Abstract
The molecular basis for the different roles of IL-2 and IL-15 in lymphocyte function has been poorly defined. Searching for differences that underlie the distinct T cell responses to the two cytokines, we observed a marked susceptibility of the IL-15-induced but not of the IL-2-induced proliferation to rapamycin despite a decrease of p70S6 kinase (p70S6K) activation by the drug in response to both cytokines. Activated splenic T lymphocytes deficient in the FK506-binding protein (FKBP) 12, a target of rapamycin activity, had reduced proliferation in response to IL-15 but not to IL-2. This decreased proliferation was accompanied by reduced activation of p70S6K and of the extracellular signal-regulated kinases (ERK) after IL-15 treatment. In contrast to FKBP12–/– cells, splenic FKBP12.6–/– T cells exhibited a decreased proliferative response to IL-2 in the presence of rapamycin without affecting p70S6K or ERK activation. Thus, IL-15 induces T cell proliferation mainly via FKBP12-mediated p70S6K activation. In contrast, IL-2 signaling involves multiple pathways that include at least one additional pathway that depends on FKBP12.6.
Many but not all of the responses to IL-2 are similar to those of IL-15 in T lymphocytes and natural killer cells in vitro by virtue of the fact that the two cytokines share two receptor subunits, IL-2Rβ and γc (1). However, studies in mice deficient in IL-2, IL-15 or their private receptor α chains revealed different effects of the two cytokines. IL-2 was found to play a role in the induction of immune tolerance by means of the elimination of self-reactive T cells, whereas IL-15 inhibited this process (2, 3). In this regard, IL-15 but not IL-2 was shown to inhibit activation-induced cell death in vitro (4–6). Mice with deficiencies in either IL-15 or IL-15Rα had reduced numbers of CD8-positive memory cells, natural killer cells, and natural killer T cells, characteristics not seen in IL-2-deficient mice (7, 8). Signaling pathways that define these functional differences between both cytokines are poorly understood.
The most proximal event during the classical IL-2/15-signaling pathway is the activation of Janus kinases 1 and 3 that mediates signal transducer and activator of transcription (STAT) 5 phosphorylation and signaling (9). In addition, Janus kinase-induced phosphorylation of IL-2Rβ is necessary to generate docking sites for signaling molecules. Binding of shc to phosphorylated Tyr-338 on IL-2Rβ links the IL-2/15 receptor to the Ras-Raf-mitogen-activated protein kinase (MAPK) cascade (10–13). A pathway of particular importance for cell proliferation seems to be the IL-2/15-driven phosphatidylinositol 3-kinase (PI3-K) activation that regulates the transcriptional activity of E2F (14). IL-2-induced activation of PI3-K also mediates the phosphorylation of the 40S ribosomal subunit S6 via mammalian target of rapamycin (mTOR) and p70S6 kinase (p70S6K) leading to the translational control of mRNA crucial for cell proliferation (15).
FK506-binding proteins (FKBP) represent a group of peptidyl propyl cis/trans isomerases that mediate conformational changes in target proteins (16–20). Two immunosuppressive drugs affect the activation mechanisms of FKBP: the presence of FK506 mediates an inhibition of the calcium-activated phosphatase calcineurin (21). Rapamycin forms inhibitory complexes with FKBP that regulate mTOR. The best-studied members of the FKBP family are the closely related FKBP12 and -12.6. These single-domain isomerases are known to associate with receptors such as the transforming growth factor β and ryanodine receptors. FKBP12 is ubiquitously expressed and interacts with proteins in several signaling cascades (22). Mice with targeted disruption in FKBP12 die in utero with multiple cardiac defects (23). The activity of FKBP12 seems to be necessary for the immunosuppressive effect of FK506 in T lymphocytes (24). The phenotype of FKBP12.6–/– mice includes cardiac hypertrophy (25). The effects of mutations in FKBP12 or -12.6 on hematopoietic cells have not been described.
Here, we present data that show a critical role mediated by FKBP12 and -12.6 in the IL-2- and IL-15-driven proliferation of T lymphocytes and define signaling differences in response to these related cytokines. In particular, FKBP12 is required for proliferation induced by IL-15 but not by IL-2. Multiple pathways contribute to IL-2-induced proliferation, including one that involves FKBP12.6.
