Abstract
The ability of a remedy to modulate the pathological process in the target organ is crucial for its therapeutic activity. Glatiramer acetate (GA, Copaxone, Copolymer 1), a drug approved for the treatment of multiple sclerosis, induces regulatory T helper 2/3 cells that penetrate the CNS. Here we investigated whether these GA-specific T cells can function as suppressor cells with therapeutic potential in the target organ by in situ expression of T helper 2/3 cytokines and neurotrophic factors. GA-specific cells and their in situ expression were detected on the level of whole-brain tissue by using a two-stage double-labeling system: (i) labeling of the GA-specific T cells, followed by their adoptive transfer, and (ii) detection of the secreted factors in the brain by immunohistological methods. GA-specific T cells in the CNS demonstrated intense expression of the brain-derived neurotrophic factor and of two antiinflammatory cytokines, IL-10 and transforming growth factor β. No expression of the inflammatory cytokine IFN-γ was observed. This pattern of expression was manifested in brains of normal and experimental autoimmune encephalomyelitis-induced mice to which GA-specific cells were adoptively transferred, but not in control mice. Furthermore, infiltration of GA-induced cells to the brain resulted in bystander expression of IL-10 and transforming growth factor β by resident astrocytes and microglia. The ability of infiltrating GA-specific cells to express antiinflammatory cytokines and neurotrophic factor in the organ in which the pathological processes occur correlates directly with the therapeutic activity of GA in experimental autoimmune encephalomyelitis/multiple sclerosis.
Neurotrophic factors and antiinflammatory cytokines have been shown to play a crucial role in the modulation of multiple sclerosis (MS) and its experimental model, experimental autoimmune encephalomyelitis (EAE) (1–3). Yet, the information available on their expression within the CNS is limited. Various current therapies for MS attempt to interfere with the pathological processes by immune intervention and upregulation of these regulatory substances. However, whereas the peripheral effect of these treatments has been extensively studied, little information exists on their effect in the target organ, namely the brain. The study of CNS diseases is particularly complicated, because the accessibility of immune factors and regulatory cells is strictly controlled by the blood–brain barrier, constituting a distinct and unique microenvironment.
Glatiramer acetate (GA, Copaxone, Copolymer 1), a drug approved for the treatment of MS, exerts a marked suppressive effect on EAE induced by various encephalitogens in several species (4). We previously demonstrated that the therapeutic effect of GA is mediated by regulatory cells that suppress disease and secrete high amounts of antiinflammatory T helper (Th) 2/3 cytokines (5). These GA-induced suppressor cells crossreact with the autoantigen myelin basic protein by secreting Th2/3 cytokines and induce bystander suppression of processes mediated by other encephalitogens (6). GA-specific Th2/3 cells have been found in the periphery, in the spleens and lymph nodes of experimental animals, and in peripheral blood mononuclear cells of humans treated with GA, suggesting that these cells are involved in the therapeutic effect induced by GA on MS as well (5–7). Furthermore, we have recently demonstrated that GA-specific regulatory cells, induced in the periphery either by injection or by oral treatment with GA, pass the blood–brain barrier and accumulate in the CNS. This finding was manifested by their isolation from brains of actively sensitized GA-treated mice and by localization of GA-specific cells in the brain after their passive transfer to the periphery (8, 9).
Although the presence of GA-specific Th2 cells in the CNS was confirmed, their ability to actually secrete Th2 cytokines and induce therapeutic effect in situ has not been verified. Some reservation concerning the capability of T cells to secrete Th2 cytokines in the CNS was raised after finding that the CNS environment induces bias toward the Th1 pathway and that myelin basic protein-specific Th2 cells can aggravate EAE (6, 10). It was therefore essential to elucidate whether the GA-specific cells accumulating in the brain actually function as suppressor cells in the diseased organ and secrete antiinflammatory cytokines in situ.
