Abstract
The Cu-binding β-amyloid precursor protein (APP), and the amyloid Aβ peptide have been proposed to play a role in physiological metal regulation. There is accumulating evidence of an unbalanced Cu homeostasis with a causative or diagnostic link to Alzheimer's disease. Whereas elevated Cu levels are observed in APP knockout mice, APP overexpression results in reduced Cu in transgenic mouse brain. Moreover, Cu induces a decrease in Aβ levels in APP-transfected cells in vitro. To investigate the influence of bioavailable Cu, transgenic APP23 mice received an oral treatment with Cu-supplemented sucrose-sweetened drinking water (1). Chronic APP overexpression per se reduced superoxide dismutase 1 activity in transgenic mouse brain, which could be restored to normal levels after Cu treatment (2). A significant increase of brain Cu indicated its bioavailability on Cu treatment in APP23 mice, whereas Cu levels remained unaffected in littermate controls (3). Cu treatment lowered endogenous CNS Aβ before a detectable reduction of amyloid plaques. Thus, APP23 mice reveal APP-induced alterations linked to Cu homeostasis, which can be reversed by addition of dietary Cu.
Alzheimer's disease (AD) is characterized pathologically by neuronal degeneration and the deposition of amyloid plaques and neurofibrillary tangles in the brains of affected individuals. Amyloid deposits are primarily composed of the Aβ amyloid peptide, of which Aβ1–40 is the predominant soluble species in biological fluids, and Aβ1–42 is the major species found in developing plaque deposits (1). The amyloid deposits in AD brains originate from the larger β amyloid precursor protein (APP) (2, 3). APP and its α-secretory forms are involved in the viability, growth, and morphological, functional plasticity of nerve cells, and in learning or memory processes (4).
APP has a primary N-terminal Cu-binding domain (CuBD) that binds Cu with nanomolar affinity (5), and a secondary CuBD, which is generated in Aβ after proteolytic processing of APP (6). N-terminal CuBDs of APP family paralogs and orthologs were found to have antioxidant activities, whereas the recently evolved human APP exerts unique redox properties, suggesting an overall conservation in its function or activity (7–9). The underlying reaction, a reduction of Cu(II) to Cu(I), and the structural homology of the CuBD of APP to Cu chaperones suggests APP to function as a Cu(I)-binding neuronal metallochaperone (7, 10). Potentially, APP–Cu(I) complexes may reduce hydrogen peroxide by forming an APP–Cu(II)-hydroxyl radical intermediate, or may modulate neurotoxicity and APP fragmentation (11–13) when Cu is allowed to accumulate beyond cellular needs. In addition, Cu has been reported to bind to Aβ (6) and increase its aggregation in vitro (14), potentiating its neurotoxic effects (15, 16).
Animal model systems revealed that APP is actively involved in balancing Cu concentrations in cells. In APP- and APLP2-knockout mice, Cu levels were found increased in cerebral cortex and liver, whereas overexpression of APP was reported to result in significantly reduced Cu levels in the Tg2576 line (17–19). Depending on the conservation of the CuBD, Cu was also found to influence APP processing in cells when Cu greatly reduced the levels of amyloid Aβ, and caused an increase in the secretion of the APP ectodomain (20, 21). This beneficial influence of Cu on APP metabolism is best explained by a role for APP in Cu efflux. Moreover, Cu may influence homooligomerization of holo-APP, which is of critical importance for the amyloidogenic pathway of APP (22).
The contrasting in vitro observations and the lack of in vivo data on the influence of Cu on APP processing and function prompted us to study APP23 transgenic mice (23) and littermate nontransgenic controls, which received Cu-supplemented sucrose-containing drinking water (treatment solution) or sucrose-containing drinking water without supplemented Cu (vehicle solution) ad libitum. Mice were treated for a period of 3 months, starting at the age of 12, 15, and 18 months, when all APP23 mice had already developed amyloid plaques. A second group started at the age of 2 months for a treatment period of 12 months. By studying the influence of bioavailable Cu on APP-overexpressing mice, our data revealed beneficial effects on brain Cu levels, Aβ production, and superoxide dismutase 1 (SOD-1) activity.
Methods
Animals and Treatment Protocol. APP23 [B6-Tg(Thy1APP)23SdZ] mice were obtained from the Novartis Pharma breeding facilities (Stein, Switzerland). The generation of the mouse line APP23, which is transgenic for human APP carrying the Swedish mutation has been described elsewhere (23).
