Abstract
Among the HIV-associated pulmonary complications, opportunistic pneumonias are major causes of morbidity and mortality. The spectrum of HIV-associated opportunistic pneumonias is broad and includes bacterial, mycobacterial, fungal, viral, and parasitic pneumonias. Bacterial pneumonia is the most frequent opportunistic pneumonia in the United States and Western Europe while tuberculosis (TB) is the dominant pathogen in sub-Saharan Africa. With the use of combination antiretroviral therapy and prophylaxis, the incidence of Pneumocystis pneumonia (PCP) has declined. Nevertheless, PCP continues to occur in persons who are unaware of their HIV infection, those who fail to access medical care, and those who fail to adhere to antiretroviral therapy or prophylaxis. Although pneumonias due to Cryptococcus neoformans, Histoplasma capsulatum, Coccidioides immitis, cytomegalovirus (CMV), and Toxoplasma gondii are less frequent, their presence in the lung is often indicative of disseminated disease and is associated with significant mortality.
Keywords: Human immunodeficiency virus (HIV), Acquired immune deficiency syndrome (AIDS), Opportunistic infection, Bacterial pneumonia, Tuberculosis, Pneumocystis pneumonia
Introduction
The lungs are a principal target of human immunodeficiency virus (HIV)-associated complications and persons with HIV infection are at an increased risk for a wide spectrum of opportunistic pneumonias, neoplasms, and pulmonary conditions (Table 1). Among the HIV-associated pulmonary complications, opportunistic pneumonias are major causes of morbidity and mortality and are frequent reasons for referral to respiratory specialists for diagnostic evaluation and treatment. The spectrum of HIV-associated opportunistic pneumonias is broad and includes bacterial, mycobacterial, fungal, viral, and parasitic pneumonias. This review will provide an overview of the epidemiology of HIV-associated opportunistic pneumonias and describe important features of the diagnostic evaluation. The review will also describe the classical clinical and radiographic presentation, diagnosis, treatment, and prevention of the most common HIV-associated opportunistic pneumonias.
Table 1.
Spectrum of HIV-associated Pulmonary Diseases
| Opportunistic Infections |
| Bacteria |
| Streptococcus pneumoniae |
| Haemophilus species |
| Pseudomonas aeruginosa |
| Other bacteria |
| Mycobacteria |
| Mycobacterium tuberculosis |
| Mycobacterium avium complex |
| Mycobacterium kansasii |
| Other mycobacteria |
| Fungi |
| Pneumocystis jirovecii (formerly P. carinii) |
| Cryptococcus neoformans |
| Histoplasma capsulatum |
| Coccidioides immitis |
| Penicillium marneffei |
| Aspergillus species (most often A. fumigatus) |
| Other fungi |
| Viruses |
| Cytomegalovirus |
| Other viruses |
| Parasites |
| Toxoplasma gondii |
| Other parasites |
| Malignancies |
| Kaposi sarcoma |
| Non-Hodgkin lymphoma |
| Bronchogenic carcinoma |
| Interstitial Pneumonitides |
| Lymphocytic interstitial pneumonitis (LIP)¶ |
| Nonspecific interstitial pneumonitis (NSIP) |
| Other |
| Chronic obstructive pulmonary disease (COPD) |
| Pulmonary arterial hypertension (PAH) |
| Immune reconstitution inflammatory syndrome (IRIS) |
Review
The HIV/AIDS epidemic has had a major impact throughout the world. In December 2007, the World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS) estimated that there are 33 million people living with HIV.(1) (http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.a sp) Most of these people are unaware of their HIV infection and, as a result, unknowingly contribute to the spread of the infection.
The epidemic has disproportionately affected people residing in areas of the world that have fewer resources to combat the disease. The WHO/UNAIDS estimated that there were 2.7 million people who were newly infected with HIV in 2007 and greater than 95% of these new infections occurred among persons residing in low and middle income countries (LMIC).(1) Sub-Saharan Africa accounts for an estimated 22 million cases of HIV/AIDS and has an estimated prevalence of 5% in adults ages 15-49. In these LIMC, the HIV/AIDS epidemic has often over-burdened the under-resourced health care infrastructure.
The epidemic has been a major cause of morbidity and mortality worldwide. The WHO/UNAIDS has estimated that 25 million people have died from HIV/AIDS, including 2 million people who died in 2007.(1) A significant proportion of these deaths were due to opportunistic pneumonias. For example, the WHO has estimated that TB was the cause of death in 200,000 persons with HIV/AIDS in 2006.(2) (http://www.who.int/tb/publications/global_report/2008/pdf/fullreport.pdf) The magnitude of the HIV/AIDS epidemic has led to an unprecedented worldwide effort to provide life-saving antiretroviral therapy and has involved partnerships between governmental, non-governmental, philanthropic, medical, and pharmaceutical organizations. In addition to providing antiretroviral therapy to those with HIV infection, accurate diagnosis and appropriate treatment and prevention of HIV-associated opportunistic pneumonias are both important strategies for reducing the morbidity and mortality from HIV/AIDS.
Epidemiology
In the United States, Pneumocystis pneumonia (PCP), tuberculosis (TB), and recurrent bacterial pneumonia (defined as two episodes occurring within a 12-month period) are 3 of the 10 most frequent AIDS-defining diseases.(3) In the WHO European western region, PCP and TB are the 2 most common AIDS-indicator diseases and TB is the most common AIDS-indicator disease in their center and eastern region (2006). Although comparable data outside of the US and Europe are limited, TB is clearly a dominant pathogen in persons residing in LIMC. In addition, PCP has been increasingly reported in areas of the world such as sub-Saharan Africa where it had previously been thought to be a rare pathogen.(4) In endemic regions, Coccidioides immitis and Histoplasma capsulatum are major causes of HIV-associated disease, while Cytomegalovirus and Toxoplasma gondii are the most frequent HIV-associated viral and parasitic pneumonias reported, respectively.
Evaluation
Ideally, the evaluation of respiratory symptoms and suspected pneumonia in a person with HIV infection is aimed at establishing a definitive diagnosis. However, definitive diagnosis may require invasive diagnostic procedures such as bronchoscopy and may require sophisticated laboratory techniques, which are frequently unavailable in many clinical settings throughout the world. Regardless of the setting, the goal of any evaluation is to narrow the differential diagnosis to a single probable diagnosis such that appropriate therapy can be initiated and, depending on available resources, appropriate diagnostic testing can be obtained. The challenge of HIV infection is that the clinical and radiographic presentations of HIV-associated opportunistic pneumonias overlap and also that persons with HIV infection may present with more than one concurrent pneumonia.
