Abstract
OBJECTIVE
To investigate whether magnesium sulfate (MgSO4) prevents fetal brain injury in inflammation-associated preterm birth (PTB).
STUDY DESIGN
Utilising a mouse model of PTB, LPS or normal saline (NS)-exposed mice via intrauterine injection, were randomized to intraperitoneal treatment with MgSO4 or NS. From the 4 treatment groups, 1)NS+NS; 2)LPS+NS; 3)LPS+MgSO4; and 4)NS+MgSO4, fetal brains were collected for QPCR studies and primary neuronal cultures. mRNA expression of cytokines, cell death, and markers of neuronal and glial differentiation were assessed.
Immunocytochemistry and confocal microscopy were performed.
RESULTS
There was no difference between LPS+NS and LPS+MgSO4 groups in expression of pro-inflammatory cytokines, cell death markers as well markers of pro-oligodendrocyte and astrocyte development (P>0.05 for all). Neuronal cultures from LPS+NS demonstrated morphological changes and this neuronal injury was prevented by MgSO4 (P<0.001).
CONCLUSION
Amelioration of neuronal injury in inflammation-associated PTB may be a key mechanism by which MgSO4 prevents cerebral palsy.
Keywords: magnesium sulfate, mouse model of preterm birth, neuroinflammation, neuronal injury
INTRODUCTION
Cerebral palsy (CP) is a non-progressive motor impairment syndrome which occurs in 1 to 3.6 per 1000 live births.1–3 Almost 8% of ex-preterm children born at less than 28 weeks of gestation are affected by CP.2, 4, 5 Despite the advances in perinatology and neonatology and the dramatic reduction in the mortality of high-risk infants, there has been no reduction in prevalence of CP.6
Magnesium sulfate (MgSO4) has been investigated in several clinical trials as well as in systemic reviews and meta-analyses as a possible therapeutic agent to reduce CP in “at-risk“ fetuses.7–11 In most of these trials, “at risk” fetuses were those likely to be born preterm from spontaneous preterm birth—a condition that is frequently associated with intrauterine inflammation. While these studies demonstrated that MgSO4 may prevent CP at 2 years of life, there have been some concerns raised with the use of antenatal MgSO4. Although a meta-analysis did not show an increase in neonatal death associated with MgSO4,8 there still has been some concern raised regarding a possible increased risk of neonatal death from MgSO4 12, 13 Furthermore, in an in vivo model, maternal administration of high doses of MgSO4 was shown to lead to cell death in a developing mouse brain.14
Based on the latest Cochrane review,9 the neuroprotective role for antenatal MgSO4 given to women at risk of preterm birth for the preterm fetus is “now established.” Despite these recommendations, the mechanism by which MgSO4 serves as a neuroprotective agent in the preterm brain has not been elucidated.
From models of hypoxic-ischemic (HI) and traumatic brain injury, the protective effect of MgSO4 is believed to be through its action as a non-competitive antagonist of the N-methyl-D-aspartic acid (NMDA)-receptor.15–19 However, other animal data suggest that MgSO4 may serve an anti-apoptotic role and prevent neuronal cell loss.20, 21 To date, there are no animal trials investigating the use of MgSO4 as a neuroprotective agent in the setting of prenatal inflammation.
These studies sought to determine whether MgSO4 administered to the mother can prevent fetal brain injury as a possible mechanism by which it appears to be neuroprotective in human clinical trial of preterm infants. Using a mouse, we have demonstrated that intrauterine inflammation results in a cytokine response in the fetal brain, white matter damage (WMD) as well as neuronal injury.22–25 Using this established model,26 we investigated the ability of MgSO4, administered to the mother, to prevent fetal brain injury. The objectives of these studies were to investigate: 1) whether administration of MgSO4 altered the pro-inflammatory response in fetal brain; 2) whether administration of MgSO4 altered apoptotic or necrotic pathways in the fetal brain and, most importantly, 3) whether MgSO4, administered in vivo, could prevent fetal brain injury and, specifically, neuronal injury.
MATERIALS AND METHODS
Mouse model of intrauterine inflammation
CD-1 out-bred, timed pregnant mice (Charles River Laboratories, Wilmington, MA) were utilized in an established model of inflammation-induced preterm birth.23, 26 As approximately 85% of spontaneous preterm births at less than 28 weeks are associated with intrauterine inflammation as demonstrated by the presence of histological chorioamnionitis,27 this mouse model aptly mimics this common clinical scenario which occurs in many cases of spontaneous preterm birth. Furthermore, using this model of preterm birth, we have demonstrated that prior to preterm delivery, exposure to intrauterine inflammation results in fetal brain injury and, thus, this model is also useful to assess interventions that might ameliorate or reduce adverse neonatal outcomes from inflammation-associated PTB.23–25 Survival surgery and intrauterine injections of lipopolysaccharide (LPS, 250 µg/dam in 100 µL PBS; from Escherichia coli, 055:B5, Sigma Chemical Co., St. Louis, MO) were performed on embryonic day 15 (E15) of gestation (term is 19 days) as previously reported.26 Briefly, anesthesia was obtained by a continuous flow of isofluorane/oxygen (air O2), supplied by a mask that fits over the mouse’s head. After deep anesthesia was reached, a mini-laparotomy was performed in the lower abdomen. The right uterine horn was identified and LPS or saline was infused into the uterus between the first and the second gestational sacs. Routine closure was performed and the dams recovered in 3–5 minutes. Dams were humanely euthanized 6 hours after surgery by utilizing carbon dioxide (CO2). Three dams were utilized per each treatment group. Immediately after euthanasia, four fetuses per dam were taken from lower uterine horns; as such, all fetuses from all dams were in the same proximity to where LPS was infused. Fetal brains were collected for message RNA (mRNA) studies and for primary cortical neuronal cultures. Guidelines for the care and use of animals were approved by the University of Pennsylvania.
Treatment groups
After intrauterine (IU) infusion of LPS or NS (as previoulsy described)23, dams were randomized to intraperitoneal (IP) treatment with MgSO4. The maternal MgSO4 injection protocol involved an IP dose of 270 mg/kg followed by 27 mg/kg every 20 minutes for 4 hours; injections were given in a volume of 0.1 ml. A second dose of 270 mg/kg was given at the end of the 4 hour period. An average dam weight at E15 is 40 grams. Control mice were injected with same volume of normal saline and at the same timing schedule. The selected protocol followed Hallak et al.28, 29 A prior report, using this protocol in mice, demonstrated that 30 minutes after the injection of MgSO4, the magnesium values in the mothers’ blood samples were approximately double the normal values.30 The use of this protocol in rats resulted in a 125% increase in magnesium level in the fetal forebrain after 4 hours.31 This level of MgSO4 was demonstrated to prevent fetal brain damage associated with hypoxic-ischemic brain injury in a rodent model.28–30 Hence, using this protocol, the following 4 treatment groups were compared in these studies: 1) NS and NS (negative control); 2) LPS and NS (positive control); 3) LPS and MgSO4; and 4) NS and MgSO4. Three dams from each treatment group were utilized for these experiments; from each dam, 4 fetal brains were used.
