Abstract
Background
Low-intensity pulsed ultrasound stimulation (LIPUS) reportedly increases osteogenesis in fracture models but fails in intact bone, suggesting LIPUS does not act on mechanotransduction and growth factor pathways of intact bone.
Questions/Purposes
We asked whether daily 20-minute LIPUS applied to intact tibias would act on bone proteins involved in mechanotransduction (focal adhesion kinase [FAK], and extracellular signal-regulated kinase-1/2 [ERK-1/2]), and growth factor signaling (insulin receptor substrate-1 [IRS-1]) pathways at 7, 14, and 21 days of treatment.
Methods
Immunoblotting was performed to detect FAK, ERK-1/2, and IRS-1 expression and activation from the stimulated intact tibias at 7, 14, and 21 days of daily 20-minute LIPUS.
Results
LIPUS increased FAK expression (at 7 days), ERK-1/2 (at 14 days), and IRS-1 (at 7 days), but expression decreased 7 days later, indicating a noncumulative effect of LIPUS. As only FAK expression was detected at 21 days, these observations suggest LIPUS influences nuclear reactions that may be modulated by a major cellular mechanism preferentially inhibiting IRS-1 expression and not FAK expression. Increased ERK-1/2 expression at 14 days suggests the differing mechanisms for promoting ERK-1/2, FAK, and IRS-1 syntheses. IRS-1 expression behaved similarly to FAK expression; therefore, LIPUS may modulate growth factor pathways. LIPUS increased sustained FAK and ERK-1/2 activation, but not IRS-1, suggesting sustained ERK-1/2 activation is not the result of mechanically induced growth factor activation.
Conclusions
LIPUS acts on mechanotransduction and growth factor pathways in intact bone in a noncumulative manner.
Clinical relevance These data suggest LIPUS applied to intact bone acts on proteins involved in osteogenesis.
Introduction
Among the factors that influence fracture healing, LIPUS distinguishes itself by being noninvasive and easy to apply. LIPUS reportedly accelerates bone healing in animals [2, 10, 33] and humans [15, 16, 24] from the first week of stimulation onward [18, 40]. Despite its pronounced effects during fracture repair, LIPUS does not increase bone mineral density or prevent bone loss of intact bone [21, 35, 41, 42, 44]. These observations suggest LIPUS cannot activate proteins that act in mechanotransduction signaling pathways or growth factor signaling pathways as both pathways lead to bone formation of intact bone.
Although the underlying molecular mechanisms of action of LIPUS, and of other modalities of mechanical stimulation, remain unclear, in vitro studies suggest mechanical loading on bone cells induces integrin clustering at focal adhesions in bone cells, leading to FAK autophosphorylation at Tyr-397 (P-FAK). FAK is considered one of the main proteins responsible for integrin-mediated mechanically induced bone formation. P-FAK couples with various proteins to activate mechanotransduction signaling pathways. P-FAK/ERK-1/2 is one important mechanotransduction signaling pathway that promotes bone formation [4, 25, 36].
In vitro mechanical stimulation can act synergistically with growth factors such as parathyroid hormone and insulinlike growth factor-1 (IGF-1) to induce osteogenesis [17, 22]. IGF-1 incites bone formation through the IRS-1/ERK-1/2 signaling pathway [30]. However, it is unclear whether LIPUS stimulates IGF-1 expression in bone cells. Some authors report relatively young osteoblasts responded to LIPUS by transiently upregulating message levels of IGF-1 [28], whereas others suggest LIPUS apparently had no effect on IGF-1 expression in clonal rodent osteosarcoma cells [43].
We therefore asked whether (1) LIPUS modulates the activation and expression of bone proteins involved in mechanically induced bone formation such as FAK and ERK-1/2, and (2) LIPUS modulates the activation and expression of bone proteins involved in growth factor-induced bone formation such as IRS-1 and ERK-1/2.
Materials and Methods
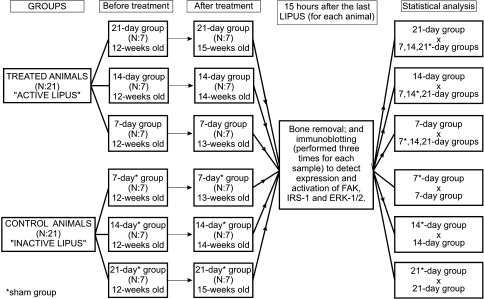
Forty-two male Unib: WH rats, aged 12 weeks, obtained from the CEMIB—Multidisciplinary Center for Biological Investigation, were assigned randomly to a treatment or control (sham treatment) group. A daily 20-minute LIPUS was applied to the lateral aspect of both hind limbs of each animal in the treatment groups, whereas sham stimulation was performed with inactive LIPUS (the device was turned off) to the control groups (sham groups). Animals were followed until the 7th, 14th, or 21st day of LIPUS. Fifteen hours after the last LIPUS of each animal, each pair of tibias and fibulas was harvested and used to detect the expression and activation of FAK, IRS-1, and ERK-1/2 by immunoblotting to determine if there were any differences between treated and control groups. Immunoblotting to detect activation or expression of each protein was performed three times (triplicate) for each sample (Fig. 1). Throughout the experiment, all animals were maintained in the same location in a 25°C environment with 12-hour/12-hour artificial light/dark cycles and received drinking water and a standard diet. The experimental procedures were approved by the Institutional Animal Care and Use Ethics Committee (protocol 873-1) and were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (National Institutes of Health publication number 85–23, revised in 1996).
