Abstract
We examined the ability of sphingosine-1-phosphate (S1P) to desensitize extracellular signal–related kinase (ERK), a mitogen-activated protein kinase linked to antiapoptotic responses in the heart. In isolated adult mouse cardiomyocytes, S1P (10 nM–5 μM) induced ERK phosphorylation in a time- and dose-dependent manner. S1P stimulation of ERK was completely inhibited by an S1P1/3 subtype receptor antagonist (VPC23019), by a Gi protein inhibitor (pertussis toxin) and by a mitogen-activated protein kinase/ERK kinase inhibitor (PD98059). A selective S1P3 receptor antagonist (CAY10444) had no effect on S1P-induced ERK activation. The selective S1P1 agonist SEW2871 also induced ERK phosphorylation. Activation of ERK by restimulation with 100 nM S1P was suppressed after 1 hour of preincubation with 100 nM S1P but recovered fully the next day, suggesting receptor recycling. Similar results were obtained in protein kinase Cε-null cardiomyocytes. Treatment with the nonselective S1P receptor agonist FTY720 for 1 hour also reduced phospho-ERK expression in response to subsequent S1P stimulation. In contrast to S1P, some desensitization to FTY720 persisted after overnight exposure. Cell death induced by hypoxia/reoxygenation was reduced by pretreatment with exogenous S1P. This enhanced survival was abrogated by pretreatment with PD98059, VPC23019, or pertussis toxin. Thus, exogenous S1P induces rapid and reversible S1P1-mediated ERK phosphorylation. S1P-induced adult mouse cardiomyocyte survival requires ERK activation mediated via an S1P1–Gi pathway.
Keywords: adult cardiomyocytes, S1P receptors, ERK, signal transduction, receptor recycling, desensitization
INTRODUCTION
Sphingosine-1-phosphate (S1P) is a bioactive lysophospholipid that regulates many important cellular processes including growth, survival, differentiation, cytoskeletal rearrangements, motility, angiogenesis, and calcium mobilization.1,2 It is now accepted that many S1P actions are mediated through subtypes of S1P G protein–coupled receptors, which comprise S1P1–5.3–5 These receptor-associated signaling pathways may have important roles in many physiological and pathological events.
Recent biochemical evidence supports this notion. S1P1 interacts with Gαi, whereas S1P2 and S1P3 couple with Gαq, Gα13, and Gαi in a ligand-dependent manner.6 The existence of S1P receptors in the heart was first reported by Bünemann et al7 in 1995. Subsequent studies have shown that the S1P1 receptor exhibits the most prominent expression pattern in cardiomyocytes.8,9 The S1P1 receptor mediates proliferative/survival and migratory signaling in many different cell types.10–12 Our laboratory has recently reported that the S1P1 receptor is associated with activation of Akt, inactivation of glycogen synthase kinase 3 beta, and reduction of cytochrome c release from mitochondria in cardiac myocytes subjected to prolonged hypoxia.13
Protein kinase C (PKC) is a family of 11 serine/threonine kinases that transduce multiple signals in the regulation of differentiation, migration, and a variety of other cellular functions.14 We and others have shown that S1P-mediated cardioprotection against ischemia/reperfusion injury involves a PKCε-independent pathway.15,16 Graeler et al17 focused on cell chemotactic responses to demonstrate that recovery from downregulation of S1P1 G protein–coupled receptors in T lymphocytes is PKCε dependent.
Acute ischemia/reperfusion injury leads to myocyte cell death and in humans to either acute or long-term cardiac decompensation. Cardioprotective interventions can prevent or ameliorate such outcomes. As S1P and agonists that act at S1P receptors are cardioprotective and can be given over prolonged periods both to animals and to humans, we wished to determine whether their signaling effects would undergo desensitization, which might impair their effectiveness.
We used an adult mouse cardiac myocyte culture model for in vitro studies and measured extracellular signal–related kinase (ERK) as an important signaling molecule not previously studied in the heart in response to S1P receptor agonism. We employed different S1P receptor antagonists and PKCε-null myocytes to test the hypothesis that S1P-mediated phosphorylation of ERK, a mitogen-activated protein kinase (MAPK) linked to prosurvival responses in the heart,18–21 is desensitized after agonist exposure. This culture model also allowed us to measure the effects of S1P-stimulated ERK activation on cardiac myocyte viability during hypoxia–reoxygenation stress.
MATERIALS AND METHODS
Materials
S1P, PD98059, and pertussis toxin (PTX) were from BIOMOL International (Plymouth Meeting, PA). VPC23019 was from Avanti Polar Lipids, Inc (Alabaster, AL). FTY720 and CAY10444 were from Cayman Chemical (Ann Arbor, MI). Antibodies directed against ERK and MAPK/ERK kinase (MEK) were from Cell Signaling Technology (Danvers, MA).
Animals
Wild-type C57BL6 mice were from Charles River Laboratories (Hollister, CA). PKCε-null mice were provided by Dr. Robert Messing (Gallo Research Center, Emeryville, CA). Only male mice were used for all studies. Genotyping using polymerase chain reaction to confirm the absence of PKCε DNA was routinely performed on tail samples as described.22 The background of these mice is C57BL6. All studies were approved by the Institutional Animal Care and Use Committee of the San Francisco Veterans Affairs Medical Center. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Adult Mouse Cardiac Myocyte Isolation and Culture
Cardiac myocytes were isolated and cultured from mice 2–3 months of age weighing 25–30 g using a modification of the collagenase dissociation method reported by Zhou et al,23 as previously described in our laboratory.13 Isolated cardiac myocytes were plated for 2 hours on 35- and 60-mm tissue culture dishes coated with 10 μg/mL laminin. The cells were suspended in minimum essential medium (MEM) with Hanks buffered salt solution (HBSS), 10 μg/mL penicillin, 1.5 μM vitamin B12, and 10 mM 2,3-butanedione monoxime (BDM). After this period of attachment, the medium was changed to MEM/HBSS containing 10 μg/mL penicillin, 1.5 μM vitamin B12, and 1 mM BDM and incubated overnight at 37°C in a humidified atmosphere of 1% CO2 and air. The culture protocol yielded an average of 80% rod-shaped myocytes at a plating density of 50 cells per square millimeter that were viable at pH 7.2 for 48 hours. Experiments were performed the day after isolation and culture at which time medium was changed to contain no BDM.
