Abstract
Malalignment of the cup in total hip arthroplasty (THA) increases the risks of postoperative complications such as neck cup impingement, dislocation, and wear. We asked whether a tailor-made surgical guide based on CT images would reduce the incidence of outliers beyond 10° from preoperatively planned alignment of the cup compared with those without the surgical guide. We prospectively followed 38 patients (38 hips, Group 1) having primary THA with the conventional technique and 31 patients (31 hips, Group 2) using the surgical guide. We designed the guide for Group 2 based on CT images and fixed it to the acetabular edge with a Kirschner wire to indicate the planned cup direction. Postoperative CT images showed the guide reduced the number of outliers compared with the conventional method (Group 1, 23.7%; Group 2, 0%). The surgical guide provided more reliable cup insertion compared with conventional techniques.
Level of Evidence: Level II, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Improper acetabular cup alignment in THA correlates with an increased risk of neck cup impingement, dislocation, and wear [15, 21, 26]. To reduce these complications, a safe range of cup alignment or safe zone was defined by Lewinnek et al. as an abduction angle of 40° ± 10° and anteversion angle of 15° ± 10° [15]. With this range, the dislocation rate was reportedly 1.5% and outside this range increased to 6.1%. However, a correct preoperatively estimated orientation often is not achievable because surgeons cannot readily determine the intraoperative pelvic orientation [6].
To reduce malalignment of the cup, various sorts of computer-assisted surgery have been introduced. Such systems can reduce the number of outliers, ie, the number of cases outside some previously defined safe zone [12, 22, 26]. However, most of these systems require an additional 15 to 46 minutes over conventional methods and considerable costs for installation and use per arthroplasty case [1, 14, 30]. The total cost of the equipment ranges from $125,000 to $277,000 [14]. One cost analysis shows the expenses for operating room and anesthesia professional fee are approximately $150 and $40, respectively, if the operation takes an additional 15 minutes [14].
To address the additional time of the concerns, we developed a tailor-made surgical guide produced using a rapid prototyping (RP) technique for cup insertion surgery [10]. Like other tailor-made surgical guides [2, 3, 9, 23], the surgical guide has a base part that fits on the bone intraoperatively and a guide part to achieve preoperatively planned alignment of the component on the basis of preoperative computed tomography (CT) images.
We asked whether (1) a tailor-made surgical guide based on CT images would reduce the incidence of outliers beyond 10° from preoperatively planned alignment of the cup compared with those without the surgical guide; (2) “alignment accuracy of the cup placement” (defined as the absolute difference between the preoperative and postoperative cup orientations) was better with the surgical guide than with the conventional technique; and (3) operating time and blood loss differed without and with the surgical guide.
Materials and Methods
From January to October 2008, one surgeon (MS) performed primary THAs on 69 patients (nine males, 60 females) with an average age of 63.9 years (range, 39–87 years) using the acetabular component (Trilogy; Zimmer, Warsaw, IN). The preoperative diagnosis was osteoarthritis in 54 patients, osteonecrosis of the femoral head in nine patients, and rheumatoid arthritis in six patients. We compared 38 patients (38 hips, Group 1) using the manual technique with 31 patients (31 hips, Group 2) using the surgical guide (Table 1). This is because we considered more than 30 hips in each group was sufficient to address our first research question on the basis of some previous studies comparing an outlier of the range within 10° from the preoperatively estimated alignment of the cup between the cases with and without surgical intervention [12, 22]. Selection of the patients for the surgical guide was not randomized. We used the surgical guide in only the first scheduled case in each operating day when we had two or three THAs so as to allow sufficient time to prepare for the cases with a surgical guide. We compared the number of cases postoperatively in each group that were outside a range of 10° from the preoperatively estimated alignment of the cup. We also compared alignment accuracy of cup placement, operating time, and amount of blood loss between the two groups. This study was approved by the Institutional Review Board, and all patients provided written informed consent.
Table 1.