Primary cortical neuronal cultures
Using sterile technique, E15 fetal brains were harvested 4.5–6 hours after the IU randomization and placed into Petri dishes containing cold Ca++/Mg++-free Hanks Balanced Salt Solution (HBSS; Invitrogen, Carlsbad, CA), pH 7.4. The cortex, a part of the fetal brain, was separated from meninges, olfactory bulbs, brain stem and cerebellum. Each cortex was minced, placed in 4 ml neurobasal medium (NBM; Invitrogen, Carlsbad, CA) containing 0.03% trypsin (Invitrogen, Carlsbad, CA) and incubated for 15 minutes at 37°C and 5% CO2. Brain tissue was removed and placed in 4.5 ml NBM containing 10% fetal bovine serum (FBS) and allowed to settle to inactivate the trypsin. The medium was decanted and replaced with NBM supplemented with B-27 vitamin (Invitrogen, Carlsbad, CA) and 0.5mM L-glutamine and cells were dissociated by trituration. This media combination, NBM in the absence of fetal bovine serum, allows for the select growth of neurons and not glia (astrocytes or microgia).32, 33 Cells were plated at low density (104 cells/ml) on poly-L-lysine (1 mg/ml; Sigma-Aldrich, St. Louis, MO) coated glass coverslips, using 12-well culture plates. Twelve fetal brains (n=12) from three dams (4 fetal brains per dam) per treatment group were utilized for the analysis of neuronal morphology per each treatment group. Cells were plated to equal density for each experiment. All experiments were performed in triplicate to assure the consistency of the results.
Immunocytochemistry
Cortical cell cultures were fixed and stained at days in vitro (DIV) 3 and 10 to assess morphologic changes between the treatment groups, using double immunofluorescence as previously reported.24 A mouse monoclonal antibody to Microtubule-associated protein 2 (MAP2; Sigma-Aldrich, St. Louis, MO) was used to identify dendrites and cell bodies at dilution of 1:100. A rabbit polyclonal antibody to 200 kDa Neurofilament protein (NF-200, Sigma-Aldrich, St. Louis, MO) was used to label the entire cell at dilution of 1:400. Confocal microscopy (Leica SP2 Confocal) was utilized for the morphological evaluation of the neurons.
Quantitative analysis of dendritic processes from cortical cultures experiments
Dendritic processes emanating from neuronal cell body were analyzed at DIV 3 using previously described techniques.24, 34 Briefly, cells were selected at random using at least 3 coverslips for each condition (3 different cultures or 3 different dams). At least 3 experiments were performed for the condition. To quantify processes emanating from each cell body, 30 neurons from each treatment group were evaluated at a final image magnification of 400×. Individual neurons were selected if they were clearly defined and not overlapping with other neurons. Fluorescent images were recorded and analyzed using a Dell Latitude D620, using an image processing program (Image J 1.37v).
Quantitative PCR (QPCR) for expression of pro-inflammatory cytokines, neuronal and glial differentiation markers and cell death-associated genes
Whole E15 fetal brains were harvested after the IU randomization for mRNA, using the Trizol method (Invitrogen, Carlsbad, CA). Fetal brains from each dam were pooled, RNA was extracted and cDNA was created as per protocol.24, 25 QPCR was performed as previously reported 23–25 for evaluation of: 1) pro-inflammatory cytokines (interleukin (IL)-1β, IL6 and tumor necrosis factor (TNF)-α); 2) neuronal differentiation markers (MAP2 and nestin); 3) markers of white matter damage, glial fibrillary acidic protein (GFAP) and pro-oligodendrocyte marker (PLP1/DM20); and 4) cell death-associated genes (caspase1, 3, 8 and 9). Twelve fetal brains (n=12) from three dams (4 fetal brains per dam) per treatment group were utilized for the analysis and the comparison of the mRNA expression.
Statistical Analysis
Statistical analyses were performed using the SigmaStat software program (Aspire Software International, Ashburn, VA). For the comparison of the mRNA expression results and the number of dendritic processes between groups, One-way ANOVA or ANOVA on ranks (for non-parametric data) was used. If significance was reached, pair-wise comparison was then performed using Student-Newman-Keuls (SNK) or Dunn methods. One-way ANOVA was used for all of the comparisons of the mRNA expression as the data for these studies were normally distributed. ANOVA on ranks was used for the comparison of the dendritic processes.
RESULTS
Quantitative PCR (QPCR) for expression of pro-inflammatory cytokines, neuronal and glial differentiation markers and cell death-associated genes
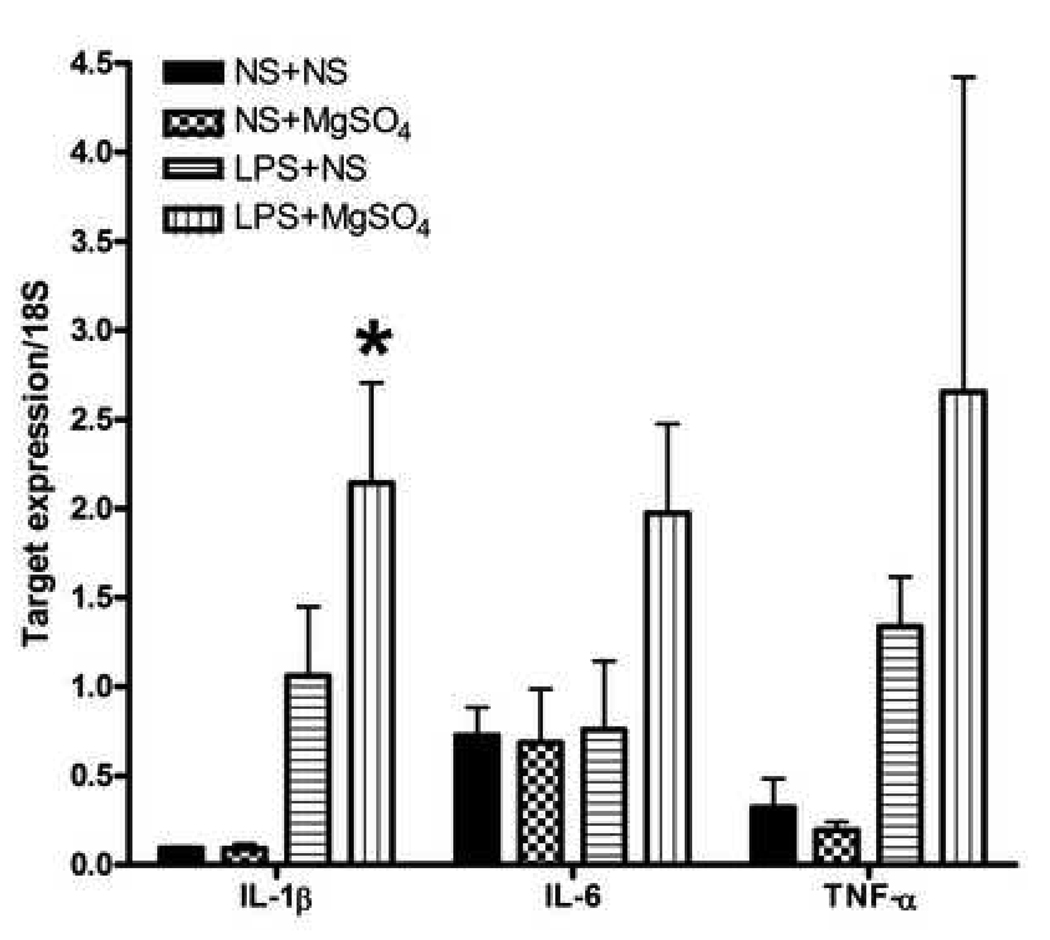
In whole fetal brains, IL-1β mRNA levels were significantly different between the treatment groups (P=0.008, One–way ANOVA; Figure 1). In LPS+NS fetal brains, IL-1β mRNA was increased 9-fold compared to NS+NS (P=0.184, SNK). Similarly, IL-1β mRNA levels in LPS+MgSO4 were 23-fold increased compared to NS+NS (P=0.007, SNK) and 24-fold increased compared to NS+MgSO4 (P= 0.012, SNK) treatment groups. IL-1β mRNA expression was not significantly different between LPS+NS and LPS+ MgSO4 groups (P=0.06, SNK). The pro-inflammatory cytokines, IL-6 and TNF-α, were not differentially expressed between groups (P>0.05, One-way ANOVA for both; Figure 1).
