Abstract
T helper (Th) cell activation, differentiation, and immune function are regulated by costimulatory molecules. Inducible costimulator (ICOS) is a recently identified costimulatory receptor expressed on activated T cells. A ligand for ICOS, B7h, is expressed on B cells and other types of antigen-presenting cells (APC). Although ICOS has been shown to be essential in T cell activation and differentiation, the regulatory roles of B7h at different stages of T cell immune responses have not been examined genetically. In this study, we generated and analyzed B7h-deficient mice. We present evidence that B7h is the only ligand for ICOS, and ICOS, its only corresponding receptor. Th cells, when activated with B7h-deficient APC, exhibited reduced proliferation and IL-2 production. In addition, Th cells produced significantly reduced amounts of IL-4 and -13 after differentiation at the presence of B7h–/– APC. This cytokine defect was associated with a deficiency in c-Maf expression and could be rescued completely by c-Maf overexpression in T cells. Furthermore, we showed that effector T cells, when restimulated in the presence of B7h-deficient APC, exhibited reduced Th2 cytokine production. Therefore, B7h is required for proper Th cell activation, differentiation, and effector cytokine expression.
CD4+ T helper (Th) cells are the major regulatory cell type to orchestrate immune and autoimmune responses. Activated by antigen-presenting cells (APC), T cells produce IL-2, undergo clonal expansion, and differentiate into Th1 or -2 functional subsets, which are specialized in secreting distinct profiles of cytokines to mediate different types of immune responses (1–4). Th1 cells make IFN-γ and regulate cellular immunity and inflammatory reactions. Th2 cells, on the other hand, produce IL-4, -5, -10, and -13 to regulate humoral as well as allergic responses.
T cell activation and differentiation require not only T cell receptor recognition of the antigen–MHC complex but also costimulation through the interaction of accessory molecules on APC and their corresponding receptors on T lymphocytes. CD28 is the most important costimulatory receptor on T cells. It binds to B7.1 (CD80) and B7.2 (CD86) on activated APC. Studies using T cells derived from CD28-deficient mice demonstrated its role in T cell proliferation and survival (5). Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) is a homologue of CD28 and binds to B7.1 and -.2 molecules with much higher affinity than CD28. CTLA-4, induced after T cell activation, down-regulates T cell proliferation and cytokine production (6). Mice deficient in CTLA-4 displayed polyclonal T cell activation and a lymphoproliferative disorder that resulted in neonatal lethality. CTLA-4 therefore plays a role in down-regulating T cell responses.
Inducible costimulator (ICOS) is the third member of the CD28 family. It is not expressed by naïve Th cells but is induced after T cell activation (7, 8). Recently, we and others constructed and analyzed mice deficient in the Icos gene (9–11). ICOS–/– T cells exhibited reduced proliferation and IL-2 production, indicating a role of ICOS in T cell activation (9, 11). Furthermore, ICOS–/– T cells were selectively impaired in IL-4 expression after in vitro differentiation or in vivo priming by protein antigen in complete Freund's adjuvant (CFA) or Alum; on the other hand, they were capable of secreting IL-5 (9). In vivo, ICOS-deficient mice exhibited greatly impaired humoral immunity and germinal center reactions (9, 10, 12); on the other hand, we found that ICOS–/– mice were extremely sensitive to experimental autoimmune encephalomyelitis (9) but completely resistant to collagen-induced arthritis (13). Therefore, ICOS is important for T cell activation and effector function.
A ligand for ICOS, B7h (also named as B7RP-1, etc.), has been described to be expressed on B cells, macrophages, and in nonlymphoid tissues and cells (7, 14). Despite the important roles of ICOS, the function of B7h in immune responses or whether it mediates its action solely through ICOS is relatively unclear. In this study, we generated B7h-deficient mice and found B7h as the only ligand for ICOS and ICOS, its only receptor. We further analyzed the roles of B7h in different phases of Th cell immune responses and showed here that B7h is required for proper T cell activation, differentiation, and effector cytokine expression.