Materials and Methods
FKBP12- and FKBP12.6-Deficient Mice and Isolation of Mature T Cells. Both FKBP12- and FKBP12.6-deficient mice had been generated (23, 24). Homozygous FKBP12-deficient embryos die in utero. Therefore, mature T cells were obtained by adoptive transfer. Homozygous or FKBP12–/– heterozygous fetal liver cell suspensions (embryonic day 18.5) were injected into C57BL/6J recombinase-activating gene 1-deficient mice (The Jackson Laboratory) conditioned with 400 rad of γ irradiation (Gamma Cell 40 Extractor, MDS Nordion, Kanata, ON, Canada). Mature T cells were harvested from spleens of host mice 4–8 weeks after reconstitution. Spleen cells were mechanically separated. Red blood cells were removed by Ficoll-Paque centrifugation. The genotypes of reconstituted lymphocytes were confirmed by Western blotting. Mature FKBP12.6-deficient T cells were obtained from spleens of 8-week-old FKBP12.6-deficient mice. The genotype was confirmed by RT-PCR.
Tissue Culture. Cells were grown in RPMI medium 1640 and 10% FBS supplemented with antibiotics and IL-2 (15R-Kit and CTLL-2, 0.5 nM; splenic T cells, 1 nM). CTLL-2 is a murine T cell line that responds to both IL-2 and IL-15. 15R-Kit was derived from the human IL-2-responsive T cell line Kit-225. IL-15 responsiveness was achieved by a stable expression of human IL-15Rα in these cells (26). Splenic T cells were activated for 2 days by T cell receptor cross-linking using plates that had been coated with antibodies against CD3 (10 μg/ml) and CD28 (5 μg/ml), which was followed by an expansion for an additional 4-day period. Before stimulation with IL-2 or IL-15, cells were incubated in the absence of cytokine for various periods: CTLL-2, 2 h; 15R-Kit, 48 h; activated splenic T cells, 4 h. Rapamycin (Sigma), LY294002 (Calbiochem), or FK506 (Fujisawa Pharmaceutical, Deerfield, IL) were added 30 min before cytokine stimulation and were present for identical periods in all samples in any given experiment.
Cell Proliferation Assay. Cells were plated at various concentrations (15R-Kit and splenic T cells at 5 × 104 per well, CTLL-2 at 2 × 104 per well) and incubated for 24 h (15R-Kit and CTLL-2) or for 48 h (splenic T cells). For the final 6 h of culture, 1 μCi (1 Ci = 37 GBq) [3H]thymidine (Perkin–Elmer) was added to the cells. Cells were harvested, and [3H]thymidine incorporation was measured as an assessment of proliferation.
Western Analyses. Cells were lysed in 100 mM Tris (pH 6.8), 4% SDS, and 20% glycerol, sonicated, and subjected to SDS/PAGE. The following antibodies were used: phospho-p70 (no. 9206) and p70 (no. 9202) (Cell Signaling Technology, Beverly, MA); phospho-ERK (no. SC-7383) and ERK (no. SC-94) (Santa Cruz Biotechnology); phospho-STAT5, no. 71-6900 (Zymed); and STAT5, no. 06-588 (Upstate Biotechnology, Lake Placid, NY). Antibodies against FKBP12 were generated by immunizing rabbits with full-length FKBP12 fused to GST.
