Abstract
Introduction:
This study evaluated the effects on blood pressure (BP) of valsartan 160 mg or losartan 100 mg addition to amlodipine 5 mg in hypertensive patients.
Methods:
221 patients with inadequately controlled BP (DBP ≥ 90 mmHg) after 4 weeks of treatment with amlodipine 5 mg were randomized to receive losartan/amlodipine combination therapy or valsartan/amlodipine combination therapy for 4 weeks in a cross-over study design. At the end of the wash-out period and of each treatment period, clinic and ambulatory BP measurements were recorded.
Results:
166 patients completed the study. Both combination treatments induced a greater ambulatory BP reduction than did monotherapy. However, the further mean reductions in BP versus monotherapy were significantly greater with the valsartan/amlodipine combination (SBP/DBP: −7.9 ± 3.4/−6.5 ± 2.6 mmHg for 24-hour, −8.0 ± 3.4/−6.6 ± 2.7 mmHg for daytime; −7.7 ± 3.3/−6.4 ± 2.7 mmHg for nighttime) than with the losartan/amlodipine combination (SBP/DBP: −5.5 ± 2.8/−4.2 ± 2.1 mmHg for 24-hour, −5.7 ± 2.9/−4.4 ± 2.2 mmHg for daytime; −4.8 ± 2.8/−3.7 ± 2.2 mmHg for nighttime; P < 0.01 vs valsartan/amlodipine). The incidence of adverse events with valsartan/amlodipine (8%) and losartan/amlodipine (9%) was lower than that observed with amlodipine monotherapy (17%; P < 0.05 vs combinations).
Conclusion:
Valsartan 160 mg plus amlodipine 5 mg produced greater BP reductions than losartan 100 mg plus amlodipine 5 mg.
Keywords: angiotensin receptor blocker, ambulatory blood pressure monitoring, valsartan, losartan, amlodipine, combination therapy
Introduction
Current hypertension management guidelines advocate a blood pressure (BP) goal of <140/90 mmHg in the general population with uncomplicated hypertension.1,2 Lower BP goals are recommended for high-risk patients, such as those with concomitant diabetes mellitus, renal disease, or evidence of other target organ damage. These recommendations are supported by evidence accumulated from long-term trials suggesting that lower BP values are associated with better outcomes in a broad range of patients.3,4 Major studies have shown that most patients with hypertension need two or more antihypertensive drugs to achieve their BP goals, regardless of the medication chosen as initial therapy.5–8 Advantages of combination therapy include the following: (1) greater BP reduction and higher response rates than with monotherapy, probably caused by the simultaneous effect on several regulatory systems involved in abnormal BP elevation; (2) favorable alterations in pharmacokinetics; (3) fewer adverse effects with consequent better tolerability and improved compliance with treatment; and (4) possibly lower costs of treatment.9,10
Given the vast array of available antihypertensive agents, the number of potential combinations is large; however, rational choice must be based on the characteristics of each agent and their complementary mechanisms of action.9,10
Treatment guidelines suggest that the combination of an angiotensin receptor blocker (ARB) and a calcium channel blocker (CCB) provides an effective option for patients with hypertension.11
The use of these drugs in combination has the potential to achieve additive BP reduction by targeting multiple mechanisms involved in BP regulation.12 ARBs that block the angiotensin II type 1 receptors act to promote vasodilation and sodium excretion.13 CCBs blocking calcium channels in vascular smooth muscle cells thereby reduce peripheral vascular resistance.12 Targeting multiple systems has benefits in terms of overcoming potential counter-regulatory mechanisms, eg, the compensatory activation of the renin-angiotensin system induced by CCBs.12,14 Further, such a combination of drugs provides advantages in enhancing tolerability, in that ARBs prevent or attenuate some of the adverse events of CCBs, such as ankle edema and headache.15,16
Given the general validity of these pharmacodynamic considerations, the efficacy of the CCB/ARB combination must be assessed in a clinical setting, specifically compared with ARB monotherapy, because the different pharmacologic properties of the various ARBs might have an important clinical impact, and might produce different interactions with CCBs, with a consequent possible influence on clinical efficacy.
