Abstract
The lupus-like autoimmune syndrome of MRL/Mp-Tnfrsf6lpr (lpr) mice is characterized by progressive lymphadenopathy and autoantibody production, leading to early death from renal failure. Activation of T helper lymphocytes is one of the events in the pathogenesis of the disease in these mice and likely in human systemic lupus erythematosus. Among T helper lymphocytedependent cytokines, IFN-γ plays a pivotal role in the abnormal cell activation and the fatal development of the lpr disease. IL-18, an inducer of IFN-γ in T lymphocytes and natural killer cells, may contribute to the disease because cells from lpr mice are hypersensitive to IL-18 and express high levels of IL-18. To assess the contribution of IL-18 to the pathogenesis in the animal model, in vivo inhibition of IL-18 was attempted. Young lpr mice were vaccinated against autologous IL-18 by repeated administration of a cDNA coding for the murine IL-18 precursor. Vaccinated mice produced autoantibodies to murine IL-18 and exhibited a significant reduction in spontaneous lymphoproliferation and IFN-γ production as well as less glomerulonephritis and renal damage. Moreover, mortality was significantly delayed in anti-IL-18-vaccinated mice. These studies support the concept that IL-18 plays a major role in the pathogenesis of the autoimmune syndrome of lpr mice and that a reduction in IL-18 activity could be a therapeutic strategy in autoimmune diseases.
The severe autoimmune syndrome of MRL/Mp-Tnfrsf6lpr (lpr) mice closely resembles human systemic lupus erythematosus and the autoimmune lymphoproliferative syndrome. The disease model is characterized by progressive lymphadenopathy, hypergammaglobulinemia, autoantibody production, and immune complex formation, which leads to vasculitis, arthritis, and fatal renal failure (1, 2). Disease in lpr mice is accelerated by a mutation in the tumor necrosis factor receptor superfamily 6 (Tnfrsf6) gene, which impairs Fas-dependent apoptotic functions (3). Thus, lpr mice show accumulation of self-reactive T lymphocytes (4, 5) associated with lymphadenopathy and expansion of abnormal CD4- CD8- double negative (DN) T cells (6). Despite the central role of CD4+ T cells in the initiation and progression of the disease (7, 8), the relative role of T helper 1 (Th1) and Th2 cells and their respective cytokines in the human and the murine disease remains controversial (9). Both IFN-γ and IL-4 take part in disease development, because knockout mice for either the IFN-γ or IL-4 gene show reduction in the disease parameters of lymphadenopathy, organ failure, and early mortality (10). Several studies point to a major pathogenic role for Th1 cytokines. For example, IL-12 and IL-12-induced nitric oxide play a significant pathogenic role in the lpr disease (11). Administration of IFN-γ exacerbates the disease both in humans and mice (12, 13), whereas animals deficient for expression of IFN-γ or IFN-γ receptor type I develop a less acute disease and exhibit less renal histopathological changes (14, 15). DN T cells and autoantibodies are absent in IFN-γ-deficient mice (10), and the renal damage is dramatically reduced in IFN-γR knockout mice (14). Other studies indicate that IFN-γ is at the basis of the fatal kidney disease in lpr mice (16).
The cytokine IL-18, formerly known as IFN-γ-inducing factor, induces IFN-γ production in IL-2, IL-12, or IL-15 primed T and natural killer (NK) cells and increases the activity and proliferation of Th1, NK, and CD8+ cells (17, 18). Because of the ability to induce IFN-γ production and Th1 cell activation and regulate the synthesis of inflammatory cytokines (19, 20), IL-18 likely participates in autoimmune diseases. A correlation between expression of IL-18 and the active stage of the disease has been observed in the development of autoimmune Th1-dependent insulitis in nonobese diabetic mice (21). The development of experimental autoimmune encephalomyelitis can be prevented by the administration of neutralizing anti-IL-18 antibodies (22), whereas in IL-18-deficient mice administration of exogenous IL-18 restores autoreactivity by means of IFN-γ induction (23). IL-18 also promotes collagen-induced inflammatory arthritis (24), whereas the incidence and severity of the disease are strongly reduced in IL-18 knockout mice (25). Elevated concentrations of IL-18 have been measured in the affected tissues of patients with Crohn's disease (26, 27) and rheumatoid arthritis (28, 29).
Increased levels of circulating IL-18 also correlate with disease activity in human systemic lupus erythematosus (30–33). In the lpr lupus-like syndrome lymph node (LN) cells and LN-derived autoreactive T cell lines are hyperreactive to IL-18 in terms of proliferation and IFN-γ production, possibly because of the constitutive expression of the IL-18 receptor β chain (34). Prolonged IL-18 administration exacerbates the lupus-like disease of lpr mice, although it is unable to induce the disease in normal +lpr littermates (35). Nevertheless, a causative role for IL-18 in the autoimmune pathogenesis of this and other models remains to be established.
In this present study, the involvement of IL-18 in the development of autoimmune murine lupus was investigated by reducing the activity of endogenous IL-18 using sustained neutralization in the lpr mice. To accomplish this, a cDNA vaccination procedure was used to trigger a response to autologous IL-18. Auto anti-IL-18 antibodies induced by vaccination could significantly decrease IFN-γ production as well as LN and spleen lymphoproliferation in this slowly evolving model. Eventually, vaccinated mice may exhibit a decrease or delay in the autoimmune renal damage and show a significant prolongation of life.
