Abstract
The locus coeruleus (LC)-norepinephrine system is a target of both cannabinoid and opioid actions. The present study investigated the anatomical distribution of cannabinoid-1 receptors (CB1r) in the LC and its association with mu-opioid receptors (MOR). Immunoreactivity for CB1r was localized to pre- and postsynaptic cellular profiles in the LC, 82% of which were dual-labeled for tyrosine hydroxylase (TH). Of the CB1r-immunoreactive structures, 66% were somatodendritic profiles, 22% were axon terminals, and the remaining 12% were associated with glial and small unmyelinated axon-like structures. CB1r immunoreactivity (-ir) in somatodendritic profiles was more often localized to the cytoplasm, whereas CB1r-ir located in axon terminals was more commonly localized on the plasma membrane. Somatodendritic profiles with CB1r-ir typically received input from axon terminals forming asymmetric-type synapses. In contrast, presynaptic profiles with CB1r-ir typically formed symmetric synaptic specializations. Anatomical studies confirmed the co-existence of MOR and CB1r-ir in common somatodendritic compartments of catecholaminergic neurons in the LC, and also revealed CB1r-positive axon terminals forming synaptic contact with MOR-containing dendrites. Our results provide evidence for a heterogeneous distribution of CB1r in the LC and demonstrate that CB1r and MOR co-exist in cellular profiles in this region. These data suggest important potential interactions between cannabinoid and opioid systems in LC neuronal profiles that may impact noradrenergic tone.
Keywords: cannabinoid receptor localization, electron microscopy, opioid receptor, locus coeruleus, noradrenergic neurons, cannabinoid-opioid interactions
1. Introduction
G-protein coupled receptors are abundant transmembrane proteins that comprise approximately 50% of all pharmacological targets. Cannabinoid-1 receptors (CB1r) and mu-opioid receptors (MOR) are G-protein coupled receptors that are widely expressed throughout the brain. The opioid and cannabinoid receptors are major targets for many drugs of abuse and widely-used analgesics (Demuth and Molleman, 2006; Manzanares et al., 1999; Pasternak, 2005). These receptors preferentially signal through the Gi/Go alpha subunit of the heterotrimeric G-proteins, effectively decreasing cyclic adenosine monophosphate levels (Childers et al., 1992; Dhawan et al., 1996; Howlett et al., 2002; Massi et al., 2003).
A brainstem nucleus targeted by both opioids and cannabinoids is the locus coeruleus (LC), a norepinephrine (NE) enriched region that is involved in modulating arousal, stress, and anxiety (Espana and Berridge, 2006; Harburg et al., 2006; Rios et al., 2006; Van Bockstaele et al., 2000; Wang and Burns, 2006). The LC projects throughout the neuroaxis and serves as the primary source of NE to forebrain regions. This region regulates arousal, attention, pain and stress via projections to multiple limbic, cortical and autonomic-related brain areas (Aston-Jones et al., 1996; Aston-Jones et al., 2001; Carrasco and Van de Kar, 2003; Dunn et al., 2004; Nestler et al., 1999). The neuroanatomical substrates of the LC-NE axis and noradrenergic signaling in the brain implicate this system in the regulation of tolerance, addiction, sensitization and withdrawal from substances of abuse (Aston-Jones and Kalivas, 2008; Weinshenker and Schroeder, 2007).
The LC, a pivotal structure in opiate addiction and withdrawal, has been well-characterized in its sensitivity to opiate exposure and withdrawal (Koob et al., 1992; Nestler, 1993; Nestler et al., 1994; Nestler et al., 1999; Rasmussen and Aghajanian, 1989; Rasmussen et al., 1990; Van Bockstaele et al., 2001). This nucleus is comprised of a fairly homogeneous cluster of noradrenergic neurons that possess a high density of postsynaptic MOR (Van Bockstaele and Commons, 2001). In addition to the LC's role in opioid signaling, evidence also supports modulation of noradrenergic activity and function by cannabinoids, suggesting a potential role for local cannabinoid signaling in the LC. Using a radiolabeled cannabinoid receptor agonist, regions with low to moderate cannabinoid receptor binding were noted in noradrenergic brainstem nuclei such as the LC and nucleus of the solitary tract (Herkenham et al., 1991). Electrophysiological evidence also suggests a role for cannabinoids in the LC. Studies have demonstrated that systemic administration of cannabinoid agonists increases spontaneous firing rates of neurons in the LC and blocks evoked inhibition (Mendiguren and Pineda, 2006a; Muntoni et al., 2006). From electrophysiology experiments using brain slices, there is evidence for cannabinoid receptor activity directly within the LC, where cannabinoid receptor activation was shown to suppress the glutamatergic component of potassium chloride-evoked excitation, and to enhance N-methyl-D-aspartic acid-induced excitation of these neurons (Mendiguren and Pineda, 2004; Mendiguren and Pineda, 2007).
Recent data from our laboratory and others provide additional evidence for cannabinoid modulation of the LC. Systemic and local administration of WIN55,212-2 has been shown to increase forebrain NE release as well as increase indices of noradrenergic activity (Corchero et al., 1997; Corchero et al., 2002; Oropeza et al., 2005; Page et al., 2007; Page et al., 2008; Paldy et al., 2008; Valverde et al., 2001). In further support of such modulatory actions, cannabinoid receptors have been localized to noradrenergic axon terminals in the frontal cortex (Oropeza et al., 2005; Oropeza et al., 2007; Page et al., 2007; Page et al., 2008). Combined, these data have led us to further examine the anatomical substrates underlying cannabinoid effects on the LC by investigating the immunohistochemical localization of CB1r with respect to noradrenergic neurons in the brainstem. In addition, modulation of MOR and CB1r have both been found to alter indices of noradrenergic activity (Nestler, 1993; Nestler et al., 1999; Oropeza et al., 2005; Szabo and Schlicker, 2005) and may interact in the LC to regulate noradrenergic function. We investigated the dual localization of cannabinoid and opioid receptors in the LC to provide an anatomical substrate for potential interactions. Taken together, these studies contribute to advancing our understanding of the functional implications of cannabinoid signaling in the LC and reveal important cannabinoid-opioid receptor interactions that may impact noradrenergic activity.
2. Results
Antisera Specificity
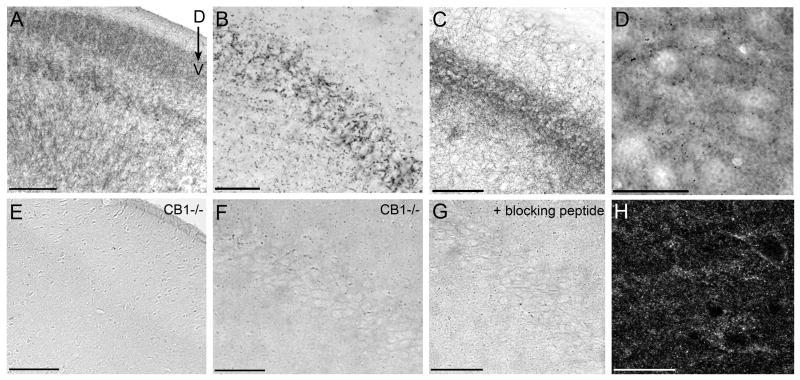
The specificity of the tyrosine hydroxylase (TH) and MOR antisera used in this study has been thoroughly established for detection of these antigens in the LC. Several control experiments were performed to verify the specificity of the CB1r antibody for detection of cannabinoid receptors in this region. Immunoperoxidase labeling for CB1r was carried out in CB1r-/- mice in multiple brain regions to verify the lack of immunoreactivity in knockout animals. In tissue sections from wild type CD1 mice, immunoperoxidase labeling for CB1r was visible in the cerebral cortex and hippocampus (Fig. 1A & B, respectively). CB1r immunohistochemistry performed simultaneously using tissue sections from the cerebral cortex and hippocampus of a CB1r-/- mouse revealed complete absence of CB1r immunoreactivity (Fig. 1E & F). Additional controls were performed in rat hippocampus sections processed for CB1r immunoperoxidase labeling (Fig. 1C), demonstrating similar immunoreactivity patterns to mouse CB1r. Preadsorption of the primary antisera with the corresponding blocking peptide abrogated CB1r immunoreactivity as compared to rat hippocampus sections in which standard CB1r immunohistochemistry was performed (Fig. 1G & C, respectively).
Figure 1.