The objective of this study was to evaluate the antihypertensive effect, evaluated by ambulatory BP monitoring, of losartan 100 mg added to amlodipine 5 mg monotherapy compared with the addition of valsartan 160 mg to the same amlodipine dose in moderately hypertensive patients who were nonresponders to amlodipine monotherapy.
Patients and methods
This was a prospective, randomized, open-label, blinded end point (PROBE)17 evaluation, cross-over study. Consecutive outpatients of both sexes, aged 35 to 75 years, were eligible for recruitment if they had a sitting diastolic BP (DBP) of ≥ 99 mmHg and <110 mmHg at the end of an initial 2-week wash-out period. Patients with sitting DBP ≥ 110 mmHg or sitting systolic BP (SBP) > 200 mmHg at the end of the washout period were excluded from the study, as were those with secondary or malignant hypertension, type 1 or type 2 diabetes mellitus, myocardial infarction or cerebrovascular accident within the preceding 6 months, heart failure, clinically significant valvular heart disease or arrhythmia, renal or hepatic insufficiency, pregnancy, or known hypersensitivity to the drugs used in the study.
The study protocol was approved by the local ethical committee and written informed consent was obtained from all patients before they were included in the study.
According to the study design, after a 2-week washout period, during which any previous antihypertensive therapy was discontinued, eligible patients were treated with amlodipine 5 mg once daily for 4 weeks. Thereafter, the non-responder patients (DPB > 90 and/or SBP > 140 mmHg) were randomized to receive either additional valsartan 160 mg or losartan 100 mg for a further 4 weeks, with the additional therapies to be taken at the same hour in the morning (approximately between 8 am and 9 am) in 2 cross-over periods, each separated by a 4-week amlodipine 5 mg monotherapy period. At the end of each study period (placebo, monotherapy, or combination), BP was measured in both the clinic environment and through noninvasive ambulatory BP monitoring. Clinic BP was obtained with a standard mercury sphygmomanometer with the patient in the sitting position, 24 hours after last drug intake. Three measurements, taken at 2-minute intervals after 10 minutes of sitting, were averaged, and these averages were used as clinic BP reference values. Heart rate (HR) was measured after each BP measurement through the palpatory method at the radial artery level.
Ambulatory BP monitoring was performed over 24 hours with the use of a clinically validated device (Spacelabs 90207 ambulatory BP monitor; Spacelabs Inc., Redmond, Washington, USA) that was programmed to measure BP every 15 minutes during the entire course of the recording. Each recording was started in the morning, immediately after clinic BP assessment and drug administration. Patients were instructed to remain motionless each time a reading was taken. Analysis of 24-hour BP recordings was preceded by removal of artefacts, according to previously described editing criteria.18 Recordings were considered valid when no more than 2 nonconsecutive hours were missing over 24 hours. For each patient, the following data related to SBP, DBP, and HR were obtained through analysis of the recordings: 24-hour mean values, as well as daytime (7 am–11 pm), nighttime (11 pm–7 am), and hourly mean values. The trough-to-peak (T/P) ratio, computed after peak and trough changes were selected, was calculated for each individual subject.19 To calculate peak changes, the clinician selected the hour in which maximal reduction in BP was noted after treatment between the second and eighth hours after drug administration and averaged this change with data from the immediately adjacent hour in which reduction was most evident. Trough BP changes were calculated by averaging the last 2 hours of the recordings.19 Data were averaged (mean) for all patients.
The smoothness index (SI) was computed by dividing the average of the 24-hour BP changes after treatment by the corresponding standard deviation.20,21 This calculation has been shown to reflect whether treatment smoothly reduced BP during the 24-hour period more accurately than the T/P ratio.20,21
At each visit, adverse events spontaneously reported or elicited by indirect questioning were recorded.
Statistical analysis
All analyses were conducted with the SAS system, version 6.12 (SAS Institute, Inc., Cary, North Carolina, USA). Analysis of variance was used for BP results. Differences in T/P ratios between treatments were evaluated with nonparametric tests (univariate signed rank test), whereas the paired Student’s t test was used to assess differences in SI. The level of statistical significance was kept at 0.05. Data are shown as mean ± standard deviation (SD).