Fig. 1.
A schematic representation of the study design is shown.
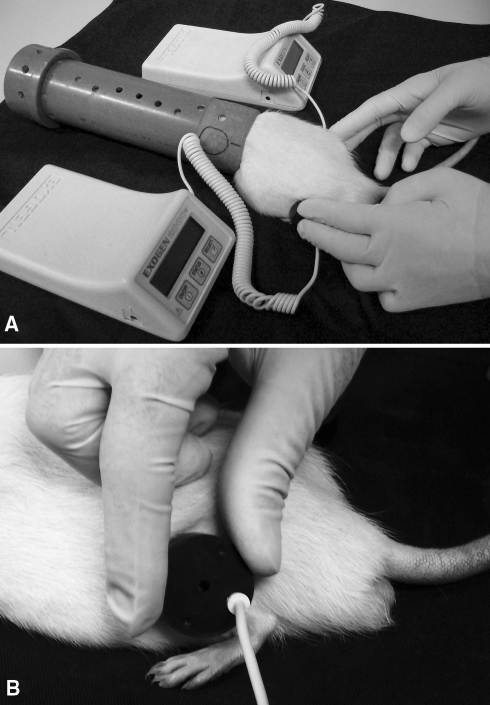
To simultaneously stimulate the pair of tibias and fibulas, we used two noninvasive devices generating a 1.5-MHz ultrasound in a pulse wave mode (200-μs burst width with repetitive frequency of 1 KHz at an intensity of 30 mW/cm2), Exogen 2000+™ (Smith & Nephew Inc, Memphis, TN). These were activated for 20 minutes daily. An integral timer monitored length of treatment and automatically turned the unit off after 20 minutes of stimulation. During the LIPUS, each rat was kept in a polyvinyl chloride tube, closed at one end to prevent escape of the animal. Only the hind limbs were kept outside the tube to allow contact of the transducer of the device on the lateral aspect of the hind limb. The tube was hollow so that the animal could breathe (Fig. 2). Control animals received sham LIPUS, which consisted of identical handling procedures with the exception of turning the device off.
Fig. 2A–B.
These photographs show low-intensity pulsed ultrasound stimulation (LIPUS) on rats’ hind limbs. (A) During LIPUS, each rat was maintained in a hollowed tube, (B) and the lateral aspects of both hind limbs were simultaneously stimulated.
Reagents and equipment for sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) electrophoresis and immunoblotting were obtained from Bio-Rad (Richmond, CA). Tris[hydroxymethyl]amino-methane (Tris), aprotinin, dithiothreitol (DTT), phenylmethylsulfonyl fluoride (PMSF), Triton X-100, Tween 20, and glycerol were obtained from Sigma Chemical Co (St Louis, MO). Nitrocellulose paper (Hybond ECL, 0.45 μm) was obtained from Amersham Pharmacia Biotech (Buckinghamshire, UK). Rabbit polyclonal antibodies specific for FAK (sc-557), FAK-Tyr(P)-397 (sc-11765-R), IRS-1-Tyr(P)-941 (sc-17199-R), ERK-2 (sc-154), and actin (sc-7210) and mouse polyclonal antibodies specific for ERK-1/2-Tyr(P)-204 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody specific for IRS-1 (#2382) was obtained from Cell Signaling Technology Inc (Danvers, MA). Horseradish peroxidase-conjugated goat antirabbit and antimouse and enhanced chemiluminescence detection reagents were obtained from Pierce Biotechnology Inc (Rockford, IL).
Fifteen hours after its last sonication (seventh stimulus for each animal of the 7-day and 7-day sham groups, 14th stimulus for each animal of the 14-day and 14-day sham groups, and 21st stimulus for each animal of the 21-day and 21-day sham groups), each rat was anesthetized by intraperitoneal injection of sodium thiopental, 50 mg/kg, and both tibias and fibulas were harvested. After this, the rats were euthanized by heart puncture (consistent with the recommendations of the Institutional Animal Care and Use Ethics Committee) [1]. Each group of four bones (a pair of tibias and a pair of fibulas) harvested from each animal was subjected to the following procedures.