Multiple methods were used to examine antibody specificity. CB1 receptor immunoreactivity (-ir) is absent in CB1r knockout mice. Immunoperoxidase labeling for CB1r in tissue sections through the cortex (A) and hippocampus (B) of wild-type CD1 mice. Immunoperoxidase staining for CB1r is absent in CB1r knockout mice in tissue sections through the cortex (E) and hippocampus (F). Preadsorption with a blocking peptide reduces CB1r immunoreactivity in the rat hippocampus. Incubation of the primary antibody in blocking peptide significantly reduced CB1r immunoperoxidase labeling in the rat hippocampus (G), as compared to control tissue run in parallel (C). Immunofluorescence and immunoperoxidase labeling of CB1r show similar patterns of immunoreactivity in the rat locus coeruleus (LC). Peroxidase labeling for CB1r in the rat locus coeruleus (D) is shown above immunofluorescence labeling for CB1r in a similar LC slice (H). Scale bars for A & E = 200μm, B & F = 50μm, C & G = 100μm, and D & H = 25μm.
Visualizing CB1r using Light and Fluorescence Microscopy
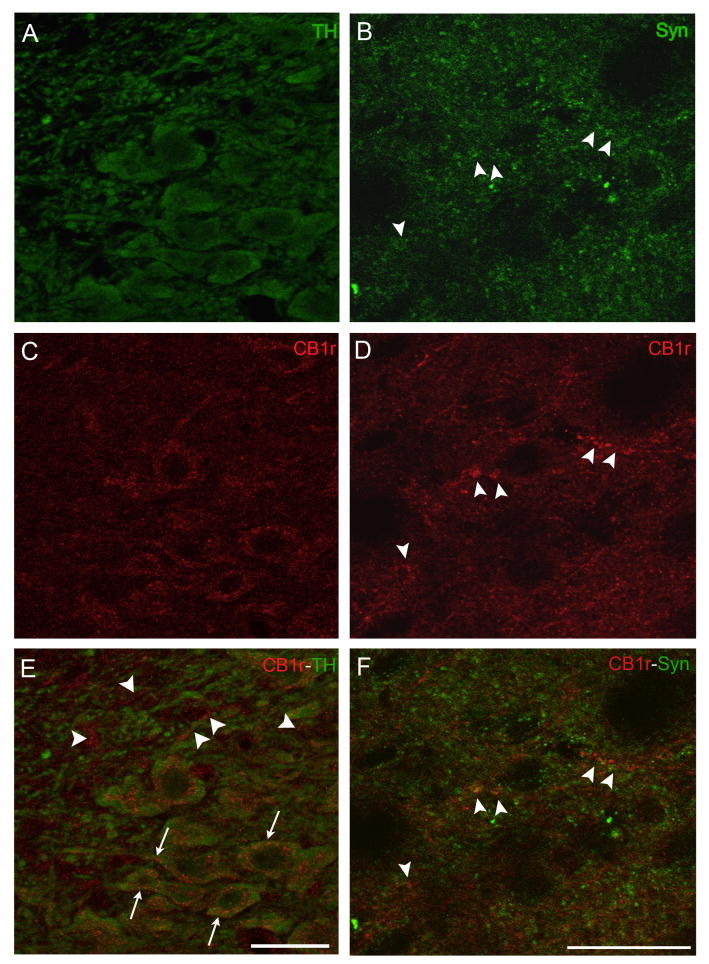
CB1r immunoreactivity was visualized using fluorescence and peroxidase labeling in rat brainstem tissue sections through the LC. In comparable rostrocaudal levels of the LC, single-labeling for CB1r using immunoperoxidase (Fig. 1D) displays a similar pattern to CB1r immunofluorescence labeling (Fig. 1H). Dual immunofluorescence studies were performed to examine the co-localization of CB1r immunoreactivity with TH, the rate limiting enzyme for norepinephrine synthesis. In LC sections labeled for CB1r and TH, TH immunoreactivity (green) reveals the localization of noradrenergic neurons in the LC (Fig. 2A). CB1r immunofluorescence (Fig. 2C, red) is scattered throughout the LC in what appears to be a mix of somatodendritic and presynaptic patterns of immunoreactivity. A merged image (Fig. 2E) reveals a considerable portion of CB1r immunoreactivity co-localized to TH-positive cells (arrows), but also CB1r labeling that is distinctly separate from TH immunoreactivity (arrowheads). To determine whether CB1r-positive structures that lack TH-labeling were indeed presynaptic, dual immunofluorescence studies for CB1r and synaptophysin, a vesicular membrane protein, were performed. Labeling for synaptophysin (green) can be visualized in a punctate pattern (Fig. 2B) and select areas of punctate CB1r immunoreactivity (Fig. 2D, red) co-localize with synaptophysin labeling in a merged image (Fig. 2F, arrowheads). Control experiments to test the specificity of the secondary antibodies used in these experiments were performed in parallel with omission of the primary antisera for CB1r, MOR, TH and synaptophysin. Incubation with the fluorescence-conjugated secondary antibodies yields minimal background labeling in the absence of primary antibody (Supplemental Fig. 1).
Figure 2.
Visualizing CB1r localization in the LC using immunofluorescence. Left column: Dual immunofluorescence confocal micrographs reveal labeling for TH (green) depicting catecholaminergic neurons (A) and CB1r immunoreactivity (C, red) within the LC. A merged image (E) reveals co-localization of TH and CB1r immunoreactivity (arrows). Arrowheads indicate presynaptic-like structures with CB1r immunoreactivity that lack TH staining. CB1r immunoreactivity appears to be both cytoplasmic and membrane-delimited. Right column: Dual immunofluorescence labeling for CB1r and synaptophysin reveal a presynaptic pattern of CB1r immunoreactivity scattered throughout the LC. Labeling for synaptophysin (Syn) in green (B), with CB1r labeling (D) in red. A merged image (F) reveals the co-localization of CB1r-positive fibers and terminal-like structures with synaptophysin immunoreactivity (arrowheads). Scale bars = 25μm.
Ultrastructural localization of CB1r
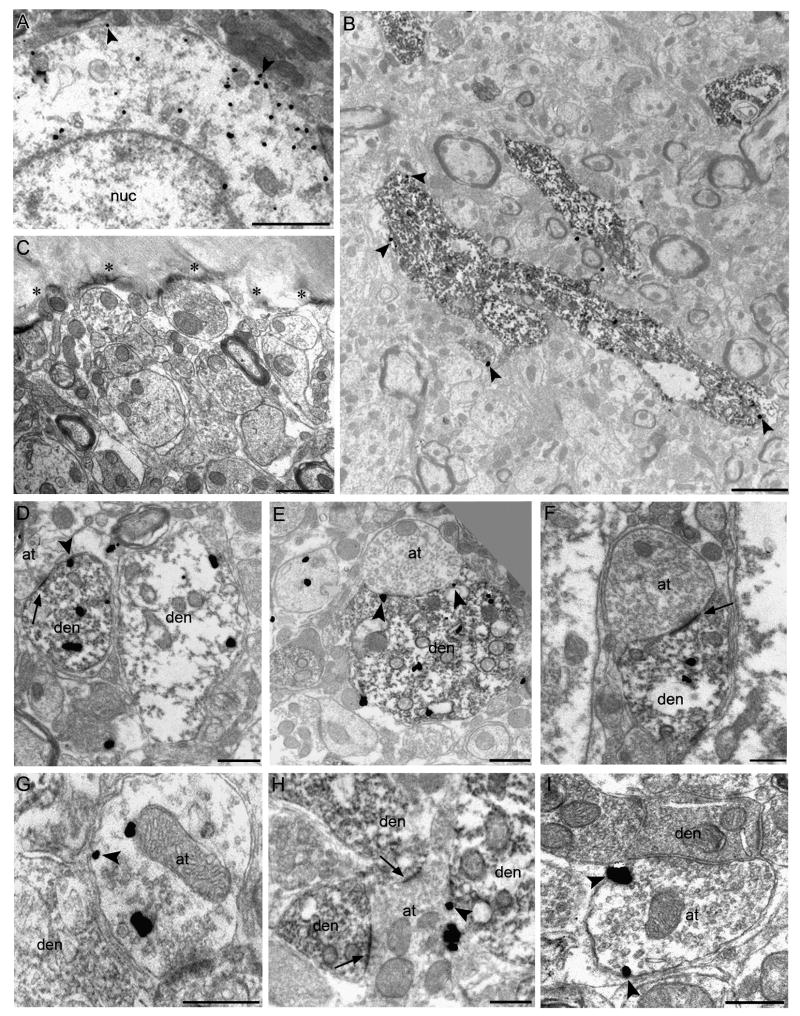
Electron microscopy (EM) was used in order to more clearly determine the subcellular distribution of CB1r in the LC using either silver-enhanced immunogold-labeling or immunoperoxidase detection. To ensure specificity of the biotinylated and gold-conjugated secondary antibodies for quantification at the ultrastructural level, control tissue taken directly from the plastic-tissue interface was processed in parallel, with omission of the primary antisera for CB1r and TH. No peroxidase or immunogold-non-specific background labeling was detected at the interface (Fig. 3C). As an added control, structures containing myelin, which is known to be devoid of CB1r, was examined for the presence of immunogold particles. Out of 450 myelinated structures analyzed, only 19 (4.2%) contained ≥1 gold particles present within the myelin.