Materials and Methods
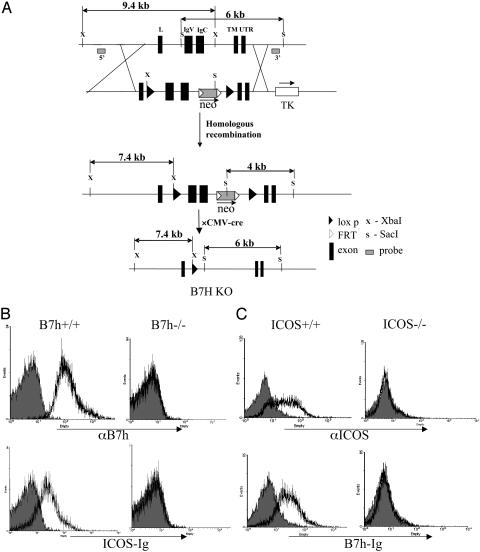
Generation of B7h-Deficient Mice. We identified a phage artificial chromosome clone from the 129 strain containing the B7h gene and characterized the genomic organization of the mouse B7h gene. A conditional gene-targeting construct was generated in which two loxp sites flank the two exons of the B7h gene that encode extracellular Ig-like domains and a neomycin-selecting gene between a pair of flp recombinase recognition sites (kind gift of Phillipe Soriano, Fred Hutchinson Cancer Research Center, Seattle) (Fig. 1). This construct was transfected into TC-1 mouse embryonic stem (ES) cells (gift of Philip Leder, Howard Hughes Medical Institute, Harvard Medical School, Boston) by electroporation. The ES cells that underwent homologous recombination were enriched by selection with G418 and gancyclovir and further identified by Southern blot analysis. Of six ES clones with proper homologous recombination, two were injected into C57BL/6 blastocysts and successfully gave germ-line transmission. We subsequently created a germ-line B7h knockout mouse by crossing B7h+/– mice with a cytomegalovirus-cre transgenic mouse (gift of Bryce Sopher, University of Washington, Seattle), in which the neo cassette, as well as the two loxp-flanked exons, was eliminated.
Fig. 1.
Generation of B7h knockout mice. (A) Diagram of B7h gene-targeting vector. Two loxp sites flank the two exons of the B7h gene that encode two extracellular Ig-like domains (IgV and IgC) and a neomycin-selecting gene between two flp recombinase recognition (FRT) sites. The germ-line B7h knockout mouse was created by eliminating the neo cassette as well as the two exons. L, leader peptide; TM, transmembrane. (B) B220+ spleen B cells from B7h+/+ and B7h–/– mice were stained with biotinylated B7h-specific antibody and ICOS-Ig fusion protein, revealed by streptavidin-R-phycoerythrin. (C) Lymph node cells from ICOS+/+ and ICOS–/– mice were activated by Con A for 48 h, and ICOS expression was assayed with an ICOS-specific antibody or a B7H-Ig protein followed by flow cytometry analysis.
Production of B7h-Ig and ICOS-Ig Fusion Proteins and Their Staining on Immune Cells. cDNA sequences encoding the extracellular portion of mouse B7h and ICOS protein were amplified by PCR and subcloned into the DES-Ig vector we described earlier (15). Stable lines secreting B7h-Ig and ICOS-Ig were constructed, and the Ig fusion proteins they produced were purified on a protein A-Sepharose column (Sigma) and labeled with Sulfo-NHS-LC-Biotin (Pierce). To examine B7h-Ig binding on T cells, lymph node cells from ICOS+/+ and ICOS–/– mice activated 2 days with Con A (2.5 μg/ml) were first incubated with a human IgG1 (10 μg/ml; Sigma) to block nonspecific binding and then stained with biotinylated B7h-Ig fusion protein. To examine ICOS-Ig staining of APC, spleen cells preblocked with human IgG1 were incubated with ICOS-Ig fusion protein. Staining of these biotin-labeled reagents was revealed by streptavidin-R-phycoerythrin (Southern Biotechnology Associates).
Th Cell Isolation and Stimulation. Th cells from lymph nodes and spleens of 6- to 8-week-old mice were isolated and stimulated as we previously reported (9). Briefly, the CD4+ T cells were first enriched by immunomagnetic depletion by using antibodies against CD8+, MHC class II+, and NK1.1+ cells, followed by goat antimouse and anti-rat Ig magnetic beads (Polysciences). The CD62L+ naïve cells were further purified by an AutoMACS sorter (Mitenyi Biotec, Auburn, CA). Splenic APC from B7h+/+ and B7h–/– mice were prepared by complement-mediated lysis of Thy1+ T cells. For primary T cell activation, purified naïve CD4+ T cells were stimulated in triplicates with different concentrations of plate-bound anti-CD3 at the presence of WT or B7h knockout APC. Culture supernatant after 24 h was collected to determine IL-2 expression. Proliferation was assayed after 3 days of treatment by adding [3H]thymidine to the culture for the last 8 h. For polyclonal Th cell differentiation, naïve T cells (1 × 106/ml) were treated with plate-bound anti-CD3 (3 μg/ml), irradiated WT or B7h–/– APC (1 × 106/ml), and IL-2 (30 units/ml, kind gift of Linda Burkly, Biogen) for 4 days. After 24-h restimulation of differentiated T cells with plate-bound anti-CD3, effector cytokine production was measured by ELISA (Pharmingen). To analyze the role of B7h in regulation of effector cytokine production, CD4+ T cells from OT-II T cell receptor transgenic mice were isolated and stimulated with Ova peptide (10 μg/ml), irradiated WT APC, and IL-2. Four days later, differentiated T cells were restimulated with Ova peptide (10 μg/ml) and irradiated splenic APC from B7h+/+ or B7h–/– mice for 24 h, and Th cytokine production was analyze by ELISA (Pharmingen).