The bones were immediately immersed in a mortar containing liquid nitrogen and then enclosed in a plastic bag and fragmented with a hammer. The resulting fragments were placed in the mortar containing liquid nitrogen and ground with a pestle until the fragments became a powder. The powder was immersed in liquid nitrogen and ground again. The resulting powder was introduced in a test tube containing 2 mL of ice-cold buffer (100 mmol/L Tris, pH 7.4; 10 mmol/L EDTA; 10 mmol/L sodium pyrophosphate; 100 mmol/L sodium fluoride; 10 mmol/L sodium orthovanadate; 2 mmol/L PMSF; 0.1 mg/mL aprotinin) followed by two homogenization sessions with Polytron PTA 20S (PT 10/35; Brinkmann Instruments, Westbury, NY) for 5 seconds at maximum speed and with an interval of 20 seconds between each session. After that, 10% Triton X-100 was added to the samples. Forty minutes later, the insoluble material was removed by centrifugation at 11,000 rpm for 30 minutes at 4°C, and the supernatant was transferred to fresh Eppendorf tubes (Eppendorf, Oldenburg, Germany) and stored with Laemmli buffer containing 200 mmol/L DTT 1:4 (one portion of Laemmli buffer containing DTT four portions of volume sample). Another portion of the supernatant was used to determine the protein concentration of each sample by the Biuret colorimetric method.
Aliquots corresponding to 250 μg of total protein were run on a 12% SDS-PAGE of 1.5 mm width with electrophoresis buffer (200 mmol/L Tris; 1.52 mol/L glycin; 7.18 mmol/L EDTA; 0.4% SDS) previously diluted in H2O (1:3). Subsequently, the proteins were transferred to nitrocellulose membranes in loading buffer (25 mmol/L Tris; 192 mmol/L glycin; 20% methanol; 0.02% SDS) at 120 V for 2 hours under ice refrigeration. The membranes were blocked with 5% nonfat dried milk in PBS-Tween 20 solution (150 mmol/L sodium chloride; 10 mmol/L Tris; 0.02% Tween 20) at room temperature for 2 hours followed by three washes with phosphate-buffered saline for 10 minutes and were probed overnight (12 hours) at 4°C under continuous shaking with specific antibodies for FAK, IRS-1, IRS-1-Tyr(P)-941, ERK-2, or ERK-1/2-Tyr(P)-204 (1:1000), or specific for FAK-Tyr(P)-397 (1:3000). For normalization purposes, the same blot also was probed with actin antibody (1:1000). The membranes then were washed again three times for 8 minutes with phosphate-buffered saline and probed with horseradish peroxidase-conjugated goat-antirabbit or antimouse (1:7500) for 2 hours at room temperature. The membranes were washed three times with phosphate-buffered saline for 10 minutes, and the immunoreactive bands were detected using enhanced chemiluminescense detection reagents. The findings were observed by autoradiography using preflashed Kodak XAR film (Eastman Kodak, Rochester, NY).
Band intensities were quantified by optical densitometry of developed autoradiographs on an imaging densitometer (UN-SCAN-IT gel™; Silk Scientific Corporation, Orem, UT). For calculations, the band intensities were calibrated automatically against the background by the software using regions adjacent to each spot. ERK has two bands that correspond to ERK-1 (44 kDa) and ERK-2 (42 kDa). Because it is not established which ERK isoform is more important for bone mechanotransduction, the sum of band intensities of both ERK isoforms was used to indicate ERK-1/2 expression.
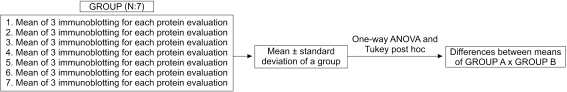
Means of the band intensities obtained from three independent immunoblottings (triplicate) to detect expression and activation of a protein of each sample were used to determine the mean ± standard deviation of each group. One-way ANOVA (α = 0.05) followed by the Tukey post hoc test was performed using Minitab® 15.1 (Minitab Inc, State College, PA) to determine the differences in FAK, IRS-1, and ERK-1/2 expression and activation between treated groups and their respective sham groups, and between the treated groups (Fig. 3).
Fig. 3.
For each rat of a group, the mean of the results obtained from three independent immunoblottings (triplicate) performed to evaluate a protein (focal adhesion kinase [FAK], insulin receptor substrate-1 [IRS-1], or extracellular signal-regulated kinase-1/2 [ERK-1/2]) expression or activation was used to determine the mean ± standard deviation of each group. One-way ANOVA and Tukey’s post hoc test were used to determine differences of the expression or activation of FAK, IRS-1, or ERK-1/2 between groups.