Results
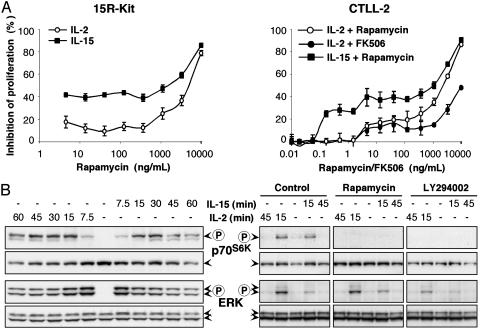
Distinct Sensitivities of IL-2- and IL-15-Induced Proliferation to Rapamycin. In an attempt to define differences in the signaling pathways in the contrasting T lymphocyte responses to IL-2 and IL-15, we noticed a marked difference in the inhibitory capacity of the immunosuppressive drug rapamycin (Fig. 1A). In two T cell lines (the murine CTLL-2 and the human 15R-Kit, which was made IL-15 responsive by the overexpression of human IL-15Rα), rapamycin strongly inhibited the proliferation of cells stimulated by low doses of IL-15. The inhibitory effect of rapamycin was much weaker if cells were responding to IL-2. Increasing the concentration of IL-15 decreased the effect of rapamycin, a phenomenon that is presumably due to a loss of receptor specificity for IL-15 (data not shown). High doses of IL-15 can activate T cells via binding to the β- and γ-subunits of the IL-2/15 receptor without a requirement for the presence of IL-15Rα, which is needed at low concentrations. In contrast to rapamycin, the immunosuppressive drug FK506, which regulates the calcineurin pathway, affected T cell proliferation only at high concentrations in response to either IL-2 (Fig. 1 A) or IL-15 (data not shown). These data indicate that the response of T cells to IL-15 seems to be more sensitive to inhibitory effects of rapamycin than the response to IL-2.
Fig. 1.
Distinct sensitivities of IL-2- and IL-15-induced proliferations to rapamycin. (A) The two T cell lines 15R-Kit (Left) and CTLL-2 (Right) were stimulated by 100 pM IL-2 or 100 pM IL-15 in the presence of various concentrations of rapamycin. The proliferative response to IL-15 as assessed by thymidine incorporation was much more effectively inhibited when compared with that of IL-2 over a wide dose range of rapamycin in both cell lines. The presence of FK506 (shown only for IL-2-stimulated CTLL-2) inhibited both IL-2- and IL-15-driven proliferation only at high concentrations, with little difference observed between IL-2 and IL-15. (B) Western analyses revealed similar phosphorylation responses of p70S6K and ERK to IL-2 and IL-15 in 15R-Kit (Left). The activation of either kinase was of shorter duration in CTLL-2 (Right). The presence of rapamycin (500 ng/ml) or LY294002 (30 μM) inhibited the activation of p70S6K but had no effect on ERK.
Rapamycin Inhibits p70S6K Phosphorylation. To define pathways that are involved in the distinct effects of rapamycin on the two cytokines, we studied IL-2 and IL-15 signaling in various cell types. A known mediator of IL-2/15 activation is mTOR, with p70S6K as a downstream target. 15R-Kit responded to either cytokine with a lasting p70S6K phosphorylation (Fig. 1B). In CTLL-2 cells, the effect was transient, with maximum phosphorylation observed 15 min after IL-2 or IL-15 stimulation.
A second pathway that is induced by IL-2/15 leads to the activation of ERK via the Ras-Raf-MAPK cascade. ERK had been suggested to be a substrate for mTOR (19). Again, we observed a lasting phosphorylation of both ERK p42 and p44 in 15R-Kit (Fig. 1B). The effect was temporary in CTLL-2, with IL-2 eliciting a stronger response than IL-15.
We then investigated the effects of various drugs with defined targets. The presence of rapamycin virtually abolished Thr-389 phosphorylation of p70S6K in CTLL-2 cells in response to both IL-2 and IL-15 (Fig. 1B). In contrast, FK506 did not inhibit IL-2- or IL-15-driven p70S6K phosphorylation (data not shown). The presence of the PI3-K inhibitor LY294002 abolished p70S6K phosphorylation similar to rapamycin. None of the three inhibitors had any detectable effect on the phosphorylation of ERK. Attempts to detect the phosphorylation of mTOR or of 4E-BP, another target of mTOR, were unsuccessful in cells stimulated with either cytokine (data not shown). These data suggest that the activities of both PI3-K and mTOR are necessary for the phosphorylation of p70S6K in response to IL-2 and IL-15.
