Abstract
Aims/Hypothesis
We previously used an integrative genetics approach to demonstrate that 5-lipoxygenase (5-LO; ALOX5) deficiency in mice (Alox5−/−) protects against atherosclerosis despite increasing lipid levels and fat mass. In the present study, we sought to further examine the role of 5-LO in adiposity and pancreatic function.
Methods
Alox5−/− and wildtype (WT) mice were characterized for adiposity and glucose/insulin metabolism using in vivo and in vitro approaches. The role of ALOX5 on pancreatic function in human islets was assessed through siRNA knockdown experiments.
Results
Beginning at 12 wks of age, Alox5−/− mice had significantly increased fat mass, plasma leptin levels, and fasting glucose levels, but lower fasting insulin levels (P < 0.05). Although Alox5−/− mice did not exhibit insulin resistance, they had impaired insulin secretion in response to a bolus glucose injection. Histological analyses revealed that Alox5−/− mice had increased islet area, beta-cell nuclear size, and numbers of beta-cells/mm2 islet (P < 0.05), indicative of both hyperplasia and hypertrophy. Basal and stimulated insulin secretion in isolated Alox5−/− islets was significantly lower than WT (P < 0.05) and accompanied by 3–5-fold decreased expression of the insulin and pancreatic duodenal homeobox 1 (Pdx1) genes. Direct perturbation of ALOX5 in isolated human islets with siRNA decreased insulin and PDX1 gene expression by 50% and insulin secretion by 3-fold (P < 0.05).
Conclusions
These results provide strong evidence for pleiotropic metabolic effects of 5-LO on adiposity and pancreatic function and may have important implications for therapeutic strategies targeting this pathway for the treatment of cardiovascular disease (CVD).
Keywords: 5-lipoxygenase, insulin secretion, obesity, leukotrienes, beta-cell function, inflammation, type 2 diabetes
Introduction
The 5-LO pathway, which generates pro-inflammatory leukotrienes (LTs) from arachidonic acid, has recently garnered a great deal of attention for its potential role in CVD-related traits. This stems from a series of studies over the last few years, which collectively have provided strong evidence for the pro-atherogenic role of LTs [1, 2]. Importantly, multiple lines of evidence support this concept, ranging from biochemical, genetic, and pharmacological studies in both mice and humans [2]. For example, we demonstrated that Alox5 deficiency in mice protects against the development of aortic lesions [3, 4] and that promoter variants of the human ALOX5 gene are associated with increased carotid intima-media thickness [5]. Other groups have also provided evidence for the involvement of the 5-LO/LT pathway in CVD traits [6–13].
More recently, we used an integrative genomics approach with inbred mouse strains, gene-targeted mice, and microarray profiling to demonstrate that 5-LO also contributes to metabolic phenotypes, such as body fat, bone density, and lipid levels [14]. These observations raise important questions regarding the potential pleiotropic effects of the 5-LO pathway on components of the metabolic syndrome. To further explore these inter-relations, we characterized Alox5−/− mice for traits related to obesity and type 2 diabetes and demonstrate that 5-LO deficiency increases adiposity and negatively affects pancreatic function.
Materials and Methods
Animal Husbandry
Alox5−/− mice were purchased from the Jackson Laboratories (Bar Harbor, Maine) and backcrossed onto a C57BL/6J (B6) background for more than 10 generations. WT B6 littermates were either bred in house or B6 mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Since no differences were observed between WT mice from either source, the data from both types of control mice were combined together. All animals were housed 4 per cage at 25°C on a 10hr dark/14hr light cycle and maintained on a chow diet (Purina #5015). All procedures were in accordance with current the National Research Council, Guide for the Care and Use of Laboratory Animals and were approved by the UCLA Animal Research Committee.
Plasma Measurements
Mice were bled retro-orbitally under isoflurane anesthesia or through the tail vein while restrained and conscious. Glucose levels were determined using commercially available kits from Sigma (St. Louis, MO) or the OneTouch Ultra Blood Glucose Monitoring System (LifeScan, Inc, Johnson & Johnson). We previously established the accuracy of glucose values using this glucometer compared to the Sigma kit (Mehrabian, unpublished observations). Insulin and leptin levels were measured using commercial ELISA kits from Crystal Chemical (Chicago, IL) and R&D Systems (Minneapolis, MN), respectively. All measurements were performed in duplicate or triplicate according to the manufacturer’s instructions.
Body Composition
Whole body fat, fluids, and lean tissue mass of isoflurane anesthetized mice were determined using a Bruker Optics Minispec NMR analyzer (The Woodlands, TX) according to the manufacturer’s recommendations. The mass of individual fat depots (retroperitoneal, epididymal, subcutaneous, and omental) were determined by dissecting and weighing each fat pad separately after the mice were euthanized.
Intraperitoneal Glucose and Insulin Tolerance Tests
Intraperitoneal insulin tolerance tests (IPITTs) were performed by administering an intraperotineal injection (0.75U/kg body weight) of recombinant human insulin (Novolin, Novo Nordisk, Bagsværd, Denmark) to conscious mice fasted for 5hrs. Plasma glucose levels were measured in blood samples obtained through the tail vein using a glucometer, as described above. For intraperitoneal glucose tolerance tests (IPGTTs), mice were fasted 12hrs overnight and injected with a bolus (1mg/g body weight) of glucose (10% wt/vol in sterile H2O) into the peritoneal cavity. Blood samples were obtained from conscious mice through the tail vein or from isoflurane-anesthetized mice through the retro-orbital plexus at 0, 15, 30, 60, 90 and 120min post injection. Plasma glucose and insulin levels were measured as described above.
Immunohistochemistry
After euthanization, the pancreas was dissected, weighed, fixed in formalin, and embedded in paraffin. Tissue sections were deparaffinized, rehydrated, and incubated with guinea pig anti-insulin antibody (Dako, Glostrup, Denmark) followed by detection with a fluorescein-conjugated rabbit anti-guinea pig antibody (Jackson ImmunoResearch Laboratories, West Grove, PA). Subsequently, the samples were labeled for glucagon with rabbit anti-glucagon or with a mixture of rabbit anti-somatostatin and anti-pancreatic polypeptide antibodies (Dako), followed by detection with donkey anti-rabbit Cy3-conjugated antibody (Jackson ImmunoResearch Laboratories). Beta-cell mass was analyzed using OpenlabTM software. The relative area of beta-cells (green fluorescence) was determined by quantification of the cross-sectional beta-cell area divided by the cross-sectional area of total tissue. The beta-cell mass per pancreas was estimated as the product of the relative cross-sectional area of beta-cells per total tissue and the weight of the pancreas. Beta-cell number/per mm2 islet, nuclear size, nuclear distance, and islet size were quantified using an Olympus IX70 inverted system microscope (Olympus America, Melville, NY) and Image-Pro Plus software (Media Cybernetics, Silver Springs, MD). The number of beta-cells/islet was calculated by multiplying mm2 of islet area by the number of beta-cells/mm2. Ten representative 20μm sections from each pancreas (spanning the width of the pancreas) were used in these analyses and results are from 10 male mice of each genotype from three litters at 12wks of age.