Nuclear Extract Preparation and Immunoblot Analysis. A nuclear fraction of Th cells was prepared as described (16). The amounts of nuclear protein were determined by Bio-Rad protein assay (BioRad) to ensure equal protein loading before Western blot analysis with antibodies to GATA-3, β-actin (Santa Cruz Biotechnology), T-bet, and c-Maf [kind gifts of Laurie Glimcher (Harvard School of Public Health, Boston) and I-Cheng Ho (Brigham and Women's Hospital, Boston)].
Keyhole Limpet Hemocyanin (KLH) Immunization. B7h+/+ and B7h–/– mice (6–8 wk old; three per group) were immunized with KLH (0.5 mg/ml) emulsified in CFA (0.5 mg/ml) at the base of the tail (100 μl each mouse). Eleven days after immunization, these mice were killed and analyzed individually. Sera from immunized mice were collected, and KLH-specific IgM, IgG1, IgG2a, and IgE antibodies were measured by using ELISA. Briefly, serum samples were added in a 3-fold serial dilution onto plates precoated with 10 μg/ml KLH. KLH-specific antibodies were detected with biotinylated goat antimouse IgM, rat anti-mouse IgG1, IgG2a, and IgE antibodies (Southern Biotechnology Associates). To measure T cell responses in vivo, spleen cells from KLH-immunized mice were stimulated in 96-well plates as triplicates with or without KLH. IL-2 was measured 1 day and effector cytokines (IFN-γ, IL-4) 4 days later by ELISA (Pharmingen). Proliferation was assayed after 3 days of treatment by adding [3H]thymidine to the culture for the last 8 h. To analyze the role of B7h in regulation of effector cytokine production, CD4+ T cells from spleens of KLH-immunized B7h+/+ mice were isolated and stimulated with 50 μg/ml KLH together with APC from B7h+/+ or B7h–/– naïve mice for 4 days. Then the supernatant of stimulated cells was harvested, and Th cytokine production was measured by ELISA (Pharmingen).
Results
Construction of B7h-Deficient Mice. To investigate the role of B7h in T cell regulation, we constructed a knockout mouse model for the B7h gene. A conditional gene-targeting construct was first generated to flank 2 exons of the B7h gene, encoding the extracellular Ig-like domains, and a neomycin-selecting gene with two loxp sites (Fig. 1A). This construct was transfected into mouse embryonic stem cells, and homologous recombinants were identified by Southern blot analysis. Two of these clones were injected into C57BL/6 blastocysts and successfully gave germ-line transmission. A germline B7h knockout mouse was created by crossing the F1 mice with a cytomegalovirus-cre transgenic mouse in which the neo cassette as well as the two Ig-encoding exons were eliminated, as determined by analysis of mouse tail DNA (data not shown). B7h+/– mice were intercrossed to generate homozygous knockout mice.
To confirm the absence of B7h protein in B7h–/– mice, spleen cells from WT and knockout mice were stained with an anti-B7H antibody [gift of William Sha (University of California, Berkeley) (17)]. WT B220+ B cells expressed B7h, but B7h–/– cells did not (Fig. 1B), indicating that a B7h-null mutant mouse was obtained. To test whether B7h is the only ligand for ICOS, an ICOS-Ig fusion protein was used in flow cytometry analysis. It bound to WT, but not to B7h–/– B cells (Fig. 1B), suggesting that B7h is the only ICOS ligand on these cells.
We also examined whether ICOS is the only receptor for B7h on activated T cells. ICOS+/+ and ICOS–/– lymph node cells were activated with Con A for 48 h. B7h-Ig fusion protein, as well as an anti-ICOS antibody, was found to stain WT-activated T cells but not ICOS–/– cells (Fig. 1C), indicating that ICOS is the only B7h receptor on T cells. These results indicate that unlike the B7.1/B7.2-CD28/CTLA4 and PDL1/PDL2-PD-1 systems, B7h and ICOS are the only ligand and receptor for the other, respectively.