Aged mice were distributed into two groups of sex-matched littermate controls with 120 mice in the Cu-treatment group and 120 in the control group. Three age-matched groups of mice were then treated for a period of 3 months, starting at 12, 15 and 18 month of age. The mice were kept under identical conventional housing conditions and all mice were housed singly (22°C, 45% relative humidity and fluorescent light for a 12-h light/12-h dark/ light cycle; Novartis Pharma). Mice were fed with Nafag (Gossau, Switzerland) rodent breeding diet no. 850. Young mice were treated for a period of 12 months, with 12 mice in the Cu-treatment group and 12 in the control group. The mice were kept under identical conventional housing conditions and fed SSniff V1185-300 (SSniff, Soest, Germany) at the animal facility of the University of Heidelberg.
All mice were given distilled deionized water (Milli-Q) ad libitum from drinking flasks containing either vehicle solution with 50 g/liter sucrose or treatment solution with 50 g/liter sucrose and 0.25 g/liter Cu-sulfate 5× H2O. Water was changed twice weekly to prevent bacterial contaminations. To detect any possible influence of the carbohydrate diet on a diet-influenced body weight increase of young mice, weight was recorded weekly during the long-term treatment period.
The concentration of sucrose added and the tolerated optimum concentrations of Cu were tested in a pilot study to exclude effects on weight and general health during the treatment (data not shown). Mice were killed by decapitation or overdose of anesthesia (aged animals) and organs were removed and stored at –70°C or in 4% formalin before analyses.
Immunohistochemistry. One hemisphere per mouse was immersionfixed by using 4% buffered formalin and embedded in paraffin for immunohistochemical analysis, and the other hemisphere was snap-frozen for biochemical analysis. Immunohistochemistry was performed on 4-μm-thick paraffin sections according to standard protocols. In brief, sections were deparaffinized in xylene and rehydrated. After treatment with 1% H2O2 in methanol to block endogenous peroxidase activity, sections were heated in a microwave oven in 10 mM citrate buffer (pH 6.0). Pretreatment with 88% formic acid enhanced Aβ immunoreactivity. Sections were treated with 10% nonfat dry milk before the addition of the primary antibodies to block nonspecific binding sites. The sections were then incubated overnight with the primary antibodies at room temperature. Staining was visualized by using the vectastain ABC method (Vector Laboratories) and diaminobenzidine as chromogen. Immunohistochemistry was performed with monoclonal antibodies, which specifically recognize Aβ40 (diluted 1:500; ABETA, Heidelberg) or Aβ42 (diluted 1:100; ABETA, Heidelberg).
Quantification of Aβ. For the quantification of human PBS- and formic acid-soluble Aβ40 and Aβ42, a microplate enzyme immunoassay (TK-Set) was used (ABETA, Heidelberg). The brain samples were homogenized (1/10, wt/vol) in 1× PBS (prepared with Milli-Q water and superpure grade reagents from Merck) by 12 strokes with a Potter Teflon pestle. The homogenate was centrifuged at 10,000 × g for 10 min at 4°C. The pellet was solubilized in 98% HCOOH by vortexing (60 s) and sonication (10 min). Centrifugation of the samples at 13,000 × g did not yield a pellet. When the pH was adjusted to 7.0 with Tris base, samples were diluted 1/15 and Aβ40, and Aβ42 levels were analyzed by a microplate enzyme immunoassay. Alternatively, samples were diluted in 65% HNO3 for microwave digestion and subsequent inductively coupled plasma MS (ICP-MS) analysis.