The evaluation begins with a history and physical examination (Table 2). The history should include information on the most recent CD4 cell count, the person's HIV risk factors and habits, history of prior opportunistic infections and current use of opportunistic infection prophylaxis and combination antiretroviral therapy, and information on residence in or travel to regions prevalent for TB and endemic fungi. The presenting complaints and the tempo and duration of these complaints should be obtained. The physical examination should look for signs suggesting extrapulmonary or disseminated disease that may tie together the respiratory complaints and pulmonary findings. Based on the history and physical examination, chest radiography is indicated for persons with suspected pneumonia. Often, the specific chest radiographic findings - combined with the CD4 cell count - evoke a differential diagnosis and plan for management and treatment (Figures 1 and 2). Selected laboratory testing may be indicated to assess for specific diseases (e.g., serum Cryptococcal antigen, CrAg) or disease severity (e.g., arterial blood gas, ABG). In some cases, chest tomography (CT) may be indicated. Where feasible, further evaluation should seek to establish a definitive diagnosis, with microbiologic evaluation of sputum for stains and cultures, and bronchoscopy in certain cases.
Table 2.
Evaluation of HIV-associated Opportunistic Pneumonias
| CD4 cell count | |
| Any | Bacterial pneumonia (especially Streptococcus pneumoniae and Hemophilus species) |
| Tuberculosis (TB) | |
| <200 cells/μL | Pneumocystis pneumonia (PCP) |
| Cryptococcus neoformans pneumonia | |
| Bacterial pneumonia accompanied by bacteremia or septicemia | |
| Extrapulmonary or disseminated TB | |
| <100 cells/μL | Bacterial pneumonia due to Pseudomonas aeruginosa |
| Toxoplasma gondii pneumonia | |
| <50 cells/μL | Coccidioides immitis, usually accompanied by disseminated disease |
| Histoplasma capsulatum, usually accompanied by disseminated disease | |
| Cytomegalovirus, usually accompanied by disseminated disease | |
| Mycobacterium avium complex, usually accompanied by disseminated disease |
HIV Risk Factors Injection drug use: increased incidence of bacterial pneumonia and TB
Habits Cigarette smoking: increased incidence of bacterial pneumonia
Prior Opportunistic Infections Increased incidence of recurrence
Use of Opportunistic Infection Prophylaxis Decreased incidence of disease
Sustained Combination Antiretroviral therapy Decreased incidence of disease
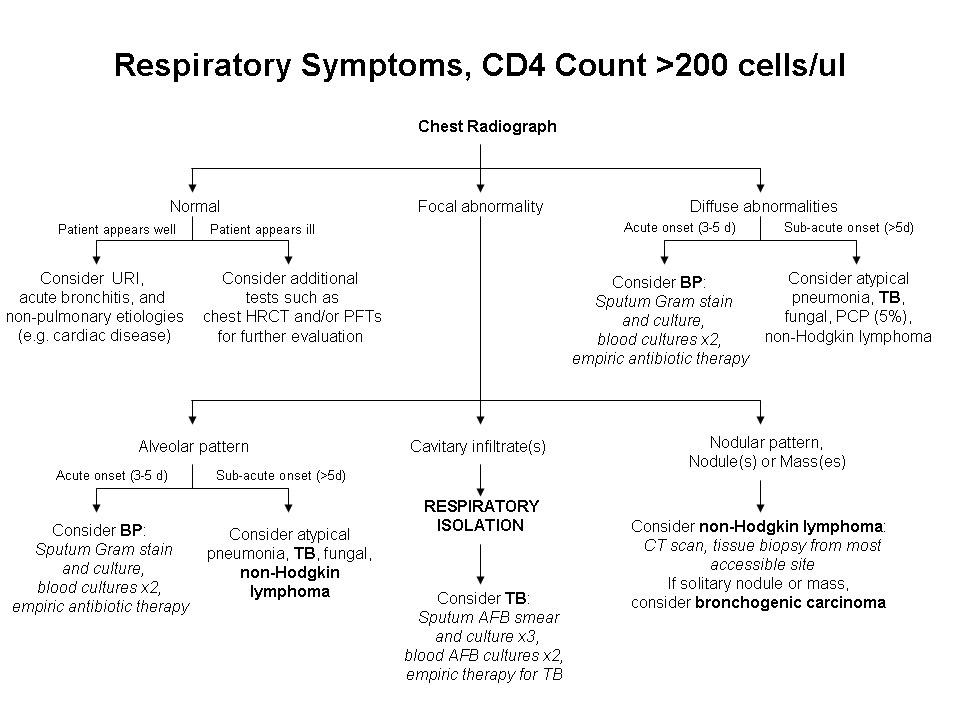
Figure 1.
Diagnostic assessment for an HIV-infected patient with respiratory symptoms and CD4 cell count >200 cells/μl. AFB, acid-fast bacilli; BP, bacterial pneumonia; CT, chest computed tomography; HRCT, chest high-resolution computed tomography; PCP, Pneumocystis pneumonia; PFTs, pulmonary function tests; TB, tuberculosis; URI, upper respiratory tract infection. Adapted from: Huang, L. Respiratory Disease. In Dolin, R, Masur, H, and Saag, M, eds. AIDS Therapy. Third edition. Philadelphia: Churchill Livingstone Elsevier, Inc., 2007. pp. 1225-1252.
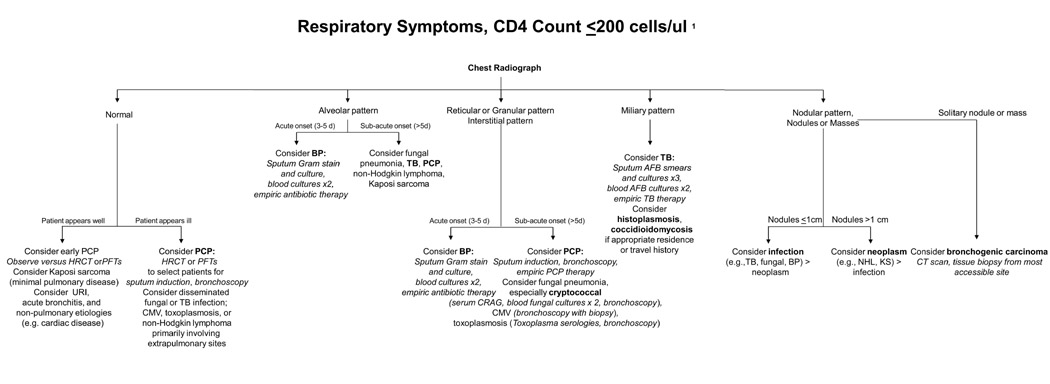
Figure 2.
Diagnostic assessment for an HIV-infected patient with respiratory symptoms and CD4 cell count of <200 cells/μl. *Some of the diagnoses occur when the CD4 cell count is ≤100 cells/μl or even ≤50 cells/μl. AFB, acid-fast bacilli; BP, bacterial pneumonia; CMV, Cytomegalovirus; CRAG, cryptococcal antigen; HRCT, chest high-resolution computed tomography; KS, Kaposi sarcoma; NHL, non-Hodgkin lymphoma; PCP, Pneumocystis pneumonia; PFTs, pulmonary function tests; TB, tuberculosis; URI, upper respiratory tract infection. Adapted from: Huang, L. Respiratory Disease. In Dolin, R, Masur, H, and Saag, M, eds. AIDS Therapy. Third edition. Philadelphia: Churchill Livingstone Elsevier, Inc., 2007. pp. 1225-1252.