B7h Is Required for Optimal Th Cell Activation by APC. ICOS has been shown by us and others to be important for optimal T cell activation and IL-2 production (9, 11). To test the role of B7h in T cell activation, we activated naïve WT or ICOS knockout Th cells by different doses of plate-bound anti-CD3 together with WT or B7h–/– splenic APC. T cells activated at the presence of B7h-deficient APC, similar to ICOS–/– T cells activated with WT or B7h knockout APC, exhibited reduced proliferation when measured by [3H]thymidine incorporation (Fig. 2), indicating that B7h expressed on splenic APC is required for optimal T cell proliferation. The hallmark of Th cell activation is the expression of IL-2, which serves as a growth factor to mediate T cell clonal expansion. Naïve CD4 cells activated with B7h knockout APC or ICOS-deficient naïve CD4 cells activated with WT or B7h knockout APC produced greatly reduced amounts of this cytokine after activation (Fig. 2). These data indicate that at least in vitro, B7h–ICOS interaction plays an important role in T cell activation and proliferation. Interestingly, absence of both ICOS on T cells and B7h on APC did not result in greater reduction of T cell proliferation or IL-2 production, further supporting that ICOS and B7h are the only binding partners to each other.
Fig. 2.
Defective Th cell activation in the absence B7h. Naïve WT (WT Th) or ICOS knockout (KO Th) Th cells (1 × 106/ml) were activated with the indicated amounts of plate-bound anti-CD3 plus irradiated WT (WT APC) or B7h knockout (KO APC) APC (1 × 106/ml). IL-2 production was determined 24 h after T cell activation by ELISA. Proliferation was assayed after 3 days of treatment by adding [3H]thymidine to the culture for the last 8 h.
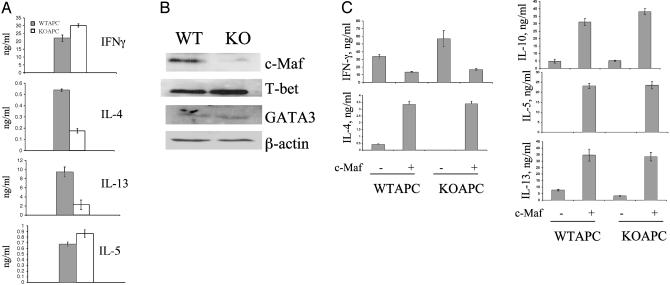
Regulation of Th Differentiation by B7h. ICOS plays a significant role in Th differentiation, most importantly by regulating IL-4 and -13 expression (9). To assess the role of B7h in Th differentiation, we purified naïve CD4 T cells from WT mice and activated them by anti-CD3, irradiated WT or B7h knockout APC for 4 days. IL-2 was added in the culture to compensate the proliferation of T cells activated at the presence of knockout APC. Th cells differentiated under this condition were restimulated with anti-CD3, and effector cytokine production was measured by ELISA (Fig. 3A). T cell activated with B7h knockout APC produced normal levels of IFN-γ and IL-5, suggesting that Th differentiation was not globally impaired. However, their production of IL-4 and -13, when restimulated, was greatly reduced. Therefore, B7h regulates Th differentiation and allows these two cytokines to be produced by effector T cells.
Fig. 3.
B7h regulates Th cell differentiation. (A)Naïve T cells (1 × 106/ml) were treated with plate-bound anti-CD3, irradiated WT, or B7h knockout (KO) APC (1 × 106/ml) and IL-2 (30 units/ml) for 4 days. Differentiated T cells were restimulated with anti-CD3 for 24 h, and effector cytokine production was measured by ELISA. (B) Naïve T cells from WT mice with or without a c-Maf transgene were differentiated, and effector cytokine expression was measured after restimulation with anti-CD3. (C) Naïve T cells (1 × 106/ml) were treated with plate-bound anti-CD3, irradiated WT or B7h knockout (KO) APC (1 × 106/ml) and IL-2 (30 units/ml) for 4 days. Differentiated T cells were restimulated with anti-CD3 for 4 h, and nuclear extracts were prepared. The amount of c-Maf, T-bet, and GATA-3 was examined by Western blot analysis. β-Actin was used to indicate equal protein loading.
It is noteworthy that in the above experiment, B7h costimulation is provided only by APC during primary but not in secondary stimulation. B7h therefore somehow, during Th differentiation, reprograms the T cell receptor signaling pathway to allow effector Th cells to produce IL-4 and -13, consistent with our analysis on ICOS–/– T cells (9). Recently we found that the defective IL-4 expression in ICOS–/– effector T cells was functionally associated with a selective deficiency of c-Maf transcription factor (18). To examine whether B7h also mediates Th differentiation through regulation of c-Maf, we differentiated WT naïve T cells with WT or B7h knockout APC, as described. Then the differentiated T cells were restimulated for 4 h with anti-CD3 and nuclear extraction prepared. The expression of several key lineage-specific transcription factors was analyzed by Western blot, including c-Maf, T-bet, and GATA-3 (Fig. 3B). Correlating with our cytokine data in Fig. 3A, we did not observe any impairment of T-bet or GATA-3 expression when T cells were differentiated with B7h–/– APC, supporting the notion that B7h–ICOS interaction is not required for Th1/-2 differentiation. The only factor we found significantly reduced in expression was c-Maf (Fig. 3B), which was reported to be expressed in Th2 cells and to specifically regulate IL-4 gene expression (19, 20).
