There is a growing interest in the potential therapeutic effect of mesenchymal stromal (stem) cells (MSCs) in many disease states (reviewed in [1-4]). MSCs can be isolated from various tissues of mammals; the blood, bone marrow, and adipose tissue have to date formed the major sources. The number of cells that can be isolated from any tissue is small. For example, MSCs represent only 0.001-0.01% of the total nucleated cells in bone marrow. However, they grow readily in vitro and can be expanded to significant numbers in culture. After in vivo administration, they have the ability to migrate to sites of inflammation and also to sites of allograft rejection (2).
MSCs are known to have regenerative, anti-inflammatory, and immunomodulatory effects, and these have all been identified both in vitro and in vivo. Immunomodulation is achieved through the effects of the MSCs on T cells, dendritic cells, B cells, and NK cells through direct cell-to-cell contact and by humoral factors. The successful treatment of patients with severe acute graft-vs-host disease by the administration of third-party haploidentical human MSCs in 2004 (5,6) created a surge of interest in the potential therapeutic effects of these cells.
Today, although relatively little is known about their in vivo biology, human MSCs are commercially available, and have been introduced into clinical medicine as potential treatments for a wide variety of pathological conditions. Currently, no fewer than 86 clinical trials are being undertaken worldwide (www.clinicaltrials.gov), at least four of which relate to organ transplantation. Their therapeutic potential is being investigated in sub-clinical rejection, chronic allograft nephropathy, and in the induction of tolerance to renal allografts.
However, there is as yet no consensus on (i) how to prepare MSCs, (ii) how to identify them accurately, and (iii) when and how many to infuse to obtain the desired therapeutic effect. The small number of human MSCs that can be obtained from tissue such as bone marrow necessitates prolonged ex vivo expansion in order to obtain sufficient numbers for therapeutic efficacy. Currently, it is considered that at least 2×106 MSCs/kg are needed for a rational clinical therapy (7). Prolonged expansion, however, may alter the function of the MSCs, e.g., with a loss of immunomodulatory function, and may increase the risk of differentiation into unwanted tissues and/or of replicative senescence.
Although allogeneic MSCs have been demonstrated to have immunosuppressive capacities in vitro, they can lose this capacity in immunocompetent animals and, indeed, can induce an antibody response. To avoid this response, it would seem essential that some immunosuppressive therapy is required at least during the early phase after MSC infusion.
Of considerable interest to those in the field of xenotransplantation research is the observation that MSCs can function, e.g., regenerate and differentiate across species barriers (8-10). This has been demonstrated in diverse animal models, e.g., human/pig-to-mouse, human/pig-to-hamster, and human-to-pig. However, to date there are no data from a pig-to-primate model.
Pig MSCs (pMSCs) might have considerable potential, particularly in xenotransplantation, but also possibly in allotransplantation. In view of the size of the pig in relation to that of the average human, pMSCs can be obtained in very large numbers, thus reducing the necessity for prolonged ex vivo expansion with its potential complications. The data available in the literature from other animal models strongly suggest that pMSCs would have a potent regenerative effect if administered to humans.
However, it is not known (i) whether pMSCs would be sufficiently immunogenic to prevent their clinical use - in other words, would they be rejected - or (ii) whether they would have a significant immunomodulatory effect on the human immune response. Of particular relevance to xenotransplantation, it is not known whether pMSCs will immunomodulate the primate response to other pig cells, e.g., islets.
The potential advantages of pMSCs extend beyond their easy availability (11,12). For example, in pig islet transplantation into primates, the MSCs could be obtained from the same islet ‘donor’, i.e., the same source pig, and could possibly provide a preferable micro-environment for the islets, resulting in more rapid stimulation of local growth factors and revascularization, and even regeneration of the islets. MSCs from genetically-engineered pigs, e.g., α1,3-galactosyltransferase gene-knockout (GTKO) pigs that are additionally transgenic for a complement-regulatory protein and possibly other beneficial genes, might be of particularly low immunogenicity, increasing their potential as a therapeutic ‘agent’ in xenotransplantation and possibly also in allotransplantation.
We have successfully isolated MSCs from GTKO pigs (manuscript submitted), and have confirmed their phenotype and differentiation capacity. They have identical or very similar characteristics to MSCs from other mammals. As anticipated, GTKO pMSCs do not express Galα1,3Gal antigens, and are significantly less immunogenic than WT pMSCs. Furthermore, there is significantly less human antibody binding (IgM and IgG) to GTKO pMSCs than to GTKO pig aortic endothelial cells (pAECs).
The human proliferative cellular responses to GTKO pMSCs, allogeneic MSCs, and to allogeneic PBMCs were compared. The response of human PBMCs to GTKO pMSCs was not significantly different from that to human MSCs (p=0.08) (Figure1), both of which were significantly lower than to allogeneic PBMCs (p<0.01).