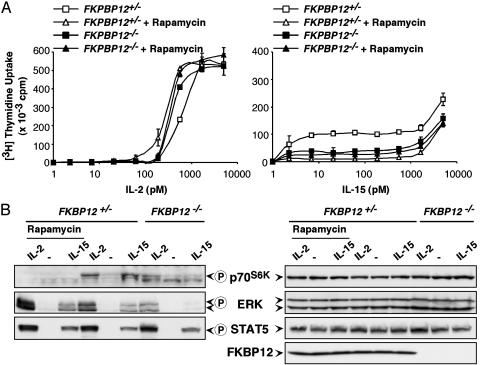
FKBP12 Deficiency Has a Marked Inhibitory Effect on IL-15-Driven but Not on IL-2-Driven Proliferation. FKBP12 represents a well-studied target of rapamycin activity. We investigated its involvement in IL-2- and IL-15-induced proliferation by using splenic T lymphocytes that had been derived from FKBP12-deficient mice. The response of CD3/28-activated splenic T cells to IL-15 generally resulted in a lower maximum thymidine uptake when compared with that of IL-2 (Fig. 2A). This phenomenon may be due to a low expression of IL-15Rα. The proliferative response of FKBP12–/– cells to IL-2 was only modestly decreased when these cells were compared with heterozygous cells. The presence of rapamycin had no effect on the IL-2 response in these cells. In contrast to the observations with IL-2, stimulation by IL-15 was strongly reduced by the lack of FKBP12 (Fig. 2 A). Contrary to the inhibition mediated by rapamycin in FKBP12+/– cells, rapamycin had no further effect on IL-15-driven thymidine incorporation in the absence of FKBP12. This result indicates that the inhibitory effect of rapamycin on the IL-15 proliferative response depends mainly on the presence of FKBP12.
Fig. 2.
FKBP12 deficiency has a major inhibitory effect on IL-15-induced but not IL-2-induced proliferation and signaling. (A) The responses of FKBP12–/– T lymphocytes that had been activated by anti-CD3 and anti-CD28 to various concentrations of IL-2 were similar to those of heterozygous cells (Left). The presence of 500 ng/ml rapamycin only modestly affected the proliferation in response to IL-2. Thymidine incorporation was more profoundly decreased compared with controls in low to moderate dose IL-15-stimulated T cells, both by the presence of rapamycin and by the deletion of FKBP12 (Right). (B) A reduced activation of both p70S6K and ERK was observed in FKBP12–/– T cells after stimulation with either IL-2 or IL-15. The presence of rapamycin decreased the p70S6K phosphorylation but increased ERK activation in heterozygous cells. STAT5 phosphorylation was unaffected either by the presence of rapamycin or by FKBP12 deficiency. The presence of FKBP12 is shown as a control.
FKBP12 Deficiency Strongly Affects ERK and p70S6K Phosphorylation in Response to IL-15 but Not to IL-2. The presence of rapamycin and the FKBP12 deficiency had similar effects on T cell proliferation in response to IL-15. We, therefore, analyzed p70S6K phosphorylation as a marker of mTOR activity in FKBP12–/– cells. T lymphocytes from heterozygous FKBP12+/– mice responded with a p70S6K Thr-389 phosphorylation to both IL-2 and IL-15 (Fig. 2B). This phosphorylation was virtually undetectable in the presence of rapamycin. In FKBP12–/– cells, p70S6K phosphorylation was strongly reduced in response to IL-15 whereas significant amounts of phosphorylated p70S6K could be detected after IL-2 stimulation. The difference between FKBP12+/– and FKBP12–/– cells cannot be explained by changes in the expression of the cytokine receptor proteins because STAT5 activation, which requires the presence of all three subunits of the IL-2/15 receptors at the concentrations of the cytokines used, was not affected by the FKBP12 deficiency. In addition, the amounts of the IL-2 and IL-15 receptor α subunits were normal in FKBP12–/– cells as assessed by FACS and Western analyses (data not shown).
Surprisingly, we also detected reduced ERK phosphorylation in FKBP12–/– cells after stimulation with either IL-2 or IL-15 (Fig. 2B). Again, the FKBP12 deletion had a stronger effect on the IL-15 response. Conversely, the presence of rapamycin positively affected ERK phosphorylation induced by either cytokine, indicating a positive feedback mechanism. Thus, FKBP12 seems to be required for the full effects of IL-15 but not of IL-2 on the induction of phosphorylated p70S6K and ERK.