Figure 1. Expression of pro-inflammatory cytokines.
A bar graph demonstrating mean mRNA levels of pro-inflammatory cytokines normalized to 18S rRNA expression in different treatment groups. IL-1β mRNA levels were significantly different between the treatment groups (P=0.008, One–way ANOVA). In LPS+NS fetal brains, IL-1β mRNA was increased 9-fold compared to NS+NS (P=0.18, SNK). Similarly, IL-1β mRNA levels were 24-fold increased in LPS+MgSO4 compared to NS+MgSO4 treatment group (P= 0.012, SNK). IL-1β mRNA expression was not different between LPS+NS and LPS+MG groups (P=0.06, SNK). The pro-inflammatory cytokines, IL-6 and TNF-α, were not differentially expressed between groups (P>0.05, One-way ANOVA for both). While 2.9-fold increase in IL-6 mRNA was observed in LPS+MgSO4 group compared with NS+ MgSO4 group, this did not reach statistical significant (P=0.09, One-way ANOVA). Similarly, TNF-α expression was increased 4.2-fold in the LPS+NS group compared with NS+NS, and 13.8-fold in LPS+MgSO4 group compared with NS+MgSO4 group (P=0.261, One-way ANOVA). Each bar represents n=12 fetal brains, from three dams (4 fetal brains per dam) per each treatment group. * P <0.05
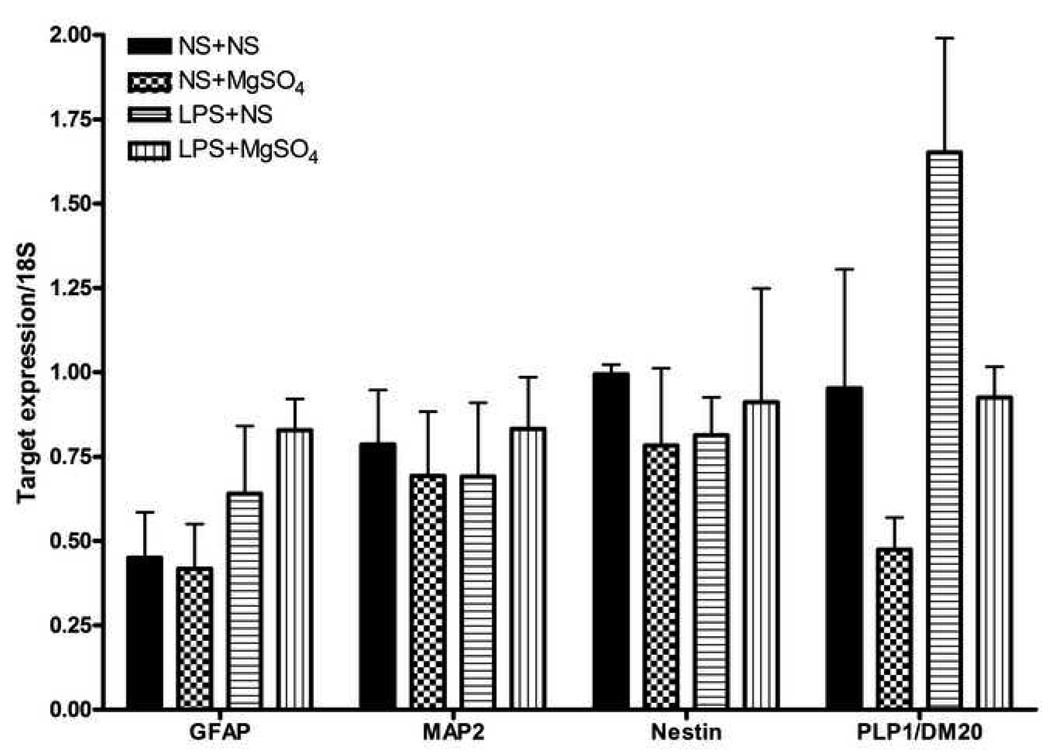
While there was variable expression of GFAP, PLP/DM20 and neuronal markers across treatment groups, these differences did not reach statistical significance (Figure 2).
Figure 2. Expression of markers of neuronal and glial differentiation.
A bar graph demonstrating mean mRNA levels of markers of neuronal and glial differentiation, normalized to 18S rRNA expression in different treatment groups. In the whole fetal brains, marker of pro-oligodendrocyte development, PLP1/DM20, was differentially expressed but did not reach statistical significance (P=0.06, One-way ANOVA). MAP2 and nestin (neuronal markers), and GFAP (astrocyte marker) were not different between groups (P>0.05, One-way ANOVA for all markers). Each bar represents n=12 fetal brains, from three dams (4 fetal brains per dam) per each treatment group.
