Abstract
Purpose
The cancer/germline antigen NY-ESO-1 is variably expressed in epithelial ovarian cancer (EOC), with most tumors showing low or heterogeneous expression, which limits patient responses to NY-ESO-1 vaccine therapy. We tested the hypothesis that promoter and global genomic DNA methylation status correlates with inter- and intra-tumor NY-ESO-1 expression status in EOC.
Experimental Design
We utilized 78 EOC tumors and 10 normal ovary controls for quantitative DNA methylation analyses and NY-ESO-1 expression analysis by immunohistochemistry (IHC) and quantitative reverse transcriptase PCR (qRT-PCR). A subset of EOC tumors were used to perform microdissections of NY-ESO-1 IHC-positive and NY-ESO-1 IHC-negative tissue regions, followed by DNA methylation analyses. EOC cell lines were treated in vitro with decitabine to determine the functional contribution of DNA methylation to NY-ESO-1 gene regulation in EOC.
Results
Compared to normal ovary, bulk EOC tissues display increased NY-ESO-1 expression, reduced NY-ESO-1 promoter methylation, and reduced LINE-1 DNA methylation. However, NY-ESO-1 expression is not significantly associated with NY-ESO-1 promoter methylation status in bulk tumors. We hypothesized that this resulted from heterogeneous intra-tumor NY-ESO-1 expression. Supporting this idea, experiments using microdissected material revealed that inter- and intra- tumor NY-ESO-1 expression heterogeneity is significantly correlated with promoter and global DNA methylation status in EOC. Moreover, decitabine treatment functionally restored NY-ESO-1 expression in non-expressing EOC cell lines.
Conclusion
DNA methylation status is associated with both inter- and intra- tumor NY-ESO-1 expression status in EOC. These findings support a novel chemo-immunotherapy approach using decitabine to augment NY-ESO-1 vaccine therapy for treatment of recurrent EOC.
Keywords: DNA methylation, Cancer germline antigens, Cancer testis antigens, decitabine, ovarian cancer, NY-ESO-1
Introduction
DNA methylation changes are recognized as a key oncogenic mechanism. Abnormalities include CpG island promoter hypermethylation, which silences tumor suppressor genes, and global genomic DNA hypomethylation, which is associated with genomic instability (1-3). In addition, a specific class of genes, known as Cancer-testis (CT) or Cancer-germline (CG) antigens, undergoes promoter hypomethylation and gene activation in a variety of cancers (4, 5). Interestingly, hypomethylation of CG antigen genes may also be linked to global genomic DNA hypomethylation in cancer (6).
Recent studies have established promoter methylation and histone H3 tail modifications as important determinants of CG antigen gene expression status in human cancer cells (7-9). In human tissues, DNA methylation inversely correlates with CG antigen gene expression (5). A key characteristic of CG antigen gene expression in vivo is that it is heterogeneous both across the tumor population and within individual tumors (4). This finding is clinically relevant, as cell and antibody-mediated responses to CG antigen-targeted vaccines are dependent on host antigen presentation (10). One previous study has addressed the mechanism of intra-tumor heterogeneous CG antigen gene expression (11). This study found that CG antigen genes display a heterogeneous expression pattern in clonal cell lines established from a melanoma tumor, and observed that MAGE-A3 expression status in the isolated cell lines is associated with promoter DNA methylation levels (11). These data suggest that DNA methylation can regulate intra-tumor heterogeneity of CG antigens; however, this conclusion is tentative, as DNA methylation changes are known to occur during the in vitro cultivation of cell lines (12). Furthermore, only one melanoma tumor was used to derive the cell lines in this study, precluding significance testing (11).
Of CG antigens under clinical investigation, NY-ESO-1 appears to be the most immunogenic and clinically important (13). NY-ESO-1 is aberrantly expressed in several human malignancies, including epithelial ovarian (EOC) cancer (14, 15). In EOC, we previously reported that NY-ESO-1 was expressed in approximately 40% of tumors, while 30% of patients expressing the antigen display circulating antibodies against NY-ESO-1 (15). Another key finding was that a sizable majority of NY-ESO-1 positive EOC tumors display either focal or heterogeneous expression of the antigen (15). This is consistent with the results of IHC studies of other CG antigens, including MAGE-A1 and MAGE-A3 (4). Thus, it is likely that tumor heterogeneity presents a critical restriction to both immunological and clinical responses to CG antigen-directed vaccines.
In a recent Phase I clinical trial in EOC using vaccination of the NY-ESO-1 peptide epitope ESO157-170, we found that repeated vaccination of EOC patients in remission led to integrated humoral and T cell responses, as well as encouraging clinical outcomes (10). However, only patients with NY-ESO-1 positive tumors are eligible for NY-ESO-1 vaccine therapy, limiting its general utility. In addition, antigen loss occurred in subset of vaccinated patients, which correlated with disease progression despite induction of immune responses (10). These data suggest that antigen expression is a key aspect of clinical responses to NY-ESO-1 vaccines. To investigate the mechanism underlying NY-ESO-1 tumor heterogeneity in EOC, we quantitatively measured NY-ESO-1 promoter methylation and global genomic DNA methylation within microdissected regions of individual EOC tumors showing variable NY-ESO-1 expression in vivo. Our findings establish DNA methylation as a critical mechanism associated with heterogeneous inter- and intra-tumor NY-ESO-1 expression. Furthermore, they direct a novel chemo-immunotherapy approach for the treatment of recurrent EOC.