Results
A total of 233 consecutive outpatients with moderate hypertension who were referred to the hypertension center of our clinic were screened for eligibility. At the end of the wash-out period 221 patients (106 males and 115 females), aged 39 to 75 years, fulfilled the inclusion/exclusion criteria and were treated with amlodipine 5 mg once daily for 4 weeks. One hundred eighty-five patients whose clinic BP was not adequately controlled (DBP ≥ 90 mmHg and or SBP > 140 mmHg) were admitted to the study and randomized to the cross-over design. Their main demographic and clinical characteristics are shown in the Table 1. They did not significantly differ from those of the 36 patients who normalized their BP with amlodipine monotherapy, with the exception of baseline SBP/DBP levels, which were lower in these latter patients (158.6 ± 10.4/101.2 ± 3.4 mmHg vs 169.8 ± 13.1/104.1 ± 6.9 mmHg in the nonnormalized patients). After randomization 19 patients dropped out (11 due to nonvalid ambulatory BP monitoring recording or intolerance to the device, 4 due to excessively high BP values and 4 were lost at follow-up), while 166 patients completed the study. The results presented here pertain to this latter group of patients.
Table 1.
Demographic and clinical characteristic of the study population
| Total randomized | Completed the study | P | |
|---|---|---|---|
| Total randomized | 185 | 166 | ns |
| Women/men | 91/94 | 82/84 | ns |
| Age y ± SD | 59.1 ± 11.7 | 58.9 ± 11.6 | ns |
| SBP, mmHg | 169.8 ± 13.1 | 169.5 ± 13.0 | ns |
| DBP, mmHg | 104.1 ± 6.9 | 103.9 ± 6.8 | ns |
| Heart rate, beats/min | 75.8 ± 7.1 | 75.7 ± 7.1 | ns |
| Smoking habit, n (%) | 41 (22.2 %) | 36 (21.6 %) | ns |
| Hypercholesterolemia, n (%) | 53 (28.6 %) | 47 (28.3 %) | ns |
| ECG-LVH, n (%) | 16 (8.6 %) | 14 (8.4 %) | ns |
Averaged 24-hour, daytime, and nighttime ambulatory SBP and DBP values are shown in Table 2. Amlodipine significantly reduced ambulatory BP values compared with baseline values: mean decreases in 24-hour, daytime, and nighttime SBP/DBP were: −13.2 ± 7.6/−9.3 ± 4.2 mmHg, −14.1 ± 7.9/−9.7 ± 4.8 mmHg, and −11.1 ± 6.8/−7.3 ± 3.9 mmHg, respectively (all P < 0.001). A further decrease in ambulatory BP was observed at the end of each combination treatment (Table 2). However, with the valsartan/amlodipine combination, the further mean reduction in BP (SBP/DBP: −7.9 ± 3.4/−6.5 ± 2.6 mmHg for 24-hour, −8.0 ± 3.4/−6.6 ± 2.7 mmHg for daytime, and −7.7 ± 3.3/−6.4±2.7 mmHg for nighttime) was greater than that seen with the losartan/amlodipine combination (SBP/DBP: −5.5 ± 2.8/−4.2 ± 2.1 mmHg for 24-hour, −5.7 ± 2.9/−4.4 ± 2.2 mmHg for daytime, and −4.8 ± 2.8/−3.7 ± 2.2 mmHg for nighttime); and the difference between the 2 treatments was statistically significant (P < 0.01; Table 2 and Figure 1). Calculation of hourly averaged SBP and DBP values (Figure 2) showed that BP reduction attained with both combinations was more consistent than that observed with amlodipine monotherapy, with no negative influence on the circadian BP profile.
Table 2.