Islet Isolation and Culture
Human islets were isolated from the pancreas of five healthy organ donors at the University of Illinois at Chicago, as described previously [15]. Mouse islets were isolated by bile duct perfusion and collagenase digestion as described elsewhere [16]. The islets were cultured on matrix-coated plates derived from bovine corneal endothelial cells (Novamed, Jerusalem, Israel), allowing the cells to attach to the dishes and spread [17]. Human islets were cultured in CMRL 1066 medium containing 5.5mmol/l glucose and mouse islets in RPMI 1640 medium containing 11.1mmol/l glucose, both supplemented with 100U/ml penicillin, 100μg/ml streptomycin, and 10% FCS (Invitrogen, Carlsbad, CA).
Insulin Release and Content Measurements
Chronic insulin release over 48hrs was evaluated in the culture medium collected before the termination of the experiment. For acute insulin release in response to glucose, mouse islets or transfected human islets were washed and pre-incubated for 30min in Krebs-Ringer bicarbonate buffer (KRB) containing 2.8mmol/l glucose and 0.5% BSA. KRB was then replaced with KRB containing 2.8mmol/l glucose for 60min (basal), followed by an additional 60min in KRB with 16.7mmol/l glucose (stimulated). Thereafter, islets were incubated in 1ml 0.18 N HCl in 70% ethanol and left overnight at 4° C. Insulin was determined using a mouse insulin ELISA kit (ALPCO Diagnostics, Salem, NH). The stimulatory index was calculated by dividing stimulated insulin secretion levels by basal insulin secretion. Beta-cell area of the stimulated islets was calculated under the microscope using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD). All experiments were performed in three independent rounds using islets isolated from three litters of 12wk-old male Alox5−/− and WT mice, respectively, (n = 10 for each strain) with three dishes for each genotype group and 20 islets per dish.
RNA Interference Experiments
Short interfering RNAs (siRNA) designed for the specific knockdown of ALOX5 expression or as a non-specific control were synthesized by Ambion (Austin, Texas). Isolated human islets were plated on extracellular matrix coated dishes and cultured in medium containing 5.5mmol/l glucose for 24hrs. The islets were then transfected with 100nmol/l of siRNA using 2.5μl/ml Lipofectamine 2000 in OptiMEM media (Invitrogen), according to the manufacturer’s protocols. The transfection medium was replaced after 12hrs with antibiotic-free culture media and 96hrs later the islets were harvested for analysis. All experiments were performed in triplicate with three dishes for each treatment group with 20 islets per dish from five donors in five independent rounds.
RNA Extraction and Real-time Quantitation
Total RNA was extracted from adipose tissue or pancreatic islets using RNeasy Mini kits (Qiagen, Valencia, CA). RNA was isolated from 50 mouse islets of each genotype group that were obtained from 10 12-wk old mice whereas 100 islets from each treatment group were used to extract RNA for the human islet experiments. cDNA was prepared from 1μg of total RNA using reverse transcription kits (Life Technologies, Gaithersburg, MD; Applied Biosystems, Foster City, CA; or Invitrogen) according to the manufacturers’ protocols. Real-time expression assays were performed either on the Light Cycler quantitative PCR system (Roche, Basel, Switzerland) or an ABI 7900HT instrument. Real-time reactions were carried out in triplicate with either SYBR green assays as described previously [18, 19] or pre-developed assays from ABI [20]. For the SYBR green assays, RNA levels for each sample were calculated using standard curves generated from serial 10-fold dilutions of pooled RNA from WT mice. For the pre-developed assays, RNA levels for each sample were calculated using pooled WT mice as the reference sample. Each sample was normalized to endogenous controls, such as GAPDH/Gapdh (glyceraldehyde-3-phosphate dehydrogenase), ACTB (beta actin), or TUBA1B/Tuba1b (alpha tubulin), and the replicates were averaged to determine differences between Alox5−/− and WT mice or between human islets treated with control or ALOX5-specific siRNAs. A list of the primer sequences used for the SYBR green assays is provided in the electronic supplementary material (ESM; ESM Table 1).
Table 1.
Measures of Body Weight and Adiposity in Alox5−/− and WT Mice
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Weight (g) | Fat (g) | % Body Fat | Weight (g) | Fat (g) | % Body Fat | |
| 10 weeks | ||||||
| Alox5−/− | 19.9 ± 0.3 | 2.5 ± 0.3 | 12.4 ± 1.2 | a26.0 ± 2.3 | a2.8 ± 0.2 | a10.8 ± 0.5 |
| WT | 18.6 ± 0.1 | 2.0 ± 0.3 | 12.2 ± 1.3 | 23.8 ± 1.7 | 1.9 ± 0.4 | 7.9 ± 1.0 |
| 14 weeks | ||||||
| Alox5−/− | a24.1 ± 0.4 | a2.8 ± 0.8 | a11.7 ± 1.4 | a29.0 ± 1.6 | a3.5 ± 0.2 | a12.2 ± 0.2 |
| WT | 21.6 ± 0.3 | 2.0 ± 0.1 | 9.5 ± 0.5 | 26.4 ± 1.7 | 2.0 ± 0.4 | 7.5 ± 0.9 |
| 16 weeks | ||||||
| Alox5−/− | a27.5 ± 0.2 | a2.9 ± 0.6 | a10.6 ± 0.24 | a30.0 ± 1.7 | a3.9 ± 0.4 | a12.9 ± 0.5 |
| WT | 22.8 ± 0.1 | 2.0 ± 0.01 | 9.3 ± 0.2 | 26.4 ± 1.5 | 2.0 ± 0.1 | 7.6 ± 0.2 |
| 19 weeks | ||||||
| Alox5−/− | a28.7 ± 0.1 | a3.4 ± 0.4 | a11.9 ± 1.3 | a31.2 ± 1.9 | a3.9 ± 0.5 | a12.4 ± 1.2 |
| WT | 23.4 ± 0.2 | 2.0 ± 0.2 | 8.8 ± 0.9 | 27.5 ± 1.5 | 2.0 ± 0.2 | 7.3 ± 0.7 |
Note: Five mice of each genotype group were weighed at the indicated ages and adiposity was determined by NMR. Data are shown as mean ± SE and statistical comparisons were only made within each gender group.
P < 0.05 between Alox5−/− and WT mice.
Statistical Analyses
Differences in measured variables between Alox5−/− and WT mice or the siRNA treatments in human islets were determined by Student’s t-test (Statview v5.0). Values are expressed as mean ± SE and differences were considered statistically significant at P < 0.05.