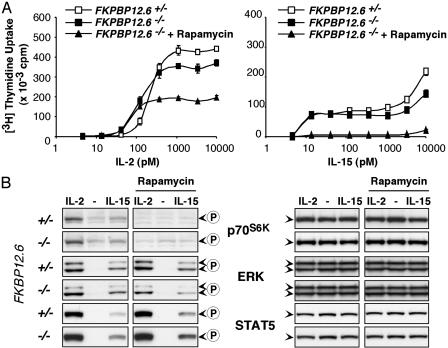
FKBP12.6 Is Involved in the T Cell Response to IL-2. To address whether IL-2 or IL-15 signaling proceeds via FKBP12.6, we analyzed splenic T cells from FKBP12.6–/– mice. The lack of FKBP12.6 was associated with a reduction of IL-15-driven proliferation that was much less than that observed when rapamycin was added to heterozygous cells (Fig. 3A). We observed a similarly modest but statistically significant effect of FKBP12.6 deficiency on IL-2-stimulated T cells (Fig. 3A Left). The reduction of IL-2-driven proliferation by the presence of rapamycin was much stronger in FKBP12.6-deficient as compared with heterozygous cells, suggesting that FKBP12.6 was involved in the IL-2 response (compare Fig. 3A with Fig. 2 A). No differences in the activation patterns of STAT5, ERK, or p70S6K between FKBP12.6–/– and FKBP12.6+/– T cells were observed independent of the presence of rapamycin (Fig. 3B). Our observation that rapamycin had only modest effects in heterozygous cells but strongly decreased proliferation in FKBP12.6–/– cells supports the view that FKBP12.6 is involved in the IL-2 pathway. Because no differences in the activation of mTOR, ERK, or STAT5 in response to IL-2 were detected between FKBP12.6-deficient and heterozygous cells, FKBP12.6 seems to be involved in a pathway that is not mediated by these signaling molecules.
Fig. 3.
Involvement of FKBP12.6 in the T cell response to IL-2. (A) FKBP12.6-deficient cells responded to both IL-2 (Left) and IL-15 (Right) with a slight decrease in thymidine uptake. The presence of rapamycin further reduced the maximum IL-2-driven proliferation, a phenomenon that had not been observed in wild-type cells (compare with Fig. 2). All three conditions of cells stimulated with 1–10 nM IL-2 were statistically different, with P values <0.0005. (B) No differences in the phosphorylation patterns of p70S6K, ERK, or STAT5 were observed between homo- and heterozygous FKBP12.6–/– T cells after 15-min stimulations with either IL-2 or IL-15 in the absence or presence of rapamycin (500 ng/ml).
Discussion
In the present study, we show that FKBP12 is a crucial element, in the proliferation-inducing cascade of IL-15-responding T lymphocytes, that is not required during the stimulation with IL-2. Both the presence of the inhibitor rapamycin and the genetic deletion of FKBP12 strongly reduced p70S6K Thr-389 phosphorylation and also severely suppressed the T cell proliferative response to IL-15. In contrast, cells deficient in FKBP12.6 were only modestly affected in their proliferative response to IL-15. These data indicate that, contrary to the situation with IL-2, mTOR activation via FKBP12 and to a lesser degree via FKBP12.6 is a necessary regulatory step in the IL-15-induced proliferation of activated T cells.
Signaling pathways that are active in IL-2-responding T cells seem to be more complex. Previous experiments showed a correlation between rapamycin-induced inhibition of p70S6K activation and T cell proliferation in response to IL-2 (27). However, when lower concentrations of rapamycin were used, we now showed that IL-2-driven T cell proliferation was only modestly affected by nanomolar doses of rapamycin that completely blocked p70S6K phosphorylation, indicating that IL-2-driven T cell proliferation can proceed via mTOR-independent pathways. Furthermore, we observed only a partial reduction of p70S6K activation in FKBP12–/– cells compared with a complete absence if cells were stimulated in the presence of rapamycin. This result implicates molecules other than FKBP12 as rapamycin receptors and as mediators of mTOR activation. On the other hand, the mTOR pathway seems to be one of the pathways involved in the IL-2 response because the presence of rapamycin reduced the proliferation in FKBP12.6–/– cells. The same experiment also demonstrated a role for FKBP12.6 in IL-2-induced T cell proliferation because the amplitude of thymidine uptake in response to IL-2 was affected by rapamycin only in homozygous FKBP12.6-deficient but not in heterozygous cells. However, a pathway other than those involving STAT5, mTOR, or ERK must be dependent on FKBP12.6 in IL-2-stimulated cells because no differences in their activation patterns were detected between FKBP12.6+/– and FKBP12.6–/– cells in the presence or absence of rapamycin. Lastly, even in the presence of rapamycin and the absence of FKBP12.6, T cells responded to IL-2 with a significant proliferation. This finding indicates the existence of a third activation cascade independent of mTOR and FKBP12.6 that is used during the response to IL-2 but not to IL-15.