Average 24-hour, daytime and nighttime ambulatory systolic blood pressure (SBP) and diastolic blood pressure (DBP) values of the randomized patients who completed the study (n = 166)
|
24-hour |
Daytime |
Nighttime |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Amlodipine | Combination | Baseline | Amlodipine | Combination | Baseline | Amlodipine | Combination | |
| SBP, mmHg | 152.3 ± 8.9 | 139.1 ± 5.8 | +L 133.6 ± 5.3 +V 131.2 ± 5.5 |
157.9 ± 9.7 | 143.8 ± 5.6 | 138.1 ± 5.3 135.8 ± 5.5 |
139.9 ± 9.3 | 128.8 ± 7.9 | +L 124.0 ± 7.2 +V 121.1 ± 7.3 |
| P (ANOVA-between treatment) | <0.01 | <0.01 | <0.01 | ||||||
| P (ANOVA-vs baseline) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| DBP, mmHg | 91.5 ± 4.2 | 82.2 ± 5.2 | +L 78.0 ± 5.8 +V 75.7 ± 5.9 |
95.2 ± 4.2 | 85.5 ± 5.2 | +L 81.1 ± 5.8 +V 78.9 ± 6.0 |
81.7 ± 5.8 | 74.4 ± 6.6 | +L 70.7 ± 6.7 +V 68.0 ± 6.8 |
| P (ANOVA-between treatment) | <0.01 | <0.01 | <0.01 | ||||||
| P (ANOVA-vs baseline) | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||
| Heart rate, beats/min | 70.7 ± 6.8 | 71.1 ± 6.1 | +L 70.7 ± 5.8 +V 70.5 ± 6.1 |
73.6 ± 7.4 | 74.2 ± 7.1 | +L 73.8 ± 7.1 +V 73.6 ± 6.9 |
64.2 ± 5.5 | 65.1 ± 5.6 | +L 64.6 ± 5.1 +V 64.2 ± 5.1 |
| P (ANOVA-between treatment) | 0.201 | 0.384 | 0.424 | ||||||
Abbreviations: L, losartan; V, valsartan.
Figure 1.
Mean ambulatory SBP and DBP reduction induced by addition of valsartan or losartan to amlodipine in the randomized patients who completed the study (n = 166).
Figure 2.
Twenty-four-hour SBP and DBP after treatment with monotherapy (4 weeks) and after losartan or valsartan added to amlodipine (8 weeks) in the randomized patients who completed the study (n = 166).
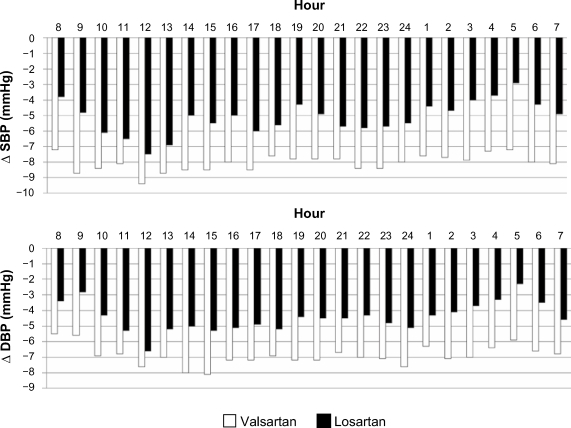
Analysis of hourly profiles also confirmed that the BP reduction attained with the addition of valsartan to amlodipine was greater than that attained with the addition of losartan to amlodipine, particularly at nighttime (Figure 3). The T/P ratio computed at the end of combination treatments was above the threshold of 0.5, which is universally regarded as clinically acceptable, however, it was significantly higher with the valsartan/amlodipine combination than with the losartan/amlodipine combination (P < 0.05 for both SBP and DBP; Table 3). As with the T/P ratio, the average SI was higher with the valsartan/amlodipine combination than with the losartan/amlodipine combination (P < 0.05 for SBP, P < 0.01 for DBP; Table 3). Clinical BP data (Table 4) showed a significant reduction in SBP/DBP levels with amlodipine monotherapy compared with baseline; this reduction was greater after combination treatments. Again, compared with amlodipine monotherapy, the changes in SBP and DBP values were significantly greater when valsartan was added to amlodipine than when losartan was added to amlodipine.
Figure 3.
Mean differences from monotherapy in hourly SBP and DBP values after 4 weeks of losartan or valsartan added to amlodipine in the randomized patients who completed the study (n = 166).
Table 3.
Mean values of trough/peak ratio and smoothed index after 4 weeks of treatment with losartan 100 mg/amlodipine 5 mg and valsartan 160 mg/amlodipine 5 mg
| Losartan/amlodipine | Valsartan/amlodipine | P | |
|---|---|---|---|
| Trough/peak ratio | |||
| SBP | 0.58 ± 0.24 | 0.65 ± 0.26 | 0.014 |
| DBP | 0.60 ± 0.22 | 0.66 ± 0.23 | 0.015 |
| Smoothed index | |||
| SBP | 2.29 ± 1.21 | 2.62 ± 1.21 | 0.014 |
| DBP | 2.15 ± 0.85 | 2.55 ± 0.97 | <0.01 |
Table 4.