HIV-associated opportunistic pneumonias can progress rapidly to respiratory failure and death without appropriate therapy. Thus, empiric therapy for the suspected diagnosis(es) should be initiated while awaiting the results of diagnostic studies. In the U.S., the National Institutes of Health (NIH), the Centers for Disease Control and Prevention (CDC), and the HIV Medicine Association of the Infectious Diseases Society of America (HIVMA/IDSA) publish “Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents” that provide recommendations for first-line and alternative therapies for treatment and prevention.(5) (http://aidsinfo.nih.gov/contentfiles/Adult_OI.pdf) Although available therapies and recommendations may differ in different areas of the world, for the purposes of this review, the NIH/CDC/HIVMA/IDSA guidelines will be provided as the basis for recommended treatment and prophylaxis regimens.
Bacterial pneumonia
The incidence of bacterial pneumonia among persons with HIV infection is greater than that among persons without HIV.(6) In those with HIV, bacterial pneumonia is frequently recurrent, and recurrent pneumonia is an AIDS-defining condition. Bacterial pneumonia may be the first manifestation of underlying HIV infection and thus the presence of HIV infection should be considered in any person presenting with bacterial pneumonia, especially if the individual has no other risk factors for pneumonia or if the pneumonia is recurrent. Similar to persons without HIV infection, Streptococcus pneumoniae and Haemophilus species are the most frequently identified causes of community-acquired bacterial pneumonia.(7) Atypical pathogens such as Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydophila species are less frequent causes of pneumonia, and are encountered with similar frequency as among those without HIV. In contrast, Pseudomonas aeruginosa and Staphylococcus aureus are both more frequently reported as community-acquired causes of pneumonia in persons with HIV infection. Risk factors for Pseudomonas include advanced HIV/AIDS (i.e., CD4 cell count at or below 50 cells/μL), underlying lung disease (bronchiectasis), neutropenia, receipt of corticosteroid therapy, and severe malnutrition. Risk factors for S. aureus include recent viral or influenza infection and use of injection drugs.
Clinical Presentation
Bacterial pneumonia can occur at any stage of HIV disease and at any CD4 cell count. However, as the CD4 cell count declines, the incidence of bacterial pneumonia increases as does the incidence of accompanying bacteremia and septicemia. The latter is especially the case with S. pneumoniae. The clinical presentation of HIV-associated bacterial pneumonia is similar to that in persons without HIV infection. Persons with bacterial pneumonia typically present with the acute onset (3 to 5 days) of fevers chills/rigors, chest pain, dyspnea, and cough that is productive of purulent sputum. Lung examination reveals evidence of consolidation and occasionally pleural effusion. Laboratory testing is usually notable for an elevated white blood cell count, often with a predominance of polymorphonuclear leukocytes (PMNs).
Chest Radiology
The radiographic presentation of HIV-associated bacterial pneumonia is similar to that in persons without HIV infection. Most persons with S. pneumoniae or Haemophilus pneumonia present with unilateral, focal, segmental or lobar consolidation, occasionally accompanied by pleural effusion. However, the frequency of these radiographic findings depends in part on the underlying bacterial pathogen. For example, a subset of persons with Haemophilus pneumonia present with bilateral interstitial or mixed interstitial-alveolar infiltrates that are indistinguishable from PCP. Also, pneumonia due to P. aeruginosa or S. aureus is often associated with cavitation. In at least one study, persons with HIV infection had an increased risk for complicated parapneumonic effusions, especially if due to S. pneumoniae or S. aureus.
Diagnosis
Current US guidelines recommend that persons hospitalized with suspected bacterial pneumonia should undergo diagnostic evaluation for specific pathogens.(5) This evaluation is optional in persons who are well enough to receive outpatient treatment. Also, persons should undergo diagnostic evaluation for specific pathogens that would alter the standard treatment (e.g., P. aeruginosa or S. aureus) whenever the presence of these pathogens is suspected on the basis of epidemiological, clinical, and radiographic clues. Typically, diagnostic evaluation of suspected bacterial pneumonia includes a pretreatment expectorated sputum specimen sent for Gram stain and culture, two blood cultures, and potentially thoracentesis if pleural effusion is present.
Treatment
The principles of treatment of HIV-associated bacterial pneumonia are similar to that in persons without HIV infection. In one study, HIV-infected persons with bacterial pneumonia had comparable time to clinical stability, lengths of hospitalization, and mortality to HIV-uninfected persons with bacterial pneumonia.(8) However, a few important differences exist. First, macrolide monotherapy is not recommended because of the increased risk of drug resistant S. pneumoniae in persons with HIV infection.(5) Instead, persons should receive a beta-lactam plus a macrolide. A respiratory fluoroquinolone is an alternative to the beta-lactam in persons who are allergic to penicillin and doxycycline is an alternative to the macrolide. One important caveat: fluoroquinolones are active against Mycobacterium tuberculosis and should only be used in cases where the presentation strongly suggests bacterial pneumonia, as this would otherwise result in TB mono-therapy in the event that TB is the etiology of the pneumonia. If TB is considered a suspected etiology, standard 4-drug TB therapy should also be used.
Prevention
According to current US Guidelines, persons with HIV infection who have a CD4 cell count greater than 200 cells/μL should be given the 23-valent polysaccharide pneumococcal vaccine.(5) Since the duration of the protective effect of vaccination is unknown, revaccination every 5 years should be considered. Persons who have a CD4 cell count less than 200 cells/μL can also be offered the vaccine. Although its efficacy may be lessened in those with advanced immunosuppression, observational studies suggest that the vaccine may still decrease risk for pneumonia in this population.(9, 10) If these persons plan to initiate antiretroviral therapy, the vaccine can be given after the CD4 cell count rises above 200 cells/μL.
Other measures may decrease the incidence of pneumonia among HIV infected persons. Generally, the Haemophilus influenzae Type B vaccine is not recommended for adults with HIV infection, given the low incidence of this infection in this group.(5) The inactivated influenza vaccine should be given to all persons with HIV infection annually prior to influenza season. Trimethoprim-sulfamethoxazole, when administered daily for PCP prophylaxis, reduces the frequency of bacterial respiratory infections. Similarly, azithromycin and clarithromycin administered for Mycobacterium avium complex (MAC) prophylaxis can reduce the frequency of bacterial pneumonia. However, given the specter of drug resistance, none of these medications should be prescribed solely for the prevention of bacterial infection. Finally, bacterial pneumonia is substantially increased among HIV infected persons who currently smoke, suggesting that efforts to improve smoking cessation could lead to substantial decreases in bacterial pneumonia.
Tuberculosis (TB)
It is estimated that one-third of the world's population is infected with M. tuberculosis. Persons with HIV infection have a substantially greater risk of progressing from latent tuberculosis infection (LTBI) to active TB compared to persons without HIV infection. In persons with HIV infection, the estimated annual risk of developing active TB ranges from 35 to 162 per 1,000 person-years, compared to 12.9 per 1,000 person-years for those without HIV infection.(5) Similar to bacterial pneumonia, TB may be the first manifestation of underlying HIV infection and thus the presence of HIV infection should be considered in any person presenting with TB.
