, showing numerous inflammatory cells surrounding the airways and streaks of mucus in the lumen. Right panel, Lung parenchyma of an OVA-sensitized Jα18−/− mouse that received 3 × 106 iNKT cells from ICOS−/− mice, showing minimal mucus production and negligible cellular infiltration. All stainings are H&E. Magnifications, ×200. D, Transfer of NKT cells from CD28 −/− mice does not restore AHR. CD1d tetramer positive cells (3 × 106) were positively selected from the spleen of BALB/c or CD28−/− mice and were adoptively transferred into OVA-sensitized Jα18−/− mice and challenged with OVA. Data are the mean ± SEM of five mice.
The absolute requirement for ICOS costimulation of iNKT cells in the induction of AHR was similar to the requirement for CD28 co-stimulation of iNKT cells in induction of AHR, as transfer of iNKT cells from CD28−/− mice also failed to reconstitute the development of AHR in OVA-sensitized Jα18−/− mice (Fig. 4D).
Adoptive transfer of WT NK T cells restores AHR in ICOS−/−
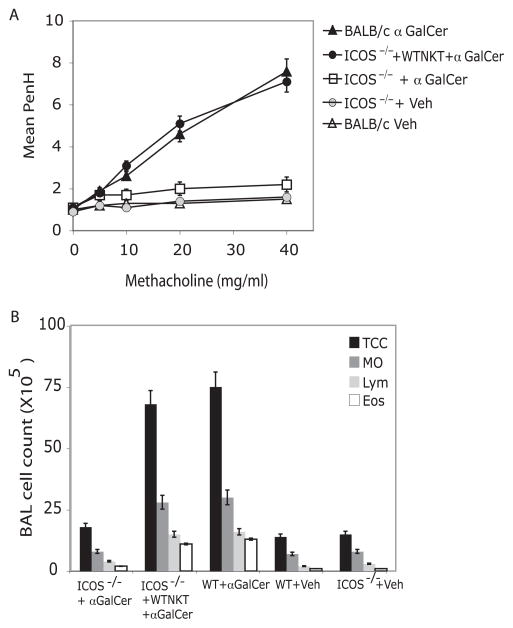
When sensitized and challenged with Ag, ICOS−/− mice do not develop airway inflammation. However, ICOS−/− mice lack ICOS expression on conventional T cells as well as NK T cells. To directly examine the role of ICOS in iNKT cell-driven AHR, we used an α-GalCer model of AHR, which highlights and isolates the role of iNKT cells. WT mice challenged with i.n. α-GalCer, a glycolipid Ag that specifically activates CD1d-restricted iNKT cells, developed severe AHR in 24 h characterized by peribronchiolar infiltrates, which include eosinophils and neutrophils (4). ICOS−/− mice failed to develop AHR or airway eosinophilia when challenged with α-GalCer i.n., whereas WT BALB/c developed severe AHR as expected (Fig. 5A, 5B). To determine whether WT iNKT cells could restore the development of AHR in ICOS−/− mice, iNKT cells were positively selected from the spleens of WT mice and transferred into ICOS−/− mice, which were subsequently challenged with α-GalCer. The WT NK T cells restored α-GalCer-induced AHR and eosinophilia in the ICOS−/− recipients to levels seen in WT BALB/c control mice (Fig. 5A,B), confirming the importance of ICOS costimulation for iNKT cell function in the development of AHR.
FIGURE 5.
Adoptive transfer of WT iNKT cells restores AHR in ICOS−/−. A, iNKT cells positively selected from spleen of BALB/c mice using CD1d tetramer were adoptively transferred (3 × 106 cells) into naive ICOS−/− recipient mice. AHR was assessed 24 h after i.n. challenge with α-GalCer or vehicle control. Data are the mean ± SEM Penh, representative of three experiments (n = 6). B, BAL fluid from the mice in A was analyzed 24 h after AHR measurement. Results are shown as the number of cells per ml in BAL fluid. TCC, Total cell number; Mo, monocyte/macrophage; Lym, lymphocytes; and Eos, eosinophils.