Plaque Count. To quantify plaque load, the left hemisphere of APP23 mice was cut sagitally in serial sections and immunostained as outlined above. Quantitative image analyses was performed by using the optimas 6.0 image analysis program (Optimas, Media-Cybernetics, Carlsbad, CA). The digitized gray level images were recorded with a charge-coupled device video camera (Sony) attached to a light microscope (Zeiss Axioskop) by using the ×10 objective. The video frame was calibrated by a 5 + 100/100 standard (Zeiss). Pixel size was measured in horizontal and vertical direction and defined in the Optimas tool, Calibrate Spatial, to calculate the areas of interest in the corresponding microscopic field. The cortical area was analyzed by taking video frames from adjacent microscopic fields with a grid size of 1,250 × 985 μm as assessed by the calibration standard. The mean gray tone of the images was measured by using the Histogram tool from Optimas. The illumination of the microscope was adapted to achieve the same mean gray value in each measurement. High-frequency gray tone variations in the image were caused by noise and eliminated by a median filter (5 × 5 pixels). To measure the amyloid plaque size, images were converted into binary images by using a threshold algorithm. The magnitude of the threshold was adapted to the gray levels defining the outer contour of the plaques. The Optimas tool, Area measurement, was applied to measure the size of the plaque deposits. Subsequently, the results of the plaque count procedure were revised in an interactive procedure and corrected for false-positive structures. Three sections per mouse at the level of the dorsal hippocampus, evenly spaced from medial to lateral, were analyzed. Finally, the results from all sections examined from an individual mouse were calculated together.
Cu,Zn-SOD (SOD-1) Activity. Frozen brain tissue was homogenized in lysis buffer (20 mM Tris·HCl, pH 7.4). The homogenates were centrifuged at 8,500 × g at 4°C for 10 min to spin down tissue fragments, nuclei, and mitochondria. The clear supernatant was used for determining SOD-1. After inactivation of Mn-SOD by ethanol-chloroform extraction, the SOD-1 activity in the upper aqueous layer was measured spectrophotometrically by using the commercial superoxide dismutase assay kit (Merck Biosciences, San Diego). Briefly, the assay is based on the SOD-1-mediated increase in the rate of autoxidation of 5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzo[c]fluorene (BXT-01050) (24). Autoxidation of BXT-01050 yields a chromophore, which absorbs maximally at 525 nm. Kinetic measurement of the 525-nm absorbance change was performed at 37°C, recording absorbance increase over time after addition of BXT-01050. Interfering mercaptans, such as glutathione, were eliminated by a rapid alkylation reaction with 1,4,6-trimethyl-2-vinylpyridinium trifluoromethane-sulfonate. Other redox activities were excluded by testing the linearity of the measured SOD-1 activity on sample dilution. The SOD-1 activity was quantified from the Vsample/Vcontrol ratio of the autoxidation rates of BXT-01050 measured in the presence (Vs), and in the absence (Vc), of brain lysate samples. One SOD-1 activity unit is defined as the activity that doubles the rate of autoxidation background (Vs/Vc = 2). Protein extracts (20 μg) were fractionated onto a 16% Tricine-SDS gel (Invitrogen) and transferred to nitrocellulose membranes [Hybond enhanced chemiluminescence (ECL), Amersham Pharmacia Biosciences, Little Chalfont, U.K.]. SOD1 was detected by using a polyclonal SOD-1 antiserum (SOD-1, C-17, Santa Cruz Biotechnology). The immunocomplex was visualized by using the ECL method (Amersham Pharmacia Biosciences). The amount of SOD-1 was normalized to β-actin. Protein-blotting analysis showed that the levels of SOD-1 polypeptide were similar to each other (data not shown).
ICP-MS. For analysis of metal concentrations, samples were prepared by HNO3 closed-vessel microwave digestion and diluted in Milli-Q water to a final concentration of 6.5% HNO3 for analysis by ICP-MS. ICP-MS was performed by using a HP4500 Series 300 ShieldTorch system instrument (Agilent, Waldbronn, Germany) in peak-hopping mode with spacing at 0.05 atomic mass units, three points per peak, three scans per replicate, and an integration time of 300 ms per point as described (8). The rate of plasma flow was 15 liters/min with an auxiliary flow of 1.0 liters/min. The replicative form power was 1.2 kW. The sample was introduced by using a crossflow nebulizer at a flow rate of 1.02 liters/min. The apparatus was calibrated by using a 6.5% HNO3 solution containing Cu at 1.95, 3.9, 19.5, 39, 97.5 and 195 parts per billion with Rh-103, the internal standard for all isotopes of Cu.
Statistical Analysis. Significance was tested by multivariate (MANOVA) and univariate (ANOVA) analyses. Correction for multiple comparisons was performed by using a two-level approach with MANOVA as first and ANOVA as second level. Student's two-tailed t tests were calculated additionally for differences between elemental groups without confounding variables.