Materials and Methods
Human tissue samples
Normal ovary (NO) and epithelial ovarian cancer (EOC) tissue samples were obtained from patients undergoing surgical resection at Roswell Park Cancer Institute under Institutional Review Board approved protocols. Of the 78 total EOC samples obtained, 62 and 77 yielded high quality RNA or DNA for downstream analyses, respectively. Flash-frozen bulk tumor tissues were homogenized using an electric homogenizer with disposable microtube pestles, and RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA). Two μg of each RNA sample were converted to cDNA using random oligo-dT primer (Fermentas, Hanover, MD) and M-MuLV Reverse Transcriptase enzyme (Fermentas). For genomic DNA extractions, flash-frozen tissue samples were crushed using mortar and pestles pre-chilled with liquid nitrogen. Upon addition of lysis buffer (Gentra Systems, Minneapolis, MN), tissues were further homogenized with an electric homogenizer, and genomic DNAs were isolated using the Puregene DNA isolation kit (Gentra Systems).
NY-ESO-1 immunohistochemical (IHC) staining and microdissection of paraffin-embedded tissue samples
IHC for NY-ESO-1 was performed as previously described (15). Microdissection of paraffin tissue blocks of EOC exhibiting homogenous NY-ESO-1 positive and homogenous NY-ESO-1 negative expression (as determined by IHC) was performed using manual dissection. Afterwards, two or more 5μM paraffin curls were cut and used for genomic DNA isolations. Microdissection of paraffin tissue blocks of EOC tumors displaying heterogeneous NY-ESO-1 staining was performed by isolating (as seen by IHC) and re-embedding positively and negatively-stained tumor regions into different blocks, after which 5μM curls were prepared from each of the new blocks. In all cases, microdissected tissues were verified to contain virtually 100% tumor cells by a board-certified gynecological pathologist (P.M-F.) Genomic DNAs were purified from two or more 5μM curls of each sample using the Puregene DNA isolation kit (Gentra Systems).
Quantitative real-time reverse transcriptase PCR (qRT-PCR)
qRT-PCR for NY-ESO-1 was accomplished as described previously (7).
Sodium bisulfite DNA sequencing
Sodium bisulfite sequencing of NY-ESO-1 was accomplished as described previously (7).
Quantitative Bisulfite-Pyrosequencing
The methylation status of the NY-ESO-1 promoter region was analyzed using quantitative pyrosequencing of sodium bisulfite converted DNA (16). Primers are reported in Supplemental Table 1. PCR cycling conditions were 95°C for 30 seconds, 55.7°C for 30 seconds, and 72°C for 1 minute, for 45 cycles. The resulting biotinylated PCR product was bound to Streptavidin Sepharose High Performance beads (Amersham Biosciences, Uppsala, Sweden), and the immobilized PCR product was purified using Pyrosequencing Vacuum Prep Tool (Biotage AB, Uppsala, Sweden), denatured with 0.2 M NaOH, and washed using Tris, pH 7.6. Pyrosequencing of the purified single-stranded PCR product was accomplished using the PSQ HS96 Pyrosequencing System (Biotage AB). Non-CpG cytosines served as internal controls to verify efficient sodium bisulfite DNA conversion, and unmethylated and methylated DNAs were also run as controls. Pyrosequencing was performed on duplicate samples, and pyrosequencing assays were performed a minimum of two times.
Global genomic DNA methylation analysis
5-methyl-deoxycytidine (5mdC) levels were determined as described previously (17). Pyrosequencing of LINE-1 was performed as described above, and the primers are reported in Supplemental Table 1.
Cell lines and drug treatments
Ovarian cancer cell lines A2780, OVCAR3, and OVCAR429 were grown in DMEM supplemented with 10% FBS, 0.5% Pen-Strep, and 2mM L-glutamine, while SKOV3 was grown in McCoy's media supplemented with 10% FBS, 0.5% Pen-Strep, 2 mM L-glutamine, and 1mM sodium pyruvate. IOSE121 cells (SV40 immortalized normal human ovarian surface epithelium cells) were grown in a 1:1 mix of 199 and MCDB105 media containing 5% FBS and 50ug/ml gentamicin. Cell lines were treated with 1μM 5-aza-2′-deoxycytidine (decitabine) (Sigma Chemical Company, St. Louis, MO) once, and RNA and DNA samples were harvested 48 hours post-treatment for mRNA expression and DNA methylation analyses.
For IHC experiments of decitabine-treated ovarian cancer cells, OVCAR3 cells were treated with 2μM decitabine at time zero and again at 24 hours post-treatment and were harvested 72 hours post-treatment. Cell pellets (∼ 50 × 106 cells) were harvested and fixed in 10% Neutral Buffered Formalin for 10 minutes, then processed and paraffin-embedded according to standard procedures. NY-ESO-1 IHC staining was performed as described previously (15).
Results
NY-ESO-1 expression and DNA methylation in bulk NO and EOC tissues
To investigate the relationship between NY-ESO-1 expression and DNA methylation in EOC, we obtained a set of flash frozen EOC tumor samples, the majority of which were of advanced stage and grade, in accordance with the typically late diagnosis of the disease (Table 1). For comparison to EOC, we obtained a set of normal ovary (NO) samples from patients undergoing hysterectomy who had no evidence of cancer. qRT-PCR analyses revealed a variable level of NY-ESO-1 expression in EOC, while NY-ESO-1 expression was uniformly low in NO (Fig. 1A). To quantitatively assess NY-ESO-1 promoter methylation status, we developed a bisulfite pyrosequencing assay that interrogated methylation over 15 CpGs contained within the 5′ CpG island of the NY-ESO-1 promoter region. This assay revealed NY-ESO-1 promoter hypermethylation in NO, and hypomethylation in many EOC samples (Fig. 1B). NY-ESO-1 promoter hypomethylation in EOC occurred at all CpG sites within the pyrosequenced region (Fig. 1C). Sodium bisulfite sequencing analysis was in agreement with the pyrosequencing data, and confirmed extensive NY-ESO-1 promoter hypomethylation in specific EOC tumors showing increased NY-ESO-1 expression (Fig. 1D).