Mean ± SD clinical blood pressure and heart rate at baseline, after amlodipine monotherapy and after its combination with losartan or valsartan in the randomized patients who completed the study (n = 166)
| Baseline | Amlodipine | Losartan/amlodipine | Valsartan/amlodipine | |
|---|---|---|---|---|
| SBP mmHg | 169.5 ± 13.0 | 154.2 ± 6.5 | 148.2 ± 6.2 | 145.7 ± 6.1* |
| DBP mmHg | 103.9 ± 6.8 | 94.1 ± 5.2 | 88.7 ± 4.8 | 84.4 ± 4.7* |
| Heart rate, beats/min | 75.7 ± 7.1 | 76.6 ± 7.2 | 75.9 ± 7.1 | 75.8 ± 6.9 |
P < 0.05 vs losartan/amlodipine.
The rate of adverse events (9% with losartan/amlodipine and 8% with valsartan/amlodipine) was not significantly different between the 2 combinations, but was lower than the rate observed with amlodipine monotherapy (17%, P < 0.05). The most frequent adverse events were ankle oedema (5.9% with losartan/amlodipine, 5.4% with valsartan/amlodipine and 12.6% with amlodipine alone), headache (2.1%, 2.6% and 3.6% respectively), flushing (0.5%, 0.5% and 0.9%) and constipation (0.5%, 0.5% and 0.4%). No serious adverse event was observed.
Discussion
The result of the present study indicate that, in patients with moderate hypertension, combination therapy with losartan 100 mg/amlodipine 5 mg or valsartan 160 mg/amlodipine 5 mg provides a clinically meaningful antihypertensive effect that is better than that attained with amlodipine monotherapy. This is consistent with findings from previous studies showing that the addition of losartan22–23 or valsartan24,25 enhances the efficacy of amlodipine.
However, the BP decrease resulting from the addition of valsartan to amlodipine was significantly greater than that observed when losartan was added to amlodipine. This was true for both SBP and DBP 24-hour mean values, as well as for daytime and nighttime mean values.
Such a difference in efficacy could be due to the different pharmacologic effect of the ARBs because of their different chemical structures and their different pharmacokinetic profiles. Losartan is an imidazole-derivative with a biphenyltetrazole side chain, while valsartan is a tetrazole-biphenyl-valine derivative and features only one heterocyclic structure; also, valsartan is an immediately active drug, whereas losartan needs to be converted into a more-active metabolite; and lastly losartan is metabolized while valsartan is excreted unchanged.26 The concept of the differing pharmacologic effects is supported by the demonstration that, in humans, the BP dose-response curves to exogenous angiotensin II with losartan pretreatment show a significant rightward shift only 4 hours after drug ingestion, while, with valsartan pretreatment, the dose-response curves show a significant rightward shift both 4 and 24 hours after drug ingestion.27
No pharmacokinetic interactions have been demonstrated when amlodipine was administrated with valsartan.22 No data are available on the pharmacokinetics of the losartan/amlodipine combination.
Clinical BP measurements confirmed that the antihypertensive effect of the valsartan/amlodipine combination was superior to that of the losartan/amlodipine combination, and that the difference (−2.5 mmHg for SBP and −2 mmHg for DBP) was due to the greater add-on effect of valsartan compared with losartan.
Because a continuous and graded relationship exists between BP values and cardiovascular risk, lower BP values are associated with better outcomes in a broad range of patients.28,29 Therefore, from a clinical point of view, even a moderate decrease in BP has the potential to significantly reduce hypertension-related morbidity and mortality, particularly in high-risk patients. Thus, for example, in the VALUE study30 apparentely minor differences in BP levels between amlodipine- and valsartan-treated patients resulted in a significantly different frequency of outcomes in hypertensive patients at high cardiovascular risk.