It was recently demonstrated that GA-specific cells of both mouse and human origin also secrete the potent brain-derived neurotrophic factor (BDNF) (3, 11), which induces axonal outgrowth, remyelination, regeneration, and rescue of degenerating neurons (12, 13). Yet, this BDNF secretion was found only in peripheral T cell lines in vitro and not in the target organ. BDNF expression by GA-specific cells in the CNS may indicate that GA activity is not restricted to antiinflammatory effect but generates actual neuroprotection and regeneration processes as well. In this study, we investigated whether GA-induced T cells that penetrate the brain do function as regulatory cells by producing Th2/3 cytokines and neurotrophic factor. We wish to report that, indeed, the cytokines IL-10, transforming growth factor β (TGF-β), and BDNF are expressed by GA-induced cells in situ.
Materials and Methods
Mice. (SJL/JxBALB/c)F1 female mice, 10–16 weeks old, were purchased from The Jackson Laboratory.
Glatiramer Acetate. GA consists of acetate salts of synthetic polypeptides, containing four amino acids: l-alanine, l-glutamate, l-lysine, and l-tyrosine (4). GA from batch no. 242990599, with an average molecular weight of 7,300, was obtained from Teva Pharmaceutical Industries (Petach Tikva, Israel) and used throughout the study.
T Cells. GA-specific cells from two T cell lines established from spleens of mice that had received daily injections (2 mg/day, 10 injections) or had been fed (250 μg/day, eight feedings) with GA, respectively, were used throughout this study. Cells were selected in vitro by repeating exposures to GA (50 μg/ml) on irradiated spleen cells, followed by propagation in T cell growth factor medium. These cells had been characterized as Th2 regulatory cells that can suppress EAE in vivo (5, 6).
T Cell Labeling. GA-specific cells were stained either by Hoechst 33342-blue fluorescent (Molecular Probes) or by PKH26-red fluorescent (Sigma), which incorporate to the cell nucleus or to the cell membrane, respectively, according to the manufacturer's instructions. Both staining methods did not interfere essentially with the biological activity of the cells, as demonstrated by the comparison of the proliferation and cytokine secretion of labeled cells to unlabeled cells and by the ability of the labeled cells to prevent EAE.
Adoptive Transfer. Activated prelabeled GA-specific T cells (30 × 106 per mouse) were injected into the peritoneum of either normal or EAE-induced mice 3 days after disease induction by whole spinal cord homogenate (5 mg in complete Freund's adjuvant) and pertussis toxin injection (0.2 μg per mouse). Brains were excised 7 days after cell injection because our previous studies indicated that this time is optimal for the accumulation of GA-specific cells, in contrast to nonspecific controls (8, 9).
Immunocytochemistry. Control EAE-induced mice injected with GA-specific T cells and normal mice were anesthetized and perfused with 2.5% paraformaldehyde. Brains were sectioned (20 μm) by cryostat or sliding microtom. Sections were preincubated in PBS with 20% horse serum and 0.05% Saponin (Sigma) for 1 h, then incubated overnight with the following primary antibodies: chicken anti-BDNF (Promega), goat anti-IL-10 (Santa Cruz Biotechnology), rabbit anti-TGF-β (Santa Cruz Biotechnology), rat anti-IFN-γ (BioSource International, Camarillo, CA), or mouse anti-glial fibrillary acidic protein (GFAP; Pharmingen), 1–10 μg/ml, diluted in PBS with 2% horse serum and 0.05% saponin. The second antibody step was performed by labeling with Cy2- or Cy3-conjugated donkey anti-chicken, donkey anti-goat, donkey antirabbit, donkey anti-rat, or donkey anti-mouse (Jackson ImmunoResearch), 1:250, for 20 min. Additional controls were incubated with secondary antibody alone. Slides of Hoechst-labeled cells were examined by fluorescence microscope (Nikon Eclipse E600). Slides of PKH26-labeled cells were examined by confocal microscope (Axiovert 100M, Zeiss). Photographs were taken by using spot software program. Images were processed with photoshop (Adobe Systems, Mountain View, CA). Most photographs presented are from EAE-induced mice injected with prelabeled GA-specific cells.