Patient data
| Parameter | Group 1, conventional (n = 38) | Group 2, tailor-made surgical guide (n = 31) |
|---|---|---|
| Gender | Males 6, females 32 | Males 3, females 28 |
| Age (years) | 64.0 (95% CI, 60.8–67.2) | 63.9 (95% CI, 59.9–67.9) |
| Height (cm) | 154.1 (95% CI, 150.3–155.5) | 152.9 (95% CI, 151.6–159.6) |
| Weight (kg) | 55.0 (95% CI, 50.1–57.9) | 54.0 (95% CI, 51.8–58.2) |
| Body mass index (kg/m2) | 23.0 (95% CI, 21.7–24.3) | 23.0 (95% CI, 22.1–24.0) |
| Underlying disease (number) osteoarthritis/osteonecrosis/rheumatoid arthritis | 31/4/3 | 23/5/3 |
| Tönnis grade [27] of patients with osteoarthritis | Grade 2, 9 cases; Grade 3, 22 cases | Grade 2, 4 cases; Grade 3, 19 cases |
CI = confidence interval.
To avoid any effect resulting from preoperative planning of the cup between the two groups, one of the authors (TH) performed three-dimensional planning of each patient’s acetabular cup using CT images. Preoperative CT images of the whole pelvis were obtained using a 2.5-mm slice thickness, slice pitch of 3 mm (0.15:1), and pixel spacing of 0.781 mm (Light Speed Plus; GE Medical Systems, Milwaukee, WI). We produced a pelvic coordinate system using a software package (3D Template; Japan Medical Materials, Osaka, Japan), which provides multiplanar (coronal, sagittal, axial) reconstructed (MPR) views and digitally reconstructed plain radiographs (DRR) on each view. A coordinate system when taking the preoperative CT images was adjusted by a line joining the bilateral bottoms of the ischium in the coronal DRR view. Then, the adjusted pelvic coordinate system was defined as the preoperative pelvic coordinate system [10]. Alignment of the cup was referenced by the radiographic definition [19]: abduction was defined as an angle difference between the face of the cup and horizontal axis in the coronal view, and anteversion was defined as the angle between the acetabular axis and the coronal plane. Alignment of each cup was planned at 40° for abduction; anteversion was planned using a range between 15°and 20°, because acetabular bony coverage and degree of femoral neck anteversion were considered [10]. These angles were defined as the preoperative cup orientation. Rotational and translational orientation matrix of the cup from the origin of the pelvic coordinate system were recorded for subsequent image processings. To match between preoperative and postoperative pelvic coordinate systems, we recorded the pelvic tilt on the sagittal DRR view, which is the angle between the line of the anatomic plane, through the bilateral anterior superior iliac spines, and the margin of the pubic symphysis and the vertical line. We also recorded pelvic rotation on the axial MPR view, which is the angle between the line joining the bilateral anterior superior iliac spines and the horizontal line [10].
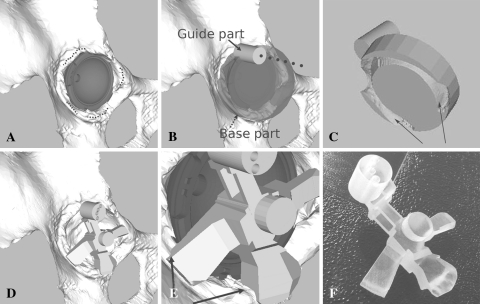
The CT images were transferred to image processing software (Virtual Place-M; Medical Imaging Laboratory, Tokyo, Japan) to reconstruct a polygonal three-dimensional pelvic model using the Marching Cubes method [17]. The surgical guide was designed by Visualization Toolkit libraries (Kitware Inc, Clifton Park, NY). The implant cup model was placed automatically in the acetabulum using the matrix data of the cup (Fig. 1A). At the same time, we could know the correlation between the cup and the acetabulum three-dimensionally and some intact parts of the acetabular edge: the inferior acetabular edge overhung the cup and the top and 2 mm around the top of the posterior and superior acetabular edge (Fig. 1A). These intact parts were used to shape the surgical guide for Group 2 in the following steps.
Fig. 1A–F.
The steps to make the surgical guide used in this study are shown. (A) A pelvic model is made from the preoperative (CT) scan. From information obtained from CT-based preoperative planning of the cup, the cup model is placed automatically on the pelvic model. The dotted lines show the intact parts of the acetabular edge for placing the surgical guide. (B) A premodel of the surgical guide, which consists of a guide (solid arrow) and base (dotted arrow) parts, is made. A broken line shows the alignment of the preoperative planned cup. (C) To fit the surgical guide on the acetabular edge intraoperatively, an imprint of the acetabular edge is made on the back side of the premodel of the surgical guide (when viewed from the acetabulum side). Two arrows indicate the area of the imprint. (D) The final model of the surgical guide is made by shaping the premodel of the surgical guide. (E) To confirm adaptation between the surgical guide and the acetabular edge, some parts of the guide are made when making the final model of the surgical guide (the arrows indicate some of these confirmation parts). (F) The surgical guide was manufactured by a rapid prototyping technique.