Materials and Methods
Mice. Homozygous MRL/MpJ +Tnfrsf6-lpr/+Tnfrsf6-lpr (+lpr) and Tnfrsf6lpr/Tnfrsf6lpr (lpr) mice (The Jackson Laboratory) were housed and bred under specific pathogen-free conditions. C57BL/6 and BALB/c mice were also obtained. Animal experimentation were reviewed by the institutional ethical board for animal research conduct authorized by the Italian Ministry of Health.
Organ and Cell Preparation. Organs were weighed, suspended in PBS with 0.1% Tween 20, and homogenized. After centrifugation at 10,000 rpm in a microfuge at 4°C for 10 min, supernatants were collected for cytokine analysis. Cell suspensions from LN and spleen were analyzed cytofluorimetrically (FACScan, Becton Dickinson) with mAbs to CD4, CD8, CD3, CD19, CD11c, B220, and IFN-γ (Pharmingen).
RT-PCR. Semiquantitative RT-PCR for determining IL-18 mRNA was performed as described (34), using the housekeeping gene HPRT for standardization.
Plasmids and Vaccination Procedure. The plasmid pEF2-IGIF expressing murine proIL-18 was kindly provided by W. M. F. Lee (University of Pennsylvania, Philadelphia). The plasmid was constructed by subcloning the murine pro-IL-18 cDNA into the pEF2 vector between the KpnI and XbaI sites. The empty vector pEF2 was used as control. Purified plasmid DNA was reconstituted in sterile saline solution at 1 μg/μl. Starting at 5–7 weeks of age, mice were immunized three to five times, at 2-week intervals with pEF2 or pEF2-IL-18 constructs (2 × 50 μg of plasmid DNA i.m. in the hind leg muscle). One week after the last injection, mice were killed by ether hyperanesthesia.
IL-18 and Anti-IL-18 Antibody Detection. Murine IL-18 concentration in organ extracts or serum samples was assessed by ELISA (R & D Systems) and an electrochemiluminescence assay (36). The two assays provided overlapping results. Anti-IL-18 antibodies were identified in dot blot. Murine recombinant IL-18 was spotted on nitrocellulose membranes, followed by saturating concentrations of BSA. After incubation with serum samples, bound anti-IL-18 antibodies were identified by using horseradish peroxidase-conjugated goat anti-mouse immunoglobulins and developing the reaction with the Super Signal West Pico system (Pierce). For indirect determination of anti-IL-18 antibodies, free IL-18 was measured before and after two acid treatments with 0.1 M glycine, pH 2.1 and centrifugation through a Microcon 100 (Amicon Millipore). A higher IL-18 concentration in the filtrate vs. unprocessed serum indicates the presence of anti-IL-18 antibodies forming complexes with IL-18. Antibody concentration was expressed in arbitrary units, defining 1 unit as the amount of putative antibodies releasing 1 pg of IL-18 after acid dissociation.
The IL-18-neutralizing capacity of serum was determined by using an assay for biologically active IL-18 in the proliferation of autoreactive lpr T cells (34). Briefly, 1 × 105 autoreactive cells were incubated with IL-18 (3–10 ng/ml) and serum samples at 37°C for 48 h and pulsed for an additional 6 h with 0.5 μCi of tritiated thymidine (Amersham Pharmacia). Results were expressed as percentage of IL-18-induced net proliferation.
Proteinuria and Determination of Renal Damage. Urine protein level was assessed by using Combur Test strips (Roche Molecular Biochemicals). Small volumes (50 μl) of urine were taken from animals at weekly intervals throughout the vaccination protocol. Renal tissue was fixed in formalin and embedded in paraffin. Sections were stained with hematoxylin and eosin, periodic acid-Schiff, trichrome, and silver-based reticulin stains. Kidney lesions were scored for severity (-, no sign of damage; +, mild alterations present in some areas; ++, clear and frequent alterations, +++, severe and widespread damage) of mesangial hypercellularity, mesangial matrix increase, lobular accentuation, and extent of staining of IgG and C3 (with FITC-labeled goat anti-mouse IgG and goat anti-mouse C3; Cappel). Sections were scored by three pathologists in a blind fashion.
Statistical Analysis. Data are presented as the mean ± SEM of replicate samples. Statistical significance for comparison between groups was determined by using a t test. Proteinuria data were analyzed by a Cochran–Mantel Haenszel test. Survival analysis was performed with the Kaplan–Meier method, and the significance of difference in survival between control and vaccinated groups was determined by using the log-rank test.
Results
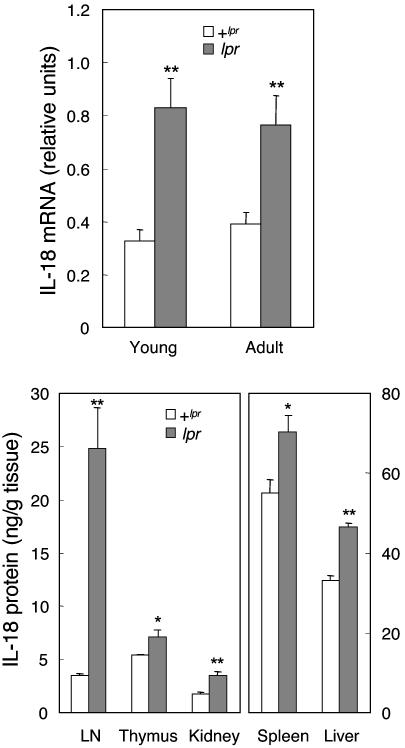
Spontaneous Production of IL-18. As shown in Fig. 1, IL-18 was significantly higher in lpr mice than in normal +lpr animals. IL-18 mRNA expression was elevated 2.5- and 2.0-fold in young (4–7 weeks old) and adult (>12 weeks old) lpr mice, as compared with +lpr counterparts. IL-18 protein levels were consistently significantly higher in lpr organs than in +lpr tissues. In LN cells, the increase in IL-18 was markedly elevated (7.3-fold vs. +lpr). In addition, IL-18 was significantly hyperexpressed in the thymus (1.5-fold), spleen (1.3-fold), kidney (2.0-fold), and liver (1.4-fold).