Figure 3.
CB1r labeling of catecholaminergic profiles at the ultrastructural level. In all panels, arrowheads depict membrane-associated CB1r immunogold. Single labeling for CB1r with immunogold particles in an LC soma demonstrates both cytoplasmic and membrane-associated CB1r localization (A). Electron micrographs with dual labeling for TH and CB1r reveal the ultrastructural localization of CB1r-ir in the LC. In a lower magnification view, CB1r immunogold is localized to large TH-positive dendrites in the LC (B). Peroxidase and gold immunoreactivity is absent in control tissue at the plastic-tissue interface (asterisks), run identically but with omission of the primary antisera (C). Co-localization of CB1r immunogold and TH peroxidase reaction product is shown in several LC dendrites (D-F). While CB1r-ir is primarily cytoplasmic in TH-positive dendrites, CB1r immunogold was observed along the membrane in proximity to axon terminals apposing these dendrites (E, arrowheads). CB1r immunogold-positive axon terminals were also observed (G-I). A CB1r-positive axon terminal formed synapses with several TH-positive dendrites (H). Arrows depict asymmetric type synaptic specializations. Scale bars for A & B = 2μm, C & F = 1μm, all others = 500nm.
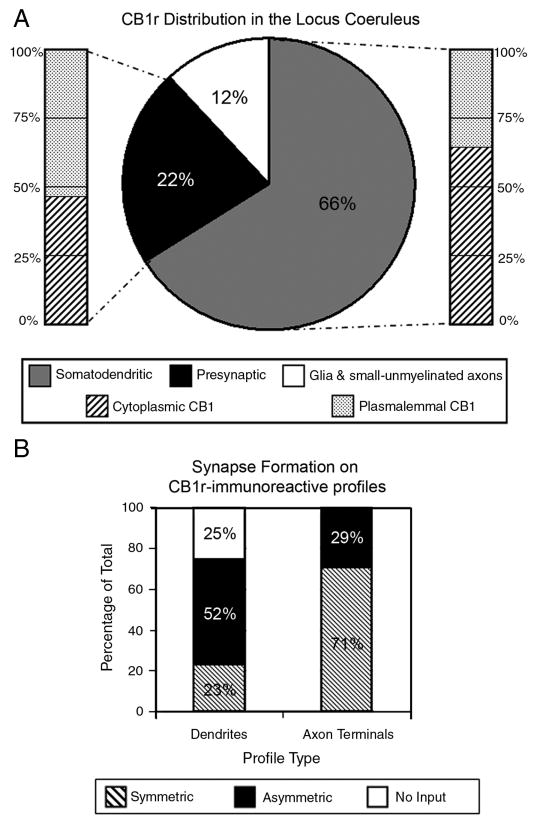
Using immunogold labeling, CB1r was observed along the plasma membrane (arrowheads) and in the cytoplasm (Fig. 3A). Dual-labeled EM was used characterize the ultrastructural localization of CB1r in relation to TH-positive cellular profiles, using a combination of immunogold and peroxidase labeling. In a lower-magnification micrograph of somatodendritic processes in the LC, qualitative assessment of CB1r and TH labeling led to the observation that a majority of CB1r immunogold was localized to TH-positive structures, with minimal labeling of the surrounding neuropil (Fig. 3B). When examining dual-labeled tissue at the ultrastructural level for the co-localization of CB1r and TH, CB1r-ir was found within the cytoplasm of TH-positive somatodendritic profiles (Fig. 3D-F). Additionally, CB1r was localized to the plasma membrane of TH-positive dendrites directly across from apposing axon terminals (Fig. 3E & F, arrowheads). CB1r distribution and immunogold localization percentages were drawn from quantification of 700 profiles (approximately 130-150 per animal, n=5 rats). Interestingly, approximately 66% (460/700) of the profiles with CB1r immunogold labeling located in the LC were somatodendritic and 59% (491/830) of the total postsynaptic CB1r immunogold particles were cytoplasmic (Fig. 4A). The frequency of TH labeling in CB1r-ir somatodendritic profiles and of CB1r-labeling in TH-ir profiles was quantified to further investigate this observation. Of the 400 CB1r-positive profiles examined, 330 profiles also contained TH immunoperoxidase, constituting 82.5% of the total CB1r-positive population analyzed. Of the 473 TH-labeled profiles, 55% (259) possessed 2 or more CB1r-immunogold particles. Analysis of CB1r synapse formation was done using a random sampling of 100 dendrites and 100 axon terminals. In profiles with a distinguishable synaptic specialization, postsynaptic CB1r-ir containing profiles typically received input from axon terminals forming asymmetric synapses (Fig. 3D & F). Out of 100 dendrites, 52 received asymmetric input, while 23 received symmetric input and the remaining 25 had no visible contact from synaptic structures (Fig. 4B).
Figure 4.
Quantification of CB1r distribution in the LC. Characterization of CB1r distribution in the LC (A). 66% of the CB1r-immunoreactive profiles quantified in the LC were somatodendritic while 22% were presynaptic axon terminals. The remaining 12% of CB1r-labeled structures were small unmyelinated axon-like structures and glial-like processes. Within the somatodendritic profiles, 59% of CB1r immunogold was located in the cytoplasm while 41% was associated with the plasma membrane. Within axon terminal profiles, 45% of CB1r immunogold was localized to the cytoplasm, with 55% located along the plasma membrane. The types of synapses formed by CB1r-immunoreactive profiles in the locus coeruleus were also characterized (B). Somatodendritic profiles containing CB1r immunogold formed asymmetric synapses with presynaptic processes 52% of the time, while 23% formed symmetric synapses. CB1r-positive somatodendritic profile received no visible synaptic contact 25% of the time. When CB1r immunogold was contained on axon terminals, they formed symmetric synapses 71% of the time, while 29% formed asymmetric synapses.
Based on immunofluorescence data, it was expected that CB1r immunoreactivity would also be present in presynaptic structures within the LC. Presynaptic profiles containing CB1r-ir were detected at the ultra-structural level (Fig. 3G-I). CB1r-labeled axon terminals contacting TH-positive dendrites were observed (Fig. 3H). Characterization of the types of synapses formed by CB1r-immunogold containing axon terminals revealed that these were comprised of both symmetric-type synapses (Fig. 3I) and asymmetric-type synapses (Fig. 3H). When CB1r-ir was present within axon terminals in the LC, CB1r-immunogold was localized to both the plasma membrane and axoplasm (arrowheads, Fig. 3G-I). Quantification of this data revealed that 22% (156/700) of the observed CB1r-ir profiles were axon terminals (Fig. 4A). Of the CB1r-positive axon terminals profiles with visible synapses, 71% (71/100) of these were symmetric synapses, while the remaining 29% (29/100) of synapse-forming CB1r-positive axon terminals resembled asymmetric-type synapses (Fig. 4B).
Anatomical Relationship of CB1r and MOR in the LC
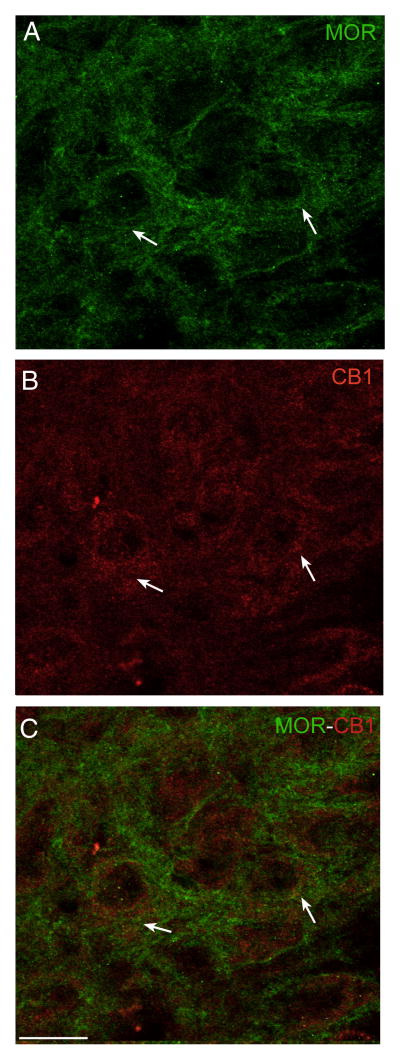
Given the array of evidence for cannabinoid-opioid interactions in the brain, and the individual presence of both cannabinoid and opioid receptors in the LC, we next moved to examine the anatomical substrate for potential CB1r-MOR interactions in the LC. Dual immunofluorescence microscopy was used to examine the possible co-localization of CB1r and MOR in the LC. In the soma and dendritic field of the LC, MOR and CB1r immunoreactivities were identified within the same neurons (Fig. 5). Although immunoreactivity for these receptors was present within common cells in the LC, in most cases, MOR (Fig. 5A, green) and CB1r fluorescence (Fig. 5B, red) did not directly co-localize to produce a yellow signal in the merged image (Fig. 5C), suggesting that most CB1r and MOR may be localized to separate areas of the same cell. However, occasional, direct co-localization of CB1r and MOR immunoreactivities was noted and may indicate close proximity or direct association of a small subset of these receptor proteins (Fig. 5C, arrows).