Western Blot Analysis
For whole cell extraction, cells were lysed in buffer (20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM ethylene glycol-bis(aminoethylether)-tetraacetic acid, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/mL leupeptin, 0.1% protease inhibitor mixture (Roche Applied Science, Indianapolis, IN), and phosphatase inhibitor cocktail (Sigma Chemical, St. Louis, MO). Protein concentration was determined using the Bradford method. Equal amounts of protein were resuspended in 4× Laemmli sample buffer, boiled for 5 minutes and subjected to sodium dodecyl (lauryl) sulfate–polyacrylamide gel electrophoresis. After transfer to a polyvinylidene fluoride membrane, the extract was blocked in 5% nonfat milk in Tris-buffered saline + 0.1% Tween-20 for 1 hour. Membranes were probed overnight with primary antibodies, washed 3 times with Tris-buffered saline/Tween-20 for 5 minutes, and probed with secondary antibodies for 1 hour. The membranes were rinsed 3 times, and the signal was detected using enhanced chemiluminescence (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions.
Hypoxia–reoxygenation Protocol
On the day after isolation and culture, cardiac myocytes were incubated in a Bactron I anaerobic chamber containing a humidified atmosphere of 1% CO2 and 99% N2 for 3 hours. Before starting the experiment, medium was changed to serum-free, glucose-free MEM with HBSS that did not contain BDM. This medium was pre-equilibrated overnight in the anaerobic chamber containing 1% CO2 and 99% N2. Immediately after hypoxia, cells were treated with pharmacological agonists under normoxic conditions for the indicated times as described in the results section. This reoxygenation period lasted for 16 hours. Normoxic and reoxygenated experimental medium were pre-equilibrated overnight in water-jacketed incubators in a humidified atmosphere of 1% CO2 and air. Cardiac myocyte survival was measured as previously described in our laboratory13 by staining cells in tissue culture dishes with trypan blue solution (Sigma Chemical).
Statistical Analysis
Data are expressed as the mean ± SEM. Mean values were compared by 1-way analysis of variance and post hoc Student–Newman–Keuls testing. P < 0.05 was considered statistically significant.
RESULTS
S1P and SEW2871 Activate ERK 1/2
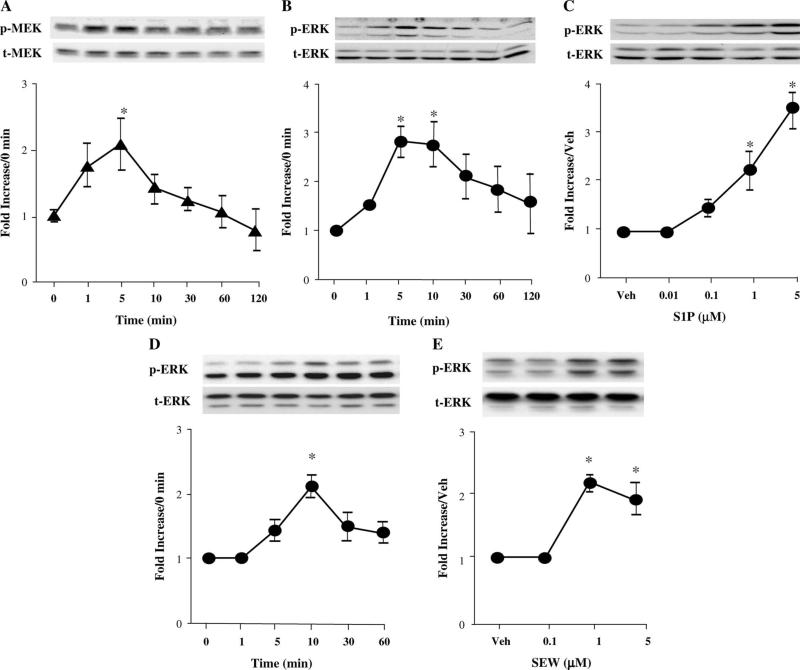
At 100 nM, S1P elicited transient phosphorylation of MEK 1/2, starting at 1 minute and reaching a maximum at 5 minutes, with no change in the level of nonphosphorylated MEK 1/2 (Fig. 1A). S1P 100 nM also stimulated time-dependent ERK 1/2 phosphorylation, starting at 1 minute and reaching a maximum between 1 and 5 minutes, with no change in the level of nonphosphorylated ERK 1/2 (Fig. 1B). Incubation of cells with 10 nM–5 μM S1P for 10 minutes resulted in a concentration-dependent increase in ERK 1/2 phosphorylation (Fig. 1C). Similar results were found for the selective S1P1 receptor agonist SEW2871 (Fig. 1, panels D and E).
FIGURE 1.
S1P induces a time- and concentration-dependent increase in the phosphorylation of MEK 1/2 and ERK 1/2 in adult mouse cardiomyocytes. Myocytes were exposed or not to 100 nM S1P for different times (1–120 minutes, A and B) or for 10 minutes with increasing concentrations of S1P (10 nM–5 μM, C). The results of concentration–response and time course data for SEW2871 stimulation of pERK 1/2 are shown in panels D and E. Western blot analyses of phospho-MEK 1/2, total MEK 1/2, pERK 1/2, and total ERK 1/2 are shown in autoradiograms representative of 3–11 independent experiments. The band intensities of phosphoproteins were normalized against total MEK or ERK and expressed as a fold increase in relation to the corresponding control. *P < 0.05 compared with control values. Veh, vehicle.
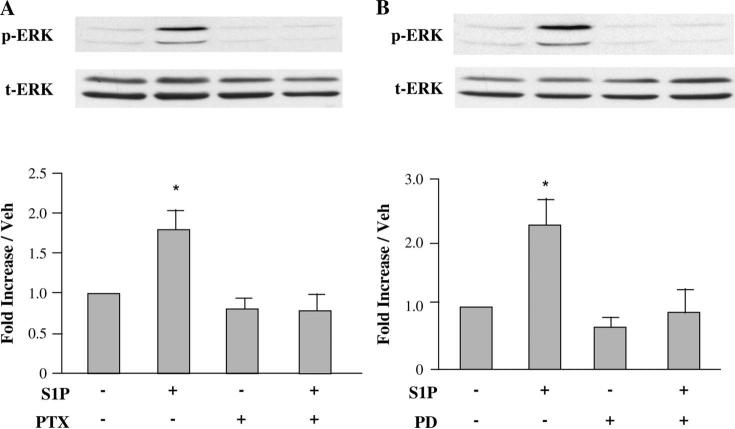
S1P-mediated ERK 1/2 Phosphorylation Is MEK 1/2 Dependent and Is Sensitive to PTX
As noted above and in Figure 1, treatment of cardiomyocytes with 100 nM S1P for 10 minutes resulted in marked phosphorylation of ERK 1/2. This phosphorylation was blocked by prior overnight incubation with PTX (Fig. 2A), indicating the involvement of a PTX-sensitive Gi-coupled receptor. Pretreatment of cells with 10 μM of the MEK inhibitor PD98059 for 60 minutes resulted in significantly less ERK 1/2 phosphorylation (Fig. 2B). These data indicate that ERK phosphorylation by S1P depends completely on the upstream phosphorylation of MEK 1/2 by S1P.