The ICOS-ICOSL pathway is required for CD4+ NK T cell homeostasis
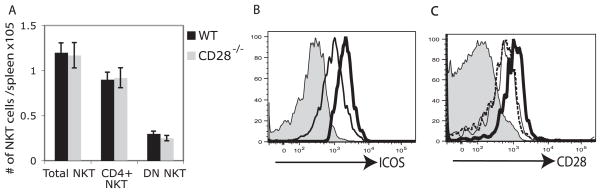
We observed that the number of iNKT cells recovered from ICOS−/− spleen was lower than that from BALB/c WT mice. Therefore, we compared the number of iNKT cells in thymus, liver, and spleen, the principal organs where NKT cells are located, in ICOS−/−, ICOSL−/−, and WT BALB/c. We found that while the number of iNKT cells in thymus were comparable among groups, the number of iNKT cells was greatly reduced in liver and spleen of ICOS−/− and ICOSL−/− compared with WT (Fig. 6A). The decrease was primarily in the CD4+ iNKT cell subset and DN iNKT were little changed. These results suggest that ICOS-ICOSL interactions have a critical role in maintaining NK T cell populations in peripheral organs. To confirm the role of ICOS in peripheral homeostasis of iNKT cells, we treated WT BALB/c mice with blocking anti-ICOSL mAb or isotype control. The number of iNKT cells in liver and spleen 48 h after anti-ICOSL mAb administration was greatly reduced in treated mice compared with recipients of isotype control, whereas mAb treatment had no effect on the number of NK T cells in thymus (Fig. 6B).
FIGURE 6.
The ICOS-ICOSL pathway is required for CD4+ NKT cell survival. A, Thymus, liver, and spleen cells from naive BALB/c, ICOS−/−, or ICOSL−/− mice were stained with α-GalCer-loaded tetramer and mAbs against Vβ and CD4. The number of total and iNKT cell subsets were calculated. Results are shown as the number of cells per organ. Data are the mean ± SEM of eight mice. B, A cohort of naive BALB/c mice received 1-mg anti-ICOSL or isotype mAb control i.p. 48 h later, thymus, liver, and spleen cells were stained with α-GalCer-loaded tetramer and mAbs against Vβ and CD4, and the number of total and NKT cell subsets were calculated. Results are shown as the total number of cells per organ. Data are the mean ± SEM of five mice.
In contrast to the reduced number of iNKT cells observed in periphery of ICOS−/− and ICOSL−/− mice, the number of iNKT cells in spleen of CD28−/− mice was comparable to that of WT mice (Fig. 7A), indicating that CD28-B7 interactions are not required for peripheral homeostasis of iNKT cells. Since up-regulation of ICOS has been reported to be dependent on CD28 signaling in conventional CD4+ T cells (21), we examined the expression of ICOS on iNKT cells in CD28−/− mice. CD4+ iNKT cells expressed higher levels of ICOS than DN iNKT cells from CD28−/− mice (Fig. 7B) as was observed in WT mice (Fig. 1). Furthermore, the level of CD28 expression on iNKT cells from ICOSL−/− and from B7-1−/−B7-2−/− mice was comparable to WT BALB/c control, indicating that surface expression of CD28 on iNKT cells is not dependent on B7-1/2 or ICOSL interaction (Fig. 7C).
FIGURE 7.
The CD28 pathway is not required for CD4+ NKT cell survival. A, Spleen cells from naive BALB/c or CD28−/− mice were stained with α-GalCer-loaded tetramer and mAbs against Vβ and CD4. The number of total iNKT cells and iNKT cell subsets was calculated. Results are shown as the number of cells per spleen. Data are the mean ± SEM of five mice. B, Spleen cells from CD28−/− mice were stained with α-GalCer-loaded PE-conjugated CD1d tetramers, anti-TCR Vβ, and anti-CD4. ICOS expression was analyzed by gating on CD4+ (thick line) or DN (thin line) iNKT cells. Shaded histogram represents the isotype control. C, Spleen cells from naive BALB/c (dotted line), B7-1−/−2−/− (thick line), and ICOSL−/− (thin line) mice were stained with α-GalCer-loaded PE-conjugated CD1d tetramers, anti-TCR Vβ. CD28 expression was analyzed by gating on iNKT cells. Shaded histogram represents the isotype control.