To assess whether c-Maf is the target transcription factor regulated by B7h, we assessed whether overexpression of c-Maf can restore the cytokine defects in T cells differentiated with B7h–/– APC. We differentiated WT T cells from c-Maf transgenic mice or their littermate controls with anti-CD3, irradiated WT, or B7h knockout APC and IL-2 for 4 days followed by restimulation with anti-CD3. As measured by ELISA, c-Maf transgene significantly increased expression of all Th2 cytokines IL-4, -5, -10, and -13 and decreased IFN-γ production in effector T cells differentiated with WT APC (Fig. 3C). In addition, c-Maf transgene completely restored the IL-4 and -13 defects in T cells differentiated at the presence of B7h knockout APC (Fig. 3C). Thus, B7h, through ICOS, regulates Th2 differentiation through induction of c-Maf transcription factor. Interestingly, c-Maf was thought to be a primary regulator of IL-4 (19, 20). Our finding presented here suggests a role of c-Maf in IL-13 regulation.
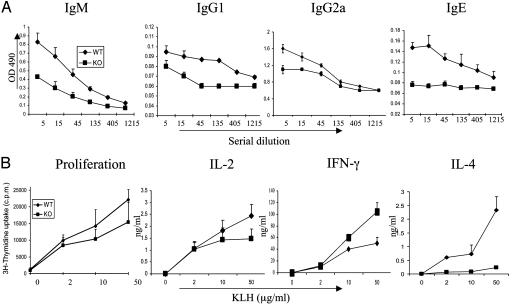
B7h Regulates T-Dependent Immune Responses in Vivo. To examine the role of B7h in immune responses to T-dependent antigens in vivo, we immunized WT or B7h knockout mice in the base of the tail with KLH in CFA, and their immune responses were examined (Fig. 4). B7h–/– mice had comparable splenic cellularity, as the WT mice before immunization (44.6 ± 7 × 106 for knockout and 49.6 ± 15 × 106 for WT), but exhibited much smaller spleens compared with those of the WT after immunization. The average number of cells collected per spleen was three times less than that obtained from WT mice (105 ± 15 × 106 cells; B7h knockout, 31 ± 10 × 106 cells), which may suggest a defect in B cell activation and proliferation in vivo. To further examine the humoral immune responses, we measured anti-KLH antibody titers in sera from immunized mice (Fig. 4A). KLH-specific IgG1 and IgG2a were very moderately reduced in sera from immunized B7h–/– mice as compared with controls. Anti-KLH IgE production was absent in the absence of B7h. Interestingly, anti-KLH IgM was also significantly reduced in B7h–/– mice. This result suggests an important role ICOS–B7h interaction in initial B cell response before Ig class-switching occurred.
Fig. 4.
B7h regulates T-dependent immune responses in vivo. B7h+/+ and B7h–/– mice (three per group) were immunized with KLH in CFA. Eleven days later, the mice were killed and analyzed. (A) Anti-KLH antibodies (IgM, IgG1, IgG2a, and IgE) were measured in the sera by ELISA. The sera from B7h+/+ and B7h–/– mice were subject to a 3-fold serial dilution, and the concentrations of KLH-specific IgM, IgG1, Ig2a, and IgE were analyzed by ELISA and averaged for each group. (B) Spleen cells from immunized mice were stimulated in 96-well plates as triplicates with the indicated concentration of KLH peptide. Proliferation was assayed after 3 days of treatment by adding [3H]thymidine to the culture for the last 8 h. IL-2 was measured 1 day later, and effector cytokines (IFN-γ and IL-4) were measured after 4 days of treatment.
Defective B cell responses in B7h–/– mice could be caused by defective T cell priming or lack of certain T cell products. To analyze antigen-specific T cell response in B7h+/+ and B7h–/– mice, spleen cells from immunized mice were restimulated with or without the KLH protein. T cell proliferation and cytokine production were examined. When equal numbers of WT or knockout spleen cells were restimulated with KLH in vitro, the B7h–/– cells exhibited very moderately reduced IL-2 production or proliferation (Fig. 4B). To examine further whether there was a defect associated with effector function in B7h-deficient mice, we measured effector cytokine production (IL-4 and IFN-γ) by spleen cells in response to different concentrations of KLH (Fig. 4B). B7h–/– spleen cells produced slightly higher levels of IFN-γ, but IL-4 production was greatly impaired (Fig. 4B), indicating a defect in Th2 effector function. Because B cell proliferation and class-switching to IgE depends on Th2 cytokines, especially IL-4, an IL-4 defect in B7h–/– mice may result in impaired humoral immunity.