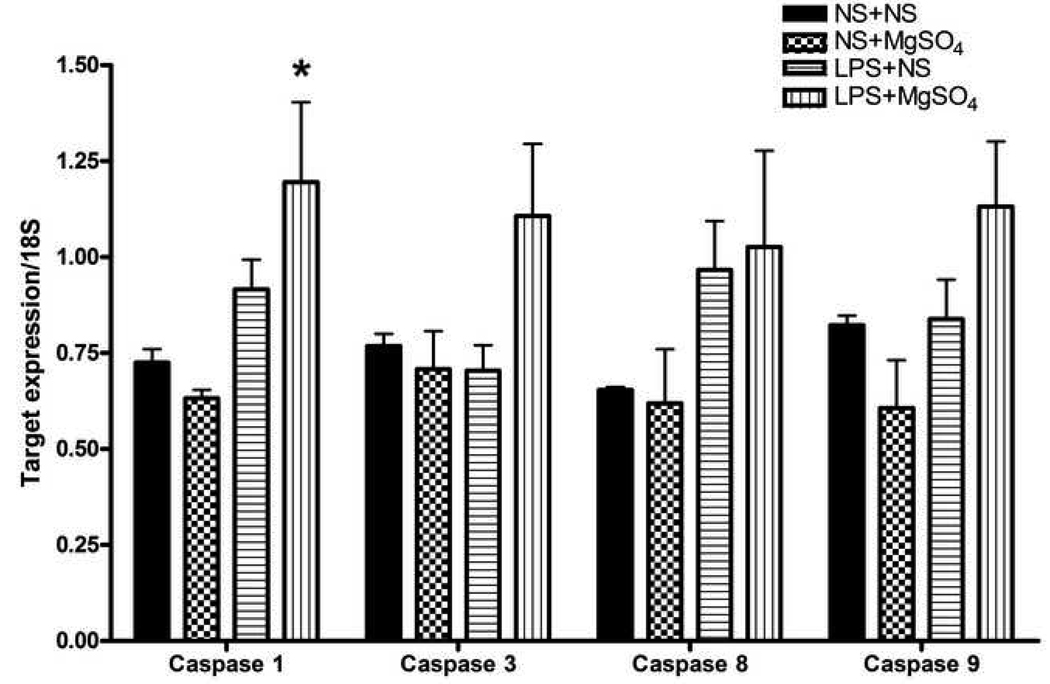
In regards to mediators of apoptosis, caspase-1 mRNA was significantly different between the treatment groups (P=0.03, One-way ANOVA; Figure 3) . In LPS+NS fetal brains, caspase-1 mRNA expression was increased 1.3-fold compared to NS+NS (P=0.27, SNK). Caspase-1 mRNA levels were increased in LPS+MgSO4 compared to NS+MgSO4 (P= 0.03, SNK) and NS+NS (P=0.04, SNK) treatment groups. Caspase-1 mRNA expression was not different between LPS+NS and LPS+ MgSO4 groups (P=0.118, SNK).
Figure 3. Expression of caspases.
A bar graph demonstrating mean mRNA levels of the cell death associated genes (caspases 1, 3, 8 and 9), normalized to 18S rRNA expression in different treatment groups. Caspase-1 mRNA was differentially expressed between groups (P=0.03, One-way ANOVA). In LPS+NS fetal brains, caspase-1 mRNA expression was increased 1.3-fold compared to NS+NS (P=0.265, SNK). Caspase-1 mRNA levels were increased in LPS+MgSO4 compared to NS+MgSO4 (P= 0.032, SNK) and NS+NS (P=0.044, SNK) treatment groups. Caspase-1 mRNA expression was not different between LPS+NS and LPS+ MgSO4 groups (P=0.118, SNK). Message expression of caspase 3, 8 and 9 was not statistically different between the groups (P>0.05, One-way ANOVA). Each bar represents n=12 fetal brains, from three dams (4 fetal brains per dam) per each treatment group. * P<0.05
Message expression of caspase 3, 8 and 9 was not statistically different between the groups (P>0.05, One-way ANOVA; Figure 3).
Neuronal morphology
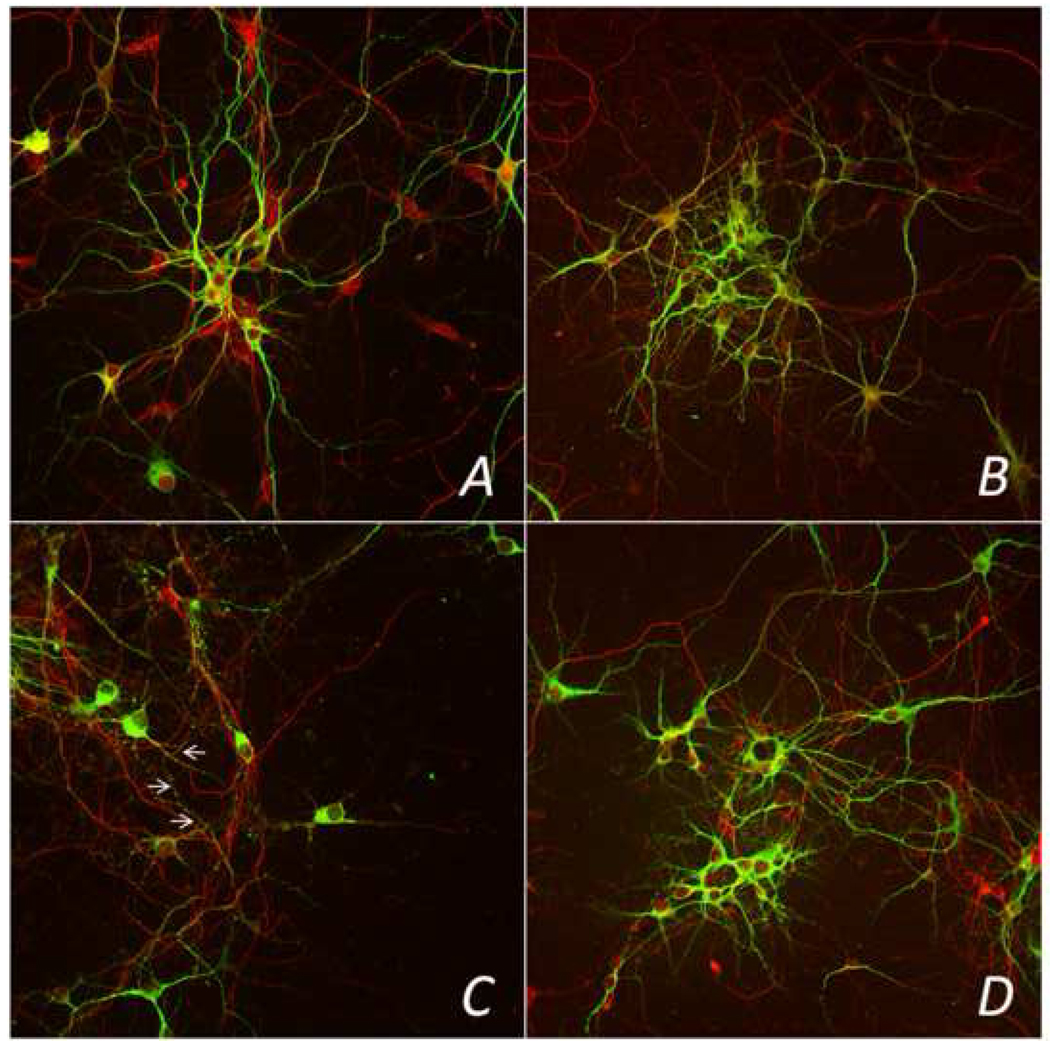
Consistent with our prior studies, primary cultures from fetal brains in the LPS+NS treatment group resulted in an abnormal neuronal morphology,24 demonstrating an overall decreased MAP2 staining (also known as MAP2 beading), neuronal fragility, decreased number of dendritic processes, and an overall degreased aggregation of neurons at DIV 10 (Figure 4). In contrast, in the LPS+MgSO4 group, neuronal morphology appeared similar to NS+NS treatment group, with normal MAP2 staining and aggregation patterns. Similarly, in NS+MgSO4 group, we observed no change in neuronal morphology or aggregation patterns (Figures 4).