Figure 5.
Dual immunofluorescence micrographs showing CB1r and MOR labeling in the LC. MOR (A, green) and CB1r labeling (B, red) in the LC. A merged image (C) reveals co-localization of CB1r and MOR in the LC (arrows). CB1r and MOR immunoreactivities were also seen in common cells in the merged image where direct overlap of the fluorescent signals was not present. Scale bar = 20μm.
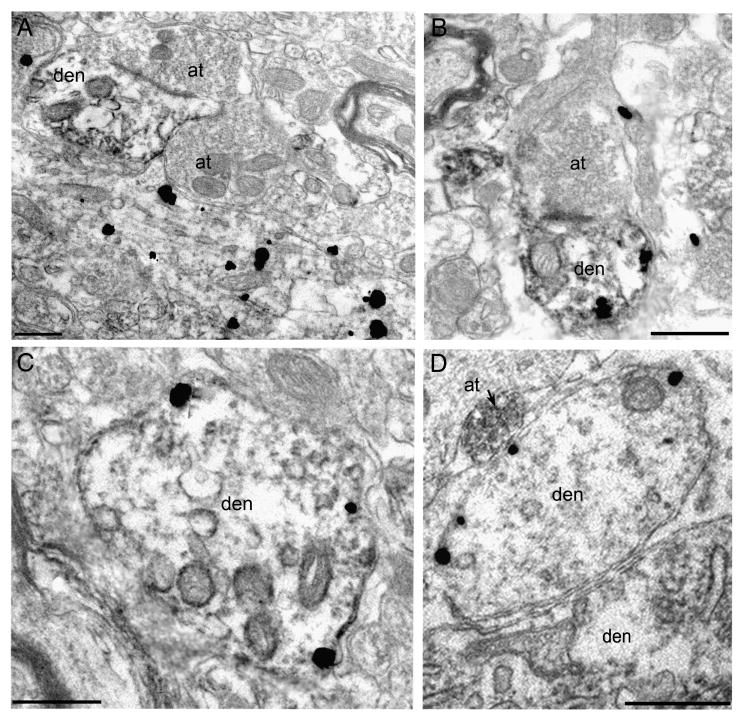
To further resolve the potential anatomical substrate of cannabinoid-opioid interactions in the LC, EM was used to analyze the ultrastructural distributions of CB1r and MOR. Using tissue from a group of 4 rats, dual-labeling of LC sections for CB1r and MOR resulted in the identification of several different substrates for receptor interactions. To verify the consistency of distribution patterns, half of the LC tissue sections were labeled with CB1r immunogold and MOR peroxidase (Fig. 6A & C) while the remaining sections were labeled with CB1r peroxidase and MOR immunogold (Fig. 6B & D). Observations of the anatomical substrate(s) for CB1r-MOR interactions were consistent across both labeling paradigms. From a randomly chosen subset of 125 electron micrographs with CB1r and MOR immunoreactivity, the various interactions were quantified. Oftentimes, MOR-containing dendrites were captured in the same micrographic field nearby, but not in contact with, CB1r-immunogold labeled dendrites or soma (Fig. 6A). In more than half of the analyzed micrographs (55%, 69/125), CB1r and MOR-ir structures within the same field fell in to this category. CB1r and MOR immunoreactivities were also found to co-localize within somatodendritic profiles (Fig. 6B& C), and this type of interaction was observed in 35% (44/125) of the micrographs. Other times, axon terminals containing immunoperoxidase labeling for CB1r were located in apposition to MOR-containing dendrites (Fig. 6D), which was noted 10% (12/125) of the time. A schematic of the various anatomical interactions are illustrated in Figure 7.
Figure 6.
Electron micrographs reveal several possibilities of an anatomical substrate for cannabinoid-opioid interactions in the LC. On the left, dual-labeling EM was performed with CB1r labeling in immunogold and MOR in immunoperoxidase. A dendrite with MOR peroxidase labeling was located next to a large CB1r-immunogold labeled profile (A). CB1r and MOR immunoreactivities were seen to co-localize within LC dendrites (C). On the right, dual-labeling EM was performed with the labels reversed: immunogold MOR labeling and immunoperoxidase CB1r labeling. A dual labeled dendrite with MOR immunogold and CB1r peroxidase received contact from an axon terminal (B). An axon terminal containing CB1r immunoperoxidase contacted a MOR-containing dendrite located near a large dendrite (D). Scale bars = 500nm.
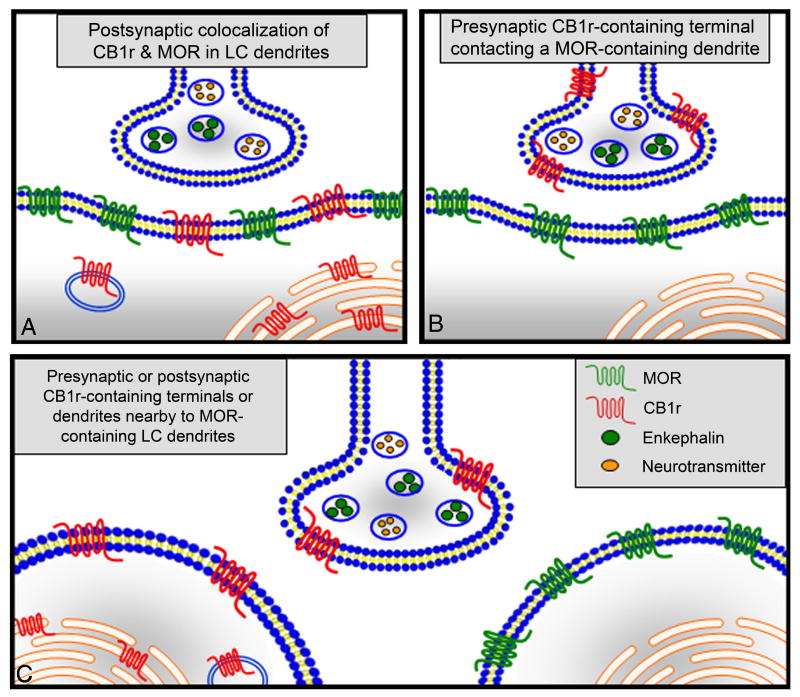
Figure 7.
Schematic demonstrating the various anatomical substrates for cannabinoid-opioid receptor interactions observed within the LC in this manuscript. CB1r and MOR were observed in common somatodendritic profiles (A). CB1r-containing axon terminals formed synaptic contacts with MOR-containing dendrites (B). Alternatively, separate CB1r-positive and MOR-positive cellular profiles were found in the proximity, but not in direct contact with one another in the same field (C).
3. Discussion
Our results provide an anatomical substrate for direct effects of cannabinoid receptor modulation in the LC. CB1r-ir was distributed both pre- and postsynaptically within the LC. Within somatodendritic profiles, CB1r-ir was primarily cytoplasmic in localization, but also showed a plasmalemmal distribution. Somatodendritic profiles containing CB1r-ir received a greater number of asymmetric versus symmetric-type synapses. CB1r-ir was also detected within presynaptic profiles, where the synaptic specializations were more commonly of the symmetric type. Potential CB1r and MOR co-localization within cellular profiles was also apparent in the LC. With respect to MOR localization, several anatomical substrates for possible interactions between the cannabinoid and opioid receptor systems were evident, including co-existence in common postsynaptic structures and apposition of presynaptic CB1r-ir structures with MOR-ir somatodendritic profiles.
Potential for direct cannabinoid effects in the LC
Over the past decade, multiple studies have revealed the sensitivity of the LC to cannabinoids (Mendiguren and Pineda, 2004; Mendiguren and Pineda, 2006a; Mendiguren and Pineda, 2006b; Muntoni et al., 2006; Page et al., 2007; Page et al., 2008). Several convergent lines of neurochemical, anatomical and electrophysiological evidence have suggested a possible role for cannabinoid modulation of the LC, but the anatomical distribution of these receptors has not been identified. Data from several studies support the role of direct local CB1r-mediated effects in the LC, demonstrating agonist-induced effects on LC activity with intracerebroventricular administration and bath application to LC tissue slices (Mendiguren and Pineda, 2004; Mendiguren and Pineda, 2007; Muntoni et al., 2006). Paradoxically, another study was unable to alter LC neuronal firing with local cannabinoid agonist application, suggesting that LC sensitivity to cannabinoid exposure may result from receptor activation at peripheral sites (Mendiguren and Pineda, 2006a). However, data supporting the localization of CB1r to noradrenergic terminals in the frontal cortex provides additional evidence for direct cannabinoid activity within the noradrenergic LC circuit (Oropeza et al., 2007).