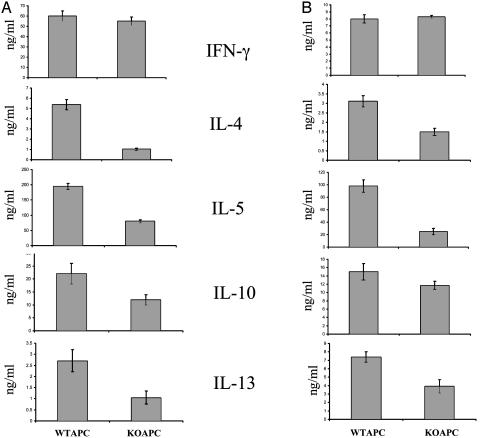
The Role of B7h in the Effector Phase. Studies using an ICOS blocker suggested a role for B7h–ICOS interaction in effector T cell reactivation by APC (21, 22), although effector T cells are generally thought to be less costimulation-dependent than naïve T cells. However, germ-line deletion of the ICOS gene has not allowed us to specifically investigate this aspect. In the current study, we examined in two experiments whether B7h is important for regulation of cytokine production in the effector stage after Th cell differentiation. In the first experiment, CD4+ T cells from spleens of KLH-immunized B7h+/+ mice were purified and restimulated with 50 μg/ml KLH together with splenic APC from B7h+/+ or B7h–/– naïve mice. Four days after restimulation, effector Th cytokine production was measured by ELISA (Fig. 5A). Th cells restimulated with WT or knockout APC produced the same levels of IFN-γ. All Th2 cytokine (IL-4, -5, -10, and -13) production, however, was reduced in different degrees when T cells were restimulated with B7h–/– APC (Fig. 5A).
Fig. 5.
B7h regulates effector Th2 cytokine production. (A) CD4+ T cells from spleens of KLH immunized B7h+/+ mice were isolated and stimulated by 50μg/ml KLH peptide with APC from B7h+/+ (WTAPC) or B7h–/– (KOAPC) naïve mice for 4 days. After 4 days of stimulation, Th cytokine production was measured by ELISA. (B) CD4+ T cells from OT-II mice (1 × 106/ml) were treated with Ova peptide (10 μg/ml), irradiated WT APC (1 × 106/ml), and IL-2 (30 units/ml) for 4 days. Differentiated T cells were then restimulated with Ova peptide and irradiated WT (WT) or B7h knockout (KO) APC. Effector cytokine production was measured by ELISA.
In the second experiment, we differentiated OT-II cells with its agonistic Ova peptide, B7h+/+ APC, and IL-2 for 4 days. The differentiated T cells were then restimulated for 24 h with Ova peptide in the presence of B7h+/+ or B7h–/– APC. Production of all Th2 effector cytokines, especially IL-4, -5, and -13, was also selectively reduced when differentiated OT-II cells were restimulated with B7h–/– APC (Fig. 5B). Taken together, our results indicate that ICOS–B7h interaction is also important for cytokine production in the effector stage after Th differentiation.
Discussion
In immune responses, Th cell activation, differentiation, and function are regulated by costimulatory molecules. ICOS, the third member of the CD28 family, is induced after T cell activation (7, 8). Studies using ICOS-deficient mice have indicated ICOS as an essential regulator of Th activation, differentiation, and effector function (9–13). A ligand for ICOS, B7h, is expressed on B cells and in nonlymphoid tissues such as lung (8, 14). Because other members of the CD28 family all have two known ligands, it is unclear whether B7h is the only ICOS ligand. The function of B7h in immune responses has not been examined genetically.
In this study, we constructed and analyzed mice deficient for the B7h gene. B7h–/– B cells lost binding by an anti-B7h antibody as well as an ICOS-Ig (Fig. 1B). On the other hand, Con A-activated ICOS–/– T cells could not be stained by a B7h-Ig (Fig. 1C). Further phenotypic analysis of B7h–/– mice presented in this paper correlated well with that of the ICOS knockout mice. This work provides a conclusive evidence to indicate that, unlike the B7.1/B7.2-CD28/CTLA4 and PDL1/PDL2-PD-1 systems, B7h and ICOS are the only ligand and receptor for the other, respectively.