We designed the premodel of the surgical guide, which consists of a base and guide parts (Fig. 1B). The base part, which is a cylindrical object, was placed overlapping the acetabular edge. The guide part is another cylindrical bore and was designed to parallel the direction of the planned cup using the matrix data of the planned cup. The guide part also contains cylindrical foramina to allow insertion of a 2-mm diameter Kirschner wire on the superior acetabulum intraoperatively. The pelvic model and the premodel of the surgical guide were exported in stereolithography format to Magics 11 (Materialise NV, Leuven, Belgium) software for spatial image processing to design the surgical guide. The guide part was combined with the base part. The base part was modified by spatially subtracting a part of the acetabular edge from itself (ie, Boolean subtraction). This modification provided an imprint of the part of the acetabular edge in the base part (Fig. 1C). Furthermore, the modified base part was shaped by removing itself partly to fit on only the aforementioned intact parts of the acetabular edge (Fig. 1D). At the same time, some confirmation points were made to investigate adaptation between the surgical guide and the acetabular edge intraoperatively (Fig. 1E). The surgical guide then was manufactured from a photosensitive medical-grade resin using an RP machine (Eden 250; Objet Geometries Ltd, Rehovot, Israel) (Fig. 1F).
The surgeon performed all operations using a modified posterolateral approach [8] with a 12- to 15-cm skin incision. In Group 1, we performed conventional THAs while observing images from the three-dimensional preoperative planning of the cup. These images simulated an overview of the shape of the acetabulum and a positional relationship between the acetabulum and the preoperative planned cup, which we could acquire if the surgeon intraoperatively observed the surgical field from the lateral position. Height of the inferior acetabular edge above the cup and the extent of the overhung part of the cup over the superoposterior acetabular edge were useful for determining cup alignment during seating of a trial cup [7]. Therefore, we used the images to avoid excessive malalignment of the cup. We also used a commercial-based mechanical guide (Lateral alignment flame; Zimmer) to avoid excessive malalignment of the cup. The angles of the mechanical guide were 45° for abduction and 20° for anteversion in operative definition (which means 46.8° for abduction, 14.0° for anteversion in the radiographic definition) [19]. In Group 2, slightly larger acetabular exposure than usual was required to place the surgical guide. The exposure was continued until we could see a 2 mm outer surface from the top of the rim of the inferior, posterior, and superior acetabulum where the guide was placed. After acetabular reaming during THA, the surgical guide was placed on the periacetabulum (Fig. 2A). Because the acetabular shape is not perfectly hemispheric and the height of the acetabular edge is asymmetric [13, 28, 29], the surgical guide could be placed using the rather more complex shape of the acetabulum captured by the customized guide. Then, the Kirschner wire was inserted on the superior acetabulum through one foramen of the guide part. After removing the surgical guide (Fig. 2B), cup fixation was performed while observing alignment of the Kirschner wire (Fig. 2C). The time from setup to removal of the surgical guide was recorded. After cup fixation, the remaining steps of the THA, including femoral rasping and stem fixation, were performed using conventional procedures. In both groups, we recorded the operative time and intraoperative blood loss.
Fig. 2A–C.
The three steps for clinical use of the tailor-made surgical guide used in this study are shown. (A) The surgical guide is placed on the acetabular edge and the Kirschner wire is inserted through the surgical guide. A broken line on the acetabulum shows the superior acetabulum edge. (B) The guide is removed after insertion of the Kirschner wire. (C) Cup fixation is performed while observing the alignment of the Kirschner wire. A broken line shows the line of the cup inserter, which is parallel to the alignment of the Kirschner wire.