Clinical Presentation
TB can occur at any stage of HIV disease and at any CD4 cell count.(11) However, as the CD4 cell count declines, the incidence of TB increases as does the incidence of accompanying mycobacteremia and extrapulmonary or disseminated disease. In persons whose CD4 cell count is above 350-400 cells/μL, the clinical presentation of TB is similar to that in persons without HIV infection. Typically, these persons have disease that is limited to the lungs. Classic symptoms include fever, chills, night sweats, anorexia, weight loss, and cough (usually present for 3 weeks' duration or more). Lung examination may reveal evidence of consolidation and occasionally pleural effusion. In persons whose CD4 cell count is below 200 cells/μL, the clinical presentation of TB may include symptoms and signs due to extrapulmonary involvement. In these persons, laboratory abnormalities such as elevated liver function tests, anemia, leukopenia, and thrombocytopenia may represent evidence of TB liver and bone marrow involvement, respectively.
Chest Radiology
In persons whose CD4 cell count is above 350-400 cells/μL, the radiographic presentation of TB is similar to that in persons without HIV infection. Most of these persons present with a classic reactivation TB radiographic pattern consisting of unilateral or bilateral upper lung zone fibronodular infiltrates with or without cavitation.(12, 13) In contrast, persons whose CD4 cell count is below 200 cells/μL often present with a primary TB pattern consisting of middle and lower lung zone infiltrates, or a miliary pattern. Occasionally, the chest radiograph may be normal. Cavitation is less common but intrathoracic adenopathy is more common in these individuals with advanced HIV/AIDS.
Diagnosis
Typically, diagnostic evaluation of suspected TB includes 2 to 3 sputum specimens sent for acid fast bacillus (AFB) smear and, if available, mycobacterial culture.(5) Ideally, specimens are collected in the morning, on separate days. Direct nucleic acid amplification tests can be performed on specimens that are AFB-smear positive and a positive nucleic acid amplification result in these cases has a high predictive value for TB. Mycobacterial blood cultures should also be obtained, especially in persons whose CD4 cell count is below 200 cells/μL and/or persons with evidence of disseminated disease. Needle aspiration or tissue biopsy of accessible extrapulmonary lesions (e.g., lymph node) can also be done. A positive AFB smear result should be presumed to represent M. tuberculosis and TB therapy should be initiated or continued, pending results from mycobacterial cultures.
Treatment
The principles of the treatment of TB are similar to that in persons without HIV infection. Current US guidelines recommend directly observed therapy (DOT) for all persons with HIV infection and TB. Initial treatment of presumed drug-sensitive TB consists of isoniazid (INH), rifampin (RIF) or rifabutin (RFB), pyrazinamide (PZA), and ethambutol (EMB) (pyridoxine should also be given).(5) Treatment of pan-sensitive TB consists of a 6-month regimen with the initial 4 drugs for 2 months, followed by INH and RIF (or rifabutin) for 4 additional months. Persons with cavitary lung disease whose 2-month repeat sputum culture remains positive should receive an additional 3 months of treatment (total of 9 months). Persons with extrapulmonary TB that involves the central nervous system (CNS), bone, or joint(s) should receive 9 to 12 months of treatment and those with extrapulmonary TB involving other sites should receive 6 to 9 months of treatment. Consultation with a TB expert should be obtained in persons with known drug-resistant (multi drug-resistant, MDR, or extensively drug-resistant, XDR) TB and in persons who are failing standard 4-drug therapy, where drug-resistance is suspected. In persons on antiretroviral therapy, drug interactions can occur particularly between the rifamycins and both protease inhibitors and non-nucleoside reverse transcriptase inhibitors. Consultation with experts in HIV should be obtained in these cases. In addition, persons receiving dual therapy for HIV infection and TB may develop the immune reconstitution syndrome, IRS (or immune reconstitution inflammatory syndrome, IRIS) [see accompanying review].
Prevention
Current US guidelines recommend that persons with HIV infection regardless of their age should be treated for LTBI if they have no evidence of active TB and (1) a positive diagnostic test for LTBI and no prior treatment for LTBI or active TB; (2) a negative diagnostic test for LTBI but are considered close contacts of persons with infectious pulmonary TB; or (3) a history of untreated or inadequately treated TB (i.e., evidence of old fibrotic lesions on chest radiograph), regardless of their LTBI test results.(5) In persons with HIV, ≥5 mm of induration at 48-72 hours is considered to represent a positive purified protein derivative (PPD) tuberculin skin test (TST). IFN-gamma release assays (IGRAs) can also be used to diagnose LTBI.(14, 15) Since HIV infection is associated with a high risk for progression to TB, current guidelines recommend that persons with either a positive TST or IGRA should be considered infected with M. tuberculosis.(5) Persons with HIV infection should receive INH either daily or twice weekly for 9 months. Persons receiving INH should also receive pyridoxine to minimize the risk of developing peripheral neuropathy.
Pneumocystis pneumonia (PCP)
PCP remains the most frequent AIDS-defining diagnosis in the United States; however, its overall incidence is declining in the US.(16) The use of combination antiretroviral therapy and PCP prophylaxis has contributed to the dramatic declines reported. Nevertheless, PCP continues to occur in persons who are unaware of their HIV infection, those who fail to access medical care, and those who fail to adhere to antiretroviral therapy or prophylaxis.(17) Furthermore, PCP has been increasingly reported in areas of the world such as sub-Saharan Africa where it had previously been thought to be a rare pathogen.(4)
Clinical Presentation
Approximately 90-95% of cases of PCP in adolescents and adults occur in persons whose CD4 cell count is below 200 cells/μL. Classically, PCP presents with fever, cough that is non-productive, and dyspnea. Symptoms are sub-acute and are usually present for 3-4 weeks. Physical examination of the chest is unremarkable in approximately 50%. Inspiratory crackles are the most common abnormal finding on lung examination. Most persons with PCP have an elevated serum lactate dehydrogenase (LDH) level. However, serum LDH is non-specific and elevations can be seen in many pulmonary and non-pulmonary conditions. Other tests, including S-adenosylmethionine levels and beta-D-glucan, have been studied as diagnostic tests for PCP.