FIGURE 2.
S1P-mediated ERK activation is PTX sensitive and requires MEK phosphorylation. Quiescent cardiomyocytes were pretreated or not for 16 hours with 100 ng/mL of the Gi/o inhibitor PTX (A) or for 1 hour with 10 μM of the MEK inhibitor PD98059 (B) before incubation with 100 nM S1P for 10 minutes. The phosphorylation of ERK 1/2 induced by S1P was abolished by PTX or PD98059. Equal gel loading was verified using an antibody against total ERK 1/2. Specific bands corresponding to phosphorylated forms of ERK 1/2 were quantified by densitometry and expressed as percentage of the corresponding control. Data are expressed as mean ± SEM of 3–4 independent experiments. *P < 0.05 compared with all other values. Veh, vehicle.
Role of S1P1 Receptors in ERK 1/2 Phosphorylation by S1P
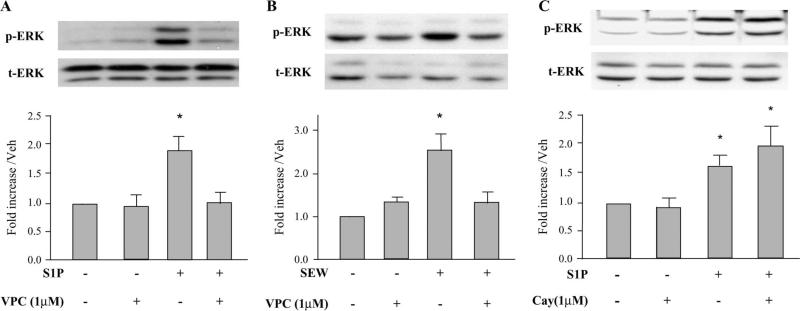
We examined the effects of S1P receptor antagonists on ERK 1/2 phosphorylation to identify the responsible S1P receptor subtype. Pretreatment of myocytes for 30 minutes with 1 μM VPC23019, an inhibitor of S1P1 and S1P3 receptors, inhibited the activation of ERK by S1P (Fig. 3A). SEW2871, a selective S1P1 receptor agonist, also stimulated ERK phosphorylation and was inhibited by 1 μM VPC23019 (Fig. 3B). Furthermore, S1P activation of ERK was not inhibited by 0.1–10 μM CAY10444, a selective S1P3 receptor antagonist (Fig. 3C), thus excluding involvement of the S1P3 receptor subtype. These data suggest that S1P-stimulated ERK 1/2 phosphorylation is mediated predominantly via S1P1 receptors. Neither VPC23019 alone (Figs. 3A, B) nor CAY10444 alone (Fig. 3C) had any effect on basal phosphorylation of ERK.
FIGURE 3.
Effect of S1P receptor antagonists on S1P-induced ERK activation in adult cardiomyocytes. Panel A. Cardiomyocytes were pretreated for 30 minutes with 1 μM of the S1P1 and S1P3 receptor antagonist VPC23019 before stimulation with S1P (1 μM) for 10 minutes. Panel B. Cardiomyocytes were pretreated for 30 minutes with 1 μM VPC23019 before stimulation with the selective S1P1 receptor agonist SEW2871 (1 μM) for 10 minutes. Panel C. Quiescent cardiomyocytes were pretreated for 30 minutes with 1 μM of the selective S1P3 receptor antagonist CAY10444 before stimulation with either 100 nM (third bar) or 1 μM (fourth bar) S1P for 10 minutes. Equal gel loading was assessed using an antibody against total ERK 1/2. Specific bands corresponding to phosphorylated forms of ERK 1/2 were quantified by densitometry and expressed as a percentage of the corresponding control. Data are expressed as mean ± SEM. *P < 0.05 compared with all other values. For panel A, n = 5 independent experiments; for panel B, n = 6 independent experiments; and for panel C, n = 4 independent experiments. Veh, vehicle.
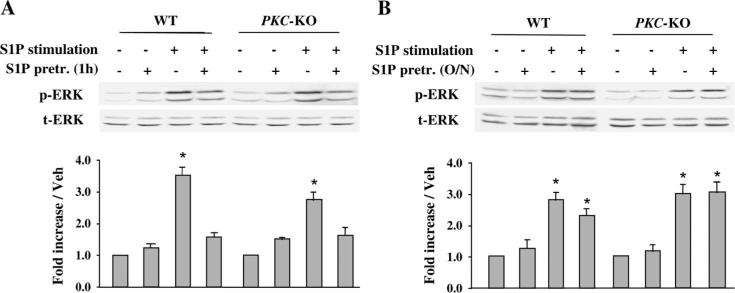
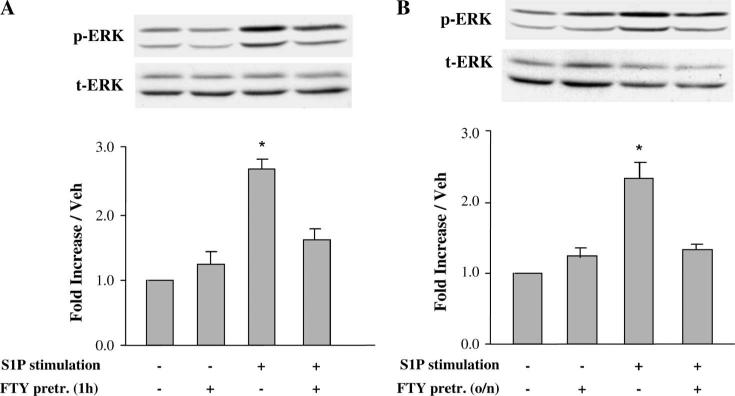
Effects of a Second Exposure to S1P and FTY720 on S1P Receptor–mediated Signaling in Wild-type and PKCε-null Adult Cardiomyocytes
Pretreatment with S1P for 1 hour led to inhibition of ERK phosphorylation in response to a second S1P stimulation in wild-type and PKCε-null adult cardiomyocytes (Fig. 4A). In contrast, when myocytes were preincubated overnight with S1P, the phospho-ERK (pERK) signal in response to short-term S1P recovered fully both in wild-type and in PKCε-null adult cardiomyocytes (Fig. 4B). These data suggest that S1P receptors may be internalized by acute exposure to S1P, but persistent downregulation does not occur. These observations also indicate that PKCε is not involved in the recovery pathway from downregulation of the S1P1 receptor subtype in adult cardiomyocytes.