Fig. 1.
Spontaneous IL-18 expression in MRL lpr mice. (Upper) Steady-state IL-18 mRNA expression was evaluated by semiquantitative RT-PCR in LN cells from young (4–7 wk) and adult (>12 wk) MRL/Mp +lpr mice (open columns) and lpr mice (solid columns). Data are the mean ± SEM of results from 5–11 mice per group.**, P < 0.01 vs. +lpr. (Lower) IL-18 protein was measured by ELISA in homogenates of different organs of 12-week-old MRL/Mp +lpr mice (open columns) and lpr mice (solid columns). Data are the mean ± SEM of results from four mice per group within a single experiment representative of two performed. *, P < 0.05 vs. + lpr; **, P < 0.01 vs. + lpr.
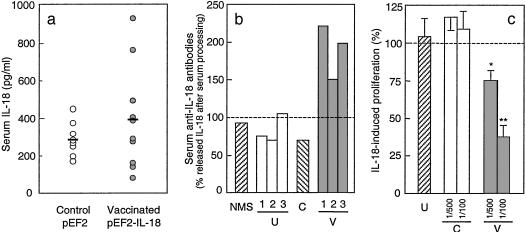
DNA Vaccination with Murine IL-18. A vaccination procedure was devised to induce an autoantibody response to mouse IL-18, which would neutralize endogenous IL-18 in lpr mice and inhibit its effects. The pEF2-IL-18 vector was inoculated repeatedly into hind legs of young lpr mice, before the onset of clinical disease. Control mice were inoculated with the empty vector pEF2 or saline. The presence of mRNA for murine IL-18 was detected in the hind leg muscle of treated mice, but not in tissues of control animals (Table 1). Measurable levels of murine IL-18 protein were found in serum of both vaccinated and control lpr mice (Fig. 2a).
Table 1. Induction of IL-18 and anti-IL-18 antibodies after cDNA vaccination: Correlation with reduced lymphadenopathy.
| Treatment | Mouse | Muscle IL-18 mRNA* | Serum anti-IL-18 immunoglobulins† | Cells/LN‡ × 10-6 | DN cells/LN,‡ × 10-6 |
|---|---|---|---|---|---|
| Control | 1 | - | - | 286 | 239 |
| pEF2 | 2 | - | - | 186 | 151 |
| 3 | - | - | 210 | 177 | |
| Vaccinated | 4 | + | + | 95 | 78 |
| pEF2-IL-18 | 5 | + | + | 95 | 70 |
| 6 | + | - | 218 | 189 | |
| 7 | + | + | 88 | 71 |
MRL/Mp Ipr mice received five administrations of plasmid pEF2 (empty vector) or pEF2-IL-18 i.m. in the hind legs (2 × 50 μg per mouse per time) every 2 weeks, starting at 7 weeks of age. Data are from single mice within one experiment of seven performed.
Expression of mRNA for murine IL-18 was examined by RT-PCR in the muscle tissue at the site of inoculation 1 week after the completion of the vaccination protocol. The result is purely qualitative and indicates absence of mRNA for murine IL-18 in the muscle of control mice and its presence in the muscle of animals inoculated with pEF2-IL-18.
For detection of anti-IL-18 immunoglobulins, a dot blot assay on recombinant murine IL-18 was performed to yield predominantly qualitative results.
Four LN were excised from each mouse 1 week after completion of the vaccination procedure, weighted, pooled, and dissociated to obtain a single-cell suspension. The number of nucleated viable cells was determined by trypan blue dye exclusion and staining with crystal violet, and the number of cells per LN was calculated. The number of DN cells (CD3+ CD4- CD8- B220+) was determined cytofluorimetrically after staining with specific antibodies.
Fig. 2.
Serum IL-18 and anti-IL-18 autoantibodies in vaccinated lpr mice. (a) IL-18 was measured by ELISA in sera of 8–10 single MRL/Mp lpr mice, 1–2 weeks after completion of the vaccination protocol with the pEF2 plasmid (control; open symbols) or the pEF2-IL-18 plasmid (vaccinated; solid symbols). Horizontal bars represent mean values. IL-18 in normal mouse serum was <200 pg/ml (data not shown). (b) Anti-IL-18 antibodies were evaluated as percentage IL-18 concentration in acid-treated vs. crude serum samples. Untreated sera (U1, U2, U3; open columns) are the preimmune sera of the same animals subjected to vaccination (V1, V2, V3; solid columns). Controls are serum from a pEF2-treated mouse (C) and normal mouse serum (NMS; striped column) spiked with 1 ng/ml recombinant murine IL-18. Antibody titers were as follows: NMS (spiked with 1 ng of IL-18), 0 units/ml; untreated controls (preimmune sera), 0, 0, and 22 units/ml; control with pEF2, 0 units/ml; vaccinated with pEF2-IL-18, 136, 282, and 452 units/ml. Data are from a single experiment representative of three performed. (c) IL-18-neutralizing activity of anti-IL-18 antibodies was assessed in an assay of IL-18-dependent proliferation of autoreactive T cells, in the presence of processed serum from vaccinated (pEF2-IL-18; V, solid columns) lpr mice or pEF2 control animals (C, open columns). Serum from untreated mice (diluted 1/100; U, striped column) was used as additional control. Data are expressed as percentage of IL-18-induced proliferation (in the absence of serum samples) and reported as the mean ± SEM of results from 3–14 animals in five experiments. *, P < 0.05 vs. IL-18-induced proliferation; **, P < 0.01 vs. IL-18-induced proliferation.