Results
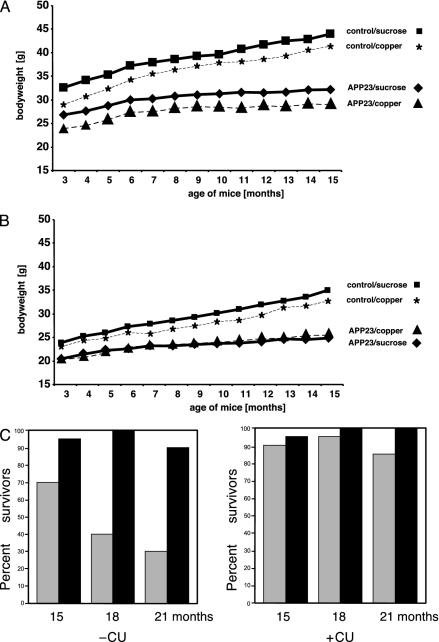
Phenotypical Analysis of Mice. To analyze the possible effects of Cu- and sucrose-supplemented drinking water on the nutritional status of the group, one-half of each group of mice was treated with Cu-supplemented sucrose-containing water, and the second half received vehicle solution (sucrose-containing water without additional Cu) ad libitum, respectively (Fig. 1). To study possible effects of long-term exposure, we monitored the body weight of 24 mice treated for 12 months (long-term treatment) and recorded the survival rate of all mice. Independent of treatment, female and male nontransgenic mice had a significantly higher body weight (MANOVA, P < 0.001) than APP 23 mice (Fig. 2 A and B). Neither a significant influence of sucrose treatment nor a significant interaction of other independent variables was observed. Thus, after 12 months of treatment, the weight of nontransgenic mice increased to a maximum of 44 g for male mice (Fig. 2A) and 35 g for female mice (Fig. 2B). In contrast, APP overexpression limited the increase of the body weight of APP23 mice to a maximum of 33 g for male mice (Fig. 2A) and 26 g for female mice (Fig. 2B). All of the mice survived the treatment period until the age of 15 months.
Fig. 1.
Scheme of APP23 transgenic mice and nontransgenic littermate controls for the short-term treatment period of aged mice. APP23, APP transgenic mice; m, month.
Fig. 2.
Body weight analyses and survival rate of treated mice. Influence of 12 months of treatment on body weight of male (A) and female (B) mice. (C) Survival rate of APP23 and nontransgenic littermate control mice at 15, 18, and 21 months of age. All mice were treated for a 3-month period (short-term treatment) starting at 12, 15, and 18 months of age, respectively. APP23 mice showed a significant reduced life duration, which was not observed in the Cu-treated group. Cu had no detrimental effect on survival of nontransgenic control mice. APP23, APP transgenic mice (gray bars); wt, nontransgenic littermate controls (black bars).
Of the 60 aged short-term-treated APP23 transgenic mice (Fig. 1) receiving vehicle solution, 34 mice died during the 3-month treatment period, whereas, of the 60 APP23 mice receiving Cu-supplemented treatment solution, only six died (Fig. 2C) (MANOVA, P < 0.001). ANOVA revealed that age had a significant influence on the death rate of APP23 mice, with an increasing rate from 15 and 21 months of age (P = 0.02). In contrast, the number of surviving mice within the groups of littermate control mice was not affected by the Cu treatment and resembled the number of APP23 mice of the Cu-supplementation group. Other confounding factors such as body weight and gender had no effect. In detail, when the numbers of surviving APP23 mice of the different age groups at the start of the experiment were compared between the treatment groups, of vehicle-treated 15-month-old mice, 14 of 20 mice survived, of 18-month-old mice, 8 mice survived, and of 21-month-old mice, 6 mice survived. In the group of Cu-treated APP23 mice, of 15-month-old mice, 18 of 20 mice survived (not significant), of 18-month-old mice, 19 mice survived (MANOVA, P < 0.001), and of 21-month-old mice, 17 mice survived (MANOVA, P < 0.001). Thus, in the present study, 5% sucrose-supplemented drinking water reduced the life expectancy of APP23 mice. This negative effect was completely absent in mice treated with 5% sucrose plus Cu.