Table 1.
| A. Tumor Stage (78 EOC samples). | ||
|---|---|---|
| FIGO1 Stage | # of tumors | % of tumors |
| IA | 4 | 5.1 |
| IB | 1 | 1.3 |
| IC | 2 | 2.6 |
| II | 2 | 2.6 |
| IIB | 1 | 1.3 |
| IIC | 1 | 1.3 |
| III | 11 | 14.1 |
| IIIB | 4 | 5.1 |
| IIIC | 39 | 50.0 |
| IV | 5 | 6.4 |
| N/d | 8 | 10.3 |
| B. Tumor Grade (78 EOC samples). | ||
| FIGO Grade | # of tumors | % of tumors |
| 1 | 2 | 2.6 |
| 2 | 7 | 9.0 |
| 3 | 59 | 75.6 |
| N/d | 10 | 12.8 |
International Federation of Gynecology and Obstetrics
N/d- no data
Fig. 1.
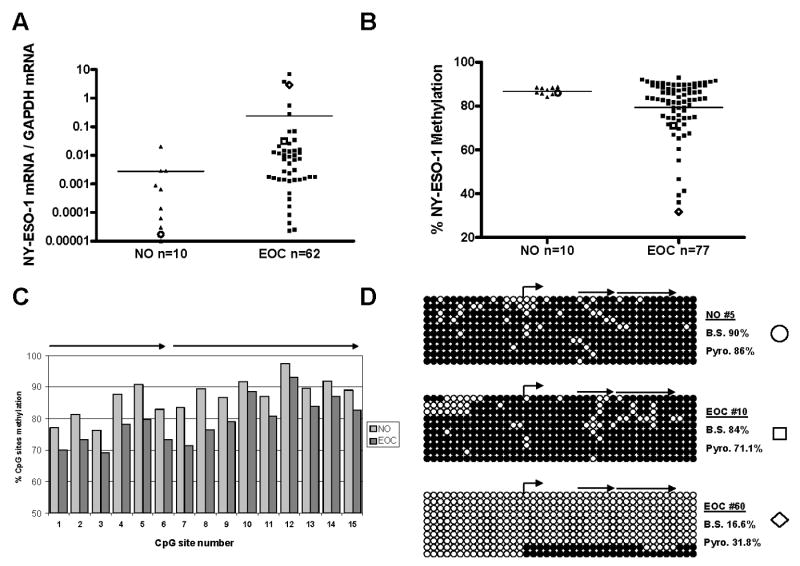
NY-ESO-1 expression and methylation in bulk normal ovary (NO) and EOC tissue samples. A, NY-ESO-1 mRNA expression was measured by qRT-PCR and normalized to GAPDH. Bars signify the mean values of the data points, and the data points indicated by open symbols correspond to the samples shown in panel D. For clarity, data points falling below the .00001 value were deemed insignificant and are not shown. The difference between the two groups was not statistically significant (unpaired, two-tailed t-test p=0.482), although many EOC tumors showed increased expression relative to NO. B, NY-ESO-1 promoter methylation was measured by quantitative bisulfite pyrosequencing. The plotted data represent the total methylation level of each sample, taking into account all 15 CpG sites analyzed. Bars signify the mean values of the data points, and the data points indicated by open symbols correspond to the samples shown in panel D. The site of the pyrosequenced region (two different primers) is shown in panel D. The difference between the two groups showed a trend towards, but did not reach, statistical significance (unpaired, two-tailed t-test p=0.089). C, Methylation levels at 15 individual CpG sites of the NY-ESO-1 promoter in NO and EOC were determined by quantitative pyrosequencing as described in B. The average methylation level of all samples in each group (NO or EOC) is plotted. The arrows indicate the regions sequenced by the two different pyrosequencing primers. D, Sodium bisulfite sequencing of the NY-ESO-1 promoter in NO and EOC. Right bent arrow indicates the transcriptional start site. Filled and open circles represent methylated and unmethylated CpG sites, respectively, and rows indicate individually sequenced alleles. The right arrows indicate the regions sequenced by the two different pyrosequencing primers (data plotted in panels B and C). Comparison of the methylation percentages obtained from bisulfite sequencing (B.S.) (for the 15 pyrosequenced CpG sites only) to those obtained from pyrosequencing (Pyro.) is shown. The symbols shown on the right of each bisulfite-sequenced sample demarcates the identity of each sample in panels A and B, as well as in Fig. 2.
As CG antigen gene expression may also be linked to genome-wide DNA hypomethylation (6), we developed and utilized two quantitative assays to follow global genomic DNA methylation, a bisulfite pyrosequencing assay for the LINE-1 repetitive DNA element, and a liquid chromatography mass spectrometry (LC-MS) method to measure total 5-methyl-deoxycytidine (5mdC) in hydrolyzed genomic DNA (17). As compared to NO, there was extensive hypomethylation of LINE-1 in EOC, but a lack of a similar effect for 5mdC, suggesting that LINE-1 methylation does not accurately predict global genomic methylation levels in this instance (Fig. 2). However, a significant increase in the variability of 5mdC levels in EOC, compared to NO, was apparent (Fig. 2B). These data suggest that loss of NY-ESO-1 promoter methylation and hypomethylation of LINE-1 are common occurrences in late stage EOC tumors, similar to other repetitive DNA elements (18).