Studies using ICOS-deficient mice have shown that ICOS is important for optimal T cell activation and IL-2 production (9, 11). In this study, we activated naïve WT or ICOS knockout CD4+ cells by different doses of plate-bound anti-CD3 together with WT or B7h knockout APC. T cells activated with B7h knockout APC and ICOS knockout T cells activated by B7h+/+ and B7h–/– APC reduced proliferation and IL-2 production (Fig. 2). An anti-B7h blocking antibody (17) also inhibited anti-CD3-activated proliferation of spleen cells (data not shown). This result suggests that in vitro, B7h–ICOS interaction plays a critical role in T cell activation and proliferation. An important role of ICOS–B7h interaction in T cell priming in vivo has been recently reported by Smith et al. (23). Using an adoptive transfer system, they showed that ICOS is involved in the initial clonal expansion of T cells after immunization; treatment of with an anti-ICOS resulted in a significantly reduced T cell response in vivo.
We previously reported that ICOS regulates Th differentiation; it is not essential for the general Th differentiation program but instead, its costimulation during Th differentiation allows IL-4 to be produced by effector T cells (9). Recently, we found that the transcription factor target for ICOS to regulate effector IL-4 expression is c-Maf (18). In the current study, we assessed the role of B7h in Th differentiation and found T cells activated at the presence of B7h knockout APC produced normal levels of IFN-γ and IL-10, but their IL-4 and -13 expression was significantly reduced (Fig. 3A). This cytokine deficiency was associated with greatly reduced c-Maf expression (Fig. 3B). c-Maf overexpression completely restored the IL-4 and -13 defects in T cells differentiated at the presence of B7h knockout APC (Fig. 3C). Thus, B7h, like ICOS, regulates IL-4 expression through regulation of the c-Maf transcription factor. Interestingly, c-Maf was thought to be a primary regulator of IL-4 (18, 19). The results presented here indicate a potential role of c-Maf in IL-13 regulation by ICOS-B7h.
Th2 cells and the cytokines they produce are important for B cell activation and class-switching. ICOS-deficient mice and human patients exhibited impaired IgG1 and IgE production and germinal center reactions (9–12, 24). In our recent analysis of ICOS function in collagen-induced arthritis, we found antigen-specific IgM production also was reduced in ICOS–/– mice on the DBA/1 background (13). To assess the role of B7h in humoral immune responses to T-dependent antigens in vivo, we immunized WT or B7h knockout mice with KLH in CFA. We found, consistent with our previous analysis with ICOS–/– mice, that B7h knockout mice exhibited impaired IgE production, whereas their IgG2a titers were less affected (Fig. 4A). Interestingly, we found that anti-KLH IgM was also reduced in the immunized B7h–/– mice (Fig. 4A). This result further supports an important role of ICOS–B7h interaction in initial B cell response before class-switching occurred.
Earlier studies using ICOS blocker suggested that B7h–ICOS interaction may regulate effector T cell reactivation by APC (20, 21). In the current work, we analyzed the role of B7h costimulation in Th effector function (Fig. 5). In these experiments, Th cells, differentiated in vitro or primed in vivo, produced the same levels of IFN-γ but reduced levels of Th2 effector cytokines (IL-4, -5, -10, and -13) when restimulated with B7h–/– APC. Coyle et al. (22) previously showed that Th2 cells express higher levels of ICOS than Th1 cells, which may lead to differential dependency of the two subsets on B7h–ICOS interaction in their effector function. Our results support this earlier finding and suggest that blocking ICOS–B7h interaction may help reduce active Th2-mediated immune diseases.
In summary, we have used B7h-deficient mice to examine the role of B7h in T cell activation, differentiation, and effector function. This work not only supports the importance of this costimulation pathway in immune responses but also indicates its potential in the treatment of immune disorders.
Acknowledgments
We thank Drs. Laurie Glimcher, I-Cheng Ho, Philippe Soriano, Philip Leder, Bryce Sopher, William Sha, and Linda Burkly for providing reagents. We are also grateful to Julie Duong for excellent technical assistance and to the entire Dong lab for help and discussions. This work is supported in part by a grant from the National Institutes of Health (to C.D.). R.I.N. is a Postdoctoral Fellowship recipient of the Arthritis Foundation, and C.D. is a Cancer Research Institute Investigator awardee.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Th cell, T helper cell; APC, antigen-presenting cell; KLH, keyhole limpet hemocyanin; CFA, complete Freund's adjuvant; ICOS, inducible costimulator.