Cu Levels in APP23 Mice. Brain samples from nontransgenic control and APP23 mice were subjected to quantitative ICP-MS measurements of Cu. After short-term treatment, nontransgenic littermate controls showed a mean Cu level of 5.58 ± 1.93 μg/g of wet weight in vehicle-treated and 5.54 ± 1.97 Cu (μg/g of wet weight) in Cu-treated mice. APP23 mice, however, elicited a reduction in net brain Cu of 4.89 ± 1.34 μg/g of wet weight in vehicle-treated mice, which was moderately increased again to normal levels after Cu treatment to a mean value of 6.10 ± 2.40 Cu (μg/g of wet weight; ANOVA, P = 0.05) at 15, 18, and 21 months of age (Fig. 3). A similar magnitude of elevated brain Cu levels was achieved in 6-month-old APP transgenic mice crossed to TxJ mice resulting in increased Cu levels in brain as reported by Phinney et al. (25). No difference in brain Zn was seen in either group investigated (data not shown). The increase of Cu levels in the brain of mice with age is consistent with findings by Maynard et al. (19).
Fig. 3.
Mean Cu levels and 95% confidence interval for mean (expressed as μg of Cu per g of wet weight) in brain homogenates of APP23 mice. Shown are Cu levels of aged APP23 mice at 15, 18, and 21 months of age, which were vehicle (–Cu)- or Cu (+ Cu)-treated for 3 months. A statistically significant difference is shown between vehicle- and Cu-treated APP23 mice (ANOVA).
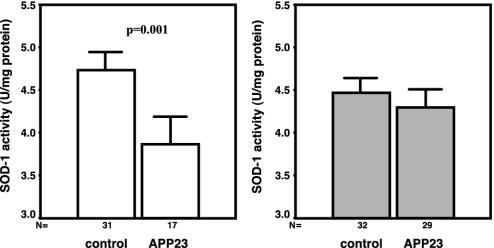
To evaluate possible changes in the activity of an abundantly occurring Cu-dependent enzyme, we quantified Cu,Zn-SOD (SOD-1) activity in brains of the short-term treatment cohort by using a quantitative SOD-1 assay (26). Whereas Western blot analyses showed that the levels of the SOD-1 polypeptide were similar between samples from all treatment groups, nontransgenic littermate controls showed a mean SOD-1 activity of 4.41 ± 0.64 units/mg protein with Cu treatment and 4.66 ± 0.76 units/mg protein after vehicle treatment. The APP23 mice, however, elicited a mean value of 3.83 ± 0.69 after vehicle treatment, which was increased again to normal levels after Cu treatment to a mean value of 4.25 ± 0.69 units/mg protein (Fig. 4). Thus, Cu supplementation seemed to provide sufficient bioavailable Cu to respond to the need of SOD-1 to reach normal levels of activity in aged APP23 brain.
Fig. 4.
Mean SOD-1 activity and 95% confidence interval for mean SOD-1 activity in brain homogenates of APP23 and nontransgenic littermate controls at 15, 18, and 21 months of age, which were vehicle (–Cu)- or Cu (+ Cu)-treated for 3 months. SOD-1 activity is expressed as mean activity (units/mg protein). A statistically significant difference was found between vehicle APP23 and wild-type mice with P = 0.001 as well as vehicle- and Cu-treated APP23 with P = 0.05 mice (ANOVA).
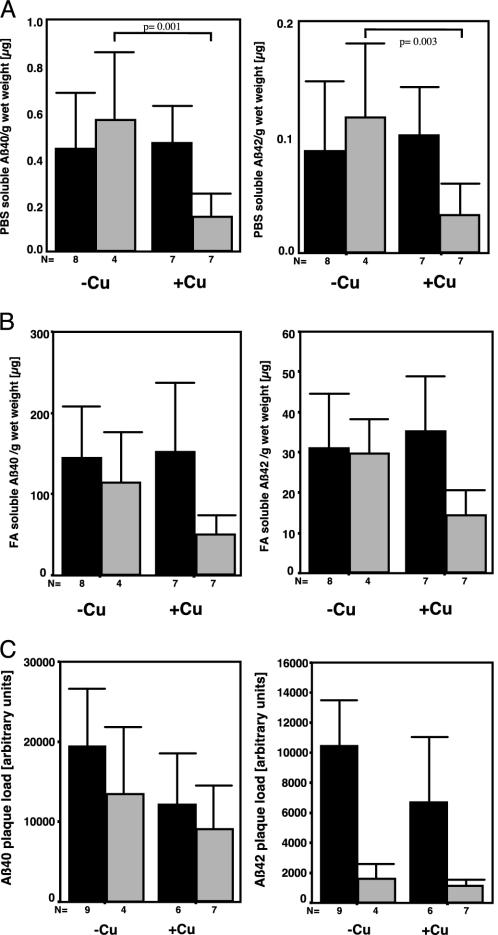
Amyloid Aβ and Plaque Burden. The amount of Aβ40 and Aβ42 are diagnostic markers for a possible effect of a given treatment as a therapy for AD. Therefore, the impact of Cu supplementation was analyzed in brain homogenates of 15-month-old APP23 mice by using an Aβ-specific ELISA. MANOVA over all groups (PBS- and formic acid-soluble Aβ40 and Aβ42, and plaque load) revealed gender (P = 0.004) and Cu treatment together with gender (P = 0.014) to have a significant effect. In detail, ANOVA showed Cu treatment to have a significant effect by lowering PBS-soluble Aβ40 (26%, P = 0.037) and PBS-soluble Aβ42 (27%, P = 0.033). This effect was due to Cu-induced lower Aβ40 (t test, P = 0.001) and Aβ42 (t test, P = 0.003) levels in male, but not in female, mice (Fig. 5A). When the influence of Cu treatment on formic acid-dissolved Aβ or plaque load was analyzed, Aβ42 was reduced, however, showing only a trend toward significance (ANOVA, P = 0.067; Fig. 5B). Plaque load was not different regarding the main effect and interaction of Cu treatment (Fig. 5C). In general, male mice responded to Cu treatment more strongly than did females, the latter showing an accelerated amyloid plaque formation. Thus, our results suggest an effect of Cu-supplemented drinking water on Aβ levels in statu nascendi in the CNS rather than on senile plaque formation.