Fig. 2.
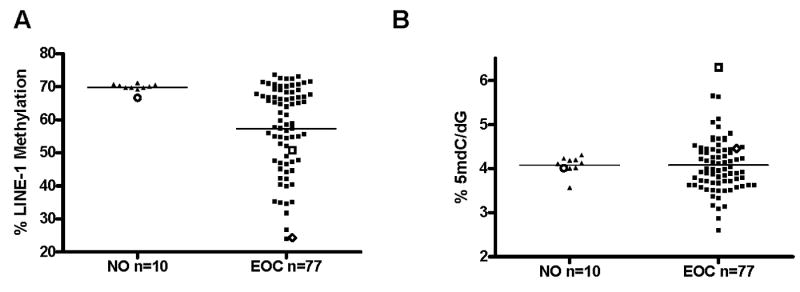
Global genomic DNA methylation in bulk NO and EOC tissue samples. A, LINE-1 repetitive element methylation was measured by quantitative bisulfite pyrosequencing. Bars signify the mean values of the data points, and the data points indicated by open symbols correspond to the samples shown in Fig. 1D. The difference between the two groups was statistically significant (unpaired, two-tailed t-test p=0.0014). B, Total genomic 5-methyl-2′-deoxycytidine (5mdC) levels were measured by LC-MS. Bars signify the mean values of the data points, and the data points indicated by open symbols correspond to the samples shown in Fig. 1D. The difference between the two groups was not significant (unpaired, two-tailed t-test p=0.7827), but sample variance was significantly greater in EOC (F test, p=0.0019).
NY-ESO-1 expression and DNA methylation in microdissected homogenously stained EOC tissues
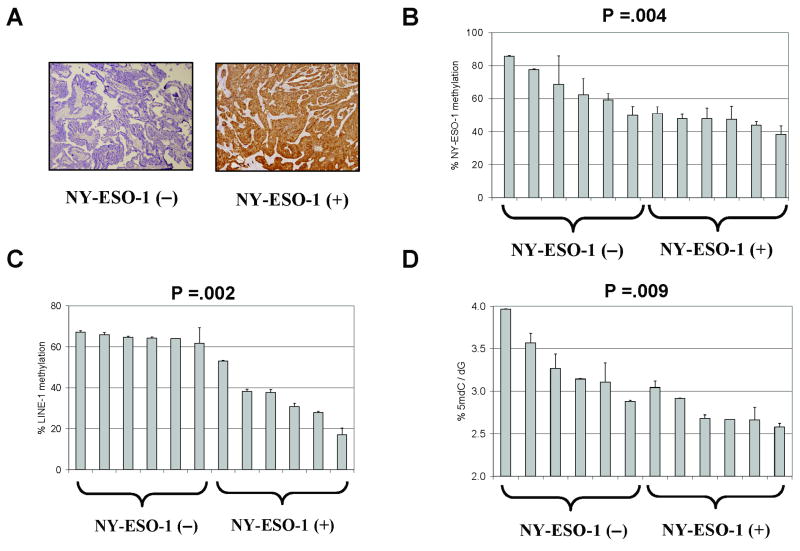
Despite the fact that increased NY-ESO-1 expression and reduced NY-ESO-1 promoter methylation occur in EOC relative to NO (Fig. 1), there was not a statistically significant association between these two parameters in EOC (Kendall's Tau, correlation = −. 0556; P =.536; n=63). We hypothesized that this may reflect, in part, intra-tumor NY-ESO-1 expression heterogeneity, which we have previously shown by IHC is frequent in EOC (15). Additionally, it is possible that the presence of other cell types, e.g. stromal, immune cells, and endothelium, in the bulk EOC tissue samples contributes to this heterogeneity. To test this hypothesis, we developed a novel approach in which we microdissected NY-ESO-1 positive and negative EOC tumor regions (as determined by IHC), and used this material to obtain genomic DNA for DNA methylation analyses (see Materials and Methods). As an initial test, we selected 12 homogeneously NY-ESO-1 IHC-staining EOC tumor specimens for analysis of DNA methylation parameters. Specifically, we obtained six tumors negative for NY-ESO-1 expression and six additional tumors showing homogeneous NY-ESO-1 staining throughout the tumor (Fig. 3A). Pathological analysis indicated that virtually all of the microdissected cells obtained were tumor in origin. Notably, both NY-ESO-1 promoter methylation, as well as both measures of global genomic DNA methylation, showed significant hypomethylation in the NY-ESO-1 positive tissues (Fig. 3B-D). Despite repeated attempts, we were unable to obtain RNA suitable for NY-ESO-1 gene expression analysis from paraffin-embedded tissues (data not shown). However, our previous data have shown a significant correlation between NY-ESO-1 mRNA expression and NY-ESO-1 IHC staining (15).
Fig. 3.
NY-ESO-1 regulation in microdissected homogenously stained EOC tissue samples. A, Representative NY-ESO-1 IHC staining in EOC tumor samples negative for NY-ESO-1 (left), or uniformly positive for NY-ESO-1 (right). B, NY-ESO-1 promoter methylation was determined by quantitative bisulfite pyrosequencing in a series of 12 EOC tumors either negative or uniformly positive for NY-ESO-1 expression by IHC. The plotted data represent the total methylation level of each sample, taking into account all 15 CpG sites analyzed. C, LINE-1 methylation was determined by quantitative bisulfite pyrosequencing using the samples described in B. D, Total genomic 5mdC levels were measured by LC-MS using the samples described in B. In panels B-D, the P-values show the results of significance testing of the difference between the NY-ESO-1 negative (−) and positive (+) tumors, using the Exact Wilcoxon Rank Sum Test. Error bars display + 1 SEM.