Chest Radiology
Classically, PCP presents with bilateral, symmetric, reticular (interstitial) or granular opacities.(18) In mild disease, these opacities are typically perihilar; whereas in severe disease, the opacities are diffuse. Less frequently, PCP may present with unilateral or asymmetrical opacities. Thin-walled cysts or pneumatoceles are seen in 10% to 20% of cases. Pleural effusions and intrathoracic adenopathy are rarely due to PCP. PCP may also present with a normal chest radiograph. In our experience, this occurs in less than 5% of cases. Persons with a normal chest radiograph may have opacities detected by high resolution chest computed tomography (HRCT) and the absence of ground glass opacities on HRCT virtually rules out the diagnosis of PCP.(19)
Diagnosis
There is no universally agreed upon approach to the management of persons with suspected PCP. Some institutions treat persons with suspected PCP empirically, reserving diagnostic procedures for the subset of people who fail to respond, while others, such as San Francisco General Hospital, pursue a definitive diagnosis.(20) Since Pneumocystis cannot be cultured, the diagnosis of PCP relies on microscopic visualization of the characteristics cysts or trophic forms on stained respiratory specimens. Typically, these specimens are obtained by sputum induction or bronchoscopy with bronchoalveolar lavage (BAL).(5) The sensitivity of induced sputum for PCP depends in part on the laboratory staining technique, with immunofluorescent staining generally more sensitive than tinctorial staining. As a general rule, a negative sputum induction cannot rule out a diagnosis of PCP. Bronchoscopy with BAL would be the next step; a BAL that is negative for Pneumocystis essentially rules out the diagnosis of PCP in studies conducted prior to combination antiretroviral therapy.(20)
Treatment
Standard treatment for PCP is 21 days; some persons will have responded well before this time and therapy can often be stopped and others will remain symptomatic and require continued therapy. Trimethoprim-sulfamethoxazole (TMP-SMX) is the recommended first-line treatment for PCP of all severities.(5) Alternatives to TMP-SMX include intravenous pentamidine and clindamycin plus primaquine for moderate to severe PCP and trimethoprim plus dapsone or atovaquone solution for milder PCP. Adjunctive corticosteroids are recommended for persons with moderate to severe PCP and a PaO2 below 70 mm Hg or an alveolar-arterial oxygen gradient greater than 35 mm Hg.(5) Similar to the case with TB, persons receiving dual therapy for HIV infection and PCP may develop IRS. Occasionally, the IRS is severe enough to cause respiratory failure.
Prevention
Primary PCP prophylaxis is recommended for persons with a CD4 cell count below 200 cells/μL or a history of oropharyngeal candidiasis, regardless of CD4 cell count. Primary prophylaxis can be considered for persons with a CD4 cell percentage below 14% or a history of a prior AIDS-defining illness. Secondary PCP prophylaxis is recommended for persons with a history of PCP. Prophylaxis is recommended for life, unless the CD4 cell count rises above 200 cells/μL for more than 3 months as a result of combination antiretroviral therapy. TMP-SMX is the recommended first-line primary and secondary prophylaxis for PCP.(5) Alternatives to TMP-SMX include dapsone with or without pyrimethamine plus leucovorin, atovaquone, and aerosolized pentamidine.
Other Fungal pneumonias
Several fungi cause pneumonia in persons with HIV infection. Cryptococcus neoformans, the most frequent cause of HIV-associated meningitis, can present with an associated pneumonia. The endemic fungi, Coccidioides immitis, Histoplasma capsulatum, and Penicillium marneffei are often among the most frequent causes of HIV-associated disease in their particular geographic regions, and all have important pulmonary presentations. Finally, Aspergillus species, especially A. fumigatus, can present with invasive pneumonia as well as the entire spectrum of Aspergillus-related pulmonary disease in persons with HIV infection (e.g., allergic bronchopulmonary aspergillosis, aspergilloma).
Cryptococcus neoformans
Cryptococcus neoformans is an encapsulated, round to oval yeast that is surrounded by a polysaccharide capsule. There are two pathogenic variants: C. neoformans var neoformans, which causes the majority of disease in persons with HIV infection, and C. neoformans var gattii.
Clinical Presentation
The majority of cases of Cryptococcal disease occur in persons with a CD4 cell count below 200 cells/μL, usually below 50 cells/μL. Cryptococcosis most frequently presents with meningitis or meningoencephalitis and with complaints of fever, fatigue or malaise, and headache.(21) Classic symptoms of meningitis such as nausea or vomiting, altered mental status, stiff neck, and photophobia are present in a minority. Pneumonia can accompany meningitis or pneumonia can be the sole manifestation of cryptococcosis with fever, cough, and shortness of breath and less commonly chest pain. Up to 10% of persons with disseminated cryptococcosis may also present with acute respiratory failure.(22) Physical examination of the chest may reveal inspiratory crackles, evidence of consolidation, or occasionally pleural effusion.
Chest Radiology
Cryptococcal pneumonia most commonly presents with diffuse bilateral interstitial infiltrates.(23) The radiographic appearance often mimics PCP. In addition to bilateral interstitial infiltrates, unilateral interstitial infiltrate, focal consolidation, nodules, nodular opacities, cavitation, pleural effusion, and hilar adenopathy have all been reported.(23) Case reports also document the occurrence of a miliary pattern, solitary pulmonary nodules, pulmonary masses, isolated pleural effusion, and pneumothorax due to C. neoformans. Finally, cryptococcal pneumonia may also present with a normal radiograph. In one review, a normal chest radiograph was seen in 11% of the 92 cases.(23)
Diagnosis
Although almost all persons with cryptococcal meningitis or disseminated cryptococcosis have a positive serum Cryptococcal antigen (CrAg), those with isolated pulmonary disease may have a negative serum CrAg.(5) Blood fungal cultures are specific and should be obtained as part of the diagnostic evaluation. The diagnosis of cryptococcal meningitis is usually established by lumbar puncture. The diagnosis of pulmonary cryptococcosis is usually established by culture of sputum or BAL fluid and occasionally from pleural fluid. Biopsy specimens from transbronchial biopsy and pleural biopsy can also be diagnostic. New cutaneous lesions may be signs of dissemination, and their sudden appearance should prompt consideration for skin biopsy.
Treatment
Most of the data on treatment of HIV-associated Cryptococcal disease involved persons with Cryptococcal meningitis, where the recommended regimen for induction therapy is Amphotericin B plus flucytosine for at least 2 weeks.(5) This is followed by consolidation therapy with fluconazole (400 mg daily) for 8 weeks, and then maintenance therapy with fluconazole (200 mg daily) for life or until the CD4 cell count rises above 200 cells/μL for at least 6 months as a result of antiretroviral therapy. Persons with meningitis and pneumonitis should be treated with this regimen. However, fluconazole, with or without flucytosine, has been used in persons with isolated pneumonia, particularly in cases of mild disease. Similar to the case with TB, persons receiving dual therapy for HIV infection and cryptococcosis may develop IRS.
Prevention
Fluconazole has been demonstrated to decrease the incidence of primary Cryptococcal disease among persons with HIV infection and a CD4 cell count below 50 cells/μL. However, most HIV specialists do not recommend its routine use as primary prophylaxis for several reasons including the low frequency of the disease, the absence of a survival benefit in randomized controlled trials, potential for toxicity, drug-drug interactions, drug resistance, and cost.(5)
Histoplasma capsulatum
Histoplasmosis is caused by a soil-dwelling dimorphic fungus, Histoplasma capsulatum. H. capsulatum is endemic to the Ohio, Mississippi, and St. Lawrence River Valleys and parts of Central and South America. Infection is caused by inhalation of organisms that become airborne via disruption of the soil.