The presence of anti-IL-18 immunoglobulins in serum of vaccinated mice was determined by dot blots (Table 1). Autoantibodies to IL-18 were found in the sera of vaccinated mice, but not in the sera of control animals (empty vector). Assessment of anti-IL-18 antibodies in the sera of vaccinated mice was inconsistent because binding of anti-IL-18 autoantibodies to IL-18 likely interferes with detection. Therefore, circulating IL-18 levels were assessed before and after a complex-dissociating treatment. A significant increase of IL-18 levels could be observed after dissociating treatment only in sera of vaccinated mice (Fig. 2b). These results indicate that the cDNA vaccination induced the production of anti-IL-18 antibodies, which were then bound to the IL-18 antigen. The IL-18-neutralizing activity of these antibodies was assessed as shown in Fig. 2c. Serum from vaccinated mice neutralized IL-18 activity, whereas serum from control mice was inactive.
LN cells from 12- to 15-week-old lpr control mice (untreated or vaccinated with the empty vector) undergo a high rate of lymphoproliferation. For example, a single LN can reach 150–200 mg (300–400 × 106 cells). As shown in Table 1, IL-18 cDNA vaccination decreased lymphoproliferation by >50% in LN cells. Spleen lymphoproliferation was also significantly reduced (≈30%; data not shown). This effect was not restricted to a particular lymphocyte population, as each subset was equally decreased (data not shown). The decrease in lymphoproliferation correlated with the presence of anti-IL-18 immunoglobulins in the serum. Table 1 also reveals that lpr mice with undetectable anti-IL-18 antibodies (control pEF2) had significant lymphadenopathy with enlarged LN and a significant number of DN cells, whereas mice with detectable anti-IL-18 antibodies (vaccinated) showed a reduction in LN cell number. Interestingly, one of the mice receiving the cDNA vaccination failed to develop antibodies to IL-18 (mouse 6, Table 1) and did not show any significant amelioration of lymphadenopathy, suggesting that the effectiveness of the cDNA treatment strictly correlated with the induction of an anti-IL-18 response.
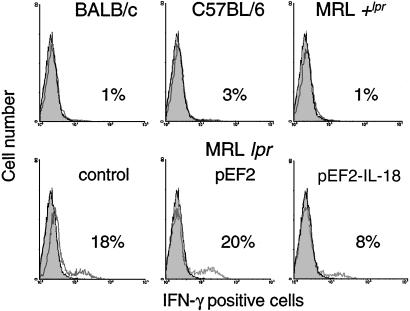
The Effect of IL-18 Vaccination on IFN-γ Production. Gated CD4+ LN cells of individual mice are shown in Fig. 3. LN cells of normal untreated mice contained low numbers of CD4+ IFN-γ-producing cells (<5%), whereas a significant percentage (18–20%) of CD4+ lymphocytes in untreated or pEF2-treated lpr LN was positive for IFN-γ. On the other hand, mice vaccinated with pEF2-IL-18 showed a drastic reduction of IFN-γ-producing CD4+ lymphocytes. Similar results were obtained with CD8+ cells (data not shown). The production of IFN-γ by LN cells was also significantly decreased in IL-18-vaccinated mice as compared with control pEF2 (1.2 ± 0.2 vs. 3.0 ± 0.5 ng per 106 cells).
Fig. 3.
IL-18 vaccination inhibits IFN-γ production in lpr LN cells. LN cells from control mice (BALB/c, C57BL/6, MRL/Mp + lpr) and 16-week-old MRL/Mp lpr mice (untreated, treated with pEF2, vaccinated with pEF2-IL-18) were analyzed cytofluorimetrically after staining with phycoerythrin-labeled anti-CD4 antibodies followed by intracellular staining with a FITC-labeled anti-murine IFN-γ antibody. Results are from gated CD4+ LN cells from individual mice (representative of three to eight animals tested in each group) in a single experiment. Dark areas: control without antibody. Clear areas: with anti-IFN-γ antibody.