Somatodendritic localization of CB1r
The current studies add to the growing literature supporting direct effects of cannabinoids on the LC and demonstrate CB1r immunoreactivity in cell bodies and dendritic processes of catecholaminergic LC neurons. Although it is not fully understood whether the population of cytoplasmic CB1r is functional and takes part in signal transduction, many endogenous and synthetic cannabinoids are lipophilic and readily cross the plasma membrane into the cytoplasmic space. It is important to note that the CB1r-labeled population detected within catecholaminergic LC cell bodies may include newly synthesized receptor en route to dendritic processes and the terminal ends of LC projections throughout the brain. However, recent in vitro studies revealed that intracellular CB1r's were able to interact with their Gi signaling subunits in endosomes and lysosomal compartments, and provided evidence that intracellular CB1r was able to mediate signal transduction through extracellular regulated kinase phosphorylation (Rozenfeld and Devi, 2008). It may be possible that both membrane-delimited and cytoplasmic CB1r are capable of taking part in signal transduction in the LC, however, whether these two receptor populations play similar or separate roles in mediating signal transduction remains to be elucidated.
The postsynaptic distribution of CB1r in the LC is consistent with several other reports of postsynaptic CB1r distribution elsewhere in the central nervous system. Ultrastructural examination of CB1r in the rat caudate-putamen revealed the presence of CB1r in cell bodies, dendrites and spines of medium spiny neurons in a cytoplasmic-to-membrane distribution very similar to that noted in the current studies (Rodriguez et al., 2001). In the rat nucleus accumbens, dendritic labeling in the shell as well as prominent presynaptic labeling in the core and shell were observed (Pickel et al., 2004). Cholinergic neuronal cell bodies and dendrites were shown to possess CB1r labeling in the basal forebrain, while in the dorsal horn, multiple groups have described postsynaptic distributions of CB1r where it may function to mediate pain transmission (Hohmann et al., 1999; Lu et al., 1999; Ong and Mackie, 1999; Salio et al., 2001). CB1r within cell bodies and proximal dendrites may be involved in a form of slow-self inhibition resulting from the calcium-dependent release of endocannabinoids (Bacci et al., 2004). In addition to the well-characterized presynaptic regulation of transmitter release by CB1r, there now exists an anatomical substrate for CB1r regulation of postsynaptic signaling in multiple brain regions.
An anatomical substrate for presynaptic modulation of catecholaminergic neurons in the LC by CB1 receptors
The phenotype of presynaptic profiles on which CB1r-ir can be found appears to be a regionally-specific phenomenon. In striatal terminals containing CB1r labeling, most formed asymmetric, excitatory type synapses (Rodriguez et al., 2001). Meanwhile, in the accumbens, CB1r-labeled terminals were found to form symmetric synapses with MOR containing terminals (Pickel et al., 2004). In the present study, the majority of CB1r-ir containing processes formed symmetric, inhibitory type synapses on TH-positive dendrites and cell bodies. Whereas postsynaptic CB1r-labeled profiles typically received input from terminals forming asymmetric synapses, presynaptic CB1r-ir within axon terminals typically formed symmetric synapses. Pre- and postsynaptic CB1r in the LC may mediate different signal transduction pathways.
The nucleus paragigantocellularis (PGi), nucleus prepositus hypoglossi (PrH), paraventricular nucleus of the hypothalamus, periaqueductal gray and preoptic areas all provide afferent input to the LC (Aston-Jones et al., 1992;Valentino and Aston-Jones, 2000). Many of these regions have demonstrated detectable levels of receptor binding when examined using a radiolabeled cannabinoid receptor agonist (Herkenham et al., 1991). The PrH, in combination with inhibitory interneurons in and around the LC, may be a source of CB1r-positive inhibitory-type presynaptic input (Aston-Jones et al., 2004). The cannabinoid receptor typically signals through inhibitory Gi/o-proteins, and CB1r-mediated inhibition within inhibitory terminals that target the LC may release inhibitory control on these neurons. This, in part, may contribute to increased LC neuronal activity following cannabinoid exposure. While more than half of presynaptic CB1r-ir was observed within axon terminals forming inhibitory synapses, a subset of CB1r-immunoreactive axon terminals did form asymmetric, excitatory type synapses that may arise from PGi processes, a medullary nucleus implicated in somatic, autonomic and visceral functions (Valentino and Aston-Jones, 2000). CB1r-ir associated with excitatory terminals may exist to counterbalance the effects of CB1r on inhibitory afferents to this nucleus.
Cannabinoid-opioid interactions in the LC
Data from resonance energy transfer and co-immunoprecipitation studies suggest a close association between CB1r and MOR (Hojo et al., 2008; Rios et al., 2006). In vitro, it was shown that CB1r and MOR signal through a common G-protein, while allosteric interactions between CB1r and MOR were demonstrated in vivo (Hojo et al., 2008; Schoffelmeer et al., 2006). At the immunofluorescence and ultrastructural levels, a variety of anatomical substrates for CB1r-MOR interactions became evident. Co-localization of CB1r and MOR labeling in somatodendritic profiles raised the possibility for potential heterodimerization of these receptors. Alternatively, CB1r and MOR may not directly associate, but possibly signal through a shared pool of second messengers and take part in convergent signal transduction. The data in this study also suggest that CB1r-containing terminals contacted MOR-containing dendrites in the LC, as has been previously reported in the accumbens (Pickel et al., 2004). In this situation, CB1r-mediated control of transmitter release from LC afferents would impact MOR-mediated signal transduction on the apposing dendrite. Finally, as was often noted during data analysis, CB1r and MOR-positive structures were observed in close proximity to one another within the same field. Without serial reconstruction, it is unknown whether these structures would form contacts with one another in different planes of the section. However, the close proximity of CB1r and MOR-positive profiles to one another creates the possibility for coordinated signaling even in the absence of direct contact. Further studies are required to fully elucidate the nature of possible CB1r-MOR interactions.
Implications of cannabinoid-opioid interactions
There are many potential benefits of cannabinoid-opioid interactions in the brain (Corchero et al., 1997; Corchero et al., 2004; Lichtman et al., 2001; Maldonado and Valverde, 2003; Manzanares et al., 1998; Manzanares et al., 1999; Rubino et al., 2000; Tham et al., 2005; Valverde et al., 2001). These interactions may be exploited in order to improve pain control, treatments for addiction, and to understand the role of these receptors systems in psychiatric dysfunction. Co-administration of cannabinoids and opiates have been shown to exert synergistic effects, causing antinociception while avoiding negative side effects in several cases (Cichewicz et al., 1999; Smith et al., 2007; Tham et al., 2005; Vigano et al., 2005; Wilson et al., 2008). Cannabinoid modulation has also been shown to attenuate opiate withdrawal signs, revealing the possibility to utilize CB1r-MOR interactions as a therapeutic target for those experiencing withdrawal during opiate detoxification and abstinence (Lichtman et al., 2001; Rubino et al., 2000).
Indeed, the LC is uniquely poised to modulate pain, arousal, anxiety, and withdrawal due to its positioning within the noradrenergic circuitry in the brain. The current findings include direct anatomical evidence and characterization of CB1r in the LC, furthering the understanding of the potential role for CB1r-signaling in this noradrenergic pathway. The anatomical substrate for cannabinoid-opioid interactions in this region also highlights the many possibilities for potential cross-modulation of the LC opioid system by cannabinoids. This wide-reaching brainstem nucleus is a pivotal structure implicated in the manifestation of opiate withdrawal. Modulation of cannabinoid signaling may be a useful target for restoring homeostasis when this region becomes dysregulated with addiction or disease states.
4. Experimental Procedure
For antibody specificity experiments, brain sections from CB1r knock-out mice (CB1r-/-) and wild type littermates were used. The CB1r-/- was generated in CD1 mice as previously reported (Ledent et al., 1999). All other experiments were performed in adult male Sprague Dawley rats (250-300g, Harlan, Indianapolis, IN) housed 3 to a cage. All animals were kept on a 12-hour light-dark cycle, with free access to food and water. All procedures were performed in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University. Animals were handled for several days prior to all experiments so as to reduce the stress response associated with human contact. Efforts were taken to minimize any pain and discomfort, and to limit the number of animals used in this study.