Results
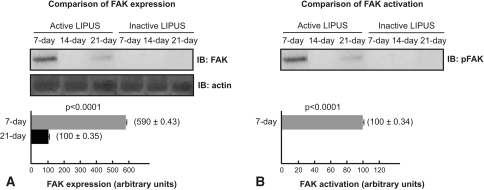
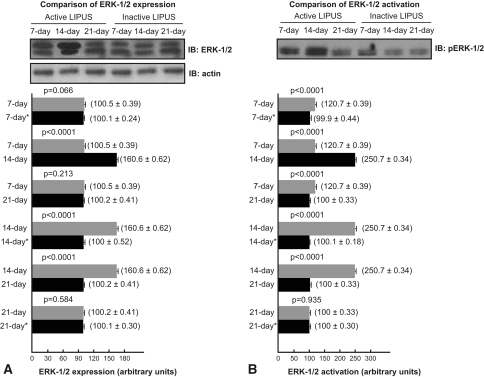
LIPUS modulated the activation and expression of FAK and ERK-1/2. We observed no FAK expression and activation by immunoblotting in control groups and in the 14-day treated group. FAK expression was observed in the 7-day and 21-day treated groups; and P-FAK was identified in the 7-day treated group 15 hours after the last LIPUS, but not in 21-day treated group. The 7-day treated group exhibited 5.9-fold higher expression (p < 0.0001) of FAK in comparison to the 21-day treated group (Fig. 4). ERK-1/2 expression and activation, in turn, were detected by immunoblotting in all treated and control groups. ERK-1/2 expression in the 7-day treated group was not increased compared with expression in the 7-day sham group (p = 0.066) and 21-day treated group (p = 0.213); but ERK-1/2 expression was 60% higher in the 14-day treated group compared with expression in the 7-day treated group (p < 0.0001), 14-day sham group (p < 0.0001), and 21-day treated group (p < 0.0001), and ERK-1/2 expression in the 21-day treated group was not increased compared with expression in the 21-day sham group (p = 0.584) (Fig. 5A). Moreover, ERK-1/2 phosphorylated (activated) at Tyr-204 (P-ERK-1/2) 15 hours after the last LIPUS in the 7-day treated group was 1.2-fold greater than in the 7-day sham group (p < 0.0001) and 21-day treated group (p < 0.0001); P-ERK-1/2 augmented 2.5-fold in the 14-day treated group compared with the 14-day sham group (p < 0.0001) and the 21-day treated group (p < 0.0001); P-ERK-1/2 in the 14-day treated group was 2.08-fold greater than in the 7-day treated group (p < 0.0001); and P-ERK1/2 in the 21-day treated group was unaltered compared with the 21-day sham group (p = 0.935) (Fig. 5B).
Fig. 4A–B.
(A) Low-intensity pulsed ultrasound stimulation (LIPUS) increased focal adhesion kinase (FAK) expression at 7 and 21 days. (B) LIPUS increased P-FAK in the 7-day group. P-FAK was not detected in the control groups, 14-day group, and 21-day group. IB = immunoblotting.
Fig. 5A–B.
(A) Low-intensity pulsed ultrasound stimulation (LIPUS) increased extracellular signal-regulated kinase-1/2 (ERK-1/2) expression at 14 days. ERK-1/2 expression was similar in the 7-day group compared with the 21-day group and compared with their respective sham groups. (B) LIPUS increased P-ERK-1/2 in the 7-day and 14-day groups. P-ERK-1/2 was similar in the 21-day group compared with its sham group. IB = immunoblotting; * = control group.
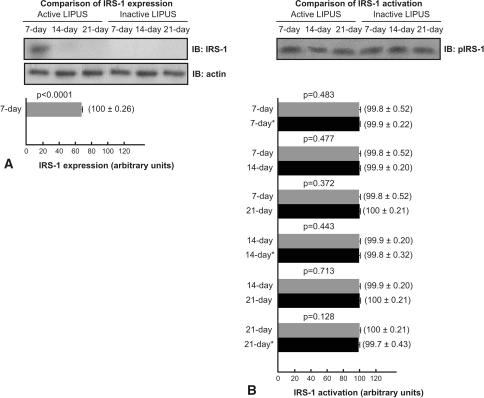
All groups showed IRS-1 phosphorylated at Tyr-941 (P-IRS-1) 15 hours after the last LIPUS. IRS-1 expression was not detected by immunoblotting in the control groups, the 14-day treated group, and the 21-day treated group, whereas IRS-1 was detected in the 7-day treated group (Fig. 6). P-IRS-1 was unaltered in the 7-day treated group compared with the 7-day sham group (p = 0.483), 14-day treated group (p = 0.477), and 21-day treated group (p = 0.372); P-IRS-1 was unaltered in the 14-day treated group compared with the 14-day sham group (p = 0.443) and 21-day treated group (p = 0.713); and P-IRS-1 was unaltered in the 21-day treated group compared with the 21-day sham group (p = 0.128).
Fig. 6A–B.
(A) Low-intensity pulsed ultrasound stimulation (LIPUS) increased insulin receptor substrate-1 (IRS-1) expression at 7 days. IRS-1 expression was not detected in control groups, the 14-day group, and the 21-day group. (B) There was no prolonged increase in IRS-1 activation in 7-day, 14-day, and 21-day groups compared with their respective controls. IB = immunoblotting; * = control group.