When the duration of hypotensive action was evaluated over 24 hours, the T/P ratios for SBP and DBP obtained with both the combinations given once daily fulfilled United States Food and Drug Administration guidelines for efficacy (T/P ratio > 50%); however, the ratio was greater with valsartan/amlodipine than with losartan/amlodipine. The SI, which provides information about the homogeneity of the antihypertensive effect,20,21 also showed significantly higher values for both SBD and DBP with the valsartan/amlodipine combination. Greater T/P ratios and SI values reflect less variability in BP, which has been demonstrated to have an independent effect on organ damage and disease prognosis.31
The losartan/amlodipine and valsartan/amlodipine combinations also were well tolerated, with a comparable incidence of adverse events between the 2 treatments, and these incidence rates were lower than the rate observed with amlodipine monotherapy. Most adverse events were of mild or moderate intensity. This is consistent with the proven tolerability profiles of ARBs when administered alone or in combination with CCBs.
Conclusion
In spite of study limitations due to the open study design and the relatively short duration of treatment, the findings of the present study indicate that the addition of valsartan 160 mg to amlodipine 5 mg produces greater ambulatory and clinic BP reductions than the addition of losartan 100 mg to the same dose of amlodipine, and this outcome probably reflects the different pharmacodynamic profiles of the 2 ARBs. These results suggest that — at least when this low dose of amlodipine is used — combination with valsartan might offer some advantage in terms of better BP response, which is of clinical relevance in high-risk hypertensive patients.
Acknowledgments and disclosures
The authors wish to thank Oxford PharmaGenesis Inc., for providing editorial support in the development of this manuscript. Editorial support was funded by Novartis Pharma AG, Basel Switzerland. The authors had full control of the contents of the manuscript.
References
- 1.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure: the JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 2.European Society of Hypertension-European Society of Cardiology Guidelines Committee 2003 European Society of Hypertension/European Society of Cardiology guidelines for the management of arterial hypertension. J Hypertens. 2003;21:1011–1053. doi: 10.1097/00004872-200306000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Turnbull E. Effects of different blood-pressure lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 4.Staessen JA, Wang JG, Thijs L. Cardiovascular prevention and blood pressure reduction: a quantitative overview updated until March 1, 2003. J Hypertens. 2003;21:1055–1076. doi: 10.1097/00004872-200306000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Hansson L, Zanchetti A, Carruthers SG, et al. for the HOT study group Effects of intensive blood pressure lowering and low-dose aspirin in patients with hypertension: principal results of the hypertension optimal treatment (HOT) randomised trial. Lancet. 1998;351:1755–1762. doi: 10.1016/s0140-6736(98)04311-6. [DOI] [PubMed] [Google Scholar]
- 6.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 7.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G, for the Heart Outcomes Prevention Evaluation Study Investigators Effects of an angiotensin converting enzyme inhibitor, ramipril, on cardiovascular events in high risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 8.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group Major outcomes in high-risk patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 9.Moser M, Black HR. The role of combination therapy in the treatment of hypertension. Am J Hypertens. 1998;11:73S–78S. doi: 10.1016/s0895-7061(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 10.Sica DA. Rationale for fixed dose combinations in the treatment of hypertension: the cycle repeats. Drugs. 2002;62:443–462. doi: 10.2165/00003495-200262030-00003. [DOI] [PubMed] [Google Scholar]
- 11.Mancia G, De Backer G, Dominiczak A, et al. for the Management of Arterial Hypertension of the European Society of Hypertension; European Society of Cardiology Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J Hypertens. 2007;2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. Erratum in: J Hypertens. 2007;25:1749. [DOI] [PubMed] [Google Scholar]