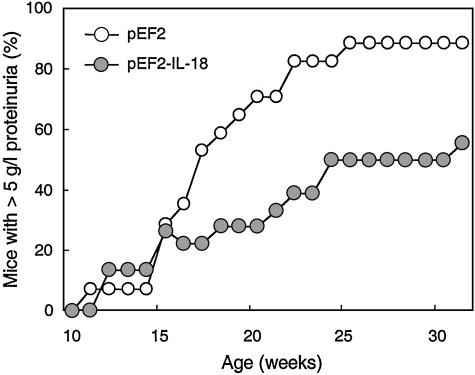
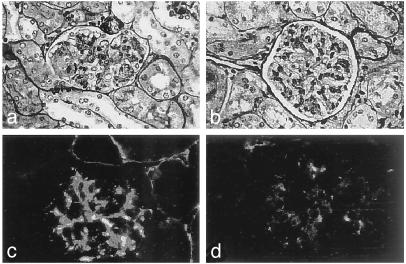
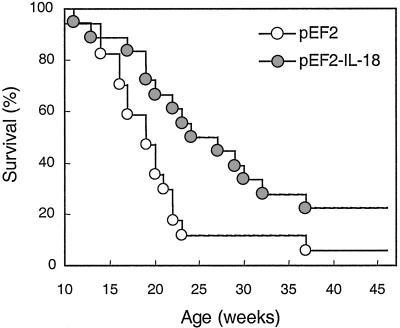
The Effect of IL-18 Vaccination on Renal Damage and Mortality. The levels of autoantibodies to the phospholipid cardiolipin and double-stranded DNA are among the main causes of immune complex deposition and the basis of the renal disease in lpr mice. There were no significant differences between control (either untreated or pEF2) and vaccinated mice at any age or within any of the antibody subclasses (data not shown). However, control pEF2 mice developed a progressive increase of proteinuria (Fig. 4), whereas IL-18 vaccinated mice showed a significant decrease and delay in the development of proteinuria. Morphologic evaluation of the renal tissue of 17-week-old control mice showed focal segmental glomerulonephritis with definite mesangial hypercellularity and segmental intracapillary and extracapillary cell proliferation, with obliteration of the capillary lumina (Fig. 5a), diffuse mesangial and peripheral granular deposition of immunoglobulins (Fig. 5c), and complement (Table 2). In contrast, in IL-18-vaccinated mice the lesions were purely mesangial, with minimal or no changes (Fig. 5b) or with moderate mesangial hypercellularity restricted to centri-lobular regions distant from the vascular pole. Immune deposits were scarce and mostly confined to the mesangium (Fig. 5d). Overall, the extensive vasculitis and infiltration of mononuclear cells was markedly reduced after IL-18 vaccination (Fig. 5 and Table 2). Because renal damage is the main cause of early death in autoimmune lpr mice, the effect of IL-18 vaccination on survival was evaluated. As shown in Fig. 6, survival of IL-18-vaccinated mice was significantly increased, with 50% of vaccinated mice still alive at 26 weeks, whereas almost 90% of control pEF2 mice died within 24 weeks. The life span of IL-18-vaccinated mice was also prolonged (median survival time 23.5 weeks in vaccinated vs. 20.0 weeks in control mice; mean survival time 25.0 weeks in vaccinated vs. 20.1 in control mice).
Fig. 4.
Anti-IL-18 vaccination inhibits proteinuria in lpr mice. Protein levels in urine of MRL/Mp lpr control mice (treated with pEF2; ○) and mice vaccinated against IL-18 (pEF2-IL-18;  ) were assessed at weekly intervals starting at 10 weeks of age. Data are from 15–24 mice, tested in three experiments. The difference between control and vaccinated mice was highly significant (P < 0.0001).
) were assessed at weekly intervals starting at 10 weeks of age. Data are from 15–24 mice, tested in three experiments. The difference between control and vaccinated mice was highly significant (P < 0.0001).
Fig. 5.
IL-18 vaccination inhibits glomerulonephritis in lpr mice. Silver-based reticulin-stained (a and b) and IgG immunofluorescence-stained (c and d) kidney sections in 17-week-old MRL/Mp lpr control mice (vaccinated with pEF2; a and c) and IL-18 vaccinated mice (b and d). (Magnifications: ×100, a and b; ×200, c and d.)
Table 2. Renal damage in vaccinated lpr mice.
| Vaccination†
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter* | pEF2 | pEF2—IL-18 | ||||||||||
| 1 | 2 | 3 | 4 | 5 | M | 1 | 2 | 3 | 4 | 5 | M | |
| Mesangial hyper-cellularity | + | ++ | ++ | ++ | +++ | ++ | + | + | + | - | - | + |
| Mesangial matrix increase | + | ++ | ++ | ++ | ++ | ++ | - | + | ++ | + | + | + |
| Lobular accentuation | + | ++ | + | ++ | ++ | ++ | - | - | - | - | - | - |
| IgG and C3 deposits | ++ | +++ | + | ++ | ++ | ++ | + | + | + | + | - | + |
| Interstitial cell infiltration and vasculitis | ++ | +++ | +++ | +++ | +++ | +++ | + | + | ++ | + | + | + |
Parameters of renal damage were evaluated on kidney sections and are expressed as follows: -, no sign of damage; +, mild alterations present in some areas; ++, clear and frequent alterations; +++, severe and widespread damage.
Tissue sections were prepared from kidneys of MRL/Mp lpr mice, dissected 2 weeks after the end of the vaccination procedure with pEF2 (control) or pEF2-IL-18 (anti-IL-18). Data reported are the scores of each mouse (indicated by numbers) with the median (M) scores of observations performed on five 17-week-old mice per group within a single representative experiment.
Fig. 6.
Anti-IL-18 vaccination prolongs survival of lpr mice. Cumulative survival data of MRL/Mp lpr control mice (treated with pEF2; ○) and mice vaccinated against IL-18 (pEF2-IL-18;  ) are reported (17–18 mice per group in four separate experiments). Survival of IL-18-vaccinated mice was significantly higher than that of control mice (P < 0.0299). Survival of untreated MRL/Mp lpr mice was fully superimposable to that of pEF2-treated mice (data not shown).