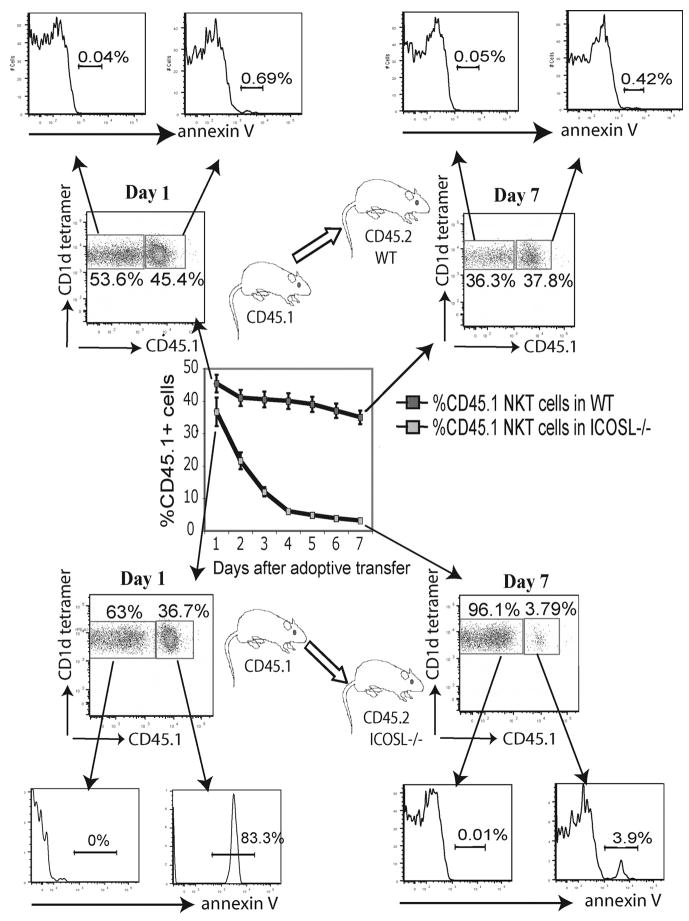
To further investigate the role of ICOS-ICOSL interactions in peripheral homeostasis of iNKT cells we adoptively transferred CD45.1 splenocytes into CD45.2 WT or ICOSL−/− mice and followed the fate of the transferred iNKT cells in the recipients. The number of iNKT cells and the development of apoptosis was assessed daily by gating on CD45.1 tetramer positive cells on the recipient splenocytes. While the number of transferred iNKT cells in WT recipient spleens decreased gradually as previously reported (33), the number of iNKT cells dramatically decreased in the ICOSL−/− recipients to ~10% of the number seen in WT recipients by 6–7 days (Fig. 8). CD45.1+ iNKT transferred cells into the ICOSL−/− recipients showed a high proportion of apoptotic cells as judged by annexin-V-FITC staining while annexin-V-FITC staining was barely detectable in cells transferred into WT recipients, suggesting that the induction of apoptosis on iNKT subsets is due to the lack of ICOS-ICOSL interactions in the periphery (Fig. 8). Together these results indicate that ICOS-ICOSL interactions have a critical role in iNKT cell peripheral homeostasis.
FIGURE 8.
ICOS/ICOSL interactions are required for NKT cell homeostasis. CD45.2 ICOSL−/− or WT control mice received 20 × 106 CD45.1 WT iNKT cell enriched splenocytes (Purity > 10%) i.v. on day 0. Splenocytes were harvested daily for 1 wk and stained with annexin-V-FITC and Abs against CD45.1, TCR Vβ, and CD1d-loaded tetramer.