Figure 4. Confocal overlay (MAP2 and NF200) images of neurons at DIV 10.
Representative images from confocal microscopy, evaluating 4 treatment groups. MAP-2 staining is seen in green and NF200 staining in red. Panel A represents normal MAP2 staining and normal neuronal aggregation in NS+NS (control group); Panel B shows normal MAP2 staining and neuronal aggregation in NS+MgSO4 group. Panel C, LPS-exposed neurons treated with NS demonstrated decreased MAP2 staining (arrows), fragility, decreased aggregation, and a reduced number of dendritic processes. In contrast, LPS-exposed neurons treated with MgSO4, LPS +MgSO4 group, in panel D, had an appearance similar to the control group, with a normal patterns of MAP2 staining and aggregation (panel A). Magnification 400×.
Quantitative analysis of dendritic processes from cortical cultures experiments
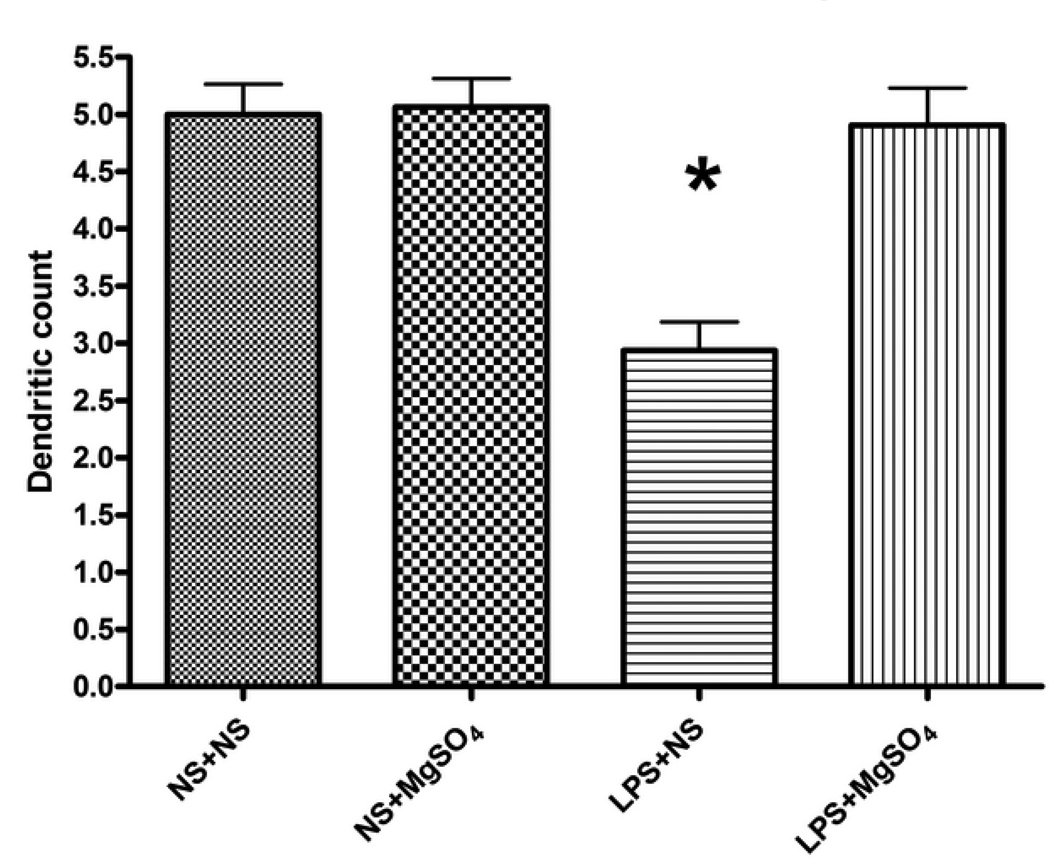
As a quantitative measure of neuronal injury, we evaluated the dendritic growth emanating from the neuronal cell bodies, a marker of synaptic connectivity,35 from the 4 experimental groups. At 48 hours after treatment (DIV3), the number of dendritic processes present on neurons was significantly different between the treatment groups (P<0.001, ANOVA on ranks; Figure 5). LPS-exposed fetal neurons in LPS+NS group had significantly less dendritic processes present on fetal neurons as compared to control, NS+NS (P<0.001, SNK). The number of dendritic processes in LPS+MgSO4 group was comparable to that present in the NS+MgSO4 treatment group (P=0.91, SNK). In fact, the dendritic count in LPS+MgSO4 group was not different from control, NS+NS (P=0.809, SNK).
Figure 5. Number of dendritic processes at DIV 3.
Means and standard errors are represented for each group. The number of dendritic processes present on neurons was significantly different between the treatment groups (P<0.001, ANOVA on ranks). There were significantly less dendritic processes present on LPS-exposed cells in LPS+NS group at DIV 3 than on NS+NS (P<0.001, SNK), LPS+MgSO4(P<0.001, SNK), or NS+MgSO4 groups (P<0.001, SNK). The number of dendritic processes in LPS+MgSO4 group was comparable to that present in the NS+MgSO4 (P=0.913, SNK) and not different than control, NS+NS (P=0.809, SNK). Each bar represents an average of thirty cells. *P<0.001
COMMENT
Prevention of neuronal injury in inflammation-associated PTB may be a key mechanism by which MgSO4 appears to be neuroprotective, specifically in decreasing CP in human studies. As we have previously demonstrated the presence of neuronal injury in fetal brains using a mouse model,24 the principle findings of this study is that this neuronal injury can be ameliorated by antenatal administration of MgSO4.