) are reported (17–18 mice per group in four separate experiments). Survival of IL-18-vaccinated mice was significantly higher than that of control mice (P < 0.0299). Survival of untreated MRL/Mp lpr mice was fully superimposable to that of pEF2-treated mice (data not shown).
Discussion
The severe and progressive autoimmune syndrome of MRL/Mp lpr mice has several characteristics in common with human systemic lupus erythematosus, in particular with the autoimmune lymphoproliferative syndrome. Among the characteristics of the autoimmune syndrome of lpr mice is the pathogenic role of Th1 activation. The importance of IL-18 in the initiation and maintenance of autoimmune alterations has been suggested by several lines of evidence. IL-18 is a potent activator of polarized Th1 cells and induces IFN-γ production and lymphocyte proliferation. A correlation between IL-18 and the autoimmune pathology has been suggested in several autoimmune syndromes, such as autoimmune diabetes in mice (21), experimental autoimmune encephalitis in rats and mice (22, 23, 37), experimental and rheumatoid arthritis (24, 25, 28, 29), human Crohn's disease and ulcerative colitis (26, 27, 38, 39), and human systemic lupus erythematosus (30–33, 35).
That elevated IL-18 may be among the pathogenic causes of autoimmune diseases, rather than a consequence of deregulated T cell activation, can be studied in the murine lpr lupus model. In a recent study, daily injections of IL-18, although unable to induce disease in +lpr littermates, could enhance disease progression and severity in lpr mice (35). Spleen and peritoneal cells from disease-affected lpr mice produce significantly higher levels of IL-18 than cells of normal animals; serum levels were also elevated (35). Increased reactivity to IL-18 can be observed in LN and spleen lymphocytes from young disease-free lpr mice as compared with +lpr cells, both in terms of IFN-γ production and proliferation (34). The data presented here show that hyperexpression of IL-18 in lpr mice is already maximal at a young age, when no pathological signs of the lupus syndrome are evident. This hyperexpression is particularly significant in LN, the organs that will be most severely affected by the disease in older age. Thus, the concomitant hyperproduction of IL-18 and enhanced sensitivity to its stimulation places young lpr mice in a state of Th1-dependent hyperactivation, which could contribute to the autoimmune pathology (9). To verify this hypothesis, a vaccination approach was designed to inhibit the prolonged excessive IL-18 in the lpr syndrome as the disease progresses. Feasibility of the approach was suggested by previous successful attempts to induce response to self-cytokines by cDNA inoculation in an autoimmune model (28). After the cDNA inoculation procedure, expression of the transgene could be detected at the inoculation site. Significant levels of circulating IL-18 were also present, although these showed high interindividual variability and were not statistically different from those of nonvaccinated mice. The presence of detectable circulating levels of IL-18 in nonvaccinated lpr mice is not unexpected, because lpr cells constitutively produce IL-18 (ref. 35 and this work). The variability that does not allow detection of significant increase of circulating IL-18 after vaccination might be explained by its masking or removal by newly formed autoantibodies or by sequestration by organ receptors.
Anti-IL-18 immunoglobulins were present in serum of vaccinated mice, but not in control mice. Quantitative direct detection of anti-IL-18 autoantibodies has been difficult, possibly holding to the same reasons as above, i.e., their masking and/or removal upon binding to IL-18. This indeed appears to be the case. After using procedures to dissociate immune complexes, significantly higher amounts of serum IL-18 were detectable as compared with those measurable in unprocessed serum. Thus, also circulating IL-18 is underestimated in vaccinated mice, conceivably because of the presence of IL-18-containing immune complexes. Despite the technical difficulties in directly measuring antibody titers, these data indicate that treatment of lpr mice with the cDNA of murine IL-18 caused overexpression of the protein that elicited a specific anti-IL-18 antibody response. This treatment can be therefore considered as a true vaccination.
The effect of the IL-18 vaccination on the progression of the autoimmune syndrome was assessed. The disease progression was significantly delayed in vaccinated mice, both in terms of lymphoproliferation and excessive IFN-γ production, suggesting an important role for IL-18 in the development of these pathological signs. The renal damage and glomerulonephritis that develop in lpr mice were also drastically delayed and decreased in IL-18-vaccinated mice, both in terms of proteinuria and by histological examination. This finding is in agreement with recent reports showing correlation between IL-18 and renal damage both in murine and human lupus (33, 40). However, anti-IL-18 vaccination did not induce any significant change in the levels of anti-double-stranded DNA and anti-phospholipid autoantibodies, among the most prominent features of the lupus disease and principal cause of immune complex-mediated renal damage (41). On the other hand, the decreased immune complex deposition in kidneys of vaccinated mice (Fig. 5) suggests that the anti-IL-18 vaccination is able to reduce the immune complex-mediated renal damage. In other studies, a lack of correlation between levels of anti-DNA autoantibodies and renal damage/survival of MRL/Mp lpr mice has been observed (42). It has been reported that lpr mice are impaired in the capacity of resolving immune complex deposits in the kidney (43). Thus the vaccination-induced decrease in renal immune complexes may be caused by restoration of effective clearance, rather than reduction of deposition. In addition, IFN-γ has a relevant and direct role in the destruction of renal cells of lpr mice (16), and it is therefore conceivable that the IL-18 vaccination can reduce renal damage by reducing IFN-γ production.