Results
Increased Adiposity in Alox5−/− Mice
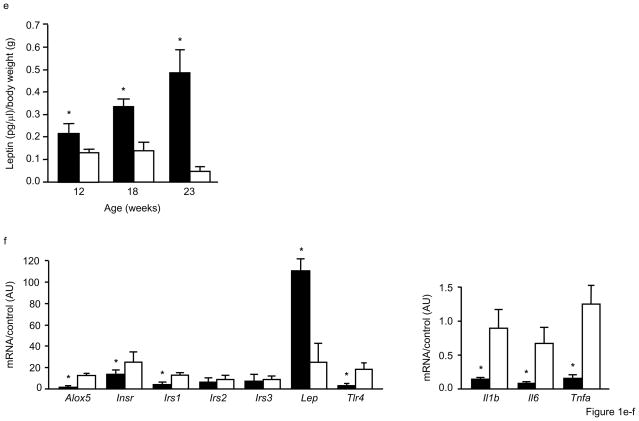
Based on our earlier observations that 5-LO deficiency led to several metabolic abnormalities, we first characterized Alox5−/− mice for measures related to obesity. Beginning at 10wks of age and out to 19wks, Alox5−/− mice had significantly higher body weight compared to age-matched WT controls (Table 1). As assessed by nuclear magnetic resonance (NMR), this difference was primarily due to increased adiposity (Table 1), which was not restricted to one anatomical region since all four adipose tissue depots were found to have increased mass (Fig. 1a). In agreement with these observations, fasting plasma leptin levels (expressed as a ratio of body weight) were also significantly higher in female Alox5−/− mice at 12wks of age, progressively increased by 23wks (Fig. 1b), and mirrored what was observed for body weight and % body fat. As we reported previously, these adiposity differences did not result from behavioral changes, such as food intake or activity levels [14]. To gain insight into the mechanism by which 5-LO deficiency leads to the observed metabolic changes, we measured mRNA levels of genes related to insulin and leptin metabolism as well as inflammation in adipose tissue from 12wk-old female mice using real-time quantitative PCR. These analyses revealed that Alox5 was expressed in adipose tissue of WT mice but, as expected, not in Alox5−/− mice (Fig. 1c). In addition, Alox5−/− mice had ~ 5-fold increased leptin gene expression, consistent with the higher plasma levels, whereas insulin receptor (Ir) and insulin receptor substrate 1 (Irs-1) mRNA levels were decreased by ~50% (Fig. 1c). Interestingly, expression of inflammatory genes, such as toll-like receptor 4 (Tlr4), interleukin-1 beta (Il-1β), Il-6, and tumor necrosis factor alpha (Tnfα), were all decreased in Alox5−/− mice compared to WT (Fig. 1c).
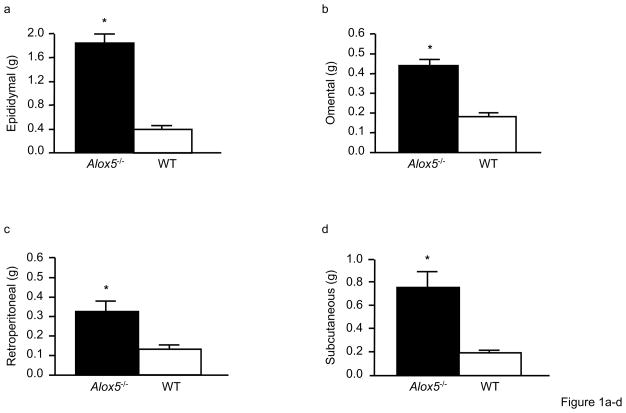
Figure 1. Alox5−/− mice have increased adiposity and altered gene expression in adipose tissue.
The mass of epididymal, retroperitoneal, omental, and subcutaneous fat depots are all increased in 23wk-old Alox5−/− mice compared to WT (a). Each fat pad from 23wk-old mice was dissected out and weighed individually. Beginning at 12wks of age, Alox5−/− mice have significantly higher fasting plasma leptin levels (expressed as a ratio of body weight), which becomes progressively more pronounced by 23wks of age (b). Gonadal adipose tissue expression of leptin is elevated ~ 5-fold in 12wk-old Alox5−/− mice compared to WT whereas the expression of insulin receptor (Ir) and insulin receptor substrate 1 (Irs-1) is decreased by ~50%. The expression of Irs-2 and Irs-3 is not significantly different. Expression of inflammatory genes, such as Tlr4, Il-1β, Il-6, and Tnfα, are also decreased in adipose from Alox5−/− mice (c). Gene expression levels were quantitated in triplicate, normalized to an endogenous control, and expressed as relative units (ru). All data are from female mice (n = 7–12 per group) and expressed as mean + SE. *P < 0.05 between Alox5−/− (filled bars) and WT (open bars) mice.
Impaired Glucose and Insulin Metabolism in Alox5−/− Mice
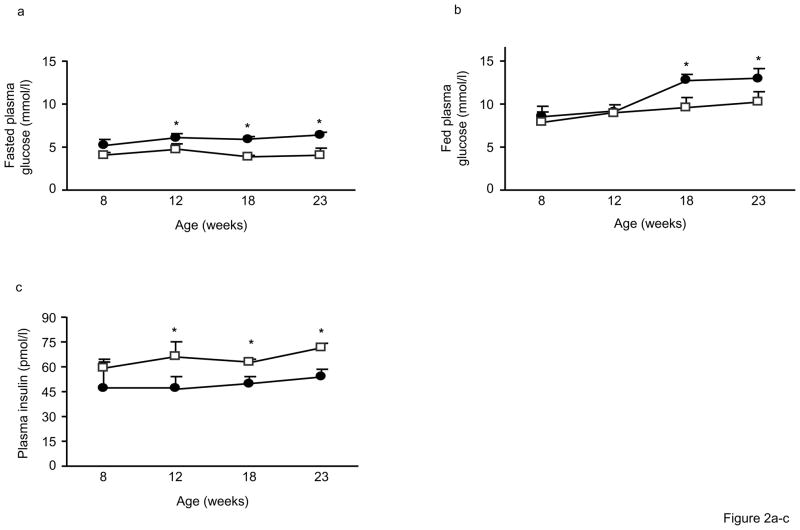
We next determined whether the increased adiposity of Alox5−/− mice was associated with other metabolic abnormalities in a series of time course experiments with mice at varying ages. As shown in Fig. 2a, fasting plasma glucose levels were not different at 8wks of age but became significantly elevated in male and female Alox5−/− mice starting at 12wks of age and remained higher out to 23wks of age. By contrast, fed plasma glucose levels became elevated at 18wks (Fig. 2b) whereas lower fasting insulin levels were observed starting at 12wks of age (Fig. 2c). These results suggest a progressive impairment of glucose homeostasis in Alox5−/− mice.