Antisera
The polyclonal primary antiserum for CB1r was raised in rabbit and directed to the last 15 amino acids of the C-terminus of the rat CB1r. Antibody specificity was verified through immunohistochemical (Fig. 1C & G) and Western blot controls (data not shown) in which immunoreactivity was abolished by incubation of the primary antibody with a peptide corresponding to the last 15 amino acids of the CB1r C-terminus. In CB1r-/- mice, immunoreactivity was abrogated in areas known to contain a high density of CB1r (Fig. 1E-F). Immunostaining in wild type mice littermates was consistent with that typically found in the brain (Fig. 1A & B).
The polyclonal MOR antibody utilized was directed to amino acids 384-398 of the C-terminus of rat MOR, and raised in guinea pig (Millipore, Billerica, MA). Antibody specificity was tested by preadsorption with the 15-residue antigenic peptide, which blocked immunoreactivity in regions known to express MOR. The antiserum was found to stain COS-7 and Neuro2A cell lines transfected with MOR, with a staining pattern consistent with that of receptor autoradiography and in situ hybridization (Arvidsson et al., 1995).
The monoclonal anti-TH antibody was raised in mouse and directed to the highly conserved mid-portion of the rat TH molecule (Immunostar, Hudson, WI). The immunogen for the mouse monoclonal antiserum was raised against denatured TH from rat pheochromocytoma and labels a single 63 kD band corresponding to TH via Western blot. The specificity of the TH antibody has been verified by preadsorption of the antibody with a high concentration of TH (Van Bockstaele and Pickel, 1993). Tissue sections processed in the absence of each primary antibody did not exhibit any detectable immunoreactivity.
The mouse monoclonal antibody for synaptophysin (Abcam, Cambridge, MA) was raised against the SY38 clone and labels a main band of approximately 38 kD corresponding to the synaptophysin membrane protein. This antibody provides punctate staining highly enriched in neuronal tissue, and has been characterized using Western Blot and immunohistochemistry (Jahn et al., 1985; Wiedenmann and Franke, 1985).
Tissue Fixation for Immunohistochemistry
Rats were deeply anesthetized with sodium pentobarbital (125mg/kg, intraperitoneal- i.p.) and perfused transcardially through the ascending aorta with heparinized saline followed by 400ml of 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4). Immediately following perfusion/fixation, brains were removed and returned to paraformaldehyde fixative for at least 30 minutes. Brains were cut in 40μm coronal sections through the forebrain, hippocampus, and LC using a vibrating microtome. Sections through the LC were made at -10.04 from bregma, 1.10mm medial-lateral, and 7.4 from the top of the skull based on the rat brain atlas of Paxinos and Watson (Paxinos, 1986).
Immunohistochemical Labeling
Standard immunohistochemistry was performed to visualize CB1r, TH and MOR as previously described (Van Bockstaele et al., 1996). Briefly, tissue sections were blocked in 0.5% bovine serum albumin in 0.1M Tris-buffered saline and incubated overnight in primary antibody in 0.1% bovum serum albumin in 0.1M TBS with 0.5% triton. For CB1r detection, tissue sections were incubated in rabbit anti-CB1r antibody at a concentration of 1:1000. For MOR detection, tissue sections were incubated at a concentration of 1:2000 in guinea pig anti-MOR (Millipore). The rate-limiting enzyme in norepinephrine synthesis was used for the localization of LC noradrenergic neurons. For detection of TH, tissue sections were incubated in a solution with mouse anti-TH at a concentration of 1:5000 (Immunostar). Labeling for synaptophysin was performed using a mouse anti-synaptophysin antibody at a concentration of 1:500 (Abcam). For dual labeling procedures, tissue sections were incubated overnight in a cocktail of primary antibodies. For immunohistochemical preadsorption experiments, equal quantities of primary antiserum targeting the CB1r were diluted into two separate vials of antibody incubation solution at a 1:1000 dilution. To one vial, blocking peptide was added at a 1μM concentration and both vials were incubated overnight at 4°C. Separate wells containing slices through the hippocampus were then incubated in one of the two antibody solutions. Standard protocol was followed identically for both wells containing tissue slices.
Immunoperoxidase detection was performed using a biotinylated donkey anti-rabbit secondary antibody for CB1r, a donkey anti-mouse antibody for TH, and donkey anti-guinea pig for MOR (Jackson Immunoresearch, West Grove, PA). Tissue sections were then incubated in an avidin-biotin complex solution (Vector Laboratories, Burlingame, CA). The peroxidase reaction product was then visualized using NovaRed Peroxidase substrate (Vector Laboratories) or 0.02% diaminobenzidine (DAB) with 10μl of 30% hydrogen peroxide in 0.1M tris-buffered saline. For light microscopy, tissue sections underwent serial dehydration, were mounted on slides, and coverslipped using Permount (Aldrich, St. Louis, MO). Tissue sections intended for dual-labeled EM were then prepared for visualization with the electron microscope. Following detection of the first antigen using immunoperoxidase, tissue sections intended for dual-labeling EM were then processed for detection of the second antigen with gold-conjugated secondary antisera. Brain sections were incubated for 2 hours in ultrasmall (<1nm) gold particle-conjugated goat anti-rabbit IgG at 1:50 for CB1r or gold-conjugated goat anti-guinea pig for MOR (Electron Microscopy Sciences, Hatfield, PA). Electron dense labeling of MOR or CB1r gold-labeling was enhanced via silver intensification of immunogold particles using a silver enhancement kit (GE/Amersham, Piscataway, NJ).
Tissue sections were prepared for visualization using the electron microscope as previously described (Van Bockstaele and Commons, 2001) with osmification, serial dehydration, flat-embedding, and tissue sectioning at 75 nm using an ultramicrotome. Control sections for each experiment were run in parallel in which one of the primary antisera was omitted, with the remaining protocol identical. Sections were collected on copper mesh grids and examined using an electron microscope (Morgani, Fei Company, Hillsboro, OR). Tissue sections were not counterstained so that gold-silver particles could be more easily discerned.
Detection of CB1r, TH and MOR for immunofluorescence utilized secondary antibodies that were fluorescein isothiocyanate (FITC: excite@492nm-emit@520nm) conjugated donkey-anti mouse IgG for TH and synaptophysin detection (Jackson Immunoresearch), Alexa Fluor 488 (excite@495nm-emit@519nm) donkey anti-rabbit IgG (Invitrogen, Carlsbad, CA) or tetramethyl rhodamine isothiocyanate (TRITC: excite@550nm-emit@570nm) conjugated donkey anti-rabbit for CB1r detection (Jackson Immunoresearch), and Rhodamine Red-X (excite@570nm-emit@590nm) conjugated donkey anti-guinea pig for MOR detection (Jackson Immunoresearch). Tissue sections underwent serial dehydration in increasing ethanol concentration, were mounted on slides, and coverslipped using DPX (Aldrich, St. Louis, MO). Sections were then viewed using a confocal microscope (Zeiss LSM 510 Meta, Carl Zeiss, Inc., Thornwood, NY). Images of control tissue processed in parallel, in which primary antibody was excluded, were captured using identical exposure settings to exclude detection of background staining. Final images were prepared using the Zeiss LSM Image Browser to assign color profiles to fluorescence images and Adobe Photoshop to adjust brightness and contrast.
Digital images were viewed and captured using the AMT advantage HR⁄HR-B CCD camera system and AMT Image Capture Engine (Advance Microscopy Techniques Corp., Danvers, MA). Profiles in electron micrographs were measured using a version of Image J software customized for AMT images (National Institutes of Health). EM images were prepared using Adobe Photoshop to adjust the brightness and contrast.
Data Analysis
To confirm dual-labeling of CB1r + TH and CB1r + MOR within LC sections for immunofluorescence studies, Z-stack analysis was performed. For better clarity of receptor distribution in the LC, all quantification of the subcellular distribution of CB1r and MOR immunoreactivities took place in ultra-thin sections using EM. Receptor quantification and analysis of receptor localization at the EM level was conducted as previously reported (Reyes et al., 2007; Van Bockstaele and Commons, 2001). Several measures were taken to prevent inclusion of spurious labeling in the quantification. First, the extent of background labeling was assessed by examining myelin (which should lack CB1r and MOR labeling) at the tissue-plastic interface where antibody penetration is maximal. These structures showed minimal immunogold labeling, as only 4.2% of myelinated structures (19/450) analyzed contained one or more gold particle. To further account for potential background, only profiles with a minimum of 2 gold particles were considered immunoreactive and used for quantification.