In the present study, we observed that the ERK-activating cascade also requires FKBP12. A role for FKBP12 in MAPK-mediated cell cycle control was described in transforming growth factor-β-stimulated fibroblasts (28). With the use of chemical inhibitors, p38 MAPK was implicated in the regulation of the cell cycle inhibitor p21. In our experiments, FKBP12–/– cells responded with a reduced phosphorylation of ERK in response to either IL-2 or IL-15, implicating FKBP12 in the ERK activation cascade. ERK activation by itself seems to be insufficient to drive proliferation because T cells that displayed an increased ERK activation in the presence of rapamycin nonetheless failed to proliferate in response to IL-15. A lack of involvement of MAPK in IL-2-driven proliferation has been shown in experiments in which cells that express certain mutants of IL-2Rβ proliferate normally without MAPK activation (10, 29, 30).
The target protein of the FKBP12 activity in the ERK-activating cascade is unclear. ERK was suggested to be a substrate for mTOR (19). In our experiments, ERK phosphorylation was not affected by the presence of inhibitors for PI3-K or mTOR. It is currently unclear whether PI3-K acts upstream of or in concert with mTOR to regulate p70S6K activity (9). Our data do not rule out an mTOR-mediated activation of ERK because both rapamycin and LY294002 could affect the target specificity of mTOR by inhibiting the pathway to p70S6K only.
The difference between the IL-2 and the IL-15 signals in their sensitivity to rapamycin could have multiple reasons. A simple difference in signaling strength could theoretically be caused by different cell surface concentrations of their private receptor α-chains. In support of this possibility, we consistently observed stronger activation of mTOR, ERK, and STAT5 if activated T lymphocytes had been stimulated by IL-2 rather than by IL-15. To define whether the receptor expression was the critical element in the differences observed between IL-2- and IL-15-driven responses, we generated the T cell line 15R-Kit with a high expression of IL-15Rα. This overexpression resulted in equal potencies of IL-2 and IL-15 in the phosphorylation of ERK and p70S6K, but the IL-15-driven proliferation in these cells was nonetheless more sensitive to rapamycin when compared with that of IL-2. Therefore, the difference in expression levels between IL-2Rα and IL-15Rα is unlikely to account for differences in the proliferation-inhibitory effect of rapamycin.
A second factor that might underlie the different sensitivities to rapamycin may be IL-2- and IL-15-induced differences in the conformation of the receptor complexes that may lead to the activation of distinct signaling pathways. Differences in IL-2- and IL-15-driven receptor subunit topology were demonstrated by fluorescence resonance energy transfer studies (31). The use of antibodies that are directed against various epitopes of IL-2Rβ revealed different binding areas for IL-2 and IL-15 on this receptor subunit (32). However, no data are available about specific signaling implications of these different receptor conformations.
Lastly, the private α-subunits of the IL-2 or IL-15 receptors by themselves could deliver intracellular signals. IL-2Rα is considered not to be directly involved in signaling in light of its short cytoplasmic tail (9). In contrast, some experiments indicate a possible association of IL-15Rα with SYK and Traf2 (33, 34).
We have shown that FKBP12 is critical in the proliferative signal induced by IL-15. Nevertheless, it is currently unclear whether FKBP12 can bind directly to the IL-2/15 receptor complexes. We had originally identified FKBP12 as an interactor with the cytoplasmic domain of IL-15Rα if both proteins were overexpressed in yeast. However, subsequent overexpression experiments in 293HEK cells revealed only a marginal association between the two proteins, and no interaction was detected between the endogenous FKBP12 and the IL-2/15 receptor complexes in T cells after stimulation with either IL-2 or IL-15 for various times. However, this finding does not exclude the existence of such a direct association under currently undefined conditions.