Fig. 5.
Mean values and 95% confidence interval for mean Aβ levels of Cu (+Cu)- or vehicle (–Cu)-treatment effects on PBS- and formic acid-soluble and plaque deposited Aβ40 and Aβ42 in APP23 mice at 15 months of age. (A) ELISA of PBS-soluble Aβ40 and Aβ42. (B) ELISA of formic acid-soluble Aβ40 and Aβ42. (C) The plaque load determined by densitometric measurement of aggregated Aβ40 and Aβ42 in senile plaques did not reveal differences between the treatment groups. The statistical difference between treatments for male mice is indicated (t test). For details see text. Open bars, female; filled bars, male.
Discussion
This study demonstrates for the first time, to our knowledge, that bioavailable Cu is beneficial to transgenic mice overexpressing human full-length APP with the Swedish mutation (APP23 mice). Whereas aged APP23 transgenic mice showed a dramatically reduced life expectancy within the observation period, Cu-treated APP23 mice did not show this premature death phenotype. In addition, at the age of 15 months, there was no significant effect on life duration, neither in the short-term nor in the long-term treatment group. Although we are unable to estimate the proportion between the caloric intake of Cu-free- or Cu-supplemented carbohydrate-containing drinking water and chow pellets, a potential difference in weight increase of such mice could be taken as an indicator of a normal nutritional status. The absence of such a difference suggests an apparent rescue of the reduced life duration by Cu-supplemented drinking water. Nevertheless, we cannot exclude that 18- and 21-month-old APP23 mice were more vulnerable to a high sucrose intake and therefore had a decrease in life duration. This occurrence could be due to a Cu deficiency as observed in the APP transgenic mice strain Tg2576 (19), possibly in conjunction with an age-associated breakdown of metal regulation (19) and the Cu-deficient sucrose-supplemented drinking water. For instance, in rats that were exclusively fed a Cu-deficient sucrose diet (27), an increased mortality had been observed.
Moreover, Cu treatment had a modulating effect on brain Cu levels and a normalizing effect on SOD-1 activity in these mice, compared with nontransgenic littermate control mice. Several studies link APP and SOD-1 function in vivo. Overexpression of APP in brain is associated with postnatal lethality (28, 29). Coexpression of high levels of human cytosolic superoxide-scavenging activity protected against the lethal effects of APP and rescued the reduced life duration (28). Moreover, SOD-1 rescues cerebral endothelial vascular dysfunction in mice overexpressing APP (30). Although there was a high correlation between age, plaque load, and life duration, our data show that plaque load is not responsible for premature death. Rather, we assume that chronic overexpression of human APP leads to metabolic dysfunctions in the mouse brain, which are related to insufficient levels of bioavailable Cu, as is evident from suppressed SOD-1 activity. This Cu deficiency in APP23 mice was also observed in other APP transgenic mouse lines (19). Because the presence of the Cu ion at the active site of human SOD-1 is essential for its enzymatic activity, we conclude that the observed rescue of the activity was due to the increasing Cu levels on dietary Cu supplementation.