Results
The Presence of Fluorescent-Labeled Adoptively Transferred GA-Specific T Cells in the Brain. Abundance of fluorescently prelabeled GA-specific cells was observed in brain sections with adoptively transferred GA-specific T cells in normal mice and particularly in brains of EAE-induced mice 7 days after their injection to the periphery. The GA-specific cells were visible in the ventricles, in particular, in the choroid plexus (Fig. 1D) and in the tissue surrounding the ventricles (Fig. 1 A). Many clusters of cells were seen around blood vessels, indicating perivascular infiltration into the brain (Fig. 1G). Individual or clustered cells penetrated various regions of the brain, including the cortex, the thalamus, the basal ganglia, and the hippocampus. Brain sections, containing the penetrating fluorescently labeled GA-specific T cells and control brain sections from EAE-induced or normal mice were immunohistochemically stained for the expression of the neurotrophic factor BDNF, the Th2 cytokines IL-10 and TGF-β, and the Th1 cytokine IFN-γ. Images were taken from various regions of the thalamus and cortex, mainly from the laterodorsal thalamus nucleus, the ventral posteromedial thalamic nucleus, the ventral posterolateral thalamic nucleus, and the somatosensory cortex.
Fig. 1.
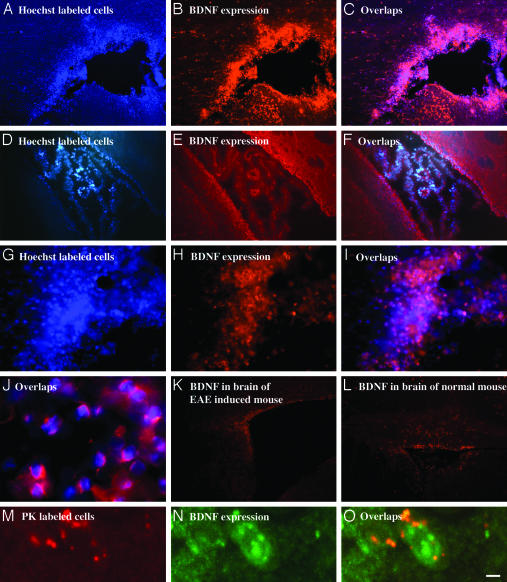
Immunohistochemical analysis of BDNF expression by GA-specific cells in the brain. Activated labeled GA-specific cells were injected into the peritoneum of either EAE-induced (A–C, G–J, and M–O) or normal (D–F) mice. After 7 days, the mice were perfused and brain sections (20 μm) were stained immunocytochemically for BDNF expression. (A–C) Area surrounding the lateral ventricle. (D–F) Choroid plexus in the lateral ventricle. (G–I) Perivascular infiltration in the cortex. (J) Overlap image of enlarged Hoechst-labeled, GA-specific cells and their BDNF secretion in the cortex. (M–O) Enlarged images of single GA-specific PK-labeled cells in the cortex. Controls demonstrate BDNF expression in corresponding brain regions of an EAE-induced mouse (K) and a normal mouse (L). (Scale bar: A–F, K, and L, 100 μm; G–I, 20 μm; J and M–O, 10 μm.)
BDNF. As shown in Fig. 1, adoptively transferred GA-specific cells that had been accumulated in the brain (Fig. 1 A, D, G, and M) expressed BDNF in situ (Fig. 1 B, E, H, and N). This finding was verified by colocalization of the corresponding fields (Fig. 1 C, F, I, and O). Furthermore, staining of GA cells in the CNS by BDNF antibodies was also demonstrated on the single-cell level in Hoechst-labeled (Fig. 1J) and PK-labeled (Fig. 1 M–O) cells. GA-specific cells expressing BDNF were observed in various regions of the brain, mainly in tissue surrounding the ventricles (Fig. 1 A–C) and in perivascular cuffs (Fig. 1 G–I). BDNF immunostaining localized both in the cytoplasm and in the membrane of the cells (Fig. 1 J and N). In contrast to the strong BDNF expression in the brains containing GA-specific cells, only marginal BDNF staining was observed within the CNS of control EAE-induced (Fig. 1K) and in normal (Fig. 1L) mice into which no GA-specific cells were injected.