PTB is enormous public health concern since many of these preterm infants survive with neurobehavioral, cognitive, and motor disabilities.36–40,41–43 Currently, the main theories regarding fetal brain injury and adverse neurological outcomes, including CP, from PTB, focus on specific structural findings of WMD.44–46 However, clinically, these structural findings of WMD do not appear to explain the majority of observed neurological and neurobehavioral outcomes in ex-preterm children.39, 41, 47–53 Consequently, as adverse neurobehavioral phenotypes can occur in the absence of notable WMD, these findings call for a new paradigm regarding the pathogenesis of adverse neurobehavioral outcomes in ex-preterm children.36, 37, 49, 54, 55 Known mechanisms leading to these neurological/neurobehavioral outcomes in other disorders include neuronal abnormalities; specifically, abnormalities in synapses and dendritic arborization.56–61 Recent work from our laboratory supports the concept that neuronal injury may be an important mechanism for adverse neurological outcomes in ex-preterm children.24, 25 This current work, suggests that this neuronal injury can be abrogated by antenatal administration of MgSO4. If neuronal injury, with or without concomitant WMD, is a critical mechanism to long-term adverse neurobehavioral outcomes in ex-preterm children, then antenatal administration of MgSO4 may hold promise for preventing a spectrum of disorders in these children. However, it remains unknown the contribution of neuronal injury and/or WMD to each specific disorder observed in these offspring.
There are notable limitations to the study. The model used for these studies is not necessarily a specific rodent model of CP. This mouse model is intended to mimic the most common clinical scenario associated with preterm birth—that being one of intrauterine inflammation. As such, this model provides a method in which to test how intrauterine inflammation may affect fetal brain development and induce brain injury.
Our studies indicate that neuronal injury, at the time point chosen, can be prevented by MgSO4 even though the message expression of cytokines remained increased. A further limitation to our study is the assessment of only mRNA expression and not protein levels of these specific cytokines. Yet, considering the short time interval to investigation, mRNA expression is likely to be altered prior to protein changes. We recognize that assessment of both mRNA and protein levels at longer intervals after exposure (from inflammation and MgSO4) may reveal different patterns of expression. Future work will be required to explore the interaction of cell death and immune mediators with neuronal injury. Understanding these limitations, our results suggest that prevention of a cytokine response does not appear to be necessary to prevent neuronal injury nor is suppression of the fetal brain cytokine response the mechanism by which MgSO4 appears to prevent acute neuronal injury.
While a meta-analysis suggest that MgSO4 is not associated with neonatal mortality,8 it has been proposed by others that MgSO4 may be implicated in increased fetal brain damage14 and possibly neonatal mortality.12 Furthermore, animal studies have demonstrated that in higher doses MgSO4 can cause cell death.14 Therefore, we also evaluated the expression of the markers of cell death (caspases) in the whole fetal brains. As the expression of these genes was not altered with the administration of MgSO4, these studies do not support the concept that MgSO4 can induce cell death in the fetal brain – at least with the dosing regimen utilized for these studies. As these studies focused on the acute effects of MgSO4 on fetal brain, 4–6 hours after exposure to both inflammation and MgSO4, these studies can only report the acute effect of MgSO4on cell death.
These studies utilized CD-1 mice which are an out-bred strain of mice and hence provide a more diverse genetic background; thus, more aptly mimicking the human condition. However, the limitation of using an out-bred strain of mice is that there is variability in the maternal and fetal response to the same stimulus, such as LPS.22, 23, 26, 62 However, despite the variability of mRNA expression, the findings of neuronal injury were consistent in fetal brains exposed to LPS as are the findings of MgSO4 preventing this injury. Thus, we doubt that increasing the sample size (and hence animal utilization) will provide more insight into the pathogenesis of fetal brain injury and/or protection of injury by MgSO4. We believe that future work investigating long term outcomes after exposure to intrauterine inflammation in the presence or absence of MgSO4 is now warranted.
If neuronal injury is the main precursor to CP in ex-preterm children, then the ability of MgSO4 to prevent neuronal changes from inflammation may be a sufficient mechanism for decreasing these adverse outcomes. Furthermore, these studies suggest that MgSO4 may primarily be involved with protection at the level of neurons. What remains unclear is the contribution of WMD compared to neuronal injury for long-term outcomes. While WMD has been implicated in CP,55, 63 CP and other adverse neurobehavioral outcomes are known to occur in the absence of WMD.36, 37, 49, 54, 55 Animal work from our laboratory and others23, 44, 45, 64 have demonstrated that intrauterine inflammation can evoke WMD. Whether WMD or neuronal injury, or both are essential for adverse neurobehavioral outcomes in ex-preterm children is not yet clear. If WMD persists despite amelioration of neuronal injury, are offspring still at risk? Future work will need to address these important questions.
We acknowledge that there are difficulties in extrapolating findings in a rodent model to the clinical realm and this is one of the limitations of this study. However, elucidating the pathways involved in fetal brain injury from preterm birth in humans is not feasible. Therefore, for these types of studies, animal models of prenatal inflammation are generally used and provide a valuable insight into the mechanisms by which inflammation promotes fetal brain injury. Supporting our findings are data from other models of neuronal injury as well as the data involving MgSO4 use in other animal species. 28–30 MgSO4 has been investigated as a potential neuroprotective agent for glutametergic, HI and traumatic brain injury in the fetal and neonatal periods. 28–30 In one model of HI brain injury, maternal treatment with MgSO4 resulted in a significant fetal protection against moderate HI-induced brain damage.28–30 Although the mechanism of the initiation of HI fetal brain injury is distinct from that following the intrauterine inflammation, a common pathway of neuronal injury may serve to unify these injuries. Despite animal and clinical studies evaluating the use of MgSO4 for neuroprotection, the precise mechanism by which MgSO4 serves to prevent neuronal injury is still under investigation. Several theories exist on possible mechanism by which MgSO4 prevents neuronal injury, which include: 1) acting as a non-competitive antagonist of NMDA-receptor, 2) preventing disruption of the blood-brain barrier permeability, and 3) inhibiting cell death.15–19, 66
Recognizing the work with MgSO4 in other models of prenatal/neonatal brain injury, our study is the first to date to investigate the use of MgSO4 in prenatal inflammation. Understanding limitations of animal models, these findings provide biological plausibility for the use of MgSO4 in clinical practice to prevent long-term adverse neurological outcomes. Future work must address whether WMD, neuronal injury or both are required for the observed adverse neurobehavioral outcomes in ex-preterm infants as this may necessitate different interventional strategies. As the prevalence of adverse neurological outcomes in ex-preterm infants is increasing, understanding the mechanism of action of potential therapeutic interventions is critical if our goal is to decrease both acute and long term adverse outcomes for these children.