Because renal failure is considered the principal cause of early death in lpr mice, the delayed appearance of renal damage in vaccinated mice should conceivably lead to a significant effect on their survival. Indeed, the IL-18 vaccination caused a highly significant prolongation of the survival time of lpr mice, with as many as 50% of vaccinated mice still alive when almost all untreated mice had died. The interpretation of these data is that neutralization of endogenous IL-18, induced with the cDNA vaccination in young disease-free mice, can significantly delay and decrease the development of the lupus-like disease of lpr mice. Thus, IL-18 appears to be among the pathogenic causes of the autoimmune syndrome. That the ameliorating effect is caused by neutralization of IL-18, rather than IL-18 itself, overexpressed after cDNA administration, is proven by a series of experimental evidence. Reduction of IFN-γ after cDNA administration is a strong indication of IL-18 inhibition. Furthermore, significant levels of anti-IL-18 antibodies can be detected in serum of vaccinated mice, but not in untreated lpr controls, and a complete correlation could be found between presence of anti-IL-18 immunoglobulins and decreased pathological signs. These findings suggest that reducing the activity of IL-18 in lupus nephritis and possibly other autoimmune disease is a therapeutic approach for reducing organ damage.
Acknowledgments
We thank Michael U. Martin (Hannover Medical School) for support and helpful discussion, Christa Urban (Hannover Medical School) for technical assistance, and Guido Fedele (Dompé SpA) for statistical analysis. This work was supported by a contract with Consorzio Autoimmunità Tardiva, Pomezia, Italy, within the “Programma Nazionale Farmaci–II” of the Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica. D.B. was partly supported by a grant from the Associazione Italiana per la Ricerca sul Cancro, Milan and European Union Contract QLK4-CT-2001-00147. C.A.D. and G.F. were supported by National Institutes of Health Grant AI-15614.
Abbreviations: DN, double negative; LN, lymph node; Th1, T helper 1.
References
- 1.Theofilopoulos, A. N. & Dixon, F. J. (1981) Immunol. Rev. 55, 179–216. [DOI] [PubMed] [Google Scholar]
- 2.Cohen, P. L. & Eisenberg, R. A. (1991) Annu. Rev. Immunol. 9, 243–269. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe-Fukunaba, R., Brannan, C. I., Copeland, N. G., Jenkins, N. A. & Nagata, S. (1992) Nature 356, 314–317. [DOI] [PubMed] [Google Scholar]
- 4.Bossù, P., Singer, G. G., Andres, P., Ettinger, R., Marshak-Rothstein, A. & Abbas, A. K. (1993) J. Immunol. 151, 7233–7239. [PubMed] [Google Scholar]
- 5.Singer, G. G. & Abbas, A. K. (1994) Immunity 1, 365–371. [DOI] [PubMed] [Google Scholar]
- 6.Mixter, P. F., Russell, J. Q., Morrissette, G. J., Charland, C., Haleman-Hoey, D. & Budd, R. C. (1999) J. Immunol. 162, 5747–5756. [PubMed] [Google Scholar]
- 7.Chesnutt, M. S., Finck, B. K., Killeen, N., Connolly, M. K., Goodman, H. & Wofsy, D. (1998) Clin. Immunol. Immunopathol. 87, 23–32. [DOI] [PubMed] [Google Scholar]
- 8.Santoro, T. J., Portanova, J. P. & Kotzin, B. L. (1988) J. Exp. Med. 67, 1713–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Or, R. L. & Segal, L. A. (1998) J. Theor. Biol. 190, 161–178. [DOI] [PubMed] [Google Scholar]
- 10.Peng, S. L., Moslehi, J. & Craft, J. (1997) J. Clin. Invest. 99, 1936–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang, F.-P., Feng, G.-J., Lindop, G., Stott, I. & Liew, F. Y. (1996) J. Exp. Med. 183, 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graninger, W. B., Hassfeld, W., Pesau, B. B., Machold, K. P., Zielinski, C. C. & Smolen, J. S. (1991) J. Rheumatol. 18, 1621–1622. [PubMed] [Google Scholar]
- 13.Nicoletti, F., Di Marco, R., Zaccone, P., Xiang, M., Magro, G., Grasso, S., Morrone, S., Santoni, A., Shoenfeld, Y., Garotta, G. & Meroni, P. (2000) Eur. J. Immunol. 30, 438–447. [DOI] [PubMed] [Google Scholar]
- 14.Haas, C., Ryffel, B. & Le Hir, M. (1997) J. Immunol. 158, 5484–5491. [PubMed] [Google Scholar]
- 15.Balomenos, D., Rumold, R. & Theofilopoulos, A. N. (1998) J. Clin. Invest. 101, 364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwarting, A., Wada, T., Kinoshita, K., Tesch, G. & Kelley, V. R. (1998) J. Immunol. 161, 494–503. [PubMed] [Google Scholar]