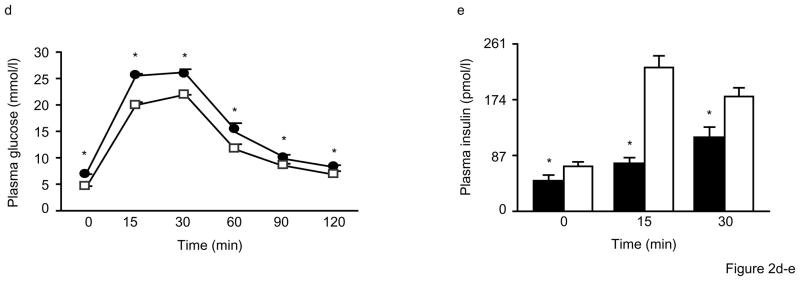
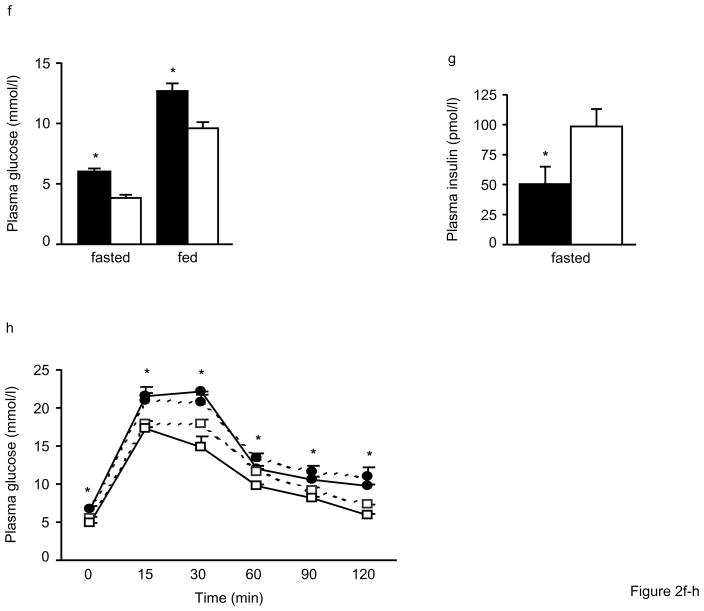
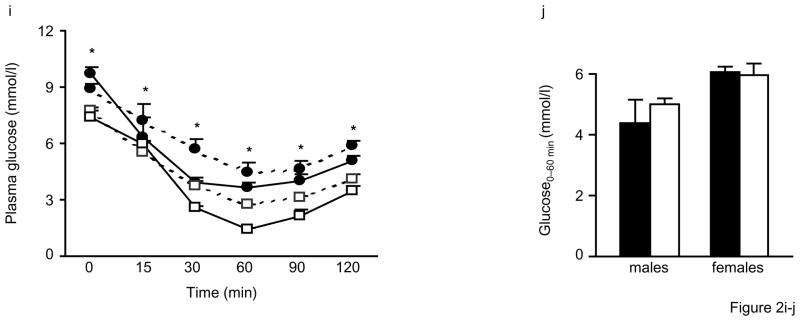
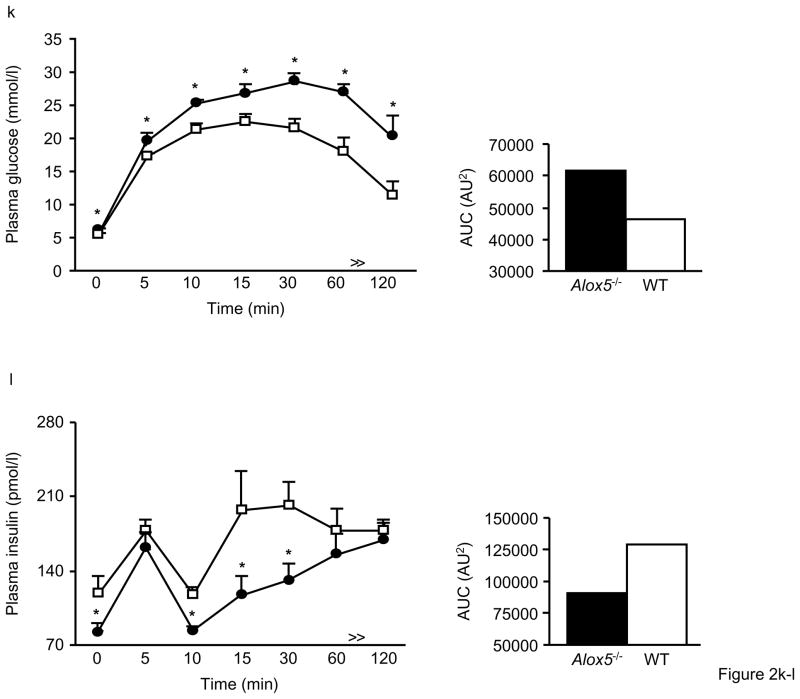
Figure 2. Impaired glucose tolerance and insulin secretion in Alox5−/− mice.
Fasting plasma glucose levels are significantly higher beginning at 12wks of age (a), fed plasma glucose levels become significantly elevated starting at 18wks (b), and fasting insulin levels are significantly lower beginning at 12wks (c) in male and female Alox5−/− mice (filled circles) compared to WT (open squares). Post glucose injection during an IPGTT, plasma glucose levels are significantly higher at all time points (d) whereas plasma insulin levels are lower at the 0, 15, and 30min time points (e) in 12wk-old male Alox5−/− mice (filled circles or bars) compared to WT (open circles or bars). Fasted and fed plasma glucose levels are higher (f) and fasting insulin levels are lower (g) in 18wk-old male and female Alox5−/− mice (filled bars) compared to WT (open bars). 18wk-old Alox5−/− male (filled circles, dashed line) and female (filled circles, solid line) mice exhibit significantly higher glucose levels at all time points after a bolus glucose injection during an IPGTT (h) compared to male (open squares, dashed line) and female (open squares, solid line)WT mice. An IPITT demonstrates that, despite starting higher at baseline, plasma glucose levels decrease at the same rate after administration of an insulin bolus in 18wk-old male (filled circles, dashed line) and female (filled circles, solid line) Alox5−/− mice compared to age-matched male (open squares, dashed line) and female (open squares, solid line) WT mice (i). This is further shown by the comparable changes in glucose levels from baseline to 60min post injection (j) in Alox5−/− mice (filled bars) compared WT (open bars). An IPGTT of 23wk-old Alox5−/− mice (filled circles) demonstrates decreased glucose clearance (k) compared to WT (open squares), which results, at least in part, from impaired insulin secretion as shown at the baseline, 10, 15, and 30min time points (l). All data are from 10–15 mice of each genotype at the indicated ages. IPITT and IPGTT experiments were performed as described in the Materials and Methods section. AUC, area under the curve; Data are shown as mean + SE. *P < 0.05 between Alox5−/− and WT mice.
To explore these differences further, we first assessed insulin sensitivity by intraperitoneal glucose tolerance tests (IPGTTs). As shown in Fig. 2d, male and female Alox5−/− mice at 12wks of age exhibited significantly higher plasma glucose levels at all time points after a bolus glucose injection compared to WT mice. This was also accompanied by significantly lower insulin levels at the 0, 15, and 30min time points (Fig. 2e). These experiments were repeated at 18wks of age where, again, we observed significantly higher fasting and fed plasma glucose (Fig. 2f) and lower fasting insulin levels (Figs. 2g) in Alox5−/− mice. In addition, an IPGTT at 18wks of age also demonstrated significantly elevated plasma glucose levels in Alox5−/− mice at all time points post injection (Fig. 2h). Given these observations, we next carried out intraperitoneal insulin tolerance tests (IPITTs) to assess insulin sensitivity. Interestingly, although Alox5−/− mice had elevated plasma glucose levels at all time points post insulin injection, including at baseline, (Fig. 2i), the rate of glucose disposal was the same as WT mice since the difference between plasma glucose from 0 to 60min were not significantly different between the two strains (Fig. 2j). These results suggest that 5-LO deficiency does not alter insulin sensitivity noticeably despite an increase in adiposity.
We next carried out another series of IPGTTs on mice at 23wks of age to gain a better understanding of both glucose and insulin dynamics simultaneously during the same experiment. Similar to the results in younger mice, 23wk-old Alox5−/− mice exhibited higher plasma glucose levels at baseline and throughout the duration of the experiment (Fig. 2k). In parallel, plasma insulin levels were significantly lower in the Alox5−/− mice at baseline and at the 10, 15, and 30min time points (Fig. 2l), suggesting an impairment of insulin secretion. We also expressed the IPGTT data by calculating mean area under the curve, which is increased for glucose and decreased for insulin levels in Alox5−/− mice compared to WT (Fig. 2k and l).