Discussion
In this study, we demonstrated that ICOS/ICOSL interactions influence the homeostatic survival of iNKT cells in the periphery, and that ICOS/ICOSL interactions are required for effective function of iNKT cells in the development of AHR. Previous studies showed that ICOS−/− mice fail to develop allergen-induced AHR (22). This failure was thought to be due to the failure of allergen-specific Th2 cells to develop, However, our data suggest that the inability of ICOS−/− iNKT cells to function and to survive in the periphery may also contribute to the failure of ICOS−/− mice to develop AHR. To separate the ICOS costimulation requirement for Th2 cell development from that for iNKT cell function, we used an adoptive transfer model, in which iNKT cells were transferred into iNKT deficient-Jα18−/− mice, which fail to develop AHR unless reconstituted with iNKT cells (2, 4, 30, 34). The OVA-sensitized Jα18−/− mice developed normal Th2 responses (1) but failed to develop AHR unless reconstituted with WT but not ICOS−/− iNKT cells. These results clearly indicate that ICOS costimulation is specifically required for iNKT cell function in the development of AHR. We confirmed the importance of ICOS costimulation in iNKT cell activation by inducing AHR with α-GalCer, using a model of AHR that bypasses the requirement for Th2 cells. ICOS−/− mice failed to develop α-GalCer-induced AHR, indicating that ICOS costimulation of iNKT cells is required for the development of AHR.
The failure of ICOS−/− iNKT cells to induce AHR was due in part to the poor capacity of ICOS−/− iNKT cells to produce cytokines on activation and for ICOS−/− iNKT cells to survive in the periphery. We showed that iNKT cells from ICOS−/− mice are impaired in production of key cytokines, such as IL-4 and IL-13, although they produce IL-2 at levels that are similar to that observed in WT iNKT cells. The production of IL-2 by iNKT cells from ICOS−/− mice indicates that ICOS−/− iNKT cells survive and are not anergized in vitro, but that they require, like conventional T cells, ICOS costimulation to produce Th1 and Th2 cytokines, as do conventional T cells. In contrast, iNKT cells from CD28−/− mice fail to produce IL-2 as well as IFN-γ, IL-4, and IL-13. These findings are consistent with previous studies of conventional CD4+ T cells, which demonstrated that CD28 induces IL-2 production, whereas ICOS does not (16, 35, 36). Importantly, ICOS but not CD28 is required for iNKT cell survival in the periphery, since we demonstrated that the number of iNKT cells in ICOS−/− or ICOSL−/− but not CD28−/− mice is greatly reduced, and survival of ICOS−/− iNKT adoptively transferred into WT mice was greatly diminished.
Previously, iNKT cell function has been shown to depend on CD28 and CD40L costimulation. In some systems, production of cytokines by iNKT cells did not occur when CD28 or CD40L cross-linking was blocked (23), but in other systems, iNKT cell expansion/proliferation but not cytokine production required CD28 and CD40L costimulation (25). In addition, ICOS costimulation of iNKT cells was shown to be required for in vivo cytotoxic activity and cytokine production by iNKT cells in a tumor model (37). However, much of the reduced in vivo activity of the iNKT cells observed in ICOS−/− mice or after treatment of WT mice with anti-ICOS mAb (37) may have been due to reduced numbers of iNKT cells, which was not considered. In contrast, we have clearly shown that ICOS critically regulates not only the function of iNKT cells in terms of cytokine production, but also the survival of CD4+ iNKT cells.