- 12.Kjeldsen SE, Aksnes TA, de la Sierra A, et al. Amlodipine and valsartan: calcium channel blockers/angiotensin II receptor blockers combination for hypertension. Therapy. 2007;4:31–40. [Google Scholar]
- 13.Dagenais NJ, Jamali F. Protective effects of angiotensin II interruption: evidence for antiinflammatory actions. Pharmacotherapy. 2005;25:1213–1229. doi: 10.1592/phco.2005.25.9.1213. [DOI] [PubMed] [Google Scholar]
- 14.Bakris GL. Combined therapy with a calcium channel blocker and an angiotensin II type 1 receptor blocker. J Clin Hypertens (Greenwich) 2008;10(1 Suppl 1):27–32. doi: 10.1111/j.1524-6175.2007.08029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogari R, Zoppi A, Derosa G, et al. Effect of valsartan addition to amlodipine on ankle oedema and subcutaneous tissue pressure in hypertensive patients. J Hum Hypertens. 2007;21:220–224. doi: 10.1038/sj.jhh.1002140. [DOI] [PubMed] [Google Scholar]
- 16.Philipp T, Smith TR, Glazer R, et al. Two multicenter, 8-week, randomized, double-blind, placebo-controlled, parallel-group studies evaluating the efficacy and tolerability of amlodipine and valsartan in combination and as monotherapy in adult patients with mild to moderate essential hypertension. Clin Ther. 2007;29:563–580. doi: 10.1016/j.clinthera.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Hansson L, Hedner T, Dahlöf B. Prospective randomized open blinded end-point (PROBE) study. A novel design for intervention trials. Blood Press. 1992;1:113–119. doi: 10.3109/08037059209077502. [DOI] [PubMed] [Google Scholar]
- 18.Parati G, Bosi S, Castellano M. Guidelines for 24 hour non invasive ambulatory blood pressure monitoring: report from a working group of the Italian Society of Hypertension. High Blood Press. 1995;4:168–174. [Google Scholar]
- 19.Omboni S, Parati G, Zanchetti A, Mancia G. Circulation of trough:peak ratio of antihypertensive treatment from ambulatory blood pressure: methodological aspects. J Hypertens. 1995;13:1105–1112. doi: 10.1097/00004872-199510000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Rizzoni D, Castellano M, Muiesan ML, Porteri E, Agabiti Rosei E. Beyond trough:peak ratio. A new index of the smoothness of the anti-hypertensive effect of a drug. High Blood Press. 1997;6:110–115. [Google Scholar]
- 21.Parati G, Omboni S, Tizzoni D, Agabiti Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens. 1998;16:1685–1691. doi: 10.1097/00004872-199816110-00016. [DOI] [PubMed] [Google Scholar]
- 22.Gokhale N, Shahani S, Pawar D. Efficacy and safety of losartan-amlodipine combination – an Indian postmarketing surveillance experience. J Indian Med Assoc. 2002;100(3):207–208. [PubMed] [Google Scholar]
- 23.Kohlmann O, Jr, Oigman W, Mion D, Jr, et al. The “LOTHAR” study: evaluation of efficacy and tolerability of the fixed combination of amlodipine and losartan in the treatment of essential hypertension. Arq Bras Cardiol. 2006;86:39–51. doi: 10.1590/s0066-782x2006000100007. [DOI] [PubMed] [Google Scholar]
- 24.Allemann Y, Fraile B, Lambert M, Barbier M, Ferber P, Izzo JL., Jr Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX-FAST) study. J Clin Hypertens (Greenwich) 2008;10:185–194. doi: 10.1111/j.1751-7176.2008.07516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greg L, Plosker GL, Robinson DM. Amlodipine/valsartan: fixed-dose combination in hypertension. Drugs. 2008;68:373–281. doi: 10.2165/00003495-200868030-00008. [DOI] [PubMed] [Google Scholar]
- 26.Chung O, Csikós T, Unger T. Angiotensin II receptor pharmacology and AT1-receptor blockers. J Hum Hypertens. 1999;13(Suppl 1):S11–20. doi: 10.1038/sj.jhh.1000744. [DOI] [PubMed] [Google Scholar]
- 27.Mazzolai L, Maillard M, Rossat J, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade in normotensive subjects: A direct comparison of three AT1 receptor antagonists. Hypertension. 1999;33:850–855. doi: 10.1161/01.hyp.33.3.850. [DOI] [PubMed] [Google Scholar]
- 28.Stamler R. Implications of the INTERALSALT. Study. J Hypertens. 1991;17(Suppl 1):116–120. [Google Scholar]
- 29.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, for the Prospective Studies Collaboration Age-specific relevance of usual blood pressure to vascular mortality. A meta analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 30.Julius S, Kjeldsen SE, Weber M, et al. for the VALUE trial Group Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or anlodipine:the value randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 31.Palatini P, Penzo M, Racioppa A, et al. Clinical relevance of nighttime blood pressure and of daytime blood pressure variability. Arch Intern Med. 1992;152:1855–1860. [PubMed] [Google Scholar]