NY-ESO-1 expression and DNA methylation in microdissected heterogenously stained EOC tissues
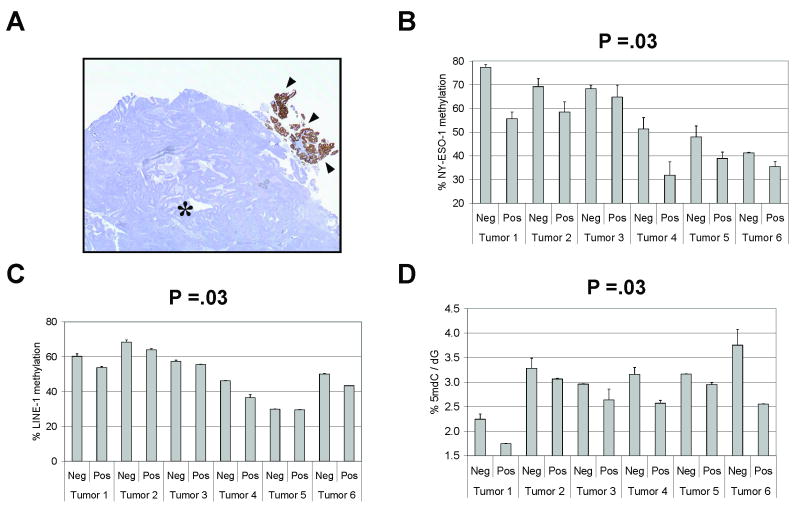
We next utilized the experimental approach described above to examine the basis for intra-tumor NY-ESO-1 expression heterogeneity in EOC. We performed microdissections of both the NY-ESO-1 positive and NY-ESO-1 negative IHC-stained areas from a set of six focal or heterogeneously NY-ESO-1 stained tumors (Fig. 4A). Again, pathological analyses confirmed that virtually all microdissected cells were tumor in origin. The resulting microdissected tissues were utilized for promoter-specific and global genomic DNA methylation analyses. Notably, in each instance, the NY-ESO-1 positive stained region of the tumor displayed NY-ESO-1 promoter hypomethylation as compared to the NY-ESO-1 negative regions of the same tumor (Fig. 4B). This trend held true despite significant variability in the absolute level of NY-ESO-1 promoter methylation in different tumors (Fig. 4B). Notably, in 5/6 tumors examined, LINE-1 methylation levels were reduced in NY-ESO-1 positive regions, while all six tumors examined showed reduced 5mdC levels in NY-ESO-1 positive regions (Fig. 4C and D). For all three measures of DNA methylation, the difference between the NY-ESO-1 negative and positive regions in the six examined tumors was statistically significant (Fig. 4B-D).
Fig. 4.
NY-ESO-1 regulation in microdissected heterogeneously stained EOC tissue samples. A, Representative example of an EOC tumor sample showing intra-tumor heterogeneous NY-ESO-1 IHC staining. The asterisk marks the NY-ESO-1 negative region and the arrows mark the NY-ESO-1 positive region of the tumor. B, NY-ESO-1 promoter methylation was determined using quantitative pyrosequencing in NY-ESO-1 positive and negative IHC-stained regions of six different heterogeneously stained EOC tumors. The plotted data represent the total methylation level of each sample, taking into account all 15 CpG sites analyzed. C, LINE-1 methylation was determined by quantitative bisulfite pyrosequencing using the samples described in B. D, Total genomic 5mdC levels were measured by LC-MS using the samples described in B. In panels B-D the P-values show the results of significance testing of the difference between the NY-ESO-1 negative (−) and positive (+) regions of the six examined tumors, using the Exact Wilcoxon Signed Rank Test. Error bars display + 1 SEM.
DNA methylation actively represses NY-ESO-1 expression in EOC cell lines
To determine whether DNA methylation plays a functional role in NY-ESO-1 gene regulation in EOC, we treated four NY-ESO-1 negative EOC cell lines (OVCAR3, SKOV3, A2780, OVCAR429), as well as an NY-ESO-1 negative SV40-transformed surface ovarian epithelial cell line (IOSE121), with the classical DNMT inhibitor 5-aza-2′-deoxycytidine (decitabine), and measured NY-ESO-1 expression after treatment using qRT-PCR. In each cell line, decitabine treatment caused a robust increase of NY-ESO-1 expression (Fig. 5A). To confirm that decitabine treatment caused concurrent NY-ESO-1 promoter hypomethylation, we performed quantitative pyrosequencing and found that decitabine treatment causes a significant reduction in NY-ESO-1 promoter methylation in each cell type (Fig. 5B). As additional controls, we examined global methylation and found that both LINE-1 methylation and 5mdC levels were also reduced (Fig. 5C and D). To determine the proportion of cells in culture responding to this treatment, we measured NY-ESO-1 expression in decitabine-treated cell cultures using IHC (Supplemental Fig. 1). We find that decitabine treatment induces a previously non-expressing cell line (OVCAR3) to display a heterogeneous pattern of NY-ESO-1 expression (Supplemental Fig. 1). Taken together, these data reveal a functional link between DNA hypomethylation and NY-ESO-1 gene activation in EOC cells.
Fig. 5.