All patients had a CT scan 3 weeks after surgery. The pelvic coordinate system of the postoperative CT images was determined using our method. The postoperative pelvic coordinate system then was adjusted to match with the preoperative pelvic coordinate system using the pelvic tilt and rotation of the preoperative pelvic coordinate system [10]. The alignment of the cup that was measured after this modification was defined as postoperative orientation of the cup. Reproducibility of measurement of postoperative orientation of the cup was evaluated previously [10]. For abduction, the intraobserver and interobserver reliability using Pearson’s correlation coefficient were 0.91 and 0.92, respectively, and for anteversion, the intraobserver and interobserver reliability were 0.94 and 0.88, respectively. The intraobserver and interobserver reliability for individual angle absolute differences were 1.6° and 1.1°, respectively, and for abduction, 1.7° and 1.7°, respectively, for anteversion. We investigated the incidence of outliers beyond 10° from preoperatively planned alignment of the cup in the two groups. We also investigated the alignment accuracy of cup placement, which was defined as the absolute angle difference between the preoperative and postoperative cup orientations.
We determined differences in the incidence of outliers beyond 10° between the two groups using the chi square test. We also determined differences in the mean alignment accuracy of the cup placement between the two groups using the Mann-Whitney U test. To determine differences in variance of alignment accuracy of the cup placement, we used the F-test. In addition, we determined differences in the operation time and intraoperative blood loss using the Mann-Whitney U test. These were analyzed using statistical software (StatView 5.0; SAS Institute Inc, Cary, NC).
Results
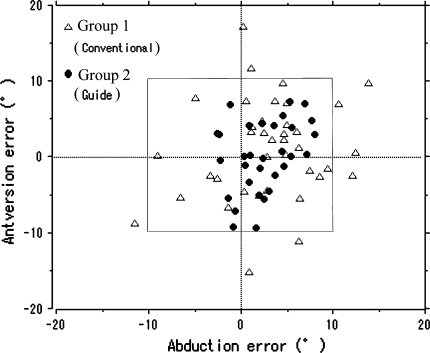
The surgical guide reduced (p = 0.003) the number of outliers compared with the conventional method (Group 1, 23.7% [nine of 38 cases]; Group 2, 0% [zero of 31 cases]) (Fig. 3; Table 2). For postoperative alignments of the cups for Group 1, the mean abduction was 38.4° (range, 27.9°–52.0°; standard deviation [SD], 5.6°) and the mean anteversion was 17.3° (range, 4.0°–35.8°; SD, 6.6°). For postoperative alignments of the cups for Group 2, the mean abduction was 38.4° (range, 33.0°–43.1°; SD, 2.9°) and the mean anteversion was 18.6° (range, 6.3°–27.5°; SD, 5.6°).
Fig. 3.
A scattergram shows deviation of postoperative alignment from the preoperatively estimated alignment of the cup in the two groups. The gray frame indicates 10° from the preoperatively estimated alignment of the cup (abduction, 40°; anteversion, 15°–20°).
Table 2.
Comparison of clinical studies on incidence of outlier beyond 10° from the preoperatively estimated alignment of the cup and alignment accuracy of the cup
| Study | Object | Incidence of outlier | Number | Control measurement | Alignment accuracy for abduction (°) | Alignment accuracy for anteversion (°) |
|---|---|---|---|---|---|---|
| Digioia et al. [6] | Manual + mechanical guide | 78.0% | 74 | CT | — | — |
| Saxler et al. [25] | Manual | 83.8% | 105 | CT | — | — |
| Kalteis et al. [12] | Manual | 53.0% | 30 | CT | 6.1 (range, 0–16) | 13.0 (range, 0–38) |
| Imageless navigation | 10.0% | 30 | CT | 3.6 (range, 1–12) | 4.2 (range, 0–10) | |
| CT-based navigation | 25.3% | 30 | CT | 4.2 (range, 0–11) | 5.3 (range, 0–14) | |
| Bosker et al. [4] | Manual (orthopaedic surgeons) | 19.7% | 85 | Radiograph | 4.1 (standard deviation [SD], 3.9) | 5.1 (SD, 4.5) |
| Manual (residents) | 36.5% | 115 | Radiograph | 6.3 (SD, 4.6) | 5.7 (SD, 5.0) | |
| Manual (total) | 29.5% | 200 | Radiograph | 5.4 | 5.5 | |
| Parrate et al. [22] | Manual | 57.0% | 30 | CT | — | — |
| Imageless navigation | 20.0% | 30 | CT | — | — | |
| Sugano et al. [26] | Manual | 27.9% | 111 | Radiograph | — | — |
| CT-based navigation | 0% | 59 | Radiograph | — | — | |
| Hananouchi et al. [11] | CT-based navigation | 0% | 40 | CT | — | — |
| Current study | Manual + mechanical guide + 3D planning | 23.7% | 38 | CT | 5.1 (range, 0.3–13.9; SD, 3.7) | 5.2 (range, 0.1–17.1; SD, 4.0) |
| Tailor-made surgical guide | 0% | 31 | CT | 3.2 (range, 0.4–8.0; SD, 2.3) | 3.7 (range, 0.1–9.3; SD, 2.7) |
CT = computed tomography; 3D = three-dimensional.