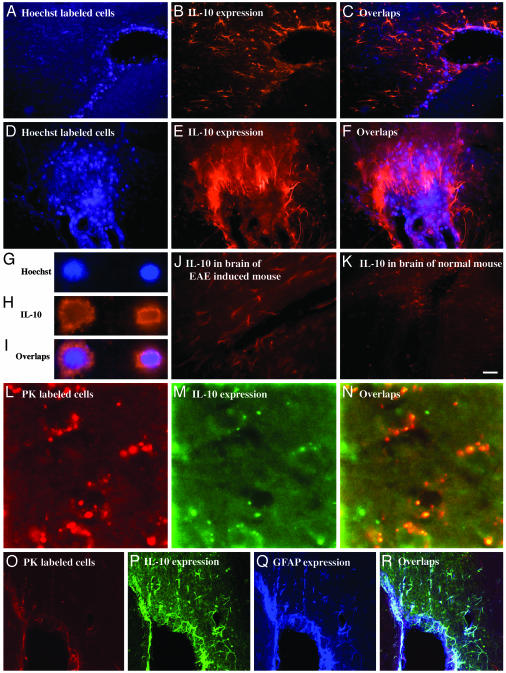
IL-10. Fig. 2 illustrates that GA-specific cells in the brain (Fig. 2 A, D, and L) demonstrated intensive staining with anti-IL-10 antibodies (Fig. 2 B, E, and M), as verified by colocalization of the corresponding fields (Fig. 2 C, F, and N). This in situ IL-10 expression by GA cells was clearly demonstrated on a single-cell level in Hoechst-labeled (Fig. 2 G–I) and PK-labeled (Fig. 2 L–N) cells. IL-10 was expressed both in the cytoplasm and in the membrane of the cells (Fig. 2 H and M).
Fig. 2.
Immunohistochemical analysis of IL-10 expression by GA-specific cells in brains of EAE-induced mice. (A–F) Perivascular infiltration in the thalamus. (G–I) Enlarged images of single cells in the thalamus. (L–N) Single cells in the cortex. (O–R) Nonoverlapping distribution of IL-10-expressing, GA-labeled cells and additional population of cells expressing IL-10 and GFAP in tissue surrounding the lateral ventricle. Controls demonstrate IL-10 expression in corresponding brain regions of an EAE-induced mouse (J) and a normal mouse (K). (Scale bar: A–C, 50 μm; D–F, 20 μm; G–I and L–N, 10 μm; J, K, and O–R, 100 μm.)
Clusters of GA-labeled cells expressing IL-10 were abundant in various regions throughout the brain. Moreover, unlabeled cells within and in the vicinity of these clusters also expressed high levels of IL-10 (Fig. 2 B, C, E, and F). These unlabeled IL-10-positive cells had elongated astrocyte-like morphology consistent with activated microglia. Triple staining of slides containing GA-specific cells (Fig. 2O) for IL-10 (Fig. 2P) and GFAP, a component of astrocyte filaments (Fig. 2Q), confirmed the astrocyte phenotype of these adjacent cells (Fig. 2R). Thus, IL-10 expression was evident not only in the labeled GA-specific cells that migrated to the brain but also in the surrounding resident CNS cells.
The intense IL-10 secretion was observed only in brains with adoptively transferred GA cells, whereas control mice expressed low to moderate IL-10 levels. IL-10 expression in brains of EAE-induced mice (Fig. 2 J) was slightly higher than that observed in brains of normal mice (Fig. 2K). Moderate IL-10 expression appeared to be associated with areas of cellular infiltrated and perivascular cuffs.