Clinical Presentation
Although the portal of entry of Histoplasma is the lung, disseminated histoplasmosis often presents as a febrile wasting illness in HIV-infected persons.(24) Most cases of disseminated histoplasmosis occur in persons with a CD4 cell count below 50-100 cells/μL but focal pneumonia can occur in persons with a CD4 cell count >250-500 cells/ μL. The clinical presentation is often nonspecific. Fever, fatigue, and weight loss are common. Occasionally, the presentation of histoplasmosis is dramatic with a sepsis-like syndrome associated with hypotension, respiratory failure, liver and renal failure, and coagulopathy.(24) Cough and dyspnea are the most frequent respiratory symptoms. These symptoms are usually found in persons with chest radiograph abnormalities and are often absent in those with normal radiographs. Physical examination is often nonspecific and most often reveals fever and wasting. Examination of the chest may reveal crackles. Hepatomegaly, splenomegaly, peripheral lymphadenopathy, central nervous system findings, and cutaneous lesions may also be seen. Frequent laboratory findings include anemia, leukopenia, thrombocytopenia, and liver function test elevations. Serum LDH and serum ferritin elevations, often pronounced, have also been reported.
Chest Radiograph
Disseminated histoplasmosis will present with a normal chest radiograph in a significant proportion of cases.(24, 25) The most common radiographic findings are diffuse, often coarse reticular, reticulonodular, or miliary infiltrates.(25) Alveolar infiltrates are occasionally seen; focal opacities are less common. Hilar and mediastinal adenopathy and calcified granulomata are each found in a minority of persons.
Diagnosis
The Histoplasma polysaccharide antigen (HPA) test is an initial test for the diagnosis of disseminated histoplasmosis.(5) The HPA test can be performed on urine, serum, cerebrospinal fluid, or BAL fluid. Although the urine and serum HPA test is sensitive in disseminated histoplasmosis, it is often negative in isolated pulmonary disease. Definitive diagnosis requires culture of the organism from clinical specimens. Blood fungal cultures are specific and should be obtained as part of the diagnostic evaluation. Occasionally, the peripheral blood smear reveals intracellular yeast. Other potential diagnostic sources include bone marrow, lymph node, and skin. The diagnosis of pulmonary histoplasmosis can be established by direct examination and culture of sputum, BAL fluid, or transbronchial biopsy.
Treatment
Amphotericin B (liposomal) is the recommended first-line treatment for disseminated histoplasmosis.(5) Persons should be treated with Amphotericin B until clinical improvement (usually at least 2 weeks). This is followed by itraconazole (200 mg three times daily for 3 days, then 200 mg twice daily) for a total of at least 12 months. Persons with severe disseminated histoplasmosis or CNS involvement and those who relapse despite appropriate therapy should receive itraconazole (200 mg daily) probably for life.
Prevention
Persons with HIV infection and a CD4 cell count below 150 cells/μL who reside in or travel to an area endemic for Histoplasma should avoid disturbing soil or areas contaminated with bird or bat droppings, or exploring caves, which are all activities known to be associated with increased risk of exposure to the organism. Itraconazole has been demonstrated to decrease the incidence of histoplasmosis among persons who reside in endemic areas. However, no survival benefit was noted. Thus, itraconazole (200 mg daily) can be considered only for persons with a CD4 cell count below 150 cells/ μL who reside in an endemic area and are at high risk for exposure to the organism.(5)
Coccidioidomycosis (Coccidioides immitis and Coccidioides posadasii)
Coccidioidomycosis is caused by a soil-dwelling dimorphic fungus consisting of two species, Coccidioides immitis and Coccidioides posadasii. C. immitis is endemic to the southwestern US, northern Mexico, and parts of Central and South America. Endemic areas are arid, warm regions. Infection is caused by inhalation of organisms that become airborne via disruption of the soil. There is no person-to-person transmission.
Clinical Presentation
Although the portal of entry of Coccidioides is the lung, coccidioidomycosis often presents with disseminated disease, diffuse pneumonia, and meningitis.(26, 27) Other frequent sites include the skin, lymph nodes, and liver. Pulmonary involvement can present as focal or diffuse pneumonia. Cases of endotracheal or endobronchial involvement have also been reported. Most cases of disseminated coccidioidomycosis occur in persons with a CD4 cell count below 50-100 cells/μL but focal pneumonia can occur in persons with a CD4 cell count >250 cells/ μL. The clinical presentation is often nonspecific. Fever, chills, night sweats, and weight loss are common. Those with meningitis can have headache and lethargy. Cough is the most frequent respiratory complaint. Physical examination findings are associated with the specific sites involved. Fever and wasting are often present. Examination of the chest may reveal crackles. Cutaneous lesions, lymphadenopathy, and hepatosplenomegaly may also be seen.
Chest Radiograph
Coccidioides pneumonia most commonly presents with diffuse reticulonodular infiltrates. Focal opacities are less common and can consist of reticulonodular infiltrates or consolidation, single or multiple pulmonary nodules, and cavities. Pleural effusion and hilar lymphadenopathy have also been reported. Finally, disseminated coccidioidomycosis can also present with a normal chest radiograph.
Diagnosis
Unlike cryptococcosis and histoplasmosis, there is no specific antigen test available for diagnosing coccidioidomycosis. Thus, definitive diagnosis requires culture of the organism from clinical specimens or identification of pathognomonic giant spherules in cytologic or histologic preparations.(5) Blood fungal cultures are positive in a minority of cases, usually in those with advanced HIV/AIDS and diffuse pneumonia or disseminated disease. Direct examination and culture of sputum, BAL fluid, or transbronchial biopsy can establish the diagnosis of pulmonary coccidioidomycosis.
Treatment
Amphotericin B is the recommended first-line treatment for disseminated coccidioidomycosis and for cases of diffuse pneumonia.(5) Persons should be treated with Amphotericin B until clinical improvement. Fluconazole or itraconazole can be used for cases of focal pneumonia.
Prevention
HIV-infected persons who reside in or travel to an area endemic for Coccidioides should avoid disturbing soil. Persons residing in an endemic area who have a positive Coccidioides IgM or IgG serologic test and a CD4 cell count below 250 cells/μL but no symptoms or signs of active disease should receive fluconazole or itraconazole.(5)
Viral pneumonias
Cytomegalovirus (CMV) is the most frequent viral pneumonia seen in persons with HIV infection. Most disease occurs in CMV-seropositive individuals and disease represents reactivation of latent infection, rather than new infection.
Cytomegalovirus
Clinical Presentation
Retinitis and gastrointestinal disease are the two most common manifestations of CMV disease; pneumonitis is uncommon. CMV is a frequent isolate from the BAL fluid of persons with advanced immunosuppression who undergo evaluation for opportunistic infections. In most of these cases, its presence represents viral shedding, rather than actual pneumonitis. CMV, however, can cause pulmonary disease, and the challenge clinicians face is to recognize these instances.(28, 29) Most cases of pneumonia occur in persons with a CD4 cell count less than 50 cells/μL. Cough, dyspnea, and fever are the most common symptoms of CMV pneumonia. Respiratory symptoms are typically present for 2-4 weeks. Physical examination of the chest may be normal or may reveal crackles or evidence of pleural effusion. The serum LDH has been reported to be elevated in CMV pneumonia.