The accuracy of cup placement with the surgical guide was better than that of conventional placement in terms of differences of its average and variance (mean accuracy: for abduction, p = 0.01; for anteversion, p = 0.08; variance: for abduction, p = 0.007; for anteversion, p = 0.03) (Table 2).
We observed no differences in operative time (p = 0.06) or blood loss (p = 0.73) between the two groups (Table 3). The mean time to use the surgical guide was 3.6 minutes (range, 2–7 minutes; SD, 1.6).
Table 3.
Operation time and blood loss in clinical studies with computer-assisted surgery
| Study | Object | Number | Operation time (minutes) | Blood loss (mL) |
|---|---|---|---|---|
| Kalteis et al. [12] | Manual | 30 | 75.1 (range, 40–120) | 399 (range, 50–1090) |
| Imageless navigation | 30 | 82.6 (range, 53–105) | 341 (range, 50–950) | |
| CT-based navigation | 30 | 92.0 (range, 61–130) | 359 (range, 20-730) | |
| Murphy et al. [18] | Manual | 189 | 178 (range, 90–335) | — |
| CT-based navigation | 185 | 177 (range, 74–348) | — | |
| Sugano et al. [26] | Manual | 111 | 111 (range, 60–225) | 751 (range, 80–1400) |
| CT-based navigation | 59 | 169 (range, 105–260) | 827 (range, 150–1800) | |
| Najarian et al. [20] | Manual | 53 | 105 | 498 |
| Imageless navigation (first series) | 49 | 128 | 520 | |
| Imageless navigation (second series) | 47 | 124 | 356 | |
| Current study |
Manual + mechanical guide + 3D planning | 38 | 116.3 (range, 79.0–158.0) | 683.9 (range, 240.0–1628.0) |
| Tailor-made surgical guide | 31 | 106.1 (range, 75.0–169.0) | 655.9 (range, 190.0–1768.0) |
CT = computed tomography; 3D = three-dimensional.
Discussion
Some efforts to reduce the malalignment of the cup are still required in conventional THAs because the conventional cases had more postoperative complications than the navigated ones [26]. We asked whether: (1) a tailor-made surgical guide based on CT images would reduce the incidence of outliers beyond 10° compared with those without the surgical guide; (2) accuracy of cup placement was better with the surgical guide than the conventional technique; and (3) operating time and blood loss differed without and with the surgical guide.
We note several limitations of our study. First, our study was not randomized. The selection of patients in whom the surgical device was used may reflect the surgeon’s selection bias. We used the surgical guide only for the first case in each operation day to allow sufficient time in the cases with the surgical guide. Second, we did not evaluate cost-effectiveness of the surgical guide and could not compare the cost-effectiveness of the surgical guide with that of navigation systems because the surgical guide we used is not available for commercial use. We also did not consider the radiation to the patient and the cost of the required CT scan. Because the mean additional time for the surgical guide was 3.6 minutes, we believe the cost for additional operation time would be saved in comparison to the additional operation time (15 to 46 minutes [1, 30]) in the navigation system. The total cost of software to make the surgical guide ranges from approximately $15,000 to $30,000. It might depend on the number of licenses or the condition of the use of the software, which was based on whether its use was for academic research or for a private purpose. The cost for material of RP per case is approximately $50 to $100. It also might change with kinds of material. The cost of a RP machine is $120,000. However, this cost might be saved if this step is outsourced to some companies that provide RP [31]. Finally, the total cost for the surgical guide per case might become reasonable if the workflow for making the surgical guide are commercialized like a RP-based surgical guide for TKA [16].