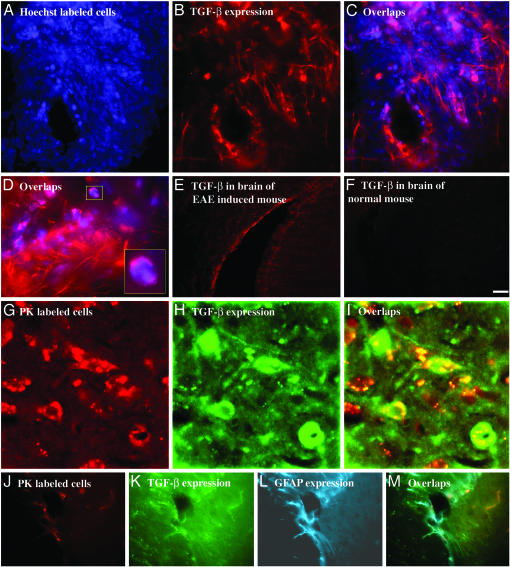
TGF-β. A similar phenomenon was observed regarding TGF-β,as shown in Fig. 3. Adoptively transferred GA-specific cells in the brain manifested extensive TGF-β expression (Fig. 3 A–D and G–L), with strong cytoplasmatic and membranal staining of individual cells (Fig. 3 D and H). The staining pattern was similar to the pattern observed for IL-10, including the positive staining of surrounding elongated cells of astrocyte morphology, which was confirmed by triple labeling with GFAP (Fig. 3 J–M). These surrounding bystander resident cells displayed similar or even higher TGF-β expression intensity compared with the staining of the GA-specific cell population (Fig. 3D).
Fig. 3.
Immunohistochemical analysis of TGF-β expression by GA-specific cells in brains of EAE-induced mice. (A–D) Perivascular infiltration in the cortex. (G–I) 3D reconstruction of PK-labeled cells in the thalamus by confocal scanning microscope. (J–M) Nonoverlapping distribution of TGF-β-expressing GA-labeled cells and additional population of cells expressing TGF-β and GFAP in tissue surrounding a blood vessel. Controls demonstrate TGF-β in corresponding brain region of an EAE-induced mouse (E) and a normal mouse (F) in the cortex. (Scale bar: A–C, 50 μm; D, 20 μm; E, F, and M–P, 100 μm; G–L, 10 μm.)
In contrast to the strong TGF-β secretion in brains with adoptively transferred GA cells, control mice expressed low to moderate TGF-β levels, which were higher in brains of EAE-induced mice (Fig. 3E) than in those of normal mice (Fig. 3F).
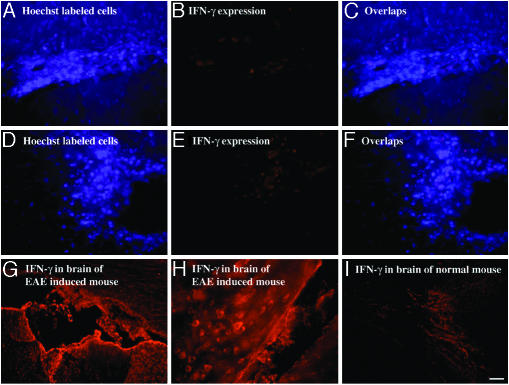
IFN-γ. The results presented hitherto pertain to the Th2-related cytokines. It was of interest to test the expression pattern of a Th1 cytokine such as IFN-γ. As shown in Fig. 4, GA-specific cells that had been accumulated in the brain (Fig. 4 A and D) did not express IFN-γ in situ (Fig. 4 B and E). It was not possible to detect any IFN-γ in the brains of mice injected with GA-labeled cells, neither in EAE-induced (Fig. 4 A–C) nor in normal (Fig. 4 D–F) mice. On the other hand, intense staining for IFN-γ was found in corresponding brain regions of EAE-induced mice that were not injected with GA-specific cells (Fig. 4 G and H). This staining was much higher than that observed in normal mice (Fig. 4I).
Fig. 4.