Impaired Beta-Cell Function in Alox5−/− Mice
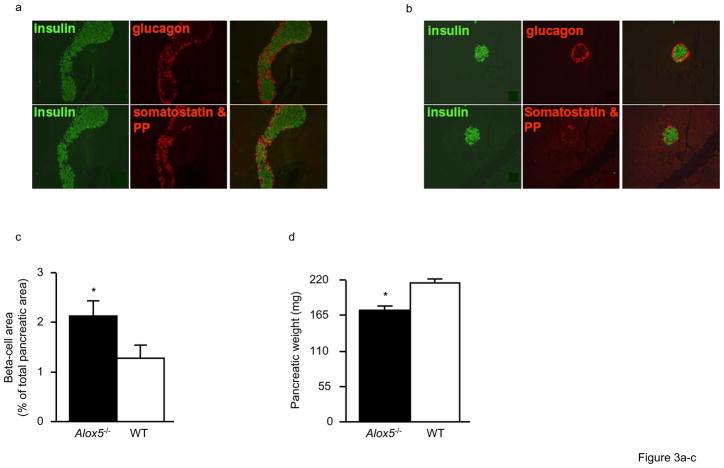
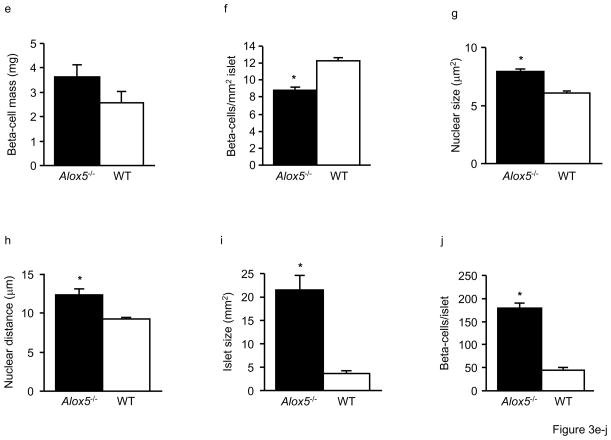
To assess whether the decreased insulin response in Alox5−/− mice was mediated by altered beta-cell mass and function, we analyzed the morphology, histology, and function of isolated pancreatic islets in vitro from 12wk-old mice. When stained for insulin, glucagon, somatostatin, and pancreatic polypeptide (PP), the islets of both Alox5−/− and WT mice contained the appropriate ratio of alpha, beta, delta, and PP-staining cells (Fig. 3a and b). However, beta-cell volume of Alox5−/− islets was increased 1.7-fold compared to WT (Fig. 3c). Since the pancreatic weight of Alox5−/− mice was decreased compared to WT (Fig. 3d), beta-cell mass was not significantly different despite the larger islets (Fig. 3e). We next analyzed the islets for the presence of hypertrophy and/or hyperplasia. As shown in Fig. 3f, Alox5−/− islets have 1.4-fold fewer beta-cells/mm2 islet area, suggesting the presence of larger beta-cells and hypertrophy. This is supported by measurements of nuclear size and nuclear distance between neighboring beta-cells, which were both 1.3-fold larger in 5-LO−/− islets compared to WT (Figs. 3g and h). To further analyze islet size, we measured mean islet area, which is 5.7-fold larger in 5-LO−/− mice (Fig. 3i), and, accordingly, leads to 4-fold more beta-cells per islet (Fig. 3j). Taken together, these data show that Alox5−/− islets exhibit both hyperplasia as well as hypertrophy, with hyperplasia being more prominent since beta-cells from Alox5−/− mice are 1.3-fold larger but are 5.7-fold more frequent per islet.
Figure 3. Morphology and histology of the pancreas in Alox5−/− and WT mice.
Double immunostaining of representative pancreatic sections (250X) for insulin (green), glucagon, somatostatin, and pancreatic polypeptide (PP) shows normal islet morphology and cellularity in both Alox5−/− (a) and WT (b) mice. The right hand panel for each genotype group shows overlaid immunostaining images. Alox5−/− mice have increased beta-cell area as a percentage of total pancreatic area (c) but decreased pancreatic weight (d). Beta-cell mass is not significantly different (e). The number of beta-cells counted per mm2 islet is decreased in Alox5−/− mice (f), suggesting the presence of hypertrophic cells. This supported by the increased nuclear size (g) and distance between nuclei of adjacent beta-cells (h) in Alox5−/− islets. Mean islet area is increased 5.7-fold (i) and there are 4-fold more beta-cells per islet (j) in Alox5−/−mice compared to WT. Ten representative 20μm sections from each pancreas (spanning the width of the pancreas) were used in these analyses and results are from 10 male mice of each genotype from three litters at 12wks of age. Data are shown as mean + SE. *P < 0.05 between Alox5−/− and WT mice.
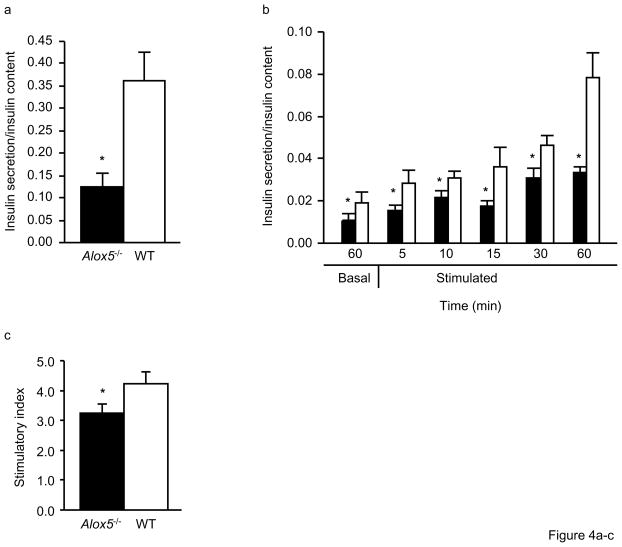
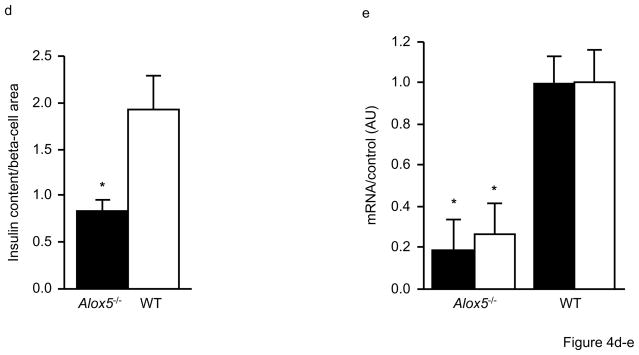
We next carried out a series of in vitro experiments to analyze insulin secretion in isolated islets from Alox5−/− and WT mice. During a 48hr culture, chronic insulin release, defined as the amount of insulin released into the media, was significantly decreased by 2.9 fold in Alox5−/− islets (Fig. 4a). Similarly, insulin secretion was significantly lower in Alox5−/− islets over a 60min pre-culture under basal conditions with 2.8mmol/l glucose (Fig. 4b) as well as during a 60min time course experiment under stimulated conditions with 16.7mmol/l glucose (Fig. 4b). Such an impairment of insulin secretion by Alox5−/− islets is further illustrated by the decreased stimulatory index as shown in Fig. 4c. Since Alox5−/− islets were larger, we also analyzed their insulin content (expressed per beta-cell area), which was significantly lower than WT (Fig. 4d). To help understand the molecular mechanisms underlying these histological and functional differences, we quantitated mRNA levels of the insulin and Pdx1 genes. PDX1 is involved in pancreatic development and differentiation and transcriptionally activates many beta-cell genes involved in glucose- stimulated insulin secretion, including glucokinase, glucose transporter 2, as well as insulin itself [21]. As shown in Fig. 4e, these analyses revealed ~ 5 and 3-fold decreased expression of insulin and Pdx1, respectively, in Alox5−/− islets compared to WT, consistent with the decreased insulin secretion and content.