Chest Radiograph
The chest radiograph findings of CMV pneumonia vary and include reticular or ground-glass opacities, alveolar infiltrates, and nodules or nodular opacities.(30) Pleural effusions may be seen as well. The latter finding may be helpful in distinguishing CMV pneumonia from PCP, where pleural effusions are rare.
Diagnosis
CMV is usually a disseminated disease and commonly involves multiple organ systems. Persons suspected of having CMV pneumonitis should undergo a careful dilated retinal examination performed by an experienced ophthalmologist, even if there are no ocular complaints.(5) Diagnosis of CMV pneumonia requires the demonstration of cytopathic inclusions and widespread specific cytopathic changes in the lungs. Neither BAL fluid nor transbronchial biopsy culture are sufficient to make the diagnosis of CMV pneumonitis.
Treatment
When suspected CMV pulmonary disease occurs in conjunction with other end-organ disease (e.g., retinitis), CMV therapy must be initiated immediately. Treatment of one end-organ CMV disease in fact treats all, although the length of therapy can differ by organ system. The therapeutic dilemma is much greater when only the lungs appear to be afflicted. Ganciclovir (intravenous) and foscarnet have been used to treat CMV pneumonia.(5)
Prevention
Oral ganciclovir has been demonstrated to decrease the incidence of CMV retinitis among persons with HIV infection and a CD4 cell count below 50 cells/μL. However, most HIV specialists do not recommend its routine use as primary prophylaxis for several reasons including the low frequency of the disease, the absence of a survival benefit in randomized controlled trials, the potential for drug resistance, and cost.(5)
Parasitic pneumonias
Toxoplasma gondii is the most frequent parasitic pneumonia seen in persons with HIV infection. Most disease occurs in Toxoplasma-seropositive individuals and disease represents reactivation of latent infection, rather than new infection.
Toxoplasma gondii
Clinical Presentation
T. gondii encephalitis is a well-recognized complication of advanced HIV disease and is an AIDS-defining condition. Although toxoplasmosis is the most common cause of focal brain abscesses in HIV-infected persons, pulmonary involvement is uncommon. Pulmonary disease may occur in persons with CNS or disseminated disease or with isolated pulmonary involvement. Toxoplasmosis presents at CD4 cell counts below 100 cells/μL. Pulmonary toxoplasmosis characteristically presents with cough that is nonproductive, dyspnea, and fever. Occasionally, disseminated toxoplasmosis may present with acute respiratory failure. Physical examination of the chest may be normal or may reveal crackles. Focal neurologic findings are common.
Chest Radiograph
The chest radiograph usually reveals bilateral infiltrates, either fine reticulonodular infiltrates indistinguishable from PCP or a coarser nodular pattern similar to that seen with tuberculosis or fungal pneumonias.(31, 32) Pleural effusions can be seen, and a variety of other radiographic findings have also been described.
Diagnosis
Almost all persons with toxoplasmosis have a positive serum Toxoplasma IgG antibody.(5) Its absence makes the diagnosis of Toxoplasma pneumonia unlikely. The diagnosis of pulmonary toxoplasmosis is usually established by bronchoscopy with BAL.
Treatment
Sulfadiazine plus pyrimethamine is the first-line recommended treatment for Toxoplasma encephalitis.(5) Leucovorin is co-administered with pyrimethamine to reduce the occurrence of hematologic toxicities from pyrimethamine. Clindamycin plus pyrimethamine (and leucovorin) and trimethoprim-sulfamethoxazole are the main alternatives.
Prevention
Persons at risk for toxoplasmosis (i.e., CD4 cell count below 100 cells/μL) are also at risk for PCP. Trimethoprim-sulfamethoxazole used for PCP prophylaxis is also effective against T. gondii.(5) Dapsone plus pyrimethamine (and leucovorin) and atovaquone with or without pyrimethamine (and leucovorin) are recommended for persons who cannot tolerate TMP-SMX.
Conclusion
Opportunistic pneumonias are major causes of morbidity and mortality in persons with HIV infection and are frequent reasons for referral to pulmonary and respiratory specialists for diagnostic evaluation and treatment. The spectrum of HIV-associated opportunistic pneumonias is broad and includes bacterial, mycobacterial, fungal, viral, and parasitic pneumonias. Accurate diagnosis, and appropriate treatment and prevention of HIV-associated opportunistic pneumonias is an important strategy for reducing the morbidity and mortality associated from HIV/AIDS.
Acknowledgments
The authors are supported by the following NIH grants: NIH K24HL087713 (LH), R01HL090335 (LH), and NIH R01HL090342 (KAC). The authors wish to acknowledge Ms. Susan Chang for her editorial assistance.