Acknowledgments
Sponsor/grant information:
This project was supported by the NIH: 5-RO1-HD046544-0 (MAE) and supported in part by the ABOG/AAOGF scholarship (IB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This research will be presented as an Oral presentation #3 at the 30th Annual Scientific Meeting of the Society for Maternal-Fetal Medicine in Chicago, IL, February 4, 2010
REFERENCES
- 1.Koman LA, Smith BP, Shilt JS. Cerebral palsy. Lancet. 2004;363:1619–1631. doi: 10.1016/S0140-6736(04)16207-7. [DOI] [PubMed] [Google Scholar]
- 2.Winter S, Autry A, Boyle C, Yeargin-Allsopp M. Trends in the prevalence of cerebral palsy in a population-based study. Pediatrics. 2002;110:1220–1225. doi: 10.1542/peds.110.6.1220. [DOI] [PubMed] [Google Scholar]
- 3.Yeargin-Allsopp M, Van Naarden Braun K, Doernberg NS, Benedict RE, Kirby RS, Durkin MS. Prevalence of cerebral palsy in 8-year-old children in three areas of the United States in 2002: a multisite collaboration. Pediatrics. 2008;121:547–554. doi: 10.1542/peds.2007-1270. [DOI] [PubMed] [Google Scholar]
- 4.Jacobsson B, Hagberg G, Hagberg B, Ladfors L, Niklasson A, Hagberg H. Cerebral palsy in preterm infants: a population-based case-control study of antenatal and intrapartal risk factors. Acta Paediatr. 2002;91:946–951. doi: 10.1080/080352502760148685. [DOI] [PubMed] [Google Scholar]
- 5.Nelson KB, Grether JK. Causes of cerebral palsy. Curr Opin Pediatr. 1999;11:487–491. doi: 10.1097/00008480-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Tyson JE, Gilstrap LC. Hope for perinatal prevention of cerebral palsy. JAMA. 2003;290:2730–2732. doi: 10.1001/jama.290.20.2730. [DOI] [PubMed] [Google Scholar]
- 7.Costantine MM, Weiner SJ. Effects of antenatal exposure to magnesium sulfate on neuroprotection and mortality in preterm infants: a meta-analysis. Obstet Gynecol. 2009;114:354–364. doi: 10.1097/AOG.0b013e3181ae98c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle LW, Crowther CA, Middleton P, Marret S. Antenatal magnesium sulfate and neurologic outcome in preterm infants: a systematic review. Obstet Gynecol. 2009;113:1327–1333. doi: 10.1097/AOG.0b013e3181a60495. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Crowther CA, Middleton P, Marret S, Rouse D. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD004661.pub3. CD004661. [DOI] [PubMed] [Google Scholar]
- 10.Crowther CA, Hiller JE, Doyle LW, Haslam RR. Effect of magnesium sulfate given for neuroprotection before preterm birth: a randomized controlled trial. JAMA. 2003;290:2669–2676. doi: 10.1001/jama.290.20.2669. [DOI] [PubMed] [Google Scholar]
- 11.Rouse DJ, Hirtz DG, Thom E, et al. A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med. 2008;359:895–905. doi: 10.1056/NEJMoa0801187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittendorf R, Pryde PG. A review of the role for magnesium sulphate in preterm labour. BJOG. 2005;112 Suppl 1:84–88. doi: 10.1111/j.1471-0528.2005.00592.x. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LW, Crowther CA, Middleton P, Marret S. Magnesium sulphate for women at risk of preterm birth for neuroprotection of the fetus. Cochrane Database Syst Rev. 2007 doi: 10.1002/14651858.CD004661.pub2. CD004661. [DOI] [PubMed] [Google Scholar]
- 14.Dribben WH, Creeley CE, Wang HH, Smith DJ, Farber NB, Olney JW. High dose magnesium sulfate exposure induces apoptotic cell death in the developing neonatal mouse brain. Neonatology. 2009;96:23–32. doi: 10.1159/000201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spandou E, Soubasi V, Papoutsopoulou S, et al. Neuroprotective effect of long-term MgSO4 administration after cerebral hypoxia-ischemia in newborn rats is related to the severity of brain damage. Reprod Sci. 2007;14:667–677. doi: 10.1177/1933719107305864. [DOI] [PubMed] [Google Scholar]
- 16.Marret S, Gressens P, Gadisseux JF, Evrard P. Prevention by magnesium of excitotoxic neuronal death in the developing brain: an animal model for clinical intervention studies. Dev Med Child Neurol. 1995;37:473–484. doi: 10.1111/j.1469-8749.1995.tb12035.x. [DOI] [PubMed] [Google Scholar]
- 17.Imer M, Omay B, Uzunkol A, et al. Effect of magnesium, MK-801 and combination of magnesium and MK-801 on blood-brain barrier permeability and brain edema after experimental traumatic diffuse brain injury. Neurol Res. 2009 doi: 10.1179/174313209X385617. [DOI] [PubMed] [Google Scholar]
- 18.Kaya M, Kucuk M, Kalayci RB, et al. Magnesium sulfate attenuates increased blood-brain barrier permeability during insulin-induced hypoglycemia in rats. Can J Physiol Pharmacol. 2001;79:793–798. [PubMed] [Google Scholar]
- 19.McDonald JW, Silverstein FS, Johnston MV. Magnesium reduces N-methyl-D-aspartate (NMDA)-mediated brain injury in perinatal rats. Neurosci Lett. 1990;109:234–238. doi: 10.1016/0304-3940(90)90569-u. [DOI] [PubMed] [Google Scholar]
- 20.Enomoto T, Osugi T, Satoh H, McIntosh TK, Nabeshima T. Pre-Injury magnesium treatment prevents traumatic brain injury-induced hippocampal ERK activation, neuronal loss, and cognitive dysfunction in the radial-arm maze test. J Neurotrauma. 2005;22:783–792. doi: 10.1089/neu.2005.22.783. [DOI] [PubMed] [Google Scholar]
- 21.Turkyilmaz C, Turkyilmaz Z, Atalay Y, Soylemezoglu F, Celasun B. Magnesium pre-treatment reduces neuronal apoptosis in newborn rats in hypoxia-ischemia. Brain Res. 2002;955:133–137. doi: 10.1016/s0006-8993(02)03395-4. [DOI] [PubMed] [Google Scholar]
- 22.Ernst LM, Gonzalez J, Ofori E, Elovitz M. Inflammation-Induced Preterm Birth in a Murine Model is Associated with Increases in Fetal Macrophages and Circulating Erythroid Precursors. Pediatr Dev Pathol. 2009 doi: 10.2350/09-05-0649-OA.1. [DOI] [PubMed] [Google Scholar]
- 23.Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–55. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- 24.Burd I, Chai J, Gonzalez J, et al. Beyond white matter damage: fetal neuronal injury in a mouse model of preterm birth. Am J Obstet Gynecol. 2009;201(279):e1–e8. doi: 10.1016/j.ajog.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burd I, Bentz A, Gonzalez J, et al. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. Journal of Neuroscience Research. 2010 doi: 10.1002/jnr.22368. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–2111. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110 Suppl 20:124–127. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 28.Hallak M, Hotra JW, Custodio D, Kruger ML. Magnesium prevents seizure-induced reduction in excitatory amino acid receptor (kainate and alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid) binding in pregnant rat brain. Am J Obstet Gynecol. 2000;183:793–798. doi: 10.1067/mob.2000.109491. [DOI] [PubMed] [Google Scholar]
- 29.Hallak M, Hotra JW, Kupsky WJ. Magnesium sulfate protection of fetal rat brain from severe maternal hypoxia. Obstet Gynecol. 2000;96:124–128. doi: 10.1016/s0029-7844(00)00844-9. [DOI] [PubMed] [Google Scholar]