Discussion
Because LIPUS stimulates fracture repair but does not increase bone mineral density on growing bone or prevent osteoporosis after spinal cord injury and estrogen deprivation [21, 35, 41, 42, 44], we questioned whether LIPUS on intact bone would regulate FAK and ERK-1/2 expression and activation, which are proteins that act on mechanotransduction signaling and induce osteogenesis [36]. In addition, because mechanical loading acts synergistically with IGF-1 to induce osteogenesis in vitro, and IGF-1 expression may be increased by LIPUS in vitro, we questioned whether LIPUS on intact bone would regulate IRS-1 and ERK-1/2 expression and activation, which are proteins that act on IGF-1 signaling and induce osteogenesis [17, 28, 30, 43].
Our study is subject to some limitations. First, because it is difficult to isolate proteins from osteocytes, osteoblasts, osteoclasts, and bone marrow cells, our findings constitute the response of all bone cells. Osteocytes are 90% to 95% of bone cells [3], and therefore we presume the main response to LIPUS may come from them. Whichever the cell type responsible for increased protein activation or expression, leading to bone formation or resorption, our objective is to determine whether intact bone responds to LIPUS. Second, we used 12-week-old Unib: WH (Wistar) rats. They are still growing because long bones of Unib: WH rats do not exhibit epiphyseal union [7, 8]. Twelve-week-old rats’ bone cell activity may differ from that of older rats. Nevertheless, LIPUS does not prevent bone loss in growing rats after sciatic neurectomy either in skeletally mature humans after spinal cord injury, suggesting mature and young bones do not exhibit different responses to LIPUS [42, 44]. Third, FAK and IRS-1 expression in intact bone subjected to habitual loading may be low; as such, immunoblotting was not sensitive enough to detect their expression in control groups. However, ERK-1/2 expression may be greater because it was detected in all groups. Greater expression of ERK-1/2 compared with FAK and IRS-1 is expected because ERK-1/2 is the target of various proteins, FAK [25], IRS-1 [37], PI3 K [13], PKC [23], Ca2+ channel [6], and others, and exerts important functions in bone, including differentiation, proliferation, and cell survival [19, 23, 25].
LIPUS augmented FAK and ERK-1/2 expression at 7 and 14 days, respectively, indicating long-term LIPUS not only influences cytoplasmic reactions [9, 36, 45], but also affects nuclear reactions, which induce FAK and ERK-1/2 synthesis. Seven days after the augmentation of FAK (at 14 days) and ERK-1/2 (at 21 days) expression, their expression decreased, showing a noncumulative effect of LIPUS on intact bone and suggesting there is a regulatory mechanism in bone cells, similar to negative feedback, that is activated after the augmentation of FAK and ERK-1/2 expression. In healthy bone, this mechanism may have antineoplastic finalities, because FAK is persistently increased in neoplasia [5, 12]. The failure of LIPUS to increase bone growth [35] may be explained by a noncumulative effect. In addition to the noncumulative effect of LIPUS, several observations may explain the failure of ultrasound to prevent bone loss in humans and animals deficient in estrogen or after neurectomy [21, 41, 42, 44]: (1) the absence of the estrogen receptor α diminishes 70% of mechanically induced bone formation and completely inhibits mechanically induced bone cell proliferation [20], and (2) neurectomized individuals exhibit muscular atrophy, which may impair osteogenesis [38, 39]. Some inflammatory mediators present during fracture healing may regulate bone cells response to LIPUS so that there is a cumulate rather than noncumulative effect on fractured bone; this being the case, LIPUS could increase osteogenesis in fractured bone [2, 15]. Nitric oxide and prostaglandin E2 are inflammatory mediators that regulate bone formation and are responsive to in vitro LIPUS [31]. Therefore, they are strong candidates to participate in the regulatory mechanism of bone cells’ response to LIPUS on intact and fractured bone. Because ERK-1/2 is a target of FAK, increased and prolonged ERK-1/2 activation at 7 days was expected. Nevertheless, ERK-1/2 expression and prolonged activation peaked at 14 days, when FAK expression and prolonged activation diminished. Probably, other ERK-1/2 upstream proteins, not FAK, have exhibited augmented activation at 14 days and increased P-ERK-1/2; and the mechanisms by which LIPUS increases FAK and ERK-1/2 expression are different, both regulated by a major regulatory mechanism. Studies suggest sustained P-ERK-1/2 more effectively regulates nuclear reactions than intermittent P-ERK-1/2 [11, 27, 32, 46]. How long-term LIPUS induced sustained increased P-FAK and P-ERK-1/2 is unknown. One mechanical stimulation increases P-FAK and P-ERK-1/2 for 4 hours [4, 14, 26, 36], whereas our long-term LIPUS not only increased P-FAK and P-ERK-1/2, but also prolonged their activation for 15 hours at 7 and 14 days (only ERK-1/2). P-FAK and P-ERK-1/2 peaks coincided with their expression peaks, supporting the observation that sustained P-FAK and P-ERK-1/2 elevation are proportional to the elevation of their expression [46]. Nevertheless, P-FAK was not detected at 21 days, suggesting the LIPUS regulatory mechanism may act more on FAK activity than on its expression.