Lack of expression of IFN-γ by GA-specific cells in the brain. Cells in the cortex of an EAE-induced mouse (A–C) and a normal mouse (D–F). Expression of IFN-γ in corresponding brain sections of controls: an EAE-induced mouse (G and H) and a normal mouse (I). (Scale bar: A–F and H,40 μm; G and I, 100 μm.)
Discussion
Immunomodulating therapies for CNS pathologies have been studied extensively and in clinical use for several years. However, the understanding of the pathway by which they actually operate in the target organ has lagged behind. Thus, neurotrophic factors and antiinflammatory cytokines are known to play a crucial role in MS and EAE modulation (1–3, 12–15), but information on their expression within the CNS is limited. In the present study we tested whether treatment with the MS drug GA results in secretion of neurotrophic factor and antiinflammatory cytokines in situ through specific Th2/3 cells that penetrate the brain. To trace the low amounts of factors secreted by a specific subset of T cells on the level of whole-brain tissue, we used the double-labeling approach in which prelabeled specific T cells were adoptively transferred and their cytokines/neurotrophic factor expression was subsequently immunohistologically detected.
As clearly demonstrated, GA-specific cells in the brain manifested intensive expression of the neurotrophic factor BDNF (Fig. 1) and of the two antiinflammatory cytokines, IL-10 (Fig. 2) and TGF-β (Fig. 3), but no trace of the inflammatory cytokine IFN-γ (Fig. 4). This expression was clearly verified on the single-cell level by labeling with two different dyes, i.e., Hoechst and PKH26, which incorporate into different cell loci (the nucleus and the membrane, respectively), indicating that these findings do not reflect an artifact caused by cell staining. It has been claimed that, because of its ability to bind cellular DNA, Hoechst labeling inhibits lymphocyte activity and interacts with bystander cells (16). We have not observed these phenomena, as demonstrated by the comparison of the proliferation and cytokine secretion of labeled cells with unlabeled cells and by the ability of the labeled cells to prevent EAE (8). Yet, to affirm staining specificity, we used, in addition, a vital labeling procedure by the membrane inserting dye PKH26. Parallel results were obtained with both staining methods.
BDNF, IL-10, and TGF-β manifested cytoplasmatic as well as membranalic expression. Positively stained GA-specific cells were apparent in various regions throughout the brain, including the cortex, the thalamus, the basal ganglia, and the hippocampus, mainly in tissue surrounding the ventricles and in perivascular locations. They were found in brains of normal and EAE-induced mice after adoptive transfer of GA-specific cells. In both cases, staining intensity on a single-cell level looked similar. Yet, the total expression on the whole-tissue level was significantly higher in EAE-induced mice, because a great deal more GA-specific cells were present in the brains of those mice than in the brains of normal mice. The difference in cell infiltration could result from blood–brain barrier injury caused by the injection of pertussis toxin during EAE induction and from the destruction typical to the disease process. In any event, an extensive expression of these modulatory substances by GA-specific cells in the CNS was manifested during the pathological process. T cells from lines induced by oral immunization or by daily injections of GA demonstrated similar CNS penetration and expression patterns of all the factors tested.
Intense staining with BDNF, IL-10, and TGF-β antibodies was found only in brains of mice that had been injected with GA-specific cells. In control mice only faint staining was noted. It is of interest that BDNF staining in brains of EAE-induced mice was marginal and similar to the staining of normal mice (Fig. 1 K and L), whereas IL-10 and TGF-β expression in brains of EAE-induced mice were somewhat higher than in brains of normal mice (Figs. 2 J and K and 3 E and F). This finding agrees with other studies that demonstrated up-regulation of these cytokines during disease (1, 2, 14, 15). In contrast to Th2/3 cytokine expression, which was increased only marginally in brains of EAE-induced mice, a significant augmentation in the expression of IFN-γ was observed in brains of EAE-induced mice (Fig. 4 G and H) in comparison with normal mice (Fig. 4I). This finding corroborates our previous results, indicating that lymphocytes isolated from brains of EAE-induced mice secrete high levels of IFN-γ (8, 9). Notably, this increase in IFN-γ was not manifested in brain lymphocytes from EAE-induced mice treated with GA (8, 9) nor in corresponding brain sections from EAE-induced mice that had been injected with GA-specific cells (Fig. 4B). In both cases, IFN-γ levels were similar to those of normal mice. IFN-γ is a principal Th1 effector cytokine, which by and large is believed to be involved in the pathological processes of EAE and MS (1). Hence, the significance of the down-regulation of this inflammatory cytokine in the target organ, induced by the MS drug GA.