The surgical guide reduced the incidence of outliers beyond 10° from the planned alignment of the cup. According to previous reports [4, 6, 12, 22, 25, 26], 19.7% to 83.8% of the cases with conventional THAs did not achieve a range within 10° from the planned alignment of the cup. We suspect the reason our data reduced outliers compared with those of a conventional technique (23.7%) relates to the use of some images and/or the use of the mechanical guide. Compared with previous reports, the surgical guide is superior to conventional techniques and comparable to navigation systems (0%–25.3%) [4, 6, 11, 12, 22, 25, 26] (Table 2).
We suggest the alignment accuracy of cup placement was better than the conventional technique in terms of differences of its average and variance. Compared with previous reports regarding alignment accuracy of cup placement, our results with the conventional technique are comparable to those of previous conventional technique groups, which are reported as 4.1° to 6.3° for abduction and 5.1° to 13.0° for anteversion [4, 12] (Table 2). Our data using the surgical guide are superior to those of previous conventional technique groups and comparable to previous navigation technique groups, which are reported as 3.6° to 4.2° for abduction and 4.2° to 5.3° for anteversion [12] (Table 2).
New surgical interventions may adversely affect operative time, subsequently increasing blood loss. However, the time for the surgical guide was, on average, 3.6 minutes. As a result, we found no difference in the operative time and blood loss between the two groups. The operation time and blood loss with the surgical guide were comparable to those reported for conventional and navigated THAs [12, 18, 20, 26] (Table 3). Two previous reports suggest the navigation systems with an optical sensor take an additional 15 to 46 minutes of operating time over conventional procedures [1, 30]. We suggest this is because the system needs additional time for intraoperative registration steps and/or for specific surgical performances during acetabular reaming and cup fixation while looking for information on the monitor of the navigation system [24]. However, the surgical guide we used maintains the conventional intraoperative procedure. After acetabular reaming, we just place the surgical guide and insert the Kirschner wire through the surgical guide. Then, we perform cup fixation while observing alignment of the Kirschner wire as the alignment guide for the planned cup. A specific skill for the surgical guide is not necessary. We consider the reason the additional operation time using the surgical guide is shorter than the time needed to use the navigation system with the optical sensor is attributable to the simplicity of our surgical guide method.
There are some drawbacks to the surgical guide. First, radiation exposure from the CT scans is a concern. However, we believe it may be justified by the benefits of the imaging information such as thickness and coverage of the acetabulum and femoral anteversion for implantation in three-dimensional planning. In addition, attempts have been made to develop low-dose radiation CT scans of the pelvis [5]. According to the previous study [5], the radiation dose for the pelvis with 1- to 1.5-mm slice thickness was 1.7 mSv as opposed to 10 mSv for a traditional pelvic CT scan. By comparison, the radiation doses from plain anteroposterior and lateral pelvic radiographs are 0.7 mSv and 0.8 mSv, respectively. Second, preoperative planning and manufacturing time for the surgical guide are necessary. The design of the surgical guide takes approximately 60 to 120 minutes and manufacture of the guide takes approximately 90 to 120 minutes. However, we believe the additional time before surgery must be separated from the additional intraoperative time because only the latter affects the status of the patients. Apart from the manufacturing time, preoperative planning time might be shortened if dedicated computer software was made for the surgical guide. Third, the surgical guide we used provides only alignments of the cup, unlike some navigation systems, which support cup position, femoral offset and alignment, and cup alignment [26]. Improvement of the design of the current surgical guide and application of a tailor-made surgical guide for femoral prostheses are necessary to address some cases that need the adjustment for severe limb length discrepancy.
We suggest a tailor-made surgical guide is useful for cup insertion as it provides more reliable cup insertion compared with conventional methods in terms of the rate of the outlier of the range within 10° from the preoperatively estimated alignment of the cup (conventional technique, 23.7%; the surgical guide, 0%) and without requiring an excessive amount of time.