Cellular elements were isolated and classified based on Fine Structure of the Nervous System (Peters et al., 1991). Somata were identified by the presence of a nucleus, Golgi apparatus, and smooth endoplasmic reticulum. Proximal dendrites contained endoplasmic reticulum, were apposed to axon terminals, and were larger than 0.7 μm in diameter. Synapses were verified by the presence of a junctional complex, a restricted zone of parallel membranes with slight enlargement of the intercellular space, and/or associated postsynaptic thickening. Asymmetric synapses were identified by prominent postsynaptic densities while symmetric synapses had thin densities on both the presynaptic and postsynaptic side of the junctional complex.
Data from 5 animals were analyzed to determine the distribution of CB1r in the rat LC using immunofluorescence and electron microscopy. Data from 4 animals were used in the characterization of the anatomical substrate for CB1r-MOR interactions. For each animal, comparable levels of the LC were selected for ultra-thin sectioning. For each animal, dendritic and axon terminal profiles were sampled from at least 5 copper grids of ultrathin tissue sections near the tissue-plastic interface. Localization of the LC was verified by the presence of a high density of TH immunoreactivity. The full somatodendritic domain of the LC contained on each grid was systematically scanned and all immunoreactive profiles with intact morphology within this domain (presynaptic or postsynaptic) were captured digitally.
Approximately 300 profiles from each animal were scanned, and approximately half of these fit all below criteria and were included in the data analysis. Dendrites with a maximal cross-sectional diameter between 0.7μm and 5μm met the criteria for analysis, and a mix of dendrites of sizes from across this range was included in the analysis. Large profiles were excluded to avoid the bias towards positive-labeling of larger structures. Extremely small, large, longitudinal and irregularly shaped profiles were excluded from the data analysis due to possibly higher perimeter/surface ratios and risk of biasing the silver grain counts towards the membrane. Any profiles containing large, irregularly shaped silver grains of more than 0.25μm were excluded from the analysis. Immunogold particles were tallied as plasmalemmal if they were clearly in contact with an intact plasma membrane, and were considered cytoplasmic only if there was a clearly discernable gap between the particle and membrane. Because the receptor antibodies used in this study target the receptor C-terminus, which is oriented towards the cytoplasmic space, only plasmalemmal CB1r and MOR immunogold particles oriented towards the cytoplasmic compartment of dendrites were included in the quantification of a profile's distribution ratio. Cellular profiles that failed to meet any of the described criteria were excluded from data analysis.
Supplementary Material
Supplementary Figure 1) Control experiments were performed to examine secondary antibody specificity. LC tissue was processed identically, with omission of the primary antibody, to assess the level of non-specific background labeling from the secondary antibodies. Using fluorescein isothiocyanate (FITC) conjugated donkey-anti mouse IgG (A), tetramethyl rhodamine isothiocyanate (TRITC) conjugated donkey anti-rabbit (B), Alexa Fluor 488 donkey anti-rabbit IgG (C), and Rhodamine Red-X conjugated donkey anti-guinea pig (D), minimal background labeling was observed. Scale bar = 25μm.
Acknowledgments
The authors would like to acknowledge Dr. Lisa Ambrose Lanci and Dr. Beverly Reyes for their critical review of the manuscript.
Abbreviations
- CB1r
cananbinoid receptor type 1
- CB1r-/-
CB1r knock out
- DMSO
dimethyl sulfoxide
- EM
electron microscopy
- FITC
fluorescein isothiocyanate
- -ir
immunoreactivity
- i.p.
intraperitoneal
- LC
locus coeruleus
- MOR
mu-opioid receptor
- NE
norepinephrine
- PGi
nucleus paragigantocellularis
- PrH
nucleus prepositus hypoglossi
- Syn
synaptophysin
- TRITC
tetramethyl isothiocyanate
- TH
tyrosine hydroxylase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jillian L. Scavone, Department of Neurosurgery, Farber Institute for Neurosciences, Thomas Jefferson University, Philadelphia, PA
Ken Mackie, Department of Psychological and Brain Sciences, Indiana University, Bloomington, Indiana.
Elisabeth J. Van Bockstaele, Department of Neurosurgery, Farber Institute for Neurosciences, Thomas Jefferson University, Philadelphia, PA
References
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–41. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Astier B, Ennis M. Inhibition of noradrenergic locus coeruleus neurons by C1 adrenergic cells in the rostral ventral medulla. Neuroscience. 1992;48:371–81. doi: 10.1016/0306-4522(92)90497-p. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Valentino RJ, Shipley MT. Role of the locus coeruleus in emotional activation. Prog Brain Res. 1996;107:379–402. doi: 10.1016/s0079-6123(08)61877-4. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chen S, Zhu Y, Oshinsky ML. A neural circuit for circadian regulation of arousal. Nat Neurosci. 2001;4:732–8. doi: 10.1038/89522. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Zhu Y, Card JP. Numerous GABAergic afferents to locus ceruleus in the pericerulear dendritic zone: possible interneuronal pool. J Neurosci. 2004;24:2313–21. doi: 10.1523/JNEUROSCI.5339-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Kalivas PW. Brain norepinephrine rediscovered in addiction research. Biol Psychiatry. 2008;63:1005–6. doi: 10.1016/j.biopsych.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacci A, Huguenard JR, Prince DA. Long-lasting self-inhibition of neocortical interneurons mediated by endocannabinoids. Nature. 2004;431:312–6. doi: 10.1038/nature02913. [DOI] [PubMed] [Google Scholar]
- Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–72. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- Childers SR, Fleming L, Konkoy C, Marckel D, Pacheco M, Sexton T, Ward S. Opioid and cannabinoid receptor inhibition of adenylyl cyclase in brain. Ann N Y Acad Sci. 1992;654:33–51. doi: 10.1111/j.1749-6632.1992.tb25954.x. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP. Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther. 1999;289:859–67. [PubMed] [Google Scholar]
- Corchero J, Avila MA, Fuentes JA, Manzanares J. delta-9-Tetrahydrocannabinol increases prodynorphin and proenkephalin gene expression in the spinal cord of the rat. Life Sci. 1997;61:PL 39–43. doi: 10.1016/s0024-3205(97)00405-0. [DOI] [PubMed] [Google Scholar]
- Corchero J, Fuentes JA, Manzanares J. Gender differences in proenkephalin gene expression response to delta9-tetrahydrocannabinol in the hypothalamus of the rat. J Psychopharmacol. 2002;16:283–9. doi: 10.1177/026988110201600401. [DOI] [PubMed] [Google Scholar]
- Corchero J, Manzanares J, Fuentes JA. Cannabinoid/opioid crosstalk in the central nervous system. Crit Rev Neurobiol. 2004;16:159–72. doi: 10.1615/critrevneurobiol.v16.i12.170. [DOI] [PubMed] [Google Scholar]
- Demuth DG, Molleman A. Cannabinoid signalling. Life Sci. 2006;78:549–63. doi: 10.1016/j.lfs.2005.05.055. [DOI] [PubMed] [Google Scholar]
- Dhawan BN, Cesselin F, Raghubir R, Reisine T, Bradley PB, Portoghese PS, Hamon M. International Union of Pharmacology. XII. Classification of opioid receptors. Pharmacol Rev. 1996;48:567–92. [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann N Y Acad Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Espana RA, Berridge CW. Organization of noradrenergic efferents to arousal-related basal forebrain structures. J Comp Neurol. 2006;496:668–83. doi: 10.1002/cne.20946. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–66. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Harburg GC, Hall FS, Harrist AV, Sora I, Uhl GR, Eisch AJ. Knockout of the mu opioid receptor enhances the survival of adult-generated hippocampal granule cell neurons. Neuroscience. 2006 doi: 10.1016/j.neuroscience.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991;11:563–83. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999;822:17–25. doi: 10.1016/s0006-8993(98)01321-3. [DOI] [PubMed] [Google Scholar]
- Hojo M, Sudo Y, Ando Y, Minami K, Takada M, Matsubara T, Kanaide M, Taniyama K, Sumikawa K, Uezono Y. mu-Opioid receptor forms a functional heterodimer with cannabinoid CB1 receptor: electrophysiological and FRET assay analysis. J Pharmacol Sci. 2008;108:308–19. doi: 10.1254/jphs.08244fp. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Jahn R, Schiebler W, Ouimet C, Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985;82:4137–41. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Maldonado R, Stinus L. Neural substrates of opiate withdrawal. Trends Neurosci. 1992;15:186–91. doi: 10.1016/0166-2236(92)90171-4. [DOI] [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–4. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- Lichtman AH, Sheikh SM, Loh HH, Martin BR. Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J Pharmacol Exp Ther. 2001;298:1007–14. [PubMed] [Google Scholar]
- Lu XR, Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in monkey basal forebrain. J Neurocytol. 1999;28:1045–51. doi: 10.1023/a:1007052507911. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O. Participation of the opioid system in cannabinoid-induced antinociception and emotional-like responses. Eur Neuropsychopharmacol. 2003;13:401–10. doi: 10.1016/j.euroneuro.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res Mol Brain Res. 1998;55:126–32. doi: 10.1016/s0169-328x(97)00371-9. [DOI] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA. Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci. 1999;20:287–94. doi: 10.1016/s0165-6147(99)01339-5. [DOI] [PubMed] [Google Scholar]