The current consensus is that activated T cells of any specificity cross the blood–brain barrier and penetrate the CNS (18), but, thereafter, T cells that are not able to recognize their specific antigen in the CNS decline to baseline level. The ability of GA-induced T cells to persist in the CNS, while cells with different specificity were completely absent, was previously shown and attributed to their crossreactivity with the myelin antigen myelin basic protein (8, 9). The present study demonstrates that GA-specific cells not only accumulate in the brain but actually function in the diseased organ as regulatory cells by the secretion of Th2/3 and neurotrophic factor. Of special interest is the finding that IL-10 and TGF-β are expressed not only by the GA-labeled cells but also by unlabeled cells within their vicinity (Figs. 2 C and F and 3D). These surrounding bystander cells have elongated astrocyte-like morphology consistent with activated microglia. Staining with GFAP, a component of astrocyte filaments, corroborated the astrocyte nature of these cells (Figs. 2 O–R and 3 J–M). The glial cells displayed similar or even higher IL-10 and TGF-β expression intensity compared with the staining of the GA specific population. Such spreading of positive staining could result either from genuine IL-10 and TGF-β secretion by the astrocytes that had been activated by the cytokines secreted from the GA-specific cells, or from the binding of IL-10 and TGF-β secreted by the GA-specific cells to their specific receptors on these astrocytes (17, 19). In both cases, this Th2/3 spreading suggests a bystander therapeutic effect of GA on the CNS resident cells.
IL-10 is a potent regulatory cytokine in autoimmunity that inhibits Th1 cells and macrophage activation in addition to its effector multifunctional therapeutic reactivity (1, 2, 14). Moreover, IL-10 can modulate glial cell responses by inhibiting MHC class II, NO, and chemokine expression (19). TGF-β suppresses cytotoxic T cell response, production of tumor necrosis factor α, IFN-γ, and factors that contribute to myelin damage, such as lysosomal enzymes and nitrogen intermediates (15). The ability of GA-infiltrating cells to express and induce the expression of these potent modulating cytokines, also by bystander CNS cells, substantiates GA therapeutic activity. Furthermore, the potent neurotrophic factor BDNF, expressed by the GA-specific cells in situ, is a key regulator of neuronal development, which supports neuronal survival and regulates neurotransmitter release and dendritic growth (12, 13). BDNF can rescue injured or degenerating neurons and induce axonal outgrowth, remyelination, and regeneration (20). The full-length BDNF receptor, tyrosine kinase receptor B, has been found in neurons in the vicinity of MS plaques and in reactive astrocytes in MS lesions (21). The BDNF secreted by the GA-infiltrating cells can therefore actually function in the MS/EAE target tissue. Thus, the expression of BDNF, IL-10, and TGF-β by GA-specific cells in the CNS directly links the therapeutic activity of GA in MS/EAE and its in situ immunomodulatory effect, namely, the antiinflammatory bystander suppression and neuroprotection induced by GA-specific T cells in the brain.
Acknowledgments
This study was supported in part by grants from Teva Pharmaceutical Industries (Israel) and Terry and Dr. Claude Oster and by a special fund of the Eugene Applebaum Family Foundation (Bloomfield Hills, MI).
Abbreviations: GA, glatiramer acetate; BDNF, brain-derived neurotrophic factor; TGF-β, transforming growth factor β; MS, multiple sclerosis; EAE, experimental autoimmune encephalomyelitis; GFAP, glial fibrillary acidic protein; Th, T helper.