- 30.Golan H, Kashtuzki I, Hallak M, Sorokin Y, Huleihel M. Maternal hypoxia during pregnancy induces fetal neurodevelopmental brain damage: partial protection by magnesium sulfate. J Neurosci Res. 2004;78:430–441. doi: 10.1002/jnr.20269. [DOI] [PubMed] [Google Scholar]
- 31.Hallak M, Cotton DB. Transfer of maternally administered magnesium sulfate into the fetal compartment of the rat: assessment of amniotic fluid, blood, and brain concentrations. Am J Obstet Gynecol. 1993;169:427–431. doi: 10.1016/0002-9378(93)90101-n. [DOI] [PubMed] [Google Scholar]
- 32.Brewer GJ. Serum-free B27/neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. doi: 10.1002/jnr.490420510. [DOI] [PubMed] [Google Scholar]
- 33.Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- 34.Monnerie H, Shashidhara S, Le Roux PD. Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: Involvement of NMDA receptors. J Neurosci Res. 2003;74:688–700. doi: 10.1002/jnr.10797. [DOI] [PubMed] [Google Scholar]
- 35.Poolos NP. Seeing the forest and the trees: dendritic injury after status epilepticus. Epilepsy Curr. 2008;8:77–79. doi: 10.1111/j.1535-7511.2008.00244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352:9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 37.Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 38.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294:318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 39.Costeloe K. EPICure: facts and figures: why preterm labour should be treated. BJOG. 2006;113 Suppl 3:10–12. doi: 10.1111/j.1471-0528.2006.01118.x. [DOI] [PubMed] [Google Scholar]
- 40.Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–F140. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Limperopoulos C, Bassan H, Sullivan NR, et al. Positive screening for autism in ex-preterm infants: prevalence and risk factors. Pediatrics. 2008;121:758–765. doi: 10.1542/peds.2007-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schendel D, Bhasin TK. Birth weight and gestational age characteristics of children with autism, including a comparison with other developmental disabilities. Pediatrics. 2008;121:1155–1164. doi: 10.1542/peds.2007-1049. [DOI] [PubMed] [Google Scholar]
- 43.Brimacombe M, Ming X, Lamendola M. Prenatal and birth complications in autism. Matern Child Health J. 2007;11:73–79. doi: 10.1007/s10995-006-0142-7. [DOI] [PubMed] [Google Scholar]
- 44.Paintlia MK, Paintlia AS, Barbosa E, Singh I, Singh AK. N-acetylcysteine prevents endotoxin-induced degeneration of oligodendrocyte progenitors and hypomyelination in developing rat brain. J Neurosci Res. 2004;78:347–361. doi: 10.1002/jnr.20261. [DOI] [PubMed] [Google Scholar]
- 45.Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Rousset CI, Chalon S, Cantagrel S, et al. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–433. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- 47.Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- 48.Reijneveld SA, de Kleine MJ, van Baar AL, et al. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Arch Dis Child Fetal Neonatal Ed. 2006;91:F423–F428. doi: 10.1136/adc.2006.093674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32:51–58. doi: 10.1053/j.semperi.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 50.Shum D, Neulinger K, O'Callaghan M, Mohay H. Attentional problems in children born very preterm or with extremely low birth weight at 7–9 years. Arch Clin Neuropsychol. 2008;23:103–112. doi: 10.1016/j.acn.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Mental health and social competencies of 10- to 12-year-old children born at 23 to 25 weeks of gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2007;120:118–133. doi: 10.1542/peds.2006-2988. [DOI] [PubMed] [Google Scholar]
- 52.Limperopoulos C, Bassan H, Gauvreau K, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. 2007;120:584–593. doi: 10.1542/peds.2007-1041. [DOI] [PubMed] [Google Scholar]
- 53.Leviton A, Gressens P. Neuronal damage accompanies perinatal white-matter damage. Trends Neurosci. 2007;30:473–478. doi: 10.1016/j.tins.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 54.Rademaker KJ, Uiterwaal CS, Beek FJ, et al. Neonatal cranial ultrasound versus MRI and neurodevelopmental outcome at school age in children born preterm. Arch Dis Child Fetal Neonatal Ed. 2005;90:F489–F493. doi: 10.1136/adc.2005.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuban KC, Allred EN, O'Shea TM, et al. Cranial ultrasound lesions in the NICU predict cerebral palsy at age 2 years in children born at extremely low gestational age. J Child Neurol. 2009;24:63–72. doi: 10.1177/0883073808321048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abuhatzira L, Shemer R, Razin A. MeCP2 involvement in the regulation of neuronal alpha-tubulin production. Hum Mol Genet. 2009;18:1415–1423. doi: 10.1093/hmg/ddp048. [DOI] [PubMed] [Google Scholar]
- 57.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biol Psychiatry. 2006;59:468–476. doi: 10.1016/j.biopsych.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 58.Hu VW, Frank BC, Heine S, Lee NH, Quackenbush J. Gene expression profiling of lymphoblastoid cell lines from monozygotic twins discordant in severity of autism reveals differential regulation of neurologically relevant genes. BMC Genomics. 2006;7:118. doi: 10.1186/1471-2164-7-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fatemi SH. The role of Reelin in pathology of autism. Mol Psychiatry. 2002;7:919–920. doi: 10.1038/sj.mp.4001248. [DOI] [PubMed] [Google Scholar]
- 60.Fatemi SH, Earle J, Kanodia R, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fatemi SH, Halt AR, Realmuto G, et al. Purkinje cell size is reduced in cerebellum of patients with autism. Cell Mol Neurobiol. 2002;22:171–175. doi: 10.1023/A:1019861721160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzalez JM, Ofori E, Burd I, Chai J, Scholler N, Elovitz MA. Maternal mortality from systemic illness: unraveling the contribution of the immune response. Am J Obstet Gynecol. 2009;200(430):e1–e8. doi: 10.1016/j.ajog.2009.01.049. [DOI] [PubMed] [Google Scholar]
- 63.Dammann O, Leviton A. Inflammatory brain damage in preterm newborns--dry numbers, wet lab, and causal inferences. Early Hum Dev. 2004;79:1–15. doi: 10.1016/j.earlhumdev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 64.Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–579. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- 65.Ning H, Wang H, Zhao L, et al. Maternally-administered lipopolysaccharide (LPS) increases tumor necrosis factor alpha in fetal liver and fetal brain: its suppression by low-dose LPS pretreatment. Toxicol Lett. 2008;176:13–19. doi: 10.1016/j.toxlet.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 66.Strecker GJ, Jackson MB, Dudek FE. Blockade of NMDA-activated channels by magnesium in the immature rat hippocampus. J Neurophysiol. 1994;72:1538–1548. doi: 10.1152/jn.1994.72.4.1538. [DOI] [PubMed] [Google Scholar]