FIGURE 4.
Effect of S1P on S1P receptor desensitization in WT and PKCε-null adult cardiomyocytes. Adult cardiomyocytes were isolated from WT and PKCε-null mouse hearts. Quiescent cardiomyocytes were preincubated with 100 nM S1P for 1 hour (A) or overnight (B), then stimulated with a second pulse of 100 nM S1P for 10 minutes. Cells were then harvested and Western blot analysis was performed with the indicated antibodies. pERK, phosphorylated p42/44 MAPK; tERK, total p42/44 MAPK; WT, wild-type cells; and PKCε KO, PKCε-null cells. Data are expressed as mean ± SEM of 3 independent experiments. *P < 0.05 compared with all other values. o/n, overnight.
To determine whether a synthetic agonist of S1P receptors has a similar effect on S1P-mediated ERK activation, FTY720 was tested. As the S1P receptor agonist FTY720 is cardioprotective and is being evaluated in human clinical trials, we wished to determine whether its signaling effects would undergo desensitization, which might impair its effectiveness. Figure 5A shows that pretreatment of adult cardiomyocytes with FTY720 for 1 hour led to reduced pERK expression in response to subsequent S1P stimulation. But, some desensitization to FTY720 persisted after overnight S1P exposure (Fig. 5B). These data suggest that FTY720-mediated S1P receptor internalization and degradation24 delays recovery of cell surface expression and function.
FIGURE 5.
Effect of FTY720 on S1P receptor desensitization in adult cardiomyocytes. Quiescent cardiomyocytes were preincubated with 100 nM FTY720 for 1 hour or overnight, then stimulated with a second pulse of 100 nM S1P for 10 minutes. Cells were then harvested and Western blot analysis was performed with the indicated antibodies. Data are expressed as mean ± SEM of 3 independent experiments. *P < 0.05 versus all other values. o/n, overnight.
Effects of S1P and Selective Pathway Inhibitors on Adult Cardiomyocyte Viability After Hypoxia–reoxygenation
ERK activation has recently emerged as an important mechanism involved in various forms of cardiac pathophysiology, including ischemia–reperfusion injury, anthracycline cardiotoxicity, and severe heart failure,18 where ERK has been shown to promote cardiac hypertrophy and to reduce cardiomyocyte apoptosis.18–21 But, whether the MEK/ERK 1/2 response is activated and effective after S1P receptor agonism in adult cardiac myocytes during hypoxia/reoxygenation has not previously been reported.
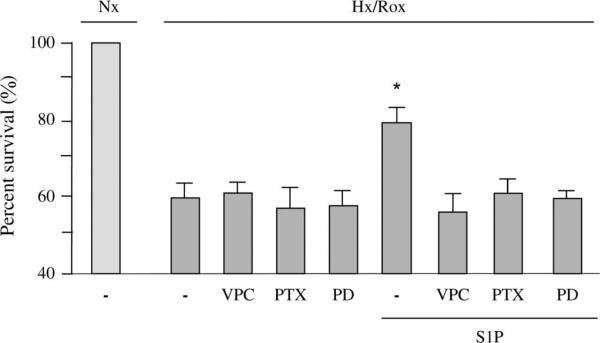
As shown in Figure 6, cardiomyocytes were exposed to 3 hours of hypoxia followed by 16 hours of reoxygenation. At the onset of reoxygenation, cells were treated with 100 nM S1P in the absence and presence of 1 μM VPC23019, 10 μM of the MEK inhibitor PD98059, or 100 ng/mL PTX, each of which had been added for 30 minutes (chemical inhibitors) or 16 hours (PTX) before the onset of hypoxia. Treatment of myocytes with 1 μM VPC23019, an inhibitor of S1P1 and S1P3 receptors at this concentration,25 abrogated cell survival stimulated by S1P subjected to hypoxia–reoxygenation. The increased cell viability induced by S1P was also abolished after treatment with 100 ng/mL PTX. It is known that PTX ribosylates adenosine diphosphate and thereby inactivates Gi through which S1P transduces signals after binding to its cognate receptors.26 The MEK inhibitor, PD98059, also suppressed the cardioprotective effect of S1P (Fig. 6). However, PD98059, VPC23019, or PTX alone had no significant effect on hypoxia–reoxygenation–induced cell death in the absence of agonist (Fig. 6). These results not only confirm a crucial role for Gi and S1P receptors in cardiomyocyte survival but also show that MEK 1/2–ERK 1/2 signaling pathways are involved in S1P-mediated cardioprotection during hypoxia–reoxygenation.
FIGURE 6.
Effect of treatment with a S1P receptor antagonist or PTX or the MEK inhibitor PD98059 on adult cardiomyocytes subjected to hypoxia–reoxygenation. Cardiomyocytes were exposed to 3 hours of hypoxia followed by 16 hours of reoxygenation. Thirty minutes before the onset of hypoxia, cells were incubated with or without the S1P1 and S1P3 receptor antagonist VPC23019 (1 μM), or the MEK inhibitor PD98059 (10 μM). For experiments involving the Gi/o inhibitor PTX, 100 ng/mL of PTX was added 16 hours before the onset of hypoxia. For all experiments, either 100 nM S1P or vehicle was added at the onset of reoxygenation and remained throughout the reoxygenation period. At this time, medium was not changed, so all inhibitors (VPC23019, PD98059, or PTX) were present for the entire duration of the experiment. Survival data are expressed as the percentage of the normoxic control values, which are set at 100%. PD98059, VPC23019, or PTX alone had no significant effect on hypoxia–reoxygenation–induced cell death in the absence of agonist. Data represent the mean ± SEM of 8 independent experiments. *P < 0.05 versus all other values. Nx, normoxia; Hx, hypoxia; Rox, reoxygenation; VPC, VPC23019; PD, PD98059.