References
- 1.Paul, W. E. & Seder, R. A. (1994) Cell 76, 241–251. [DOI] [PubMed] [Google Scholar]
- 2.Dong, C. & Flavell, R. A. (2000) Sci. STKE 49, PE1. [DOI] [PubMed] [Google Scholar]
- 3.Dong, C. & Flavell, R. A. (2000) Arthritis Res. 2, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glimcher, L. H. & Murphy, K. M. (2000) Genes Dev. 14, 1693–1711. [PubMed] [Google Scholar]
- 5.Shahinian, A., Pfeffer, K., Lee, K. P., Kundig, T. M., Kishihara, K., Wakeham, A., Kawai, K., Ohashi, P. S., Thompson, C. B. & Mak, T. W. (1993) Science 261, 609–612. [DOI] [PubMed] [Google Scholar]
- 6.Chambers, C. A. & Allison, J. P. (1999) Curr. Opin. Cell Biol. 11, 203–210. [DOI] [PubMed] [Google Scholar]
- 7.Hutloff, A., Dittrich, A. M., Beier, K. C., Eljaschewitsch, B., Kraft, R., Anagnostopoulos, I. & Krocsek, R. A. (1999) Nature 397, 263–266. [DOI] [PubMed] [Google Scholar]
- 8.Yoshinaga, S. K., Whoriskey, J. S., Khare, S. D., Sarmiento, U., Guo, J., Horan, T., Shih, G., Zhang, M., Coccia, M. A., Kohno, T., et al. (1999) Nature 402, 827–832. [DOI] [PubMed] [Google Scholar]
- 9.Dong, C., Juedes, A. E., Temann, U.-A., Shresta, S., Allison, J. P., Ruddle, N. H. & Flavell, R. A. (2001) Nature 409, 97–102. [DOI] [PubMed] [Google Scholar]
- 10.McAdam, A. J., Greenwald, R. J., Levin, M. A., Chernova, T., Malenkovich, N., Ling, V., Freeman, G. J. & Sharpe, A. H. (2001) Nature 409, 102–105. [DOI] [PubMed] [Google Scholar]
- 11.Tafuri, A., Shahinian, A., Bladt, F., Yoshinaga, S. K., Jordana, M., Wakeham, A., Boucher, L.-M., Bouchard, D., Chan, V. S. F., Duncan, G., et al. (2001) Nature 2001, 105–109. [DOI] [PubMed] [Google Scholar]
- 12.Dong, C., Temann, U. A. & Flavell, R. A. (2001) J. Immunol. 166, 3659–3662. [DOI] [PubMed] [Google Scholar]
- 13.Nurieva, R. I., Treuting, P., Duong, J., Flavell, R. A. & Dong, C. (2003) J. Clin. Invest. 111, 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Swallow, M. M., Wallin, J. J. & Sha, W. C. (1999) Immunity 11, 423–432. [DOI] [PubMed] [Google Scholar]
- 15.Sun, M., Richards, S., Prasad, D. V., Mai, X. M., Rudensky, A. & Dong, C. (2002) J. Immunol. 168, 6294–6297. [DOI] [PubMed] [Google Scholar]
- 16.Dong, C., Yang, D. D., Wysk, M., Whitmarsh, A. J., Davis, R. J. & Flavell, R. A. (1998) Science 282, 2092–2095. [DOI] [PubMed] [Google Scholar]
- 17.Liang, L., Porter, E. M. & Sha, W. C. (2002) J. Exp. Med. 196, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nurieva, R. I., Duong, J., Kishikawa, H., Dianzani, U., Rojo, J. M., Ho, I. C., Flavell, R. A. & Dong, C. (2003) Immunity 18, 801–811. [DOI] [PubMed] [Google Scholar]
- 19.Ho, I. C., Hodge, M. R., Rooney, J. W. & Glimcher, L. H. (1996) Cell 85, 973–983. [DOI] [PubMed] [Google Scholar]
- 20.Kim, J. I., Ho, I. C., Grusby, M. J.& Glimcher, L. H. (1999) Immunity 10, 745–751. [DOI] [PubMed] [Google Scholar]
- 21.Tesciuba, A. G., Subudhi, S., Rother, R. P., Faas, S. J., Frantz, A. M., Elliot, D., Weinstock, J., Matis, L. A., Bluestone, J. A. & Sperling, A. I. (2001) J. Immunol. 167, 1996–2003. [DOI] [PubMed] [Google Scholar]
- 22.Coyle, A. J., Lehar, S., Lloyd, C., Tian, J., Delaney, T., Manning, S., Nguyen, T., Burwell, T., Schneider, H., Gonzalo, J. A., et al. (2000) Immunity 13, 95–105. [DOI] [PubMed] [Google Scholar]
- 23.Smith, K. M, Brewer, J. M., Webb, P., Coyle, A. J., Gutierrez-Ramos, C. & Garside, P. (2003) J. Immunol. 170, 2310–2315. [DOI] [PubMed] [Google Scholar]
- 24.Grimbacher, B., Hutloff, A., Schlesier, M., Glocker, E., Warnatz, K., Drager, R., Eibel, H., Fischer, B., Schaffer, A. A., Mages, H. W., et al. (2003) Nat. Immunol. 4, 261–268. [DOI] [PubMed] [Google Scholar]