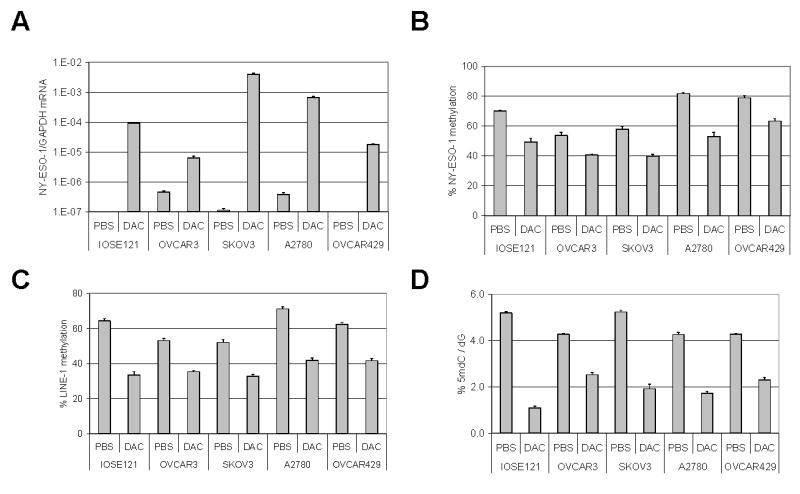
Decitabine-mediated induction of NY-ESO-1 in EOC cell lines. Cells were treated for 48 hours with 1μM decitabine or PBS (vehicle control), and RNA and DNA were harvested for analyses. A, NY-ESO-1 expression was measured by qRT-PCR and normalized to GAPDH. B, NY-ESO-1 promoter methylation was measured by quantitative bisulfite pyrosequencing. The plotted data represent the total methylation level of each sample, taking into account all 15 CpG sites analyzed. C, LINE-1 repetitive element methylation was measured by quantitative bisulfite pyrosequencing. D, Total genomic 5mdC levels were measured by LC-MS. Error bars display + 1 SEM.
Discussion
NY-ESO-1 has emerged as one of the most promising antigen targets for cancer immunotherapy, including the immunotherapy of ovarian cancer (4, 13, 15, 19). However, two limitations of NY-ESO-1 specific immunotherapy have become apparent: 1) many tumors are negative for expression of the antigen, limiting patient enrollment, and 2) the vast majority of tumors showing NY-ESO-1 expression display either focal or heterogeneous staining, which limits immune and clinical responses to NY-ESO-1 vaccine therapy (10, 15). Here we demonstrate that promoter methylation regulates NY-ESO-1 expression heterogeneity in EOC. Microdissection of either homogenously or heterogeneously stained EOC tumors, followed by quantitative DNA methylation analysis on the isolated cell populations, revealed that promoter DNA hypomethylation is directly associated with NY-ESO-1 expression. This trend held true despite significant variability in the absolute value of methylation from tumor to tumor. These data imply that individual tumors may have distinct “set points” at which DNA hypomethylation triggers NY-ESO-1 expression, which is likely regulated by other endogenous factors, including the expression of sequence specific transcription factors such as Ets and Sp1 (20-22). In additional support of this idea, we show that treatment of a polyclonal ovarian cancer cell line with decitabine results in a heterogeneous NY-ESO-1 expression pattern, suggesting that other factors besides hypomethylation likely influence NY-ESO-1 expression.
To our knowledge, this study provides the first in vivo evidence that intra-tumor heterogeneity of gene expression in cancer is associated with promoter-specific and global DNA methylation status. In important earlier studies, Gonzalgo et al. demonstrated that differential p16INK4a expression in cell clones derived from a bladder tumor correlated with promoter methylation status, and Maio et al. reported a similar finding in the context of MAGE-A3 expression in melanoma (11, 23). More recently, Rastetter et al. have reported intra-tumor heterogeneity in the methylation and expression status of a number of tumor suppressor genes in melanoma (24). In the current study we provide important new information utilizing microdissection of different regions of individual primary EOC tumors. Our data reveal a direct link between promoter DNA hypomethylation and expression of a CG antigen, NY-ESO-1, in vivo. In addition, we demonstrate for the first time that intra-tumor epigenetic heterogeneity extends to markers of global methylation status. At present, the mechanism that leads to differential DNA methylation status within individual tumors is unknown, but it may be related to the simultaneous presence of stem cells and differentiated cells within individual tumors (4).
In a seminal study, Boon and colleagues reported that global genomic DNA hypomethylation was linked to MAGE-A1 expression in cancer cell lines (6). Here we show, using two distinct parameters of global methylation, that NY-ESO-1 expression and promoter methylation correlate with global DNA hypomethylation in vivo. In both homogenously and heterogeneously NY-ESO-1-stained EOC, LINE-1 hypomethylation and reduced 5mdC showed a significant association with NY-ESO-1 expression, as well as NY-ESO-1 promoter hypomethylation. These data suggest that NY-ESO-1 promoter hypomethylation is driven by a mechanism that also affects global genomic DNA methylation status. One potential mechanism could be the expression of catalytically inactive DNMT3b isoforms, which may lead to CG antigen and/or genomic DNA hypomethylation in human cancer cells (25). Another possible mechanism involves expression of the autosomal CG antigen gene BORIS/CTCFL, which induces CG-X antigen gene expression in certain cell types, although this finding appears to be inconsistent (26-28). Interestingly, BORIS is directly regulated by promoter methylation in EOC cell lines and tumors, and is expressed in a relatively high percentage of EOC lesions (29). Based on these data, it becomes relevant to determine whether BORIS induction contributes to the expression of other CG antigens, including NY-ESO-1, in EOC.