DISCUSSION
Cardioprotective effects of S1P have been shown by us and by others both in isolated myocytes and in whole heart preparations.13,15,16,27–34 Desensitization and subsequent resensitization of S1P-activated signaling may be critical in regulating S1P-mediated cell responses. Desensitization of signaling after S1P stimulation has not previously been studied in cardiac myocytes. Once a signaling pathway is activated by a stimulus, it is desensitized to protect the cell from excessive stimulation by the initial stimulus and by other synergistically acting agonists, especially those agonists that use the same signaling pathways.35 In this study, we show that S1P-induced ERK activation undergoes desensitization but recovers rapidly and fully both in wild-type and in PKCε-null cardiomyocytes. The nonselective S1P receptor agonist FTY720 also led to reduced ERK activation in response to subsequent S1P stimulation, but desensitization persisted after overnight S1P exposure. For the first time, we demonstrate that the S1P/S1P1–Gi–MEK 1/2–ERK 1/2 cascade is an important signaling pathway that mediates cell survival during hypoxia–reoxygenation in adult cardiomyocytes. We also provide further evidence that S1P is cardioprotective through mechanisms that require S1P1 receptor activation.
S1P1 and ERK 1/2 Desensitization
A recent report in renal mesangial cells showed that S1P-mediated signaling is rapidly desensitized upon S1P receptor activation. In the latter study, there was complete loss of S1P receptors from the cell surface and receptor-mediated signaling responses after 10 minutes of S1P pretreatment, which persisted for at least 8 hours.35 In contrast, Oo et al36 reported that S1P1 recycled back to the plasma membrane within 2 hours in S1P-treated human umbilical vein endothelial cells. The immune modulator FTY720 induced internalization and degradation of S1P1 receptors.24,37 The timing and extent of S1P receptor internalization were highly dependent on FTY720 concentration.24 Unlike FTY720, the S1P1-selective synthetic agonist SEW2871 induced S1P1 internalization and recycling.38 Diverse mechanisms for S1P1 receptor downregulation and trafficking have been described. These include translocation to perinuclear vesicles,39 plasma-lemmal caveolae,40 N-glycosylation,41 and ubiquitination.42 These studies have been performed in cell lines requiring transfection of the S1P1 receptor.
In the current study, we used ERK activation as an index of S1P receptor–mediated signaling. Because radioligands for S1P receptor subtypes are not readily available and S1P1–/– mice do not survive gestation, we used 2 approaches to verify that the receptor subtype responsible for S1P-mediated ERK signaling is S1P1: chemical inhibitor studies and treatment with the selective S1P1 agonist SEW2871.38 Our data show that activation of ERK by restimulation with 100 nM S1P was suppressed after 1 hour of preincubation with 100 nM S1P but recovered fully the next day. Treatment with FTY720 for 1 hour also led to reduced ERK phosphorylation in response to subsequent S1P stimulation.
In contrast to S1P, some desensitization to FTY720 persisted after overnight exposure. These data suggest that S1P1 receptors recycled back to the plasma membrane after overnight incubation in S1P-treated cardiomyocytes, whereas recycling was not fully achieved in the FTY720-treated cells. We cannot exclude the possibility that the new receptor synthesis is involved in this process. This might apply especially to incubation with FTY720, which results in receptor degradation36,37 and might therefore require synthesis of new receptor protein. We recognize that these interpretations of our data are inferential, as we do not have direct evidence of S1P1 receptor translocation, because adult mouse cardiomyocytes cannot be readily transfected. Nevertheless, these observations may have relevance to potential therapeutic effects and dosing schedules should these agents be employed clinically.
Role of PKCε
In this study, we also sought to examine the mechanism of recovery and stabilization of S1P1 receptor cell surface expression after downregulation. Graeler et al17 reported that T-cell S1P1 receptor recovery from S1P-induced down-regulation requires PKCε-dependent late phosphorylation of S1P1 receptors and the participation of the activating protein-1 (AP-1) transcription complex. In this study, we found that activation of ERK restimulated with S1P was suppressed after 1 hour of S1P preincubation but recovered fully the next day both in wild-type and in PKCε-null cardiomyocytes. These data indicate that PKCε is not necessary for recovery of apparent downregulation of S1P1 receptors in adult cardiomyocytes. However, the role of receptor internalization in S1P-mediated signal transduction is not completely resolved. Although the mechanism is generally assumed to rely on ligand-induced desensitization of signaling molecules, it is also possible that internalization of S1P1 may facilitate transmembrane trafficking of S1P itself to specific subcellular locales such as perinuclear structures, late endosomes, or caveolae.39,40,42
S1P1 and the ERK 1/2 Pathway
Several laboratories have demonstrated that S1P can inhibit ischemia–reperfusion injury in different tissues.15,16,27,28,43 Although the coupling of S1P1 receptors to the ERK pathway has been questioned,29 Awad et al44 reported that selective S1P1 activation also reduced ischemia–reperfusion injury in mouse kidney. Studies in other cell types have also demonstrated coupling of the S1P1 receptor to ERK activation.38,42 In cultured cardiomyocytes, ischemia–reperfusion injury is best recapitulated by hypoxia followed by reoxygenation. But, whether S1P1 receptor activation can inhibit hypoxia–reoxygenation injury in adult cardiomyocytes has not been ascertained, as previous studies were performed exclusively under hypoxic conditions without prolonged reoxygenation.30,31
It is well recognized that cell or tissue reoxygenation initiates a cascade of events, including free radical generation, which results in cell death.45 Recently, we reported that S1P coupled to the S1P1 receptor activated a PI3 kinase/Akt cardioprotective pathway in adult mouse cardiac myocytes subjected to prolonged hypoxia.13 This process was mediated via S1P1 receptors.13 MEK 1/2 activates the ERK family of MAPKs, and ERK activation also is known to enhance prosurvival signaling.18–21 Thus, cardioprotection induced by both pharmacologic and ischemic preconditioning are at least in part mediated by ERK-dependent pathways.20,44,46 In this study, we used trypan blue exclusion to measure cell viability. This procedure identifies necrotic cells,47,48 but we cannot exclude the possibility that S1P also inhibits apoptosis through its ability to initiate prosurvival signaling. Such signaling is shown both in the current study and in our previous reports.13,31
However, no previous investigation has examined the role of S1P-mediated ERK 1/2 signaling in adult cardiac myocytes. We found that the MEK inhibitor PD98059 abrogated the cardioprotective effect of S1P, indicating that an ERK 1/2 signaling pathway is involved in S1P-mediated cardioprotection during hypoxia–reoxygenation. Our data also indicate that this pathway is impaired but remains active during hypoxia–reoxygenation in these cells. Whether ERK 1/2 activation is hampered because of reduced receptor number, a decline in receptor affinity, an increase in phosphatase activity, or a direct effect on ERK phosphorylation, cannot be determined from our data and will require further study.