In our studies, we found that the number of CD4+ iNKT cells was greatly reduced in the periphery but not in the thymus of both ICOS−/− and ICOSL−/− mice compared with WT mice, indicating that ICOS/ICOSL interactions are critical in homeostatic survival of CD4+ iNKT cells. In contrast, the number of iNKT cells and the level of ICOS expression in CD28−/− mice is comparable to that in WT mice (Fig. 7B), suggesting that signaling via ICOS but not via CD28 plays a unique role in regulation of CD4+ iNKT cell homeostatic survival. Moreover, the level of CD28 expression in B7-1−/−/2−/− or ICOSL−/− is comparable to that in WT mice (Fig. 7C), suggesting that the deficiency in iNKT cell function and number observed in ICOSL−/− mice is not due to the lack of CD28 expression. These observations were supported by our findings involving adoptive transfer of CD45.1+ WT iNKT cells into CD45.2+ ICOSL−/− mice. After transfer, the number of transferred iNKT cells decreased rapidly in the ICOSL−/− recipients, while the number of transferred iNKT cells in WT recipient spleens decreased very slowly. The high level of staining with annexin-V-FITC in the CD45.1+ iNKT cells indicates that this rapid decrease is due to the occurrence of apoptosis in the absence of ICOS-ICOSL interactions. Our transfer studies are consistent with prior findings that the turnover of Vα14i iNKT cells in the spleen and liver of WT is ~20% per week (38). The requirement for ICOS/ICOSL signaling by iNKT cells is continuous in the periphery, since acute disruption of this signaling, for example with the administration of anti-ICOSL blocking mAb, rapidly reduced the number of CD4+ iNKT cells in peripheral organs such as spleen and liver. In these studies, the number of iNKT cells in thymus was not affected, suggesting that iNKT cells homeostatic survival in the peripheral organs such as spleen and liver depends on ICOS signaling, but that thymus iNKT cells do not require ICOS for their homeostatic survival. These results are consistent with previous observations suggesting that while ICOS is expressed on thymocyte T cell subsets, including NK1.1+ cells (39), the survival and function of the iNKT cell population in the thymus might be independent of ICOS costimulation (40, 41).
In our studies, we noted significant differences between CD4+ and DN iNKT cells in terms of the requirement for ICOS signaling. Several groups have shown that CD4+ iNKT cells produce substantially higher levels of IL-4 and IL-13 than do DN NKT cells, but both populations produce similar levels of IFN-γ, IL-2, and IL-10 (41–43). In both ICOS−/− and ICOSL−/− mice and following anti-ICOSL mAb treatment, we noted that there was a preferential loss of CD4+ but not the DN iNKT cells in the periphery. These results suggest that the ICOS-ICOSL costimulatory pathway, which enhances IL-4, IL-13, and IL-10 production (20, 22), may preferentially support the survival of CD4+ iNKT cells in the periphery by enhancing the production of these particular cytokines. Since IL-4 and IL-13 are particularly important for the induction of AHR (44, 45), it is not surprising that the loss of ICOS-ICOSL signaling disrupts the ability of iNKT cells to induce AHR.
In summary, we demonstrated that ICOS-ICOSL interactions are required for both the function and survival of iNKT cells. During the induction of AHR, ICOS expression on iNKT cells plays an important role by allowing iNKT cells to produce proinflammatory cytokines such as IL-4, IL-13, and IFN-γ. Thus, the iNKT cells expressing ICOS contribute significantly to the development of AHR. In addition, ICOS is required for the survival and homeostasis of CD4+ iNKT cells in the periphery. Future therapies therefore, using ICOS antagonists, might be effective in treating patients with excessive CD4+ iNKT cell activity such as allergic disease and asthma.
Footnotes
Abbreviations used in this paper: AHR, airway hyperreactivity; iNKT, invariant NK T cell; WT, wild type; DN, double negative; DC, dendritic cell; MC, mononuclear cell; BMDC, bone marrow derived-dendritic cell; RL, airway resistance; Cdyn, dynamic compliance.
Disclosures
The authors have no financial conflict of interest.
This work was supported by the Eleanor and Miles Shore Scholarship for research in Medicine, Harvard Medical School (to O.A.), Children’s Hospital Career Development Grant (to O.A.), National Institutes of Health Grant R01 AI066020 (to O.A.), and National Institutes of Health Grant P01 AI56299 (to G.F., A.H.S., R.D.K., and D.U.) and RO1 HL069507 (to R.D.K.).