Using cell lines and pharmacological modulation of DNA methylation, we show that DNA methylation plays a causative role in NY-ESO-1 expression silencing in EOC. It is unlikely that decitabine-mediated NY-ESO-1 induction results from other effects of this drug, as we have previously shown that genetic targeting of DNA methylation also elicits NY-ESO-1 expression (7). Importantly, Schrump and colleagues have recently shown in a Phase I clinical trial that decitabine treatment induces NY-ESO-1 expression in tumor tissues and NY-ESO-1 specific antibodies in vivo (30). Taken together, these data suggest the use of DNA methyltransferase inhibitors to augment NY-ESO-1 vaccine therapy for the treatment of EOC. This strategy has a number of potential advantages over existing single agent NY-ESO-1 vaccine therapy. First, the use of DNMT inhibitors to induce antigen expression would broaden the patient population eligible for vaccine therapy. Second, promotion of more uniform NY-ESO-1 expression within residual metastatic disease would be expected to increase immunological responses to these cells. Third, we have recently observed an occurrence of NY-ESO-1 antigen loss following the completion of NY-ESO-1 vaccine therapy in EOC (10). If DNA methylation proves to be a mechanism for antigen loss in vivo, then DNMT inhibitors may be an effective way to combat this problem. Finally, the utility of epigenetic therapy for augmenting CG antigen vaccine efficacy is likely not limited to either NY-ESO-1 or EOC, but may have utility in the context of additional tumor antigens and cancer types (31, 32).
Supplementary Material
NY-ESO-1 IHC of decitabine-treated OVCAR3 cells. OVCAR3 cells were treated with 2μM decitabine or PBS (vehicle control), and cell pellets were harvested and processed for NY-ESO-1 IHC staining as described in the Materials and Methods. IgG1 served as an isotype-specific negative control for IHC.
Acknowledgments
We thank Dr. Jenny Black of RPCI for critical reading of the manuscript, and Smitha James, Amy Beck, and Bernadette Keitz of RPCI for excellent technical assistance. We thank Dr. Nelly Auersperg and the Canadian Ovarian Tissue Bank for IOSE121 cells and Dr. Ivan Still of RPCI for ovarian cancer cell lines. This work was supported by grants from the NCI (RO1CA11674) and the Roswell Park Alliance Foundation (to ARK), a Cancer Vaccine Collaborative Grant from the Cancer Research Institute/Ludwig Institute for Cancer Research (to KO), and NCI Center grant CA16056 (to RPCI). AWR was supported by NIH training grant 5T32CA009072.
Abbreviations
- EOC
epithelial ovarian cancer
- NO
normal ovary
- CG antigen
cancer germline antigen
- DNMT
DNA methyltransferase
- Decitabine
5-aza-2′-deoxycytidine
- qRT-PCR
quantitative reverse transcriptase PCR
References
- 1.Ehrlich M. DNA methylation in cancer: too much, but also too little. Oncogene. 2002;21:5400–13. doi: 10.1038/sj.onc.1205651. [DOI] [PubMed] [Google Scholar]
- 2.Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004;4:143–53. doi: 10.1038/nrc1279. [DOI] [PubMed] [Google Scholar]
- 3.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–92. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson AJ, Caballero OL, Jungbluth A, Chen YT, Old LJ. Cancer/testis antigens, gametogenesis and cancer. Nat Rev Cancer. 2005;5:615–25. doi: 10.1038/nrc1669. [DOI] [PubMed] [Google Scholar]
- 5.De Smet C, Lurquin C, Lethe B, Martelange V, Boon T. DNA methylation is the primary silencing mechanism for a set of germ line- and tumor-specific genes with a CpG-rich promoter. Mol Cell Biol. 1999;19:7327–35. doi: 10.1128/mcb.19.11.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Smet C, De Backer O, Faraoni I, Lurquin C, Brasseur F, Boon T. The activation of human gene MAGE-1 in tumor cells is correlated with genome-wide demethylation. Proc Natl Acad Sci U S A. 1996;93:7149–53. doi: 10.1073/pnas.93.14.7149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James SR, Link PA, Karpf AR. Epigenetic regulation of X-linked cancer/germline antigen genes by DNMT1 and DNMT3b. Oncogene. 2006;25:6975–85. doi: 10.1038/sj.onc.1209678. [DOI] [PubMed] [Google Scholar]
- 8.Loriot A, De Plaen E, Boon T, De Smet C. Transient down-regulation of DNMT1 methyltransferase leads to activation and stable hypomethylation of MAGE-A1 in melanoma cells. J Biol Chem. 2006;281:10118–26. doi: 10.1074/jbc.M510469200. [DOI] [PubMed] [Google Scholar]
- 9.Wischnewski F, Pantel K, Schwarzenbach H. Promoter demethylation and histone acetylation mediate gene expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells. Mol Cancer Res. 2006;4:339–49. doi: 10.1158/1541-7786.MCR-05-0229. [DOI] [PubMed] [Google Scholar]
- 10.Odunsi K, Qian F, Matsuzaki J, et al. Vaccination with an NY-ESO-1 peptide of HLA class I/II specificities induces integrated humoral and T cell responses in ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:12837–42. doi: 10.1073/pnas.0703342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sigalotti L, Fratta E, Coral S, et al. Intratumor heterogeneity of cancer/testis antigens expression in human cutaneous melanoma is methylation-regulated and functionally reverted by 5-aza-2′-deoxycytidine. Cancer Res. 2004;64:9167–71. doi: 10.1158/0008-5472.CAN-04-1442. [DOI] [PubMed] [Google Scholar]