Figure 4. Impaired beta-cell function and altered gene expression in isolated islets from Alox5−/− mice.
Chronic insulin secretion during a 48hr culture is significantly decreased in isolated islets from Alox5−/− mice compared to WT (a). Insulin secretion is defined as the amount secreted into the media and expressed as a ratio of secreted insulin to insulin content. Glucose-stimulated insulin secretion is impaired in Alox5−/− (filled bars) vs. WT (open bars) islets (b). Basal insulin secretion was measured after a 60min incubation in KRB with 2.8mmol/l glucose and stimulated insulin secretion was measured at 5, 10, 15, 30 and 60min after changing the medium to KRB with 16.7mmol/l glucose. Compared to WT, Alox5−/−islets have a reduced stimulatory index (c), which was calculated by dividing stimulated insulin release by basal insulin release. Despite having larger islets, the insulin content of Alox5−/− islets, expressed per beta-cell area, is lower compared to WT (d). Expression levels of the insulin (filled bars) and Pdx1 (open bars) genes are decreased in isolated islets from Alox5−/− mice compared to WT, as assessed by quantitative real-time quantitative PCR (e). The mRNA levels of each gene were measured in triplicate, normalized to an endogenous control, and are expressed in relative units (ru). All data are from three independent experiments from three litters of 12wk-old male Alox5−/− and WT mice (n = 10 for each genotype). Cell culture results are from three independent experiments from three dishes for each genotype group with 20 islets per dish. Total RNA was isolated from 50 islets of each genotype, with 10 mice in each group. Data are shown as mean + SE. *P < 0.05 between Alox5−/− and WT mice.
Direct Effect of 5-LO on Insulin Gene Expression and Secretion in Human Islets
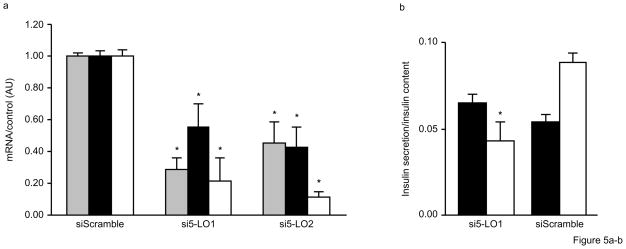
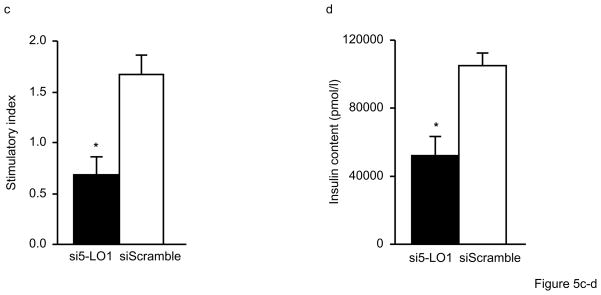
To determine whether impaired insulin secretion as a result of 5-LO deficiency is due to a direct effect in the pancreas or secondary to increased adiposity, we carried out siRNA knockdown experiments in isolated human islets. For these experiments, siRNAs were designed to be either specific for ALOX5 or a non-specific control. Transfection of control siRNA (siScramble) into islets had no effect on ALOX5, insulin, and PDX1 gene expression. By contrast, two different siRNAs specific for ALOX5 (si5-LO1 and si5-LO2) each significantly decreased ALOX5 expression by over 50% along with a concomitant decrease in insulin and PDX1 mRNA levels (Fig. 5a). Although insulin secretion under basal conditions (2.8mmol/l glucose) was not affected by a ALOX5-specific siRNA, stimulated insulin secretion after 60min with 16.7mmol/l glucose was significantly attenuated (Fig. 5b), thus leading to a significantly decreased stimulatory index (Fig. 5c). As shown in Fig. 5d, ALOX5 siRNA-treated islets also had reduced insulin content compared to islets treated with the control siRNA. Importantly, these results are consistent with the in vivo and in vitro experiments described above for Alox5−/− mice and demonstrate that 5-LO directly regulates insulin expression and secretion at the level of pancreatic islets.
Figure 5. Inhibition of 5-LO expression in human islets by siRNA decreases insulin expression and secretion.
Human islets were transfected with either one of two ALOX5-specific siRNAs (si5-LO1 or si5-LO2) or a control siRNA (siScramble) for 96hrs. ALOX5-specific siRNA significantly decreases ALOX5 (gray bars), insulin (filled bars), and PDX1 (open bars) gene expression (a). Total RNA was extracted from 100 islets for each treatment group and gene expression was carried out with real-time quantitative PCR. Glucose-stimulated insulin secretion is decreased in human islets treated with a ALOX5-specific siRNA (si5-LO1) (b). Cultured islets were incubated under basal conditions in KRB with 2.8mmol/l glucose (filled bars) for 60min followed by a successive incubation for 60min under stimulated conditions with KRB and 16.7mmol/l glucose (open bars). Treatment with a ALOX5-specific siRNA decreased the stimulatory index (c), defined as the ratio between stimulated and basal values of insulin secretion, as well as the insulin content of human islets (d). No differences were observed with a non-specific control siRNA (siScramble). Results are five independent experiments from five donors in triplicate with three dishes for each treatment group with 20 islets per dish. Data are expressed as mean + SE. *P < 0.05 between ALOX5 siRNA and scramble siRNA.
Discussion
With the exception of the genes known to cause maturity onset diabetes of the young, the genetic factors controlling insulin secretion are not well known. Notably, recent genetic studies in humans have provided evidence that genes controlling beta-cell function play an important role in adult-onset type 2 diabetes as well, suggesting that insulin secretion-related mechanisms may have more significant genetic contributions to common forms of type 2 diabetes than previously recognized [22–26]. Thus, identification of novel pathways regulating beta-cell function and/or insulin secretion may lead to a better understanding of type 2 diabetes and its underlying etiology. We now provide evidence that the 5-LO/LT pathway is another mechanism by which such processes are regulated. Importantly, our observations are in agreement with a series of earlier studies carried out by Metz et al. demonstrating that lipoxygenase inhibitors decrease glucose-stimulated insulin secretion in a dose dependent manner [27, 28] and that arachidonic acid metabolites play a role as “third messengers” for hormone release [29, 30]. More recently, Prasad and colleagues have shown that 12/15-LO, a related enzyme that also metabolizes arachidonic acid, influences beta-cell function and insulin secretion as well [31].
In this study, we demonstrate that 5-LO deficiency in mice impairs glucose/insulin homeostasis from an early age. For example, isolated islets and young Alox5−/− mice at 12wks of age had a decreased insulin response both in vitro and in vivo, respectively. These observations suggest that 5-LO deficiency alters beta-cell function relatively early in life and prior to the onset of more pronounced obesity. Such abnormalities continue as the mice age since decreased fasting and glucose-stimulated plasma insulin levels are also observed at 18 and 23wks of age. It is possible that overall pancreatic function is compromised as well given the significantly reduced weight of the pancreas and insulin content of Alox5−/− islets. Interestingly, our histological analyses suggest that Alox5−/− islets are larger and contain beta-cells that have undergone both hyperplasia and hypertrophy. Thus, these morphological changes many indicate compensatory mechanisms for the reduced insulin secretory capacity of Alox5−/− islets and/or that LTs are required for normal pancreatic development. Furthermore, siRNA knockdown experiments in human islets decreased gene expression and insulin secretion in a manner consistent with the observations in isolated Alox5−/− islets. Taken together, our results indicate a direct role for 5-LO in the regulation of insulin expression and secretion, both in an acute setting as well as over the long term.