- 17.Okamura, H., Tsutsui, H., Komatsu, T., Yutsudo, M., Hakura, A., Tanimoto, T., Torigoe, K., Okura, T., Nukada, Y., Hattori, K., et al. (1995) Nature 378, 88–91. [DOI] [PubMed] [Google Scholar]
- 18.Tomura, M., Zhou, X.-Y., Maruo, S., Ahn, H.-J., Hamaoka, T., Okamura, H., Nakanishi, K., Tanimoto, T., Kurimoto, M. & Fujiwara, H. (1998) J. Immunol. 160, 4738–4746. [PubMed] [Google Scholar]
- 19.Dinarello, C. A. (1999) J. Allergy Clin. Immunol. 103, 11–24. [DOI] [PubMed] [Google Scholar]
- 20.McInnes, I. B., Gracie, J. A., Leung, B. P., Wei, X. & Liew, F. Y. (2000) Immunol. Today 21, 312–315. [DOI] [PubMed] [Google Scholar]
- 21.Rothe, H., Jenkins, N. A., Copeland, N. G. & Kolb, H. (1997) J. Clin. Invest. 99, 469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wildbaum, G., Youssef, S., Grabie, N. & Karin, N. (1998) J. Immunol. 161, 6368–6374. [PubMed] [Google Scholar]
- 23.Shi, F.-D., Takeda, K., Akira, S., Sarvetnick, N. & Ljunggren, H.-G. (2000) J. Immunol. 165, 3099–3104. [DOI] [PubMed] [Google Scholar]
- 24.Leung, B. P., McInnes, I. B., Esfandiari, E., Wei, X. & Liew, F. Y. (2000) J. Immunol. 164, 6495–6502. [DOI] [PubMed] [Google Scholar]
- 25.Wei, X., Leung, B. P., Arthur, H. M. L., McInnes, I. B. & Liew, F. Y. (2001) J. Immunol. 166, 517–521. [DOI] [PubMed] [Google Scholar]
- 26.Pizarro, T. T., Michie, M. H., Bentz, M., Woraratanadharm, J., Smith, M. F., Jr., Foley, E., Moskaluk, C. A., Bickston, S. J. & Cominelli, F. (1999) J. Immunol. 162, 6829–6835. [PubMed] [Google Scholar]
- 27.Monteleone, G., Trapasso, F., Parrello, T., Biancone, L., Stella, A., Iuliano, R., Luzza, F., Fusco, A. & Pallone, F. (1999) J. Immunol. 163, 143–147. [PubMed] [Google Scholar]
- 28.Gracie, J. A., Forsey, R. J., Chan, W. L., Gilmour, A., Leung, B. P., Greer, M. R., Kennedy, K., Carter, R., Wei, X.-Q., Xu, D., et al. (1999) J. Clin. Invest. 104, 1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka, M., Harigai, M., Kawaguchi, Y., Otha, S., Sugiura, T., Takagi, K., Ohsako-Higami, S., Fukasawa, C., Hara, M. & Kamatani, N. (2001) J. Rheumatol. 28, 1779–1787. [PubMed] [Google Scholar]
- 30.Wong, C. K., Li, E. K., Ho, C. Y. & Lam, C. W. (2000) Rheumatology 39, 1078–1081. [DOI] [PubMed] [Google Scholar]
- 31.Amerio, P., Frezzolini, A., Abeni, D., Teofoli, P., Girardelli, C. R., De Pità, O. & Puddu, P. (2002) Clin. Exp. Rheumatol. 20, 535–538. [PubMed] [Google Scholar]
- 32.Robak, E., Robak, T., Wozniacka, A., Zak-Prelik, M., Sysa-Jedrzejowska, A. & Stepien, H. (2002) Eur. Cytokine Network 13, 364–368. [PubMed] [Google Scholar]
- 33.Wong, C. H., Ho, C. Y., Li, E. K., Tam, L. S. & Lam, C. W. (2002) Clin. Exp. Immunol. 130, 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann, D., Del Giudice, E., Ciaramella, A., Boraschi, D. & Bossù, P. (2001) J. Immunol. 166, 3757–3762. [DOI] [PubMed] [Google Scholar]
- 35.Esfandiari, E., McInnes, I. B., Lindop, G., Huang, F.-P., Field, M., KomaiKoma, M., Wei, X.-Q. & Liew, F. Y. (2001) J. Immunol. 167, 5338–5347. [DOI] [PubMed] [Google Scholar]
- 36.Fantuzzi, G., Reed, D. A. & Dinarello, C. A. (1999) J. Clin. Invest. 104, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jander, S. & Stoll, G. (1998) J. Neuroimmunol. 91, 93–99. [DOI] [PubMed] [Google Scholar]
- 38.Siegmund, B., Fantuzzi, G., Rieder, F., Robertson, F., Lehr, H. A., Hartmann, G., Dinarello, C. A., Endres, S. & Eigler, A. (2001) Am. J. Physiol. 281, R1264–R1273. [DOI] [PubMed] [Google Scholar]
- 39.Pagès, F., Lazar, V., Berger, A., Danel, C., Lebel-Binay, S., Zinzindohoué, F., Desremaux, P., Cellier, C., Thiounn, N., Bellet, D., et al. (2001) Eur. Cytokine Network 12, 97–104. [PubMed] [Google Scholar]
- 40.Faust, J., Menke, J., Kriegsmann, J., Kelley, V. R., Mayet, W. J., Galle, P. R. & Schwarting, A. (2002) Arthritis Rheum. 46, 3083–3095. [DOI] [PubMed] [Google Scholar]
- 41.Spronk, P. E., Limburg, P. C. & Kallenberg, C. G. (1995) Lupus 4, 86–94. [DOI] [PubMed] [Google Scholar]
- 42.Yasuda, T., Yoshimoto, T., Tsubara, A. & Matsuzawa, A. (2001) Cell. Immunol. 210, 77–86. [DOI] [PubMed] [Google Scholar]
- 43.Cruse, J. M., Lewis, R. E. & Dilioglou, S. (2000) Exp. Mol. Pathol. 69, 211–222. [DOI] [PubMed] [Google Scholar]