References
- 1.Liblau, R. S., Singer, S. M. & McDevitt, H. O. (1995) Immunol. Today 16, 34–38. [DOI] [PubMed] [Google Scholar]
- 2.Kennedy, M. K., Torrance, D. S., Picha, K. S. & Mohler, K. M. (1992) J. Immunol. 149, 2496–2505. [PubMed] [Google Scholar]
- 3.Ziemssen, T., Kumpfel, T., Klinkert, W. E., Neuhaus, O. & Hohlfeld, R. (2002) Brain 125, 2381–2391. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum, D., Aharoni, R., Fridkis-Hareli, M., Arnon, R. & Sela, M. (1998) in The Decade in Autoimmunity, ed. Shoenfeld, Y. (Elsevier, New York), pp. 183–188.
- 5.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1997) Proc. Natl. Acad. Sci. USA 94, 10821–10826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aharoni, R., Teitelbaum, D., Sela, M. & Arnon, R. (1998) J. Neuroimmunol. 91, 135–146. [DOI] [PubMed] [Google Scholar]
- 7.Duda, P. W., Schmied, M. C., Cook, S. L., Krieger, J. I. & Hafler, D. A. (2000) J. Clin. Invest. 105, 967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aharoni, R., Teitelbaum, D., Leitner, O., Meshorer, A., Sela, M. & Arnon, R. (2000) Proc. Natl. Acad. Sci. USA 97, 11472–11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aharoni, R., Meshorer, A., Sela, M. & Arnon, R. (2002) J. Neuroimmunol. 126, 58–68. [DOI] [PubMed] [Google Scholar]
- 10.Krakowski, M. L. & Owens, T. (1997) Eur. J. Immunol. 27, 2840–2847. [DOI] [PubMed] [Google Scholar]
- 11.Kipnis, J., Yoles, E., Porat, Z., Cohen, A., Mor, F., Sela, M., Cohen, I. R. & Schwartz, M. (2000) Proc. Natl. Acad. Sci. USA 97, 7446–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thoenen, H. (1995) Science 270, 593–598. [DOI] [PubMed] [Google Scholar]
- 13.Barde, Y. A. (1997) Nature 385, 391–393. [DOI] [PubMed] [Google Scholar]
- 14.Bettelli, E., Nicholson, L. B. & Kuchroo, V. K. (2003) J. Autoimmun. 20, 265–267. [DOI] [PubMed] [Google Scholar]
- 15.Morris, M. M., Dyson, H., Baker, D., Harbige, L. S., Fazakerley, J. K. & Amor, S. (1997) J. Neuroimmunol. 74, 185–197. [DOI] [PubMed] [Google Scholar]
- 16.Parish, C. R. (1999) Immunol. Cell Biol. 77, 499–512. [DOI] [PubMed] [Google Scholar]
- 17.Hulshof, S., Montagne, L., De Groot, C. J. A. & Van Der Valk, P. (2002) Glia 38, 24–35. [DOI] [PubMed] [Google Scholar]
- 18.Flugel, A., Berkowicz, T., Ritter, T., Labeur, M., Li, Z., Ellwart, J. W., Willem, M., Lassmann, H. & Wekerle, H. (2001) Immunity 14, 547–560. [DOI] [PubMed] [Google Scholar]
- 19.Ledeboer, A., Breve, J. P., Poole, S., Tilders, F. J. & Van Dam, A. M. (2000) Glia 30, 134–142. [DOI] [PubMed] [Google Scholar]
- 20.Gravel, C., Gotz, R., Lorrain, A. & Sendtner, M. (1997) Nat. Med. 3, 765–770. [DOI] [PubMed] [Google Scholar]
- 21.Stadelmann, C., Kerschensteiner, M., Misgeld, T., Bruck, W., Hohlfeld, R. & Lassmann, R. (2002) Brain 125, 75–85. [DOI] [PubMed] [Google Scholar]