References
- 1.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–588. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 2.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, Fourneau JM, Sidobre S, Kronenberg M, Taniguchi M, et al. Cutting edge: invariant V α 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–1641. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 3.Jin N, Miyahara N, Roark CL, French JD, Aydintug MK, Matsuda JL, Gapin L, O’Brien L, Gelfand EW, Born WK. Airway Hyperresponsiveness through Synergy of γδ T Cells and NKT Cells. J Immunol. 2007;179:2961–2968. doi: 10.4049/jimmunol.179.5.2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proc Natl Acad Sci USA. 2006;103:2782–2787. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim JO, Kim DH, Chang WS, Hong C, Park SH, Kim S, Kang CY. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J Allergy Clin Immunol. 2004;114:1332–1338. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Kim DH, Chang WS, Lee YS, Lee KA, Kim YK, Kwon BS, Kang CY. 4-1BB engagement costimulates NKT cell activation and exacerbates NKT cell ligand-induced airway hyperresponsiveness and inflammation. J Immunol. 2008;180:2062–2068. doi: 10.4049/jimmunol.180.4.2062. [DOI] [PubMed] [Google Scholar]
- 7.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, Dekruyff RH, et al. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matangkasombut P, Pichavant M, Yasumi T, Hendricks C, Savage PB, DeKruyff RH, Umetsu DT. Direct activation of NK T cells induces airway-hyperreactivity in nonhuman primates. J Allergy Clin Immunol. 2008 doi: 10.1016/j.jaci.2008.02.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 10.Sen Y, Yongyi B, Yuling H, Luokun X, Li H, Jie X, Tao D, Gang Z, Junyan L, Chunsong H, et al. Vα24-invariant NKT cells from patients with allergic asthma express CCR9 at high frequency and induce Th2 bias of CD3+ T cells upon CD226 engagement. J Immunol. 2005;175:4914–4926. doi: 10.4049/jimmunol.175.8.4914. [DOI] [PubMed] [Google Scholar]
- 11.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanovic R. Invariant natural killer T cells in asthma and chronic obstructive pulmonary disease. N Engl J Med. 2007;356:1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Vα14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 13.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Bendelac A. CD1-restricted T-cell responses and microbial infection. Nature. 2000;406:788–792. doi: 10.1038/35021233. [DOI] [PubMed] [Google Scholar]
- 15.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 16.Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, Kroczek RA. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397:263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 17.Tafuri A, Shahinian A, Bladt F, Yoshinaga SK, Jordana M, Wakeham A, Boucher LM, Bouchard D, Chan VS, Duncan G, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–109. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 18.McAdam AJ, Greenwald RJ, Levin MA, Chernova T, Malenkovich N, Ling V, Freeman GJ, Sharpe AH. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–105. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 19.Dong C, Juedes AE, Temann UA, Shresta S, Allison JP, Ruddle NH, Flavell RA. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 20.Akbari O, Freeman GJ, Meyer EH, Greenfield EA, Chang TT, Sharpe AH, Berry G, DeKruyff RH, Umetsu DT. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 21.McAdam AJ, Chang TT, Lumelsky AE, Greenfield EA, Boussiotis VA, Duke-Cohan JS, Chernova T, Malenkovich N, Jabs C, Kuchroo VK, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalo JA, Tian J, Delaney T, Corcoran J, Rottman JB, Lora J, Algarawi A, Kroczek R, Gutierrez-Ramos JC, Coyle AJ. ICOS is critical for T helper cell-mediated lung mucosal inflammatory responses. Nat Immunol. 2001;2:597–604. doi: 10.1038/89739. [DOI] [PubMed] [Google Scholar]
- 23.Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways. J Immunol. 2001;166:6012–6018. doi: 10.4049/jimmunol.166.10.6012. [DOI] [PubMed] [Google Scholar]