Obesity is now widely recognized to be associated with a heightened inflammatory state with elevated circulating and expression levels of inflammatory cytokines, such as C-reactive protein, IL-6, and TNFα [32]. Moreover, two separate reports have shown that adipose tissue is characterized by macrophage infiltration, which could affect its normal function and inflammatory properties [33, 34]. In this regard, we show that although 5-LO deficiency increases adiposity from as early as 12wks of age, Alox5−/− adipose tissue has decreased expression of inflammatory genes. Thus, the mechanism by which 5-LO and LT deficiency leads to abnormal adipose biology and increased fat mass is not entirely clear. It should be noted however that 5-LO could presumably play a direct mechanistic role given that it is expressed in both macrophages and adipose tissue.
Leptin is secreted by adipose tissue to regulate energy expenditure and food consumption. Interestingly, Alox5−/− mice exhibit hyperleptinemia but do not have increased food intake [14], suggesting that leptin signaling in the hypothalamus is either not affected by 5-LO deficiency or that there are other compensatory mechanisms. In addition, elevated plasma leptin levels in Alox5−/− mice appear to result from upregulation of the gene, although it is possible that other mechanisms such as decreased clearance and/or leptin resistance may be involved as well. Elevated leptin levels and the so-called “adipo-insular axis” may also provide another potential mechanism for the impaired insulin secretion in Alox5−/− mice. For example, leptin inhibits insulin biosynthesis and secretion [35, 36], and the increased plasma leptin in Alox5−/− mice could also contribute to the decreased expression of insulin in beta-cells and its secretion in addition to what is directly affected by 5-LO itself. This could be particularly relevant in older mice where adiposity has increased substantially.
In previous studies, we reported that 5-LO deficiency dramatically decreased aortic lesion development in two mouse models [3, 4] and that humans carrying certain ALOX5 gene variants had increased carotid atherosclerosis [5]. Moreover, recent studies in humans and mice have demonstrated that various pharmacological modifiers of the 5-LO pathway decrease inflammatory biomarkers and aortic lesion formation, respectively, [9, 13, 37], raising the possibility of developing 5-LO pathway inhibitors for the treatment of CVD. Our results suggest that it would be important for such therapeutic strategies to carefully consider potentially undesirable metabolic effects as well. While complete inactivation of 5-LO may result in developmental changes that alter adipose tissue metabolism and/or pancreatic function that would otherwise not occur if the 5-LO pathway is inhibited in adulthood, our siRNA experiments to directly perturb ALOX5 in human islets suggest that decreased 5-LO expression and/or activity can have an acute effect on islet function and insulin secretion. In addition, the LT pathway bifurcates to the LTB4 and cysteinyl LT branches after the generation of LTA4 by 5-LO, raising the possibility that deficiency of both types of LTs leads to metabolic abnormalities. For example, montelukast, which only blocks cysteinyl LT signaling, is widely used for the treatment of asthma but has not been reported to be associated with metabolic disturbances. Lastly, since arachidonic acid can also be converted to other fatty acid derivatives by the cyclooxygenase and 12/15-LO enzymes, it is possible that the metabolic phenotypes we observe with Alox5−/− mice also result from increased production of other eicosanoids due to shunting of arachidonic acid to these alternative pathways. For example, Goulet et al. reported that macrophages of Alox5−/− mice have increased production of prostaglandins and thromboxanes [38] but it is not known whether the metabolic consequences we observe with 5-LO deficiency are due directly to the lack of LTs and/or indirectly to increased production of other eicosanoids. Thus, additional studies will be required to determine whether pharmacological antagonism of the 5-LO pathway will provide beneficial CVD effects in humans without compromising adiposity and pancreatic function.
In conclusion, we provide strong evidence that the inflammatory 5-LO/LT pathway has pleiotropic effects on adiposity and type 2 diabetes-related traits. Specifically, decreased 5-LO expression leads to generalized obesity and pancreatic dysfunction in mice as well as impaired insulin secretion in human islets. These results provide a new mechanism by which metabolic abnormalities associated with obesity and type 2 diabetes can arise.
Electronic Supplementary Material Table 1. Primer Sequences for SYBR Green Real-Time Gene Expression Assays.
Acknowledgments
This work was supported by NIH grants HL079353 (HA and MM) and HL30568 (MM and AJL), American Heart Association grant 0355031Y (MM), American Diabetes Association Junior Faculty Grant 706JF41 (KM), the NIH/Older Americans Independence Center at UCLA (KM), and the Larry L. Hillblom Foundation (LLHF grant No. 2005 1C to KM). FTS is the recipient of the Swiss National Foundation Fellowship Award. Human islets were provided through the Islet Cell Resource Consortium, administered by the Administrative and Bioinformatics Coordinating Center of the City of Hope National Medical Center and supported by NCRR, NIDDK, and the Juvenile Diabetes Research Foundation. A portion of this work was conducted in a facility constructed with support from Research Facilities Improvement Program Grant Number C06 (RR10600-01, CA62528-01, RR14514-01) from the National Center for Research Resources (NCRR).
Abbreviations
- 5-LO
5-lipoxygenase ALOX5, Alox5
- ACTB
beta actin
- B6
C57BL/6J
- CVD
cardiovascular disease
- GAPDH/Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- Il
interleukin
- IPGTTs
intraperitoneal glucose tolerance tests
- IPITTs
intraperitoneal insulin tolerance tests
- Ir
insulin receptor
- Irs-1
insulin receptor substrate 1
- LT(s)
leukotriene(s)
- NMR
nuclear magnetic resonance
- PP
pancreatic polypeptide
- PDX1
pancreatic duodenal homeobox 1
- siRNA
short interfering RNA
- Tlr4
toll-like receptor 4
- Tnfα
tumor necrosis factor alpha
- TUBA1B/Tuba1b
alpha tubulin
- WT
wildtype
Footnotes
Duality of interest. The authors declare that there is no duality of interest associated with this manuscript.