Figure 1.
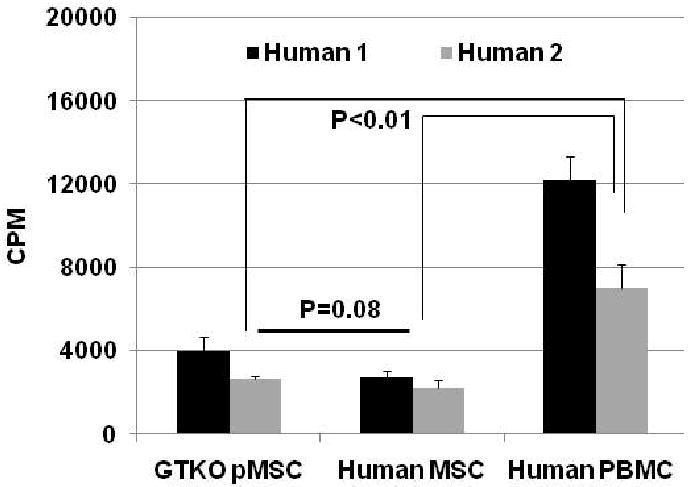
The cellular response of PBMCs from two healthy volunteers to (i) GTKO pMSCs, (ii) human MSCs, and (iii) human PBMCs in MLR (responder:stimulator ratio at 1:10). The proliferation of human PBMCs was significantly greater to allogeneic PBMCs than to both human MSCs and GTKO pMSCs (p<0.01). There was no significant difference between the response to human MSCs and that to GTKO pMSCs (p=0.08). Data representative of 2 different experiments.
In summary, therefore, we believe that genetically-modified pMSCs may have considerable potential in xenotransplantation. Not only are they available in large numbers, which reduces the necessity for prolonged ex vivo expansion, but they can be obtained from a herd of identical or almost-identical pigs, thus significantly reducing the variation seen when they are obtained from pooled human sources. This may be a significant advantage over human MSCs. Importantly, they would appear to be no more immunogenic than allogeneic MSCs, suggesting that they would not be at a disadvantage in this respect if administered clinically. Their anti-inflammatory and regenerative capacities in humans are likely to be no different from allogeneic MSCs. If it can be confirmed that their immunomodulatory effect is comparable to that of human MSCs, then their potential as a therapeutic agent may be considerable.
References
- 1.Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759. doi: 10.2337/db08-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sordi V. Mesenchymal stem cell homing capacity. Transplantation. 2009;87:S42. doi: 10.1097/TP.0b013e3181a28533. [DOI] [PubMed] [Google Scholar]
- 3.Dahlke M, Hoogduijn M, Eggenhofer E, et al. Toward MSC in solid organ transplantation: 2008 position paper of the MISOT study group. Transplantation. 2009;88:614. doi: 10.1097/TP.0b013e3181b4425a. [DOI] [PubMed] [Google Scholar]
- 4.Popp FC, Eggenhofer E, Renner P, et al. Mesenchymal stem cells can affect solid organ allograft survival. Transplantation. 2009;87:S57. doi: 10.1097/TP.0b013e3181a288aa. [DOI] [PubMed] [Google Scholar]
- 5.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 7.Bartmann C, Rohde E, Schallmoser K, et al. Two steps to functional mesenchymal stromal cells for clinical application. Transfusion. 2007;47:1426. doi: 10.1111/j.1537-2995.2007.01219.x. [DOI] [PubMed] [Google Scholar]
- 8.Davani B, Ikonomou L, Raaka BM, et al. Human islet-derived precursor cells are mesenchymal stromal cells that differentiate and mature to hormone-expressing cells in vivo. Stem Cells. 2007;25:3215. doi: 10.1634/stemcells.2007-0323. [DOI] [PubMed] [Google Scholar]
- 9.Henriksson HB, Svanvik T, Jonsson M, et al. Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 2009;34:141. doi: 10.1097/BRS.0b013e31818f8c20. [DOI] [PubMed] [Google Scholar]
- 10.Shabbir A, Zisa D, Leiker M, Johnston C, Lin H, Lee T. Muscular dystrophy therapy by nonautologous mesenchymal stem cells: muscle regeneration without immunosuppression and inflammation. Transplantation. 2009;87:1275. doi: 10.1097/TP.0b013e3181a1719b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solari MG, Srinivasan S, Boumaza I, et al. Marginal mass islet transplantation with autologous mesenchymal stem cells promotes long-term islet allograft survival and sustained normoglycemia. J Autoimmun. 2009;32:116. doi: 10.1016/j.jaut.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Brusko TM. Mesenchymal stem cells: a potential border patrol for transplanted islets? Diabetes. 2009;58:1728. doi: 10.2337/db09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]