In summary, our data show evidence for signaling differences between IL-2 and IL-15 that are involved in cytokine-mediated proliferation of T lymphocytes. Rapamycin addition caused a marked inhibition of IL-15-driven T cell proliferation but had only modest effects on that induced by IL-2. This result was explained in part by the observation that FKBP12, a target of rapamycin activity, was required in the proliferative stimulation by IL-15, as well as in IL-15-induced activation of p70S6K and of ERK, but was not essential for the proliferative activity of IL-2. The signaling pathways leading to IL-2-mediated proliferation are more complex than those of IL-15. IL-2 uses a series of alternative pathways, including those involving FKBP12 and FKBP12.6, as well as a third signaling cascade. Thus, the differences in the functions mediated by IL-2 and IL-15 are associated with differences in the signaling pathways used by these two cytokines.
Acknowledgments
We thank Hanying Chen for technical assistance.
Abbreviations: STAT, signal transducer and activator of transcription; MAPK, mitogen-activated protein kinase; PI3-K, phosphatidylinositol 3-kinase; mTOR, mammalian target of rapamycin; p70S6K, p70S6 kinase; FKBP, FK506-binding protein; ERK, extracellular signal-regulatedkinase.
References
- 1.Waldmann, T. A., Dubois, S. & Tagaya, Y. (2001) Immunity 14, 105–110. [PubMed] [Google Scholar]
- 2.Kundig, T. M., Schorle, H., Bachmann, M. F., Hengartner, H., Zinkernagel, R. M. & Horak, I. (1993) Science 262, 1059–1061. [DOI] [PubMed] [Google Scholar]
- 3.Willerford, D. M., Chen, J., Ferry, J. A., Davidson, L., Ma, A. & Alt, F. W. (1995) Immunity 3, 521–530. [DOI] [PubMed] [Google Scholar]
- 4.Bulfone-Paus, S., Ungureanu, D., Pohl, T., Lindner, G., Paus, R., Ruckert, R., Krause, H. & Kunzendorf, U. (1997) Nat. Med. 3, 1124–1128. [DOI] [PubMed] [Google Scholar]
- 5.Dooms, H., Desmedt, M., Vancaeneghem, S., Rottiers, P., Goossens, V., Fiers, W. & Grooten, J. (1998) J. Immunol. 161, 2141–2150. [PubMed] [Google Scholar]
- 6.Marks-Konczalik, J., Dubois, S., Losi, J. M., Sabzevari, H., Yamada, N., Feigenbaum, L., Waldmann, T. A. & Tagaya, Y. (2000) Proc. Natl. Acad. Sci. USA 97, 11445–11450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy, M. K., Glaccum, M., Brown, S. N., Butz, E. A., Viney, J. L., Embers, M., Matsuki, N., Charrier, K., Sedger, L., Willis, C. R., et al. (2000) J. Exp. Med. 191, 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lodolce, J. P., Boone, D. L., Chai, S., Swain, R. E., Dassopoulos, T., Trettin, S. & Ma, A. (1998) Immunity 9, 669–676. [DOI] [PubMed] [Google Scholar]
- 9.Gaffen, S. L. (2001) Cytokine 14, 63–77. [DOI] [PubMed] [Google Scholar]
- 10.Friedmann, M. C., Migone, T. S., Russell, S. M. & Leonard, W. J. (1996) Proc. Natl. Acad. Sci. USA 93, 2077–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ravichandran, K. S., Igras, V., Shoelson, S. E., Fesik, S. W. & Burakoff, S. J. (1996) Proc. Natl. Acad. Sci. USA 93, 5275–5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans, G. A., Goldsmith, M. A., Johnston, J. A., Xu, W., Weiler, S. R., Erwin, R., Howard, O. M., Abraham, R. T., O'Shea, J. J., Greene, W. C., et al. (1995) J. Biol. Chem. 270, 28858–28863. [DOI] [PubMed] [Google Scholar]