IRS-1 participates in IGF-1 signaling pathways, cellular proliferation, and extracellular matrix synthesis and is indispensable for maintaining bone turnover, growth, and repair in response to hormonal stimulation [29, 30, 34]. IRS-1 expression behaved similarly to FAK expression at 7 and 14 days, indicating LIPUS acts on IRS-1 synthesis, probably as a result of mechanically induced increased IGF-1 expression [28]. However, IRS-1 expression decreased at 14 and 21 days, suggesting the regulatory mechanism acts preferentially on IRS-1 expression than on FAK expression. Because IRS-1 acts on signaling of growth factors, the LIPUS regulatory mechanism may act preferentially on growth factor pathways than on mechanotransduction pathways. Surprisingly, we detected P-IRS-1 in all groups, but this finding does not invalidate the findings with IRS-1 expression: despite the difficulty in detecting IRS-1 expression at 14 and 21 days we did detect increased IRS-1 expression at 7 days. P-IRS-1 was unaltered in all groups, indicating long-term LIPUS does not induce prolonged P-IRS-1. Earlier evaluation of IRS-1 (less than 1 hour after LIPUS) showed increased P-IRS-1 at 7 days (data not shown). Because LIPUS did not induce prolonged P-IRS-1, it is less probable that increased ERK-1/2 expression and activation were the result of mechanically induced growth factor pathway activation.
Our data suggest long-term LIPUS acts, in a noncumulative manner, on mechanotransduction pathways through increased FAK and ERK-1/2 activation and expression and on growth factors’ pathways through increased IRS-1 expression, probably as a result of IGF-1 mechanically induced increased expression. A major regulatory mechanism may exist in bone cells and respond to long-term LIPUS by inhibiting preferentially FAK activation and IRS-1 expression to inhibit osteogenesis.
Acknowledgments
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), which granted Mr Carlos Vinícius Buarque de Gusmão a “scientific initiation” scholarship.
Footnotes
One or more of the authors (CVBG) have received funding from a “scientific initiation” scholarship from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at The State University of Campinas (UNICAMP), Campinas, São Paulo, Brazil.
References
- 1.AVMA Guidelines on Euthanasia (Formerly Report of the AVMA Panel on Euthanasia). Available at: http://www.avma.org/issues/animal_welfare/euthanasia.pdf. Accessed September 20, 2009.
- 2.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16:671–680. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 3.Bonewald LF. Mechanosensation and transduction in osteocytes. Bonekey Osteovision. 2006;3:7–15. doi: 10.1138/20060233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279:30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 5.Chatzizacharias NA, Kouraklis GP, Theocharis SE. Clinical significance of FAK expression in human neoplasia. Histol Histopathol. 2008;23:629–650. doi: 10.14670/HH-23.629. [DOI] [PubMed] [Google Scholar]
- 6.Danciu TE, Adam RM, Naruse K, Freeman MR, Hauschka PV. Calcium regulates the PI3 K-Akt pathway in stretched osteoblasts. FEBS Lett. 2003;536:193–197. doi: 10.1016/S0014-5793(03)00055-3. [DOI] [PubMed] [Google Scholar]
- 7.Dawson AB. The age order of epiphyseal union in the long bones of the albino rat. Anat Rec. 1925;31:117. doi: 10.1002/ar.1090310102. [DOI] [Google Scholar]
- 8.Dawson AB. Further studies on the epiphyses of the albino rat skeleton, with special reference to the vertebral column, ribs, sternum and girdles. Anat Rec. 1927;34:351–363. doi: 10.1002/ar.1090340506. [DOI] [Google Scholar]
- 9.Doan N, Reher P, Meghji S, Harris M. In vitro effects of therapeutic ultrasound on cell proliferation, protein synthesis, and cytokine production by human fibroblasts, osteoblasts and monocytes. J Oral Maxillofac Surg. 1999;57:409–419. doi: 10.1016/S0278-2391(99)90281-1. [DOI] [PubMed] [Google Scholar]
- 10.Duarte LR. The stimulation of bone growth by ultrasound. Arch Orthop Trauma Surg. 1983;101:153–159. doi: 10.1007/BF00436764. [DOI] [PubMed] [Google Scholar]