- 12.Smiraglia DJ, Rush LJ, Fruhwald MC, et al. Excessive CpG island hypermethylation in cancer cell lines versus primary human malignancies. Hum Mol Genet. 2001;10:1413–9. doi: 10.1093/hmg/10.13.1413. [DOI] [PubMed] [Google Scholar]
- 13.Gnjatic S, Nishikawa H, Jungbluth AA, et al. NY-ESO-1: review of an immunogenic tumor antigen. Advances in Cancer Research. 2006;95:1–30. doi: 10.1016/S0065-230X(06)95001-5. [DOI] [PubMed] [Google Scholar]
- 14.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT. Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev. 2002;188:22–32. doi: 10.1034/j.1600-065x.2002.18803.x. [DOI] [PubMed] [Google Scholar]
- 15.Odunsi K, Jungbluth AA, Stockert E, et al. NY-ESO-1 and LAGE-1 cancer-testis antigens are potential targets for immunotherapy in epithelial ovarian cancer. Cancer Res. 2003;63:6076–83. [PubMed] [Google Scholar]
- 16.Colella S, Shen L, Baggerly KA, Issa JP, Krahe R. Sensitive and quantitative universal Pyrosequencing methylation analysis of CpG sites. Biotechniques. 2003;35:146–50. doi: 10.2144/03351md01. [DOI] [PubMed] [Google Scholar]
- 17.Song L, James SR, Kazim L, Karpf AR. Specific method for the determination of genomic DNA methylation by liquid chromatography-electrospray ionization tandem mass spectrometry. Anal Chem. 2005;77:504–10. doi: 10.1021/ac0489420. [DOI] [PubMed] [Google Scholar]
- 18.Widschwendter M, Jiang G, Woods C, et al. DNA hypomethylation and ovarian cancer biology. Cancer Research. 2004;64:4472–80. doi: 10.1158/0008-5472.CAN-04-0238. [DOI] [PubMed] [Google Scholar]
- 19.Lee L, Wang RF, Wang X, et al. NY-ESO-1 may be a potential target for lung cancer immunotherapy. Cancer J Sci Am. 1999;5:20–5. [PubMed] [Google Scholar]
- 20.Karpf AR, Lasek AW, Ririe TO, Hanks AN, Grossman D, Jones DA. Limited gene activation in tumor and normal epithelial cells treated with the DNA methyltransferase inhibitor 5-aza-2′-deoxycytidine. Mol Pharmacol. 2004;65:18–27. doi: 10.1124/mol.65.1.18. [DOI] [PubMed] [Google Scholar]
- 21.De Smet C, Courtois SJ, Faraoni I, et al. Involvement of two Ets binding sites in the transcriptional activation of the MAGE1 gene. Immunogenetics. 1995;42:282–90. doi: 10.1007/BF00176446. [DOI] [PubMed] [Google Scholar]
- 22.Kang Y, Hong JA, Chen GA, Nguyen DM, Schrump DS. Dynamic transcriptional regulatory complexes including BORIS, CTCF and Sp1 modulate NY-ESO-1 expression in lung cancer cells. Oncogene. 2007;26:4394–403. doi: 10.1038/sj.onc.1210218. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalgo ML, Hayashida T, Bender CM, et al. The role of DNA methylation in expression of the p19/p16 locus in human bladder cancer cell lines. Cancer Res. 1998;58:1245–52. [PubMed] [Google Scholar]
- 24.Rastetter M, Schagdarsurengin U, Lahtz C, et al. Frequent intra-tumoural heterogeneity of promoter hypermethylation in malignant melanoma. Histology & Histopathology. 2007;22:1005–15. doi: 10.14670/HH-22.1005. [DOI] [PubMed] [Google Scholar]
- 25.Ostler KR, Davis EM, Payne SL, et al. Cancer cells express aberrant DNMT3B transcripts encoding truncated proteins. Oncogene. 2007;26:5553–63. doi: 10.1038/sj.onc.1210351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kholmanskikh O, Loriot A, Brasseur F, De Plaen E, De Smet C. Expression of BORIS in melanoma: lack of association with MAGE-A1 activation. Int J Cancer. 2008;122:777–84. doi: 10.1002/ijc.23140. [DOI] [PubMed] [Google Scholar]
- 27.Vatolin S, Abdullaev Z, Pack SD, et al. Conditional expression of the CTCF-paralogous transcriptional factor BORIS in normal cells results in demethylation and derepression of MAGE-A1 and reactivation of other cancer-testis genes. Cancer Res. 2005;65:7751–62. doi: 10.1158/0008-5472.CAN-05-0858. [DOI] [PubMed] [Google Scholar]
- 28.Hong JA, Kang Y, Abdullaev Z, et al. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–74. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- 29.Woloszynska-Read A, James SR, Link PA, Yu J, Odunsi K, Karpf AR. DNA methylation-dependent regulation of BORIS/CTCFL expression in ovarian cancer. Cancer Immun. 2007;7:21. [PMC free article] [PubMed] [Google Scholar]
- 30.Schrump DS, Fischette MR, Nguyen DM, et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res. 2006;12:5777–85. doi: 10.1158/1078-0432.CCR-06-0669. [DOI] [PubMed] [Google Scholar]
- 31.Maio M, Coral S, Fratta E, Altomonte M, Sigalotti L. Epigenetic targets for immune intervention in human malignancies. Oncogene. 2003;22:6484–8. doi: 10.1038/sj.onc.1206956. [DOI] [PubMed] [Google Scholar]
- 32.Karpf AR. A potential role for epigenetic modulatory drugs in the enhancement of cancer/germ-line antigen vaccine efficacy. Epigenetics. 2006;1:116–20. doi: 10.4161/epi.1.3.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NY-ESO-1 IHC of decitabine-treated OVCAR3 cells. OVCAR3 cells were treated with 2μM decitabine or PBS (vehicle control), and cell pellets were harvested and processed for NY-ESO-1 IHC staining as described in the Materials and Methods. IgG1 served as an isotype-specific negative control for IHC.