- 24.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–3101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagi J, Dianzani U, Kato H, Okamoto T, Katsurada T, Buonfiglio D, Miyoshi-Akiyama T, Uchiyama T. Identification of a new type of invariant V α 14+ T cells and responsiveness to a superantigen, Yersinia pseudo-tuberculosis-derived mitogen. J Immunol. 1999;163:3083–3091. [PubMed] [Google Scholar]
- 27.Van Berkel ME, Schrijver EH, Hofhuis FM, Sharpe AH, Coyle AJ, Broeren CP, Tesselaar K, Oosterwegel MA. ICOS contributes to T cell expansion in CTLA-4 deficient mice. J Immunol. 2005;175:182–188. doi: 10.4049/jimmunol.175.1.182. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y, Ueno A, Bao M, Wang Z, Im JS, Porcelli S, Yoon JW. Control of NKT cell differentiation by tissue-specific microenvironments. J Immunol. 2003;171:5913–5920. doi: 10.4049/jimmunol.171.11.5913. [DOI] [PubMed] [Google Scholar]
- 29.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, Hayakawa Y, Godfrey DI, Smyth MJ. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med. 2005;202:1279–1288. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seino K, Taniguchi M. Functionally distinct NKT cell subsets and subtypes. J Exp Med. 2005;202:1623–1626. doi: 10.1084/jem.20051600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thedrez A, de Lalla C, Allain S, Zaccagnino L, Sidobre S, Garavaglia C, Borsellino G, Dellabona P, Bonneville M, Scotet E, Casorati G. CD4 engagement by CD1d potentiates activation of CD4+ invariant NKT cells. Blood. 2007;110:251–258. doi: 10.1182/blood-2007-01-066217. [DOI] [PubMed] [Google Scholar]
- 32.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–281. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 33.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 34.Tupin E, Kronenberg M. Activation of natural killer T cells by glycolipids. Methods Enzymol. 2006;417:185–201. doi: 10.1016/S0076-6879(06)17014-7. [DOI] [PubMed] [Google Scholar]
- 35.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheicher C, Mehlig M, Zecher R, Reske K. Dendritic cells from mouse bone marrow: in vitro differentiation using low doses of recombinant granulocyte-macrophage colony-stimulating factor. J Immunol Methods. 1992;154:253–264. doi: 10.1016/0022-1759(92)90199-4. [DOI] [PubMed] [Google Scholar]
- 37.Stockinger B, Hausmann B. Functional recognition of in vivo processed self antigen. Int Immunol. 1994;6:247–254. doi: 10.1093/intimm/6.2.247. [DOI] [PubMed] [Google Scholar]
- 38.Hansen G, Berry G, DeKruyff RH, Umetsu DT. Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest. 1999;103:175–183. doi: 10.1172/JCI5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda J, Gapin L, Sidobre S, Kieper W, Tan J, Ceredig R, Surh C, Kronenberg M. Homeostasis of Vα14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]
- 41.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells secreting IL-10 mediate T cell tolerance induced by respiratory exposure to antigen. Nature Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 42.Meyer EH, Wurbel MA, Staton TL, Pichavant M, Kan MJ, Savage PB, DeKruyff RH, Butcher EC, Campbell JJ, Umetsu DT. iNKT cells require CCR4 to localize to the airways and to induce airway hyperreactivity. J Immunol. 2007;179:4661–4671. doi: 10.4049/jimmunol.179.7.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van Berkel ME, Oosterwegel MA. CD28 and ICOS: similar or separate costimulators of T cells? Immunol Lett. 2006;105:115–122. doi: 10.1016/j.imlet.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 44.Kaneda H, Takeda K, Ota T, Kaduka Y, Akiba H, Ikarashi Y, Wakasugi H, Kronenberg M, Kinoshita K, Yagita H, Okumura K. ICOS costimulates invariant NKT cell activation. Biochem Biophys Res Commun. 2005;327:201–207. doi: 10.1016/j.bbrc.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, Surh CD, Kronenberg M. Homeostasis of V α 14i NKT cells. Nat Immunol. 2002;3:966–974. doi: 10.1038/ni837. [DOI] [PubMed] [Google Scholar]