- 11.Ebisuya M, Kondoh K, Nishida E. The duration, magnitude and compartmentalization of ERK MAP kinase activity: mechanisms for providing signaling specificity. J Cell Sci. 2005;118:2997–3002. doi: 10.1242/jcs.02505. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel B, zur Hausen A, Stickeler E, Dietz C, Gitsch G, Fischer DC, Boulda J, Tempfer C, Hasenburg A. Weak expression of focal adhesion kinase (pp125FAK) in patients with cervical cancer is associated with poor disease outcome. Clin Cancer Res. 2006;12:2476–2483. doi: 10.1158/1078-0432.CCR-05-1867. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh-Choudhury N, Mandal CC, Choudhury GG. Statin-induced Ras activation integrates the phosphatidylinositol 3-kinase signal to Akt and MAPK for bone morphogenetic protein-2 expression in osteoblast differentiation. J Biol Chem. 2007;282:4983–4993. doi: 10.1074/jbc.M606706200. [DOI] [PubMed] [Google Scholar]
- 14.Guignandon G, Boutahar N, Rattner A, Vico L, Lafage-Proust M. Cyclic strain promotes shuttling of PYK2/Hic-5 complex from focal contacts in osteoblast-like cells. Biochem Biophys Res Commun. 2006;343:407–414. doi: 10.1016/j.bbrc.2006.02.162. [DOI] [PubMed] [Google Scholar]
- 15.Heckman JD, Ryaby JP, McCabe J, Frey JJ, Kilcoyne RF. Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound. J Bone Joint Surg Am. 1994;76:26–34. doi: 10.2106/00004623-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Jingushi S, Mizuno K, Matsushita T, Itoman M. Low-intensity pulsed ultrasound treatment for postoperative delayed union or nonunion of long bone fractures. J Orthop Sci. 2007;12:35–41. doi: 10.1007/s00776-006-1080-3. [DOI] [PubMed] [Google Scholar]
- 17.Kapur S, Mohan S, Baylink DJ, Lau K-HW. Fluid shear stress synergizes with insulin-like growth factor-I (IGF-I) on osteoblast proliferation through integrin-dependent activation of IGF-I mitogenic signaling pathway. J Biol Chem. 2005;280:20163–20170. doi: 10.1074/jbc.M501460200. [DOI] [PubMed] [Google Scholar]
- 18.Kristiansen TK, Ryaby JP, McCabe J, Frey JJ, Roe LR. Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound: a multicenter, prospective, randomized, double-blind, placebo-controlled study. J Bone Joint Surg Am. 1997;79:961–973. doi: 10.2106/00004623-199707000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Lai CF, Claudhary L, Fausto A, Halstead LR, Ory DS, Avioli LV, Cheng SL. ERK is essential for growth, differentiation, integrin expression, and cell function in human osteoblastic cells. J Biol Chem. 2001;276:14443–14450. doi: 10.1074/jbc.M010021200. [DOI] [PubMed] [Google Scholar]
- 20.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-α. Nature. 2003;424:389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 21.Leung KS, Lee WS, Cheung WH, Qin L. Lack of efficacy of low-intensity pulsed ultrasound on prevention of postmenopausal bone loss evaluated at the distal radius in older Chinese women. Clin Orthop Relat Res. 2004;427:234–240. doi: 10.1097/01.blo.0000137557.59228.4d. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Duncan RL, Burr DB, Gattone VG, Turner CH. Parathyroid hormone enhances mechanically induced bone formation, possibly involving L-type voltage-sensitive calcium channels. Endocrinology. 2003;144:1226–1233. doi: 10.1210/en.2002-220821. [DOI] [PubMed] [Google Scholar]
- 23.Liedbert A, Kaspar D, Blakytny R, Claes L, Ignatius A. Signal transduction pathways involved in mechanotransduction in bone cells. Biochem Biophys Res Commun. 2006;349:1–5. doi: 10.1016/j.bbrc.2006.07.214. [DOI] [PubMed] [Google Scholar]
- 24.Mayr E, Frankel V, Rüter A. Ultrasound: an alternative healing method for nonunions? Arch Orthop Trauma Surg. 2000;120:1–8. doi: 10.1007/pl00021234. [DOI] [PubMed] [Google Scholar]
- 25.Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6:56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- 26.Moalli MR, Wang S, Caldwell NJ, Patil PV, Maynard CR. Mechanical stimulation induces pp125FAK and pp60src activity in an in vivo model of trabecular bone formation. J Appl Physiol. 2001;91:912–918. doi: 10.1152/jappl.2001.91.2.912. [DOI] [PubMed] [Google Scholar]
- 27.Murphy LO, Blenis J. MAPK signal specificity: the right place at the right time. Trends Biochem Sci. 2006;31:268–275. doi: 10.1016/j.tibs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 28.Naruse K, Miyauchi A, Itoman M, Mikuni-Takagaki Y. Distinct anabolic response of osteoblast to low-intensity pulsed ultrasound. J Bone Miner Res. 2003;18:360–369. doi: 10.1359/jbmr.2003.18.2.360. [DOI] [PubMed] [Google Scholar]