Acknowledgments
We thank Dr. Keisuke Hagio, Dr. Kazuo Yonenobu, Dr. Mariko Ohshima, and Dr. Kensuke Ikuta in Osaka Minami Medical Center for supporting the operation works; Mr. Wataru Yamanashi for supporting the technical aspects for this study; Dr. Tsuyoshi Murase, Dr. Kunihiro Oka, and Mr. Ryoji Nakao for setup of the start of this study; Dr. Takashi Nishii, Dr. Takashi Sakai, and Dr. Masaki Takao at Osaka University Graduate School of Medicine, and Dr. Nobuo Nakamura, Dr. Akihiro Kakimoto, Dr. Daiki Iwana, and Dr. Makoto Kitada in Kyowakai Hospital for the preliminary study.
Footnotes
One author (TH) has received funding from grants from the Japan Hip Research Foundation, Inc, and research fellowships from the Japan Society for the Promotion of Science for Young Scientists.
Each author certifies that his institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Beringer DC, Patel JJ, Bozic KJ. An overview of economic issues in computer-assisted total joint arthroplasty. Clin Orthop Relat Res. 2007;463:26–30. [PubMed] [Google Scholar]
- 2.Berry E, Cuppone M, Porada S, Millner PA, Rao A, Chiverton N, Seedhom BB. Personalised image-based templates for intra-operative guidance. Proc Inst Mech Eng [H]. 2005;219:111–118. doi: 10.1243/095441105X9273. [DOI] [PubMed] [Google Scholar]
- 3.Birnbaum K, Schkommodau E, Decker N, Prescher A, Klapper U, Radermacher K. Computer-assisted orthopedic surgery with individual templates and comparison to conventional operation method. Spine. 2001;26:365–370. doi: 10.1097/00007632-200102150-00012. [DOI] [PubMed] [Google Scholar]
- 4.Bosker BH, Verheyen CC, Horstmann WG, Tulp NJ. Poor accuracy of freehand cup positioning during total hip arthroplasty. Arch Orthop Trauma Surg. 2007;127:375–379. doi: 10.1007/s00402-007-0294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dandachli W, Kannan V, Richards R, Shah Z, Hall-Craggs M, Witt J. Analysis of cover of the femoral head in normal and dysplastic hips: new CT-based technique. J Bone Joint Surg Br. 2008;90:1428–1434. doi: 10.1302/0301-620X.90B11.20073. [DOI] [PubMed] [Google Scholar]
- 6.Digioia AM, III, Jaramaz B, Plakseychuk AY, Moody JE, Jr, Nikou C, Labarca RS, Levison TJ, Picard F. Comparison of a mechanical acetabular alignment guide with computer placement of the socket. J Arthroplasty. 2002;17:359–364. doi: 10.1054/arth.2002.30411. [DOI] [PubMed] [Google Scholar]
- 7.Dorr LD. Standard posterior exposure for total hip replacement. In: Dorr LD, editor. Hip Arthroplasty: Minimally Invasive Techniques and Computer Navigation. Philadelphia, PA: Elsevier; 2006. pp. 30–54. [Google Scholar]
- 8.Gibson A. Posterior exposure of the hip joint. J Bone Joint Surg Br. 1950;32:183–186. doi: 10.1302/0301-620X.32B2.183. [DOI] [PubMed] [Google Scholar]
- 9.Hafez MA, Chelule KL, Seedhom BB, Sherman KP. Computer-assisted total knee arthroplasty using patient-specific templating. Clin Orthop Relat Res. 2006;444:184–192. doi: 10.1097/01.blo.0000201148.06454.ef. [DOI] [PubMed] [Google Scholar]
- 10.Hananouchi T, Saito M, Koyama T, Hagio K, Murase T, Sugano N, Yoshikawa H. Tailor-made surgical guide based on rapid prototyping technique for cup insertion in total hip arthroplasty. Int J Med Robot. 2009;5:164–169. doi: 10.1002/rcs.243. [DOI] [PubMed] [Google Scholar]
- 11.Hananouchi T, Takao M, Nishii T, Miki H, Iwana D, Yoshikawa H, Sugano N. Comparison of navigation accuracy in THA between the mini-anterior and -posterior approaches. Int J Med Robot. 2009;5:20–25. doi: 10.1002/rcs.226. [DOI] [PubMed] [Google Scholar]
- 12.Kalteis T, Handel M, Bathis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty: is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88:163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 13.Köhnlein W, Ganz R, Impellizzeri FM, Leunig M. Acetabular morphology: implications for joint-preserving surgery. Clin Orthop Relat Res. 2009;467:682–691. doi: 10.1007/s11999-008-0682-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavernia C, Hernández VH, Hommen JP. Cost analysis of navigation. In: Stiehl JB, Konermann WH, Haaker RG, DiGioia AM, editors. Navigation and MIS in Orthopedic Surgery. Heidelberg, Germany: Springer; 2007. pp. 61–67. [Google Scholar]