The addition of S1P at the time of reoxygenation in cultured myocytes may have particular clinical relevance. When subjected to short periods of ischemia/reperfusion injury before an index ischemia, tissues are protected from subsequent injury (preconditioning).49 Protection also occurs when a similar maneuver is applied at the time of reperfusion (postconditioning).49 Both pre- and postconditioning can also result from pharmacologic intervention.50 In this study, administration of S1P represents an instance of successful pharmacologic postconditioning. We have also shown that ischemic combined with pharmacologic postconditioning is highly effective in isolated hearts.51 This approach is directly relevant to clinical applications, such as treatment of patients undergoing percutaneous cardiovascular interventions (angioplasty and stent placement)52 and surgical cardiac revascularization, where reperfusion injury is common. It is also pertinent to post-coronary artery thrombolysis treatment in the heart and to any organ where there has been a cessation of blood flow leading to ischemia that is then followed by reperfusion, including the brain, the gut, and in tissues supplied by peripheral vessels.
As noted above, a recent report was unable to demonstrate prosurvival signaling mediated by the S1P1 receptor.29 In contrast, we have shown that the selective S1P1 agonist SEW2871 mediates myocyte survival during prolonged hypoxia13 and induces phosphorylation of ERK 1/2 (Fig. 3B). In parallel experiments, we have found that SEW2871 also activates Akt (n = 4, P < 0.05, data not shown). In isolated rat hearts, it was shown that the selective S1P1 receptor agonist SEW2871 given before ischemia/reperfusion at a concentration as low as 100 nM reduced infarct size significantly but did not increase the incidence or duration of reperfusion arrhythmias compared with S1P.53 In mice subjected to experimental coronary artery ligation treated with oral SEW2871 for 2 weeks, there was reduced apoptosis in the remote myocardium and enhanced fractional shortening by echocardiography compared with untreated animals.54 This was accompanied by enhanced signaling responses consisting of rescue of downregulated pAkt, enhancement of p70S6K, and increased pERK. Thus, the discrepancy regarding the efficacy of S1P1 receptor agonism is at present unclear. However, our previous and current experiments have led to the conclusion that the S1P1 receptor, which is the most abundant S1P subtype receptor in cardiac myocytes, is responsible for S1P-mediated prosurvival signaling, and for maintaining myocyte viability both during hypoxia,13 and as shown in the present study, after agonist exposure and during hypoxia/reoxygenation.
The concentration of S1P selected for treating adult cardiac myocytes at the onset of reoxygenation was empirical. We initially performed concentration–response experiments and found this concentration to be optimal. Indeed, higher concentrations (>1 μM) proved to be toxic to the cells in this in vitro model. One explanation for the efficacy of this apparently lower concentration is that most of the S1P in serum is bound to plasma lipoproteins, particularly high-density lipoprotein, and albumin.55,56 The technique previously used by others56–60 to estimate serum S1P levels involves denaturation of these proteins and so does not actually measure free S1P but rather free plus bound S1P. Our experiments were performed in albumin- and protein-free buffer so that all the added S1P remains unbound as opposed to serum.
In summary, we have shown for the first time that exogenous S1P induces rapid, specific, and reversible reactivation of S1P1-mediated signaling, which is independent of PKCε activity. We also demonstrate that S1P-induced cardiomyocyte survival requires ERK activation mediated via an S1P1–Gi pathway in adult mouse cardiomyocytes subjected to hypoxia–reoxygenation. Combined with our previous observations regarding the role of PI3 kinase and Akt in cardioprotection,13 these data suggest that both ERK 1/2 and the PI3K/Akt pathways coordinately mediate downstream S1P/S1P1 receptor–induced cardioprotection. Moreover, these pathways do not seem to be redundant because selective blockade of either pathway does not permit cell survival by the other. The absence of signaling desensitization and the elucidation of the pathways involved provide a mechanistic basis for design of experimental studies aimed at cardioprotection that employ sphingolipid agonists.