- 29.Ogata N, Chikazu D, Kubota N, Terauchi Y, Tobe K, Azuma Y, Ohta T, Kadowaki T, Nakamura K, Kawaguchi H. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone. J Clin Invest. 2000;105:935–943. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrini S, Natalicchio A, Laviola L, Cignarelli A, Melchiorre M, Stefano F, Caccioppoli C, Leonardini A, Martemucci S, Belsanti G, Miccoli S, Ciampolillo A, Corrado A, Cantatore FP, Giorgino R, Giorgino F. Abnormalities of insulin-like growth factor-I signaling and impaired cell proliferation in osteoblasts from subjects with osteoporosis. Endocrinology. 2008;149:1302–1313. doi: 10.1210/en.2007-1349. [DOI] [PubMed] [Google Scholar]
- 31.Reher P, Harris M, Whiteman M, Hai HK, Meghji S. Ultrasound stimulates nitric oxide and prostaglandin E2 production by human osteoblasts. Bone. 2002;31:236–241. doi: 10.1016/S8756-3282(02)00789-5. [DOI] [PubMed] [Google Scholar]
- 32.Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochimica et Biophysica Acta. 2007;1773:1213–1226. doi: 10.1016/j.bbamcr.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Shimazaki A, Inui K, Azuma Y, Nishimura N, Yamano Y. Low intensity pulsed ultrasound accelerates bone maturation in distraction osteogenesis in rabbits. J Bone Joint Surg Br. 2000;82:1077–1082. doi: 10.1302/0301-620X.82B7.9948. [DOI] [PubMed] [Google Scholar]
- 34.Shimoaka T, Kamekura S, Chikuda H, Hoshi K, Chung UI, Akune T, Maruyama Z, Komori T, Matsumoto M, Ogawa W, Terauchi Y, Kadowaki T, Nakamura K, Kawaguchi H. Impairment of bone healing by insulin receptor substrate-1 deficiency. J Biol Chem. 2004;279:15314–15322. doi: 10.1074/jbc.M312525200. [DOI] [PubMed] [Google Scholar]
- 35.Spadaro JA, Albanese S. Application of low-intensity ultrasound to growing bone in rats. Ultrasound Med Biol. 1998;24:567–573. doi: 10.1016/S0301-5629(98)00006-4. [DOI] [PubMed] [Google Scholar]
- 36.Tang CH, Yang RS, Huang TH, Lu DY, Chuang WJ, Huang TF, Fu WM. Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, FAK, phosphatidylinositol 3-kinase and Akt pathway in osteoblasts. Mol Pharmacol. 2006;69:2047–2057. doi: 10.1124/mol.105.022160. [DOI] [PubMed] [Google Scholar]
- 37.Tidball JG. Mechanical signal transduction in skeletal muscle growth and adaptation. J Appl Physiol. 2005;98:1900–1908. doi: 10.1152/japplphysiol.01178.2004. [DOI] [PubMed] [Google Scholar]
- 38.Utvåg SE, Grundnes O, Rindal DB, Reikerås O. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma. 2003;17:430–435. doi: 10.1097/00005131-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Utvåg SE, Iversen KB, Grundnes O, Reikerås O. Poor muscle coverage delays fracture healing in rats. Acta Orthop Scand. 2002;73:471–474. doi: 10.1080/00016470216315. [DOI] [PubMed] [Google Scholar]
- 40.Wang SJ, Lewallen DG, Bolander ME, Chao EY, Ilstrup DM, Greenleaf JF. Low intensity ultrasound treatment increases strength in a rat femoral fracture model. J Orthop Res. 1994;12:40–47. doi: 10.1002/jor.1100120106. [DOI] [PubMed] [Google Scholar]
- 41.Warden SJ, Bennell KL, Forwood MR, McMeeken JM, Wark JD. Skeletal effects of low-intensity pulsed ultrasound on the ovariectomized rodent. Ultrasound Med Biol. 2001;27:989–998. doi: 10.1016/S0301-5629(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 42.Warden SJ, Bennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD. Efficacy of Low-intensity pulsed ultrasound in the prevention of osteoporosis following spinal cord injury. Bone. 2001;29:431–436. doi: 10.1016/S8756-3282(01)00599-3. [DOI] [PubMed] [Google Scholar]
- 43.Warden SJ, Favaloro JM, Bennell KL, McMeeken JM, Ng KW, Zajac JD, Wark JD. Low-intensity pulsed ultrasound stimulates a bone-forming response in UMR-106 cells. Biochem Biophys Res Commun. 2001;286:443–450. doi: 10.1006/bbrc.2001.5412. [DOI] [PubMed] [Google Scholar]
- 44.Yang R-S, Chen Y-Z, Huang T-H, Tang C-H, Fu W-M, Lu B-Y, Lin W-L. The effects of low-intensity ultrasound on growing bone after sciatic neurectomy. Ultrasound Med Biol. 2005;31:431–437. doi: 10.1016/j.ultrasmedbio.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Yang RS, Lin WL, Chen YZ, Tang CH, Huang TH, Lu BY, Fu WM. Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. 2005;36:276–283. doi: 10.1016/j.bone.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Yee KL, Weaver VM, Hammer DA. Integrin-mediated signalling through the MAP-kinase pathway. IET Syst Biol. 2008;2:8–15. doi: 10.1049/iet-syb:20060058. [DOI] [PubMed] [Google Scholar]