References
- 1.Mehrabian M, Allayee H. 5-Lipoxygenase and atherosclerosis. Curr Opin Lipidol. 2003;14:447–457. doi: 10.1097/00041433-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Back M, Hansson GK. Leukotriene receptors in atherosclerosis. Ann Med. 2006;38:493–502. doi: 10.1080/07853890600982737. [DOI] [PubMed] [Google Scholar]
- 3.Mehrabian M, Allayee H, Wong J, et al. Identification of 5-lipoxygenase as a major gene contributing to atherosclerosis susceptibility in mice. Circ Res. 2002;91:120–126. doi: 10.1161/01.res.0000028008.99774.7f. [DOI] [PubMed] [Google Scholar]
- 4.Ghazalpour A, Wang X, Lusis AJ, Mehrabian M. Complex inheritance of the 5-lipoxygenase locus influencing atherosclerosis in mice. Genetics. 2006;173:943–951. doi: 10.1534/genetics.106.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer JH, Allayee H, Dwyer KM, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350:29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 6.Aiello RJ, Bourassa PA, Lindsey S, Weng W, Freeman A, Showell HJ. Leukotriene B4 receptor antagonism reduces monocytic foam cells in mice. Arterioscler Thromb Vasc Biol. 2002;22:443–449. doi: 10.1161/hq0302.105593. [DOI] [PubMed] [Google Scholar]
- 7.Subbarao K, Jala VR, Mathis S, et al. Role of leukotriene B4 receptors in the development of atherosclerosis: potential mechanisms. Arterioscler Thromb Vasc Biol. 2004;24:369–375. doi: 10.1161/01.ATV.0000110503.16605.15. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L, Moos MP, Grabner R, et al. The 5-lipoxygenase pathway promotes pathogenesis of hyperlipidemia-dependent aortic aneurysm. Nat Med. 2004;10:966–973. doi: 10.1038/nm1099. [DOI] [PubMed] [Google Scholar]
- 9.Jawien J, Gajda M, Rudling M, et al. Inhibition of five lipoxygenase activating protein (FLAP) by MK-886 decreases atherosclerosis in apoE/LDLR-double knockout mice. Eur J Clin Invest. 2006;36:141–146. doi: 10.1111/j.1365-2362.2006.01606.x. [DOI] [PubMed] [Google Scholar]
- 10.Helgadottir A, Manolescu A, Thorleifsson G, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 11.Helgadottir A, Manolescu A, Helgason A, et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet. 2006;38:68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 12.Iovannisci DM, Lammer EJ, Steiner L, et al. Association between a leukotriene C4 synthase gene promoter polymorphism and coronary artery calcium in young women: the Muscatine Study. Arterioscler Thromb Vasc Biol. 2007;27:394–399. doi: 10.1161/01.ATV.0000252680.72734.10. [DOI] [PubMed] [Google Scholar]
- 13.Allayee H, Hartiala J, Lee W, et al. The effect of montelukast and low-dose theophylline on cardiovascular disease risk factors in asthmatics. Chest. 2007;132:868–874. doi: 10.1378/chest.07-0831. [DOI] [PubMed] [Google Scholar]
- 14.Mehrabian M, Allayee H, Stockton J, et al. Integrating genotypic and expression data in a segregating mouse population to identify 5-lipoxygenase as a susceptibility gene for obesity and bone traits. Nat Genet. 2005;37:1224–1233. doi: 10.1038/ng1619. [DOI] [PubMed] [Google Scholar]
- 15.Oberholzer J, Triponez F, Mage R, et al. Human islet transplantation: lessons from 13 autologous and 13 allogeneic transplantations. Transplantation. 2000;69:1115–1123. doi: 10.1097/00007890-200003270-00016. [DOI] [PubMed] [Google Scholar]
- 16.Maedler K, Spinas GA, Dyntar D, Moritz W, Kaiser N, Donath MY. Distinct effects of saturated and monounsaturated fatty acids on beta-cell turnover and function. Diabetes. 2001;50:69–76. doi: 10.2337/diabetes.50.1.69. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser N, Corcos AP, Sarel I, Cerasi E. Monolayer culture of adult rat pancreatic islets on extracellular matrix: modulation of B-cell function by chronic exposure to high glucose. Endocrinology. 1991;129:2067–2076. doi: 10.1210/endo-129-4-2067. [DOI] [PubMed] [Google Scholar]
- 18.Maedler K, Sergeev P, Ehses JA, et al. Leptin modulates beta cell expression of IL-1 receptor antagonist and release of IL-1beta in human islets. Proc Natl Acad Sci U S A. 2004;101:8138–8143. doi: 10.1073/pnas.0305683101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama H, Liang G, Engelking LJ, Horton JD, Goldstein JL, Brown MS. Compensatory increase in fatty acid synthesis in adipose tissue of mice with conditional deficiency of SCAP in liver. Cell Metab. 2005;1:41–51. doi: 10.1016/j.cmet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.http://myscience.appliedbiosystems.com/navigation/mysciApplications.jsp
- 21.Brissova M, Shiota M, Nicholson WE, et al. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277:11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 22.Silander K, Mohlke KL, Scott LJ, et al. Genetic variation near the hepatocyte nuclear factor-4 alpha gene predicts susceptibility to type 2 diabetes. Diabetes. 2004;53:1141–1149. doi: 10.2337/diabetes.53.4.1141. [DOI] [PubMed] [Google Scholar]
- 23.Love-Gregory LD, Wasson J, Ma J, et al. A common polymorphism in the upstream promoter region of the hepatocyte nuclear factor-4 alpha gene on chromosome 20q is associated with type 2 diabetes and appears to contribute to the evidence for linkage in an ashkenazi jewish population. Diabetes. 2004;53:1134–1140. doi: 10.2337/diabetes.53.4.1134. [DOI] [PubMed] [Google Scholar]
- 24.Grant SF, Thorleifsson G, Reynisdottir I, et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet. 2006;38:320–323. doi: 10.1038/ng1732. [DOI] [PubMed] [Google Scholar]
- 25.Bonnycastle LL, Willer CJ, Conneely KN, et al. Common variants in maturity-onset diabetes of the young genes contribute to risk of type 2 diabetes in Finns. Diabetes. 2006;55:2534–2540. doi: 10.2337/db06-0178. [DOI] [PubMed] [Google Scholar]
- 26.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 27.Metz SA, Fujimoto WY, Robertson RP. Lipoxygenation of arachidonic acid: a pivotal step in stimulus-secretion coupling in the pancreatic beta cell. Endocrinology. 1982;111:2141–2143. doi: 10.1210/endo-111-6-2141. [DOI] [PubMed] [Google Scholar]
- 28.Metz S, VanRollins M, Strife R, Fujimoto W, Robertson RP. Lipoxygenase pathway in islet endocrine cells. Oxidative metabolism of arachidonic acid promotes insulin release. J Clin Invest. 1983;71:1191–1205. doi: 10.1172/JCI110868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metz SA, Murphy RC, Fujimoto W. Effects on glucose-induced insulin secretion of lipoxygenase-derived metabolites of arachidonic acid. Diabetes. 1984;33:119–124. doi: 10.2337/diab.33.2.119. [DOI] [PubMed] [Google Scholar]
- 30.Metz SA, Fujimoto WY, Robertson RP. Oxygenation products of arachidonic acid: third messengers for insulin release. J Allergy Clin Immunol. 1984;74:391–402. doi: 10.1016/0091-6749(84)90137-4. [DOI] [PubMed] [Google Scholar]
- 31.Prasad KM, Thimmalapura PR, Woode EA, Nadler JL. Evidence that increased 12-lipoxygenase expression impairs pancreatic beta cell function and viability. Biochem Biophys Res Commun. 2003;308:427–432. doi: 10.1016/s0006-291x(03)01418-9. [DOI] [PubMed] [Google Scholar]
- 32.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roduit R, Thorens B. Inhibition of glucose-induced insulin secretion by long-term preexposure of pancreatic islets to leptin. FEBS Lett. 1997;415:179–182. doi: 10.1016/s0014-5793(97)01115-0. [DOI] [PubMed] [Google Scholar]
- 36.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53(Suppl 1):S152–158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 37.Hakonarson H, Thorvaldsson S, Helgadottir A, et al. Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial. JAMA. 2005;293:2245–2256. doi: 10.1001/jama.293.18.2245. [DOI] [PubMed] [Google Scholar]
- 38.Goulet JL, Snouwaert JN, Latour AM, Coffman TM, Koller BH. Altered inflammatory responses in leukotriene-deficient mice. Proc Natl Acad Sci U S A. 1994;91:12852–12856. doi: 10.1073/pnas.91.26.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]