Acknowledgments
Supported by Award Number 1P01 HL068738-01A1 to J. S. Karliner. from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Maceyka M, Payne SG, Milstien S, et al. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim Biophys Acta. 2002;1585:193–201. doi: 10.1016/s1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 2.Saba JD, Hla T. Point-counterpoint of sphingosine 1-phosphate metabolism. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. Sphingosine-1-phosphate: an enigmatic signalling lipid. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 4.Hla T, Lee MJ, Ancellin N, et al. Lysophospholipid-receptor revelations. Science. 2001;294:1875–1878. doi: 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- 5.Usui S, Sugimoto N, Takuwa N, et al. Blood lipid mediator sphingosine 1-phosphate potently stimulates platelet-derived growth factor-A and -B chain expression through S1P1-Gi-Ras-MAPK-dependent induction of Kruppel-like factor 5. J Biol Chem. 2004;279:12300–12311. doi: 10.1074/jbc.M305025200. [DOI] [PubMed] [Google Scholar]
- 6.Pyne S, Pyne NJ. Sphingosine 1-phosphate signalling in mammalian cells. Biochem J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bünemann M, Brandts B, Meyer zu Heringdorf D, et al. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J Physiol. 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima N, Cavalli AL, Biral D, et al. Expression and characterization of Edg-1 receptors in rat cardiomyocytes: calcium deregulation in response to sphingosine 1-phosphate. Eur J Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazurais D, Robert P, Gout B, et al. Cell type-specific localization of human cardiac S1P receptors. J Histochem Cytochem. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- 10.Zondag GC, Postma FR, Etten IV, et al. Sphingosine 1-phosphate signalling through the G-protein-coupled receptor Edg-1. Biochem J. 1998;330:605–609. doi: 10.1042/bj3300605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Okamoto H, Takuwa N, Gonda K, et al. EDG1 is a functional sphingosine-1-phosphate receptor that is linked via a Gi/o to multiple signaling pathways, including phospholipase C activation, Ca2+ mobilization, Ras-mitogen-activated protein kinase activation, and adenylate cyclase inhibition. J Biol Chem. 1998;273:27104–27110. doi: 10.1074/jbc.273.42.27104. [DOI] [PubMed] [Google Scholar]
- 12.Kon J, Sato K, Watanable T, et al. Comparison of intrinsic activities of the putative sphingosine 1-phosphate receptor subtypes to regulate several signaling pathways in their cDNA-transfected Chinese hamster ovary cells. J Biol Chem. 1999;274:23940–23947. doi: 10.1074/jbc.274.34.23940. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Honbo N, Goetzl EJ, et al. Signals from type 1 sphingosine 1-phosphate receptors enhance adult mouse cardiac myocyte survival during hypoxia. Am J Physiol Heart Circ Physiol. 2007;293:H3150–H3158. doi: 10.1152/ajpheart.00587.2006. [DOI] [PubMed] [Google Scholar]
- 14.Churchill E, Budas G, Vallentin A, et al. PKC isozymes in chronic cardiac disease: possible therapeutic targets? Annu Rev Pharmacol Toxicol. 2008;48:569–599. doi: 10.1146/annurev.pharmtox.48.121806.154902. [DOI] [PubMed] [Google Scholar]
- 15.Jin ZQ, Zhou HZ, Zhu P, et al. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- 16.Lecour S, Smith RM, Woodward B, et al. Identification of a novel role for sphingolipid signaling in TNF alpha and ischemic preconditioning mediated cardioprotection. J Mol Cell Cardiol. 2002;34:509–518. doi: 10.1006/jmcc.2002.1533. [DOI] [PubMed] [Google Scholar]
- 17.Graeler MH, Kong Y, Karliner JS, et al. Protein kinase C epsilon dependence of the recovery from down-regulation of S1P1 G protein-coupled receptors of T lymphocytes. J Biol Chem. 2003;278:27737–27741. doi: 10.1074/jbc.C300147200. [DOI] [PubMed] [Google Scholar]
- 18.Yue TL, Wang C, Gu JL, et al. Inhibition of extracellular signal-regulated kinase enhances ischemia/reoxygenation-induced apoptosis in cultured cardiac myocytes and exaggerates reperfusion injury in isolated perfused heart. Circ Res. 2000;86:692–699. doi: 10.1161/01.res.86.6.692. [DOI] [PubMed] [Google Scholar]
- 19.Lips DJ, Bueno OF, Wilkins BJ, et al. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Tsang A, Mocanu M, et al. Ischemic preconditioning protects by activating prosurvival kinases at reperfusion. Am J Physiol Heart Circ Physiol. 2005;288:H971–H976. doi: 10.1152/ajpheart.00374.2004. [DOI] [PubMed] [Google Scholar]
- 21.Pesse B, Levrand S, Feihl F, et al. Peroxynitrite activates ERK via Raf-1 and MEK, independently from EGF receptor and p21Ras in H9C2 cardiomyocytes. J Mol Cell Cardiol. 2005;38:765–775. doi: 10.1016/j.yjmcc.2005.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray MO, Zhou HZ, Schafhalter-Zoppoth I, et al. Preservation of baseline hemodynamic function and loss of inducible cardioprotection in adult mice lacking protein kinase C epsilon. J Biol Chem. 2004;279:3596–3604. doi: 10.1074/jbc.M311459200. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YY, Wang SQ, Zhu WZ, et al. Culture and adenoviral infection of adult mouse cardiac myocytes: methods for cellular genetic physiology. Am J Physiol Heart Circ Physiol. 2000;279:H429–H436. doi: 10.1152/ajpheart.2000.279.1.H429. [DOI] [PubMed] [Google Scholar]
- 24.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Davis MD, Clemens JJ, Macdonald TL, et al. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 26.Siehler S, Manning DR. Pathways of transduction engaged by sphingosine 1-phosphate through G protein-coupled receptors. Biochim Biophys Acta. 2002;1582:94–99. doi: 10.1016/s1388-1981(02)00142-7. [DOI] [PubMed] [Google Scholar]
- 27.Jin ZQ, Zhang J, Huang Y, et al. A sphingosine kinase 1 mutation sensitizes the myocardium to ischemia/reperfusion injury. Cardiovasc Res. 2007;76:41–50. doi: 10.1016/j.cardiores.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 28.Means CK, Xiao CY, Li Z, et al. Sphingosine 1-phosphate S1P2 and S1P3 receptor-mediated Akt activation protects against in vivo myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2007;292:H2944–H2951. doi: 10.1152/ajpheart.01331.2006. [DOI] [PubMed] [Google Scholar]
- 29.Means CK, Miyamoto S, Chun J, et al. S1P1 receptor localization confers selectivity for Gi mediated cAMP and contractile responses. J Biol Chem. 2008;283:11954–11963. doi: 10.1074/jbc.M707422200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao R, Zhang J, Vessey DA, et al. Deletion of the sphingosine kinase-1 gene influences cell fate during hypoxia and glucose deprivation in adult mouse cardiomyocytes. Cardiovasc Res. 2007;74:56–63. doi: 10.1016/j.cardiores.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Karliner JS, Honbo N, Epstein CJ, et al. Neonatal mouse cardiac myocytes exhibit cardioprotection induced by hypoxic and pharmacologic preconditioning and by transgenic overexpression of human Cu/Zn superoxide dismutase. J Mol Cell Cardiol. 2000;32:1779–1786. doi: 10.1006/jmcc.2000.1212. [DOI] [PubMed] [Google Scholar]