- 13.Ravichandran, K. S. & Burakoff, S. J. (1994) J. Biol. Chem. 269, 1599–1602. [PubMed] [Google Scholar]
- 14.Brennan, P., Babbage, J. W., Burgering, B. M., Groner, B., Reif, K. & Cantrell, D. A. (1997) Immunity 7, 679–689. [DOI] [PubMed] [Google Scholar]
- 15.Brennan, P., Babbage, J. W., Thomas, G. & Cantrell, D. (1999) Mol. Cell. Biol. 19, 4729–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gingras, A. C., Raught, B. & Sonenberg, N. (2001) Genes Dev. 15, 807–826. [DOI] [PubMed] [Google Scholar]
- 17.Schiene-Fischer, C. & Yu, C. (2001) FEBS Lett. 495, 1–6. [DOI] [PubMed] [Google Scholar]
- 18.Neuhaus, P., Klupp, J. & Langrehr, J. M. (2001) Liver Transplant. 7, 473–484. [DOI] [PubMed] [Google Scholar]
- 19.Huang, S. & Houghton, P. J. (2001) Drug Resist. Updat. 4, 378–391. [DOI] [PubMed] [Google Scholar]
- 20.Schmelzle, T. & Hall, M. N. (2000) Cell 103, 253–262. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J., Farmer, J. D., Jr., Lane, W. S., Friedman, J., Weissman, I. & Schreiber, S. L. (1991) Cell 66, 807–815. [DOI] [PubMed] [Google Scholar]
- 22.Snyder, S. H., Lai, M. M. & Burnett, P. E. (1998) Neuron 21, 283–294. [DOI] [PubMed] [Google Scholar]
- 23.Shou, W., Aghdasi, B., Armstrong, D. L., Guo, Q., Bao, S., Charng, M. J., Mathews, L. M., Schneider, M. D., Hamilton, S. L. & Matzuk, M. M. (1998) Nature 391, 489–492. [DOI] [PubMed] [Google Scholar]
- 24.Xu, X., Su, B. Barndt, R., Chen, H., Heitman, J., Yan, G., Xin, H.-B., Zhuang, Y. Fleisher, S. & Shou, W. (2002) Transplantation 73, 1835–1838. [DOI] [PubMed] [Google Scholar]
- 25.Xin, H. B., Senbonmatsu, T., Cheng, D. S., Wang, Y. X., Copello, J. A., Ji, G. J., Collier, M. L., Deng, K. Y., Jeyakumar, L. H., Magnuson, M. A., et al. (2002) Nature 416, 334–338. [DOI] [PubMed] [Google Scholar]
- 26.Dubois, S., Mariner, J., Waldmann, T. A. & Tagaya, Y. (2002) Immunity 17, 537–547. [DOI] [PubMed] [Google Scholar]
- 27.Kuo, C. J., Chung, J., Fiorentino, D. F., Flanagan, W. M., Blenis, J. & Crabtree, G. R. (1992) Nature 358, 70–73. [DOI] [PubMed] [Google Scholar]
- 28.Aghdasi, B., Ye, K., Resnick, A., Huang, A., Ha, H. C., Guo, X., Dawson, T. M., Dawson, V. L. & Snyder, S. H. (2001) Proc. Natl. Acad. Sci. USA 98, 2425–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gaffen, S. L., Lai, S. Y., Ha, M., Liu, X., Hennighausen, L., Greene, W. C. & Goldsmith, M. A. (1996) J. Biol. Chem. 271, 21381–21390. [DOI] [PubMed] [Google Scholar]
- 30.Fujii, H., Ogasawara, K., Otsuka, H., Suzuki, M., Yamamura, K., Yokochi, T., Miyazaki, T., Suzuki, H., Mak, T. W., Taki, S., et al. (1998) EMBO J. 17, 6551–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damjanovich, S., Bene, L., Matko, J., Alileche, A., Goldman, C. K., Sharrow, S. & Waldmann, T. A. (1997) Proc. Natl. Acad. Sci. USA 94, 13134–13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lehours, P., Raher, S., Dubois, S., Guo, J., Godard, A. & Jacques, Y. (2000) Eur. Cytokine Network 11, 207–215. [PubMed] [Google Scholar]
- 33.Bulanova, E., Budagian, V., Pohl, T., Krause, H., Durkop, H., Paus, R. & Bulfone-Paus, S. (2001) J. Immunol. 167, 6292–6302. [DOI] [PubMed] [Google Scholar]
- 34.Bulfone-Paus, S., Bulanova, E., Pohl, T., Budagian, V., Durkop, H., Ruckert, R., Kunzendorf, U., Paus, R. & Krause, H. (1999) FASEB J. 13, 1575–1585. [DOI] [PubMed] [Google Scholar]