- Massi P, Vaccani A, Rubino T, Parolaro D. Cannabinoids and opioids share cAMP pathway in rat splenocytes. J Neuroimmunol. 2003;145:46–54. doi: 10.1016/j.jneuroim.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Cannabinoids enhance N-methyl-D-aspartate-induced excitation of locus coeruleus neurons by CB1 receptors in rat brain slices. Neurosci Lett. 2004;363:1–5. doi: 10.1016/j.neulet.2004.02.073. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. Systemic effect of cannabinoids on the spontaneous firing rate of locus coeruleus neurons in rats. Eur J Pharmacol. 2006a;534:83–8. doi: 10.1016/j.ejphar.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2006b doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Mendiguren A, Pineda J. CB(1) cannabinoid receptors inhibit the glutamatergic component of KCl-evoked excitation of locus coeruleus neurons in rat brain slices. Neuropharmacology. 2007;52:617–25. doi: 10.1016/j.neuropharm.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Muntoni AL, Pillolla G, Melis M, Perra S, Gessa GL, Pistis M. Cannabinoids modulate spontaneous neuronal activity and evoked inhibition of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2006;23:2385–94. doi: 10.1111/j.1460-9568.2006.04759.x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Cellular responses to chronic treatment with drugs of abuse. Crit Rev Neurobiol. 1993;7:23–39. [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular and cellular mechanisms of opiate action: studies in the rat locus coeruleus. Brain Res Bull. 1994;35:521–8. doi: 10.1016/0361-9230(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Alreja M, Aghajanian GK. Molecular control of locus coeruleus neurotransmission. Biol Psychiatry. 1999;46:1131–9. doi: 10.1016/s0006-3223(99)00158-4. [DOI] [PubMed] [Google Scholar]
- Ong WY, Mackie K. A light and electron microscopic study of the CB1 cannabinoid receptor in the primate spinal cord. J Neurocytol. 1999;28:39–45. doi: 10.1023/a:1007011700677. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Page ME, Van Bockstaele EJ. Systemic administration of WIN 55,212-2 increases norepinephrine release in the rat frontal cortex. Brain Res. 2005;1046:45–54. doi: 10.1016/j.brainres.2005.03.036. [DOI] [PubMed] [Google Scholar]
- Oropeza VC, Mackie K, Van Bockstaele EJ. Cannabinoid receptors are localized to noradrenergic axon terminals in the rat frontal cortex. Brain Res. 2007;1127:36–44. doi: 10.1016/j.brainres.2006.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Sparks SE, Qian Y, Menko AS, Van Bockstaele EJ. Repeated cannabinoid administration increases indices of noradrenergic activity in rats. Pharmacol Biochem Behav. 2007;86:162–8. doi: 10.1016/j.pbb.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page ME, Oropeza VC, Van Bockstaele EJ. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci Lett. 2008;431:1–5. doi: 10.1016/j.neulet.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paldy E, Bereczki E, Santha M, Wenger T, Borsodi A, Zimmer A, Benyhe S. CB(2) cannabinoid receptor antagonist SR144528 decreases mu-opioid receptor expression and activation in mouse brainstem: Role of CB(2) receptor in pain. Neurochem Int. 2008;53:309–16. doi: 10.1016/j.neuint.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Pasternak GW. Molecular biology of opioid analgesia. J Pain Symptom Manage. 2005;29:S2–9. doi: 10.1016/j.jpainsymman.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Paxinos G, W C. The Rat Brain in Sterotaxic Coordinates. Vol. Academic Press; New York: 1986. [Google Scholar]
- Peters A, Palay S, Webster H. The Fine Structure of the Nervous System. Vol. Oxford University Press; New York: 1991. [Google Scholar]
- Pickel VM, Chan J, Kash TL, Rodriguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–12. doi: 10.1016/j.neuroscience.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Aghajanian GK. Withdrawal-induced activation of locus coeruleus neurons in opiate-dependent rats: attenuation by lesions of the nucleus paragigantocellularis. Brain Res. 1989;505:346–50. doi: 10.1016/0006-8993(89)91466-2. [DOI] [PubMed] [Google Scholar]
- Rasmussen K, Beitner-Johnson DB, Krystal JH, Aghajanian GK, Nestler EJ. Opiate withdrawal and the rat locus coeruleus: behavioral, electrophysiological, and biochemical correlates. J Neurosci. 1990;10:2308–17. doi: 10.1523/JNEUROSCI.10-07-02308.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes BA, Glaser JD, Van Bockstaele EJ. Ultrastructural evidence for co-localization of corticotropin-releasing factor receptor and mu-opioid receptor in the rat nucleus locus coeruleus. Neurosci Lett. 2007;413:216–21. doi: 10.1016/j.neulet.2006.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–95. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, Mackie K, Pickel VM. Ultrastructural localization of the CB1 cannabinoid receptor in mu-opioid receptor patches of the rat Caudate putamen nucleus. J Neurosci. 2001;21:823–33. doi: 10.1523/JNEUROSCI.21-03-00823.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenfeld R, Devi LA. Regulation of CB1 cannabinoid receptor trafficking by the adaptor protein AP-3. Faseb J. 2008;22:2311–22. doi: 10.1096/fj.07-102731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Massi P, Vigano D, Fuzio D, Parolaro D. Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrome. Life Sci. 2000;66:2213–9. doi: 10.1016/s0024-3205(00)00547-6. [DOI] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M. CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12:3689–92. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Hogenboom F, Wardeh G, De Vries TJ. Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology. 2006;51:773–81. doi: 10.1016/j.neuropharm.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP. Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol. 2007;571:129–37. doi: 10.1016/j.ejphar.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo B, Schlicker E. Effects of cannabinoids on neurotransmission. Handb Exp Pharmacol. 2005:327–65. doi: 10.1007/3-540-26573-2_11. [DOI] [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE. Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol. 2005;144:875–84. doi: 10.1038/sj.bjp.0706045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino R, Aston-Jones G. Physiological and Anatomical Determinants of Locus Coeruleus Discharge: Behavioral and Clinical Implications. American College of Neuropsychopharmacology. Psychopharmacology 2000 [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP. Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur J Neurosci. 2001;13:1816–24. doi: 10.1046/j.0953-816x.2001.01558.x. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pickel VM. Ultrastructure of serotonin-immunoreactive terminals in the core and shell of the rat nucleus accumbens: cellular substrates for interactions with catecholamine afferents. J Comp Neurol. 1993;334:603–17. doi: 10.1002/cne.903340408. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Colago EE, Cheng P, Moriwaki A, Uhl GR, Pickel VM. Ultrastructural evidence for prominent distribution of the mu-opioid receptor at extrasynaptic sites on noradrenergic dendrites in the rat nucleus locus coeruleus. J Neurosci. 1996;16:5037–48. doi: 10.1523/JNEUROSCI.16-16-05037.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Peoples J, Menko AS, McHugh K, Drolet G. Decreases in endogenous opioid peptides in the rat medullo-coerulear pathway after chronic morphine treatment. J Neurosci. 2000;20:8659–66. doi: 10.1523/JNEUROSCI.20-23-08659.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Commons KG. Internalization of mu-opioid receptors produced by etorphine in the rat locus coeruleus. Neuroscience. 2001;108:467–77. doi: 10.1016/s0306-4522(01)00426-2. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Menko AS, Drolet G. Neuroadaptive responses in brainstem noradrenergic nuclei following chronic morphine exposure. Mol Neurobiol. 2001;23:155–71. doi: 10.1385/mn:23:2-3:155. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D. Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav. 2005;81:360–8. doi: 10.1016/j.pbb.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Wang HY, Burns LH. Gbetagamma that interacts with adenylyl cyclase in opioid tolerance originates from a Gs protein. J Neurobiol. 2006;66:1302–10. doi: 10.1002/neu.20286. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–51. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–28. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wilson AR, Maher L, Morgan MM. Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology. 2008;55:1219–25. doi: 10.1016/j.neuropharm.2008.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1) Control experiments were performed to examine secondary antibody specificity. LC tissue was processed identically, with omission of the primary antibody, to assess the level of non-specific background labeling from the secondary antibodies. Using fluorescein isothiocyanate (FITC) conjugated donkey-anti mouse IgG (A), tetramethyl rhodamine isothiocyanate (TRITC) conjugated donkey anti-rabbit (B), Alexa Fluor 488 donkey anti-rabbit IgG (C), and Rhodamine Red-X conjugated donkey anti-guinea pig (D), minimal background labeling was observed. Scale bar = 25μm.