- 32.Duan HF, Wang H, Yi J, et al. Adenoviral gene transfer of sphingosine kinase 1 protects heart against ischemia/reperfusion–induced injury and attenuates its postischemic failure. Hum Gene Ther. 2007;18:1119–1128. doi: 10.1089/hum.2007.036. [DOI] [PubMed] [Google Scholar]
- 33.Kim M, Kim M, Kim N, et al. Isoflurane mediates protection from renal ischemia-reperfusion injury via sphingosine kinase and sphingosine-1-phosphate-dependent pathways. Am J Physiol Renal Physiol. 2007;293:F1827–F1835. doi: 10.1152/ajprenal.00290.2007. [DOI] [PubMed] [Google Scholar]
- 34.Theilmeier G, Schmidt C, Hermann J, et al. High density lipoproteins and their constituent, sphingosine-1-phosphate, directly protect the heart against ischemia/reperfusion injury in vivo via the S1P3 lysophospholipid receptor. Circulation. 2006;114:1403–1409. doi: 10.1161/CIRCULATIONAHA.105.607135. [DOI] [PubMed] [Google Scholar]
- 35.Xin C, Ren S, Pfeilschifter J, et al. Heterologous desensitization of the sphingosine-1-phosphate receptors by purinoceptor activation in renal mesangial cells. Br J Pharmacol. 2004;143:581–589. doi: 10.1038/sj.bjp.0705980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oo ML, Thangada S, Wu MT, et al. Immunosuppressive and anti-angiogenic sphingosine 1-phosphate receptor-1 agonists induce ubiquitinylation and proteasomal degradation of the receptor. J Biol Chem. 2007;282:9082–9089. doi: 10.1074/jbc.M610318200. [DOI] [PubMed] [Google Scholar]
- 37.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 38.Jo E, Sanna MG, Gonzalez-Cabrera PJ, et al. S1P1-selective in vivo-active agonists from high-throughput screening: off-the-shelf chemical probes of receptor interactions, signaling, and fate. Chem Biol. 2005;12:703–715. doi: 10.1016/j.chembiol.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Liu CH, Thangada S, Lee MJ, et al. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10:1179–1190. doi: 10.1091/mbc.10.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Igarashi J, Michel T. Agonist-modulated targeting of the EDG-1 receptor to plasmalemmal caveolae. J Biol Chem. 2000;275:32363–32370. doi: 10.1074/jbc.M003075200. [DOI] [PubMed] [Google Scholar]
- 41.Kohno T, Wasa A, Igarashi Y. N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 2002;16:983–992. doi: 10.1096/fj.01-0809com. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Cabrera PJ, Hla T, Rosen H, et al. Mapping pathways downstream of sphingosine 1-phosphate subtype 1 by differential chemical perturbation and proteomics. J Biol Chem. 2007;282:7254–7264. doi: 10.1074/jbc.M610581200. [DOI] [PubMed] [Google Scholar]
- 43.Okazaki M, Kreisel F, Richardson SB, et al. Sphingosine 1-phosphate inhibits ischemia reperfusion injury following experimental lung transplantation. Am J Transplant. 2007;7:751–758. doi: 10.1111/j.1600-6143.2006.01710.x. [DOI] [PubMed] [Google Scholar]
- 44.Awad AS, Ye H, Huang L, et al. Selective sphingosine 1-phosphate 1 receptor activation reduces ischemia-reperfusion injury in mouse kidney. Am J Physiol Renal Physiol. 2006;290:F1516–F1524. doi: 10.1152/ajprenal.00311.2005. [DOI] [PubMed] [Google Scholar]
- 45.Li C, Jackson RM. Reactive species mechanisms of cellular hypoxia-reoxygenation injury. Am J Physiol Cell Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001. [DOI] [PubMed] [Google Scholar]
- 46.Germack R, Dickenson JM. Adenosine triggers preconditioning through MEK/ERK1/2 signalling pathway during hypoxia/reoxygenation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2005;39:429–442. doi: 10.1016/j.yjmcc.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 47.Kubasiak LA, Hernandez OM, Bishopric NH, et al. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc Natl Acad Sci U S A. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Das A, Lei X, Kukreja RC. Phosphodiesterase-5 inhibitor silendafil preconditions adult cardiac myocytes against necrosis and apoptosis. Essential role of nitric oxide signaling. J Biol Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 49.Skyschally A, Schulz R, Heusch G. Pathophysiology of myocardial infarction: protection by ischemic pre- and postconditioning. Herz. 2008;33:88–100. doi: 10.1007/s00059-008-3101-9. [DOI] [PubMed] [Google Scholar]
- 50.Andreadou I, Iliodromitis EK, Koufaki M, et al. Pharmacological pre- and post- conditioning agents: reperfusion-injury of the heart revisited. Mini Rev Med Chem. 2008;8:952–959. doi: 10.2174/138955708785132819. [DOI] [PubMed] [Google Scholar]
- 51.Vessey DA, Li L, Kelley M, et al. Combined sphingosine, S1P and ischemic postconditioning rescue the heart after protracted ischemia. Biochem Biophys Res Commun. 2008;375:425–429. doi: 10.1016/j.bbrc.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thibault H, Piot C, Staat P, et al. Long-term benefit of postconditioning. Circulation. 2008;117:1037–1044. doi: 10.1161/CIRCULATIONAHA.107.729780. [DOI] [PubMed] [Google Scholar]
- 53.Tsukada YT, Sanna MG, Rosen H, et al. S1P1-selective agonist SEW2871 exacerbates reperfusion arrhythmias. J Cardiovasc Phamacol. 2007;50:660–669. doi: 10.1097/FJC.0b013e318157a5fe. [DOI] [PubMed] [Google Scholar]
- 54.Yeh CC, Li H, Malhotra D, et al. Sphingolipid signaling and treatment during remodeling of the uninfarcted ventricular wall after myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H1193–H1199. doi: 10.1152/ajpheart.01032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okajima F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim Biophys Acta. 2002:1582l:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- 56.Murata N, Sato K, Kon J, et al. Interaction of sphingosine 1-phosphate with plasma components including lipoproteins, regulates the lipid receptor-mediated actions. Biochem J. 2000;352:809–815. [PMC free article] [PubMed] [Google Scholar]
- 57.Dalla Libera L, Sabbadini R, Renken C, et al. Apoptosis in the skeletal muscle of rats with heart failure is associated with increased serum levels of TNF-α and sphingosine. J Mol Cell Cardiol. 2001;33:1871–1878. doi: 10.1006/jmcc.2001.1453. [DOI] [PubMed] [Google Scholar]
- 58.Deutschman DH, Carstens JS, Klepper RL, et al. Predicting obstructive coronary artery disease with serum sphingosine-1-phosphate. Am Heart J. 2003;146:62–68. doi: 10.1016/S0002-8703(03)00118-2. [DOI] [PubMed] [Google Scholar]
- 59.Berdyshev EV, Gorshkova IA, Garcia JGN, et al. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography-tandem mass spectrometry. Anal Biochem. 2005;339:129–136. doi: 10.1016/j.ab.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 60.Schwab SR, Pereira JP, Matlobian M, et al. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–1739. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]