- 15.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–220. [PubMed] [Google Scholar]
- 16.Lombardi AV, Jr, Berend KR, Adams JB. Patient-specific approach in total knee arthroplasty. Orthopedics. 2008;31:927–930. doi: 10.3928/01477447-20080901-21. [DOI] [PubMed] [Google Scholar]
- 17.Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. ACM Computer Graphics. 1987;21:163–169. doi: 10.1145/37402.37422. [DOI] [Google Scholar]
- 18.Murphy SB, Ecker TM, Tannast M. THA performed using conventional and navigated tissue-preserving techniques. Clin Orthop Relat Res. 2006;453:160–167. doi: 10.1097/01.blo.0000246539.57198.29. [DOI] [PubMed] [Google Scholar]
- 19.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–232. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 20.Najarian BC, Kilgore JE, Markel DC. Evaluation of component positioning in primary total hip arthroplasty using an imageless navigation device compared with traditional methods. J Arthroplasty. 2009;24:15–21. doi: 10.1016/j.arth.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Nishii T, Sugano N, Miki H, Koyama T, Takao M, Yoshikawa H. Influence of component positions on dislocation: computed tomographic evaluations in a consecutive series of total hip arthroplasty. J Arthroplasty. 2004;19:162–166. doi: 10.1016/j.arth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Parratte S, Argenson JN. Validation and usefulness of a computer-assisted cup-positioning system in total hip arthroplasty: a prospective, randomized, controlled study. J Bone Joint Surg Am. 2007;89:494–499. doi: 10.2106/JBJS.F.00529. [DOI] [PubMed] [Google Scholar]
- 23.Radermacher K, Portheine F, Anton M, Zimolong A, Kaspers G, Rau G, Staudte HW. Computer assisted orthopaedic surgery with image based individual templates. Clin Orthop Relat Res. 1998;354:28–38. doi: 10.1097/00003086-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Rivkin G, Liebergall M. Challenges of technology integration and computer-assisted surgery. J Bone Joint Surg Am. 2009;91(suppl 1):13–16. doi: 10.2106/JBJS.H.01410. [DOI] [PubMed] [Google Scholar]
- 25.Saxler G, Marx A, Vandevelde D, Langlotz U, Tannast M, Wiese M, Michaelis U, Kemper G, Grützner PA, Steffen R, Knoch M, Holland-Letz T, Bernsmann K. The accuracy of free-hand cup positioning: a CT based measurement of cup placement in 105 total hip arthroplasties. Int Orthop. 2004;28:198–201. doi: 10.1007/s00264-004-0542-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sugano N, Nishii T, Miki H, Yoshikawa H, Sato Y, Tamura S. Mid-term results of cementless total hip replacement using a ceramic-on-ceramic bearing with and without computer navigation. J Bone Joint Surg Br. 2007;89:455–460. doi: 10.1302/0301-620X.89B4.18458. [DOI] [PubMed] [Google Scholar]
- 27.Tönnis D. Congenital Dysplasia and Dislocation of the Hip in Children and Adults. Berlin, Germany: Springer; 1987. [Google Scholar]
- 28.Vandenbussche E, Saffarini M, Delogé N, Moctezuma JL, Nogler M. Hemispheric cups do not reproduce acetabular rim morphology. Acta Orthop. 2007;78:327–332. doi: 10.1080/174536707100013870. [DOI] [PubMed] [Google Scholar]
- 29.Vandenbussche E, Saffarini M, Taillieu F, Mutschler C. The asymmetric profile of the acetabulum. Clin Orthop Relat Res. 2008;466:417–423. doi: 10.1007/s11999-007-0062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Widmer KH, Grützner PA. Joint replacemen–total hip replacement with CT-based navigation. Injury. 2004;35(suppl 1):S-A84–S-A89. [DOI] [PubMed]
- 31.Xpress3D. Rapid Prototype and Rapid Manufacture Quotes from Multiple Service Bureaus in Seconds. Available at: www.xpress3d.com. Accessed June 8, 2009.