References
- 1.World Health Organization (WHO)/Joint United Nations Programme on HIV/AIDS (UNAIDS) 2008 Report on the Global AIDS Epidemic. 2008. http://www.unaids.org/en/KnowledgeCentre/HIVData/GlobalReport/2008/2008_Global_report.asp. [Google Scholar]
- 2.World Health Organization (WHO) Global Tuberculosis Control 2008. Surveillance, Planning, Financing. 2008. http://www.who.int/tb/publications/global_report/2008/pdf/fullreport.pdf. [Google Scholar]
- 3.Jones JL, Hanson DL, Dworkin MS, Alderton DL, Fleming PL, Kaplan JE, et al. Surveillance for AIDS-defining opportunistic illnesses, 1992-1997. MMWR CDC Surveill Summ. 1999;48(2):1–22. [PubMed] [Google Scholar]
- 4.Fisk DT, Meshnick S, Kazanjian PH. Pneumocystis carinii pneumonia in patients in the developing world who have acquired immunodeficiency syndrome. Clin Infect Dis. 2003;36(1):70–8. doi: 10.1086/344951. [DOI] [PubMed] [Google Scholar]
- 5.The National Institutes of Health (NIH) the Centers for Disease Control and Prevention (CDC) the HIV Medicine Association of the Infectious Diseases Society of America (HIVMA/IDSA) MMWR. 2009. Guidelines for Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents. http://aidsinfo.nih.gov/contentfiles/Adult_OI.pdf. [Google Scholar]
- 6.Hirschtick RE, Glassroth J, Jordan MC, Wilcosky TC, Wallace JM, Kvale PA, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–51. doi: 10.1056/NEJM199509283331305. [DOI] [PubMed] [Google Scholar]
- 7.Burack JH, Hahn JA, Saint-Maurice D, Jacobson MA. Microbiology of community-acquired bacterial pneumonia in persons with and at risk for human immunodeficiency virus type 1 infection. Implications for rational empiric antibiotic therapy. Arch Intern Med. 1994;154(22):2589–96. [PubMed] [Google Scholar]
- 8.Christensen D, Feldman C, Rossi P, Marrie T, Blasi F, Luna C, et al. HIV infection does not influence clinical outcomes in hospitalized patients with bacterial community-acquired pneumonia: results from the CAPO international cohort study. Clin Infect Dis. 2005;41(4):554–6. doi: 10.1086/432063. [DOI] [PubMed] [Google Scholar]
- 9.Penaranda M, Falco V, Payeras A, Jordano Q, Curran A, Pareja A, et al. Effectiveness of polysaccharide pneumococcal vaccine in HIV-infected patients: a case-control study. Clin Infect Dis. 2007;45(7):e82–7. doi: 10.1086/520977. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Barradas MC, Goulet J, Brown S, Goetz MB, Rimland D, Simberkoff MS, et al. Impact of pneumococcal vaccination on the incidence of pneumonia by HIV infection status among patients enrolled in the Veterans Aging Cohort 5-Site Study. Clin Infect Dis. 2008;46(7):1093–100. doi: 10.1086/529201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Havlir DV, Barnes PF. Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1999;340(5):367–73. doi: 10.1056/NEJM199902043400507. [DOI] [PubMed] [Google Scholar]
- 12.Batungwanayo J, Taelman H, Dhote R, Bogaerts J, Allen S, Van de Perre P. Pulmonary tuberculosis in Kigali, Rwanda. Impact of human immunodeficiency virus infection on clinical and radiographic presentation. Am Rev Respir Dis. 1992;146(1):53–6. doi: 10.1164/ajrccm/146.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Jones BE, Young SM, Antoniskis D, Davidson PT, Kramer F, Barnes PF. Relationship of the manifestations of tuberculosis to CD4 cell counts in patients with human immunodeficiency virus infection. Am Rev Respir Dis. 1993;148(5):1292–7. doi: 10.1164/ajrccm/148.5.1292. [DOI] [PubMed] [Google Scholar]
- 14.Menzies D, Pai M, Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007;146(5):340–54. doi: 10.7326/0003-4819-146-5-200703060-00006. [DOI] [PubMed] [Google Scholar]
- 15.Steingart KR, Henry M, Laal S, Hopewell PC, Ramsay A, Menzies D, et al. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 2007;4(6):e202. doi: 10.1371/journal.pmed.0040202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas CF, Jr., Limper AH. Pneumocystis pneumonia. N Engl J Med. 2004;350(24):2487–98. doi: 10.1056/NEJMra032588. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(Suppl 1):S5–14. doi: 10.1086/313843. [DOI] [PubMed] [Google Scholar]
- 18.DeLorenzo LJ, Huang CT, Maguire GP, Stone DJ. Roentgenographic patterns of Pneumocystis carinii pneumonia in 104 patients with AIDS. Chest. 1987;91(3):323–7. doi: 10.1378/chest.91.3.323. [DOI] [PubMed] [Google Scholar]
- 19.Gruden JF, Huang L, Turner J, Webb WR, Merrifield C, Stansell JD, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169(4):967–75. doi: 10.2214/ajr.169.4.9308446. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Hecht FM, Stansell JD, Montanti R, Hadley WK, Hopewell PC. Suspected Pneumocystis carinii pneumonia with a negative induced sputum examination. Is early bronchoscopy useful? Am J Respir Crit Care Med. 1995;151(6):1866–71. doi: 10.1164/ajrccm.151.6.7767533. [DOI] [PubMed] [Google Scholar]
- 21.Chuck SL, Sande MA. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N Engl J Med. 1989;321(12):794–9. doi: 10.1056/NEJM198909213211205. [DOI] [PubMed] [Google Scholar]
- 22.Visnegarwala F, Graviss EA, Lacke CE, Dural AT, Johnson PC, Atmar RL, et al. Acute respiratory failure associated with cryptococcosis in patients with AIDS: analysis of predictive factors. Clin Infect Dis. 1998;27(5):1231–7. doi: 10.1086/514984. [DOI] [PubMed] [Google Scholar]
- 23.Meyohas MC, Roux P, Bollens D, Chouaid C, Rozenbaum W, Meynard JL, et al. Pulmonary cryptococcosis: localized and disseminated infections in 27 patients with AIDS. Clin Infect Dis. 1995;21(3):628–33. doi: 10.1093/clinids/21.3.628. [DOI] [PubMed] [Google Scholar]
- 24.Wheat LJ, Connolly-Stringfield PA, Baker RL, Curfman MF, Eads ME, Israel KS, et al. Disseminated histoplasmosis in the acquired immune deficiency syndrome: clinical findings, diagnosis and treatment, and review of the literature. Medicine (Baltimore) 1990;69(6):361–74. doi: 10.1097/00005792-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Conces DJ, Jr., Stockberger SM, Tarver RD, Wheat LJ. Disseminated histoplasmosis in AIDS: findings on chest radiographs. AJR Am J Roentgenol. 1993;160(1):15–9. doi: 10.2214/ajr.160.1.8416614. [DOI] [PubMed] [Google Scholar]
- 26.Bronnimann DA, Adam RD, Galgiani JN, Habib MP, Petersen EA, Porter B, et al. Coccidioidomycosis in the acquired immunodeficiency syndrome. Ann Intern Med. 1987;106(3):372–9. doi: 10.7326/0003-4819-106-3-372. [DOI] [PubMed] [Google Scholar]
- 27.Singh VR, Smith DK, Lawerence J, Kelly PC, Thomas AR, Spitz B, et al. Coccidioidomycosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis. 1996;23(3):563–8. doi: 10.1093/clinids/23.3.563. [DOI] [PubMed] [Google Scholar]
- 28.Wallace JM, Hannah J. Cytomegalovirus pneumonitis in patients with AIDS. Findings in an autopsy series. Chest. 1987;92(2):198–203. doi: 10.1378/chest.92.2.198. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Barradas MC, Stool E, Musher DM, Gathe J, Jr., Goldstein J, Genta RM, et al. Diagnosing and treating cytomegalovirus pneumonia in patients with AIDS. Clin Infect Dis. 1996;23(1):76–81. doi: 10.1093/clinids/23.1.76. [DOI] [PubMed] [Google Scholar]
- 30.Salomon N, Gomez T, Perlman DC, Laya L, Eber C, Mildvan D. Clinical features and outcomes of HIV-related cytomegalovirus pneumonia. Aids. 1997;11(3):319–24. doi: 10.1097/00002030-199703110-00009. [DOI] [PubMed] [Google Scholar]
- 31.Pomeroy C, Filice GA. Pulmonary toxoplasmosis: a review. Clin Infect Dis. 1992;14(4):863–70. doi: 10.1093/clinids/14.4.863. [DOI] [PubMed] [Google Scholar]
- 32.Rabaud C, May T, Lucet JC, Leport C, Ambroise-Thomas P, Canton P. Pulmonary toxoplasmosis in patients infected with human immunodeficiency virus: a French National Survey. Clin Infect Dis. 1996;23(6):1249–54. doi: 10.1093/clinids/23.6.1249. [DOI] [PubMed] [Google Scholar]