Cu treatment led to significantly decreased values of PBS-soluble Aβ40 and Aβ42. The effect after a 3-month treatment period resulted in an abundant reduction of Aβ40 and Aβ42 only in male mice, possibly due to an overlapping gender effect. We hypothesize that Cu treatment influences Aβ generation during APP proteolysis, and, hence, no effect was seen on extracellular Aβ (i.e., amyloid plaque load). Female APP23 mice elicited earlier plaque formation and much higher Aβ40 and Aβ42 levels than male mice. Although in male APP23 mice, Cu treatment attenuated levels of soluble Aβ, the plaque load remained unaffected in these mice. That finding indicates an inhibitory effect of Cu on Aβ formation in statu nascendi, rather than on plaque amyloid formation. We assume that once Aβ aggregation has started in vivo, this process is kinetically favored and renders any soluble Aβ into amyloid. Thus, bioavailable Cu may not impact amyloid formation extracellularly, but through an intracellular effect by binding to the N-terminal CuBD of APP. This finding is in agreement to our earlier findings, when Cu was found to inhibit Aβ production in a cell culture system (20). A similar tendency for reduction of human Aβ peptides was noted in a genetic approach by using TxJ mice (25). In these mice, a mutant ATPase7b Cu transporter, in combination with an APP transgenic mouse line, exhibited elevated Cu levels and beneficial effects on survival and amyloid burden with a 45% reduction in Aβ plaques. Thus, manipulations of the TxJ genotype or of dietary Cu in APP23 mice are beneficial with regard to life expectancy and plaque load.
Which mechanism is responsible for Aβ reduction in APP23 mice? The drinking water supplementation therapy seems to provide sufficient bioavailable Cu to APP23 mice. The recent observation (19, 29) that overexpression of APP in Tg2576 transgenic mice also resulted in significantly reduced brain Cu levels before the appearance of amyloid pathology, suggests that the APP ectodomain is involved in reducing brain Cu levels. This conclusion is further supported by the observation that APLP2 knockout mice, like APP knockout mice, have increased brain Cu levels (31). APLP2 has a Cu-binding site that shares structural and functional homology with the APP Cu-binding ectodomain (7, 8, 10). Thus, deleterious effects of APP or APLP2 overexpression are likely due to an interference with Cu homeostasis and intracellular Cu trafficking (32).
More evidence that APP is directly or indirectly involved in intracellular Cu homeostasis is provided by the recently revealed structural homology of its CuBD to intracellular Cu chaperones (10) and the link to the Cu chaperone for SOD-1 (CCS). The neuronal adaptor protein, X11α, interacts with the cytoplasmic domain of APP and CCS (33, 34). Overexpression of X11α inhibits SOD-1 activity through binding to CCS, which delivers and inserts Cu into SOD-1 (33, 35, 36).
Another study (37) has shown that treatment of 21-month-old Tg2576 mice with the antibiotic, clioquinol, inhibited plaque formation and concomitantly increased soluble brain Cu and Zn levels (37). This increase of metal ions in brain observed on clioquinol treatment might either be attributed to an inefficiency of the chelator with weak affinities for Zn (K1 = 7.0) and for Cu (K1 = 8.9) (38) or even more likely, due to a facilitated uptake in brain of clioquinol–Cu complexes, similarly to Cu-nitrilotriacetic acid (Cu-NTA)-treated mice (39).
At this stage, it is unknown as to whether APP secretion enhances the efflux of Cu out of the brain. However, this article, published observations (19, 20), and data from Phinney et al. (25), at least provide strong evidence that APP overexpression enables intracellular Cu to be transported out of the cell (Fig. 6). The increased Cu efflux seems to lead to a Cu deficiency and a subsequently reduced SOD-1 activity. The enzyme occurs in high intracellular concentrations (10 μM in yeast) and has a role in Zn homeostasis and Fe metabolism (40–42). Two studies (43, 44) have shown that a disturbed metal-ion homeostasis with elevated serum Cu levels occurs in AD and Down's patients, and lowered levels in postmortem AD brain (25). A relevant contribution of decreased Cu levels in the brain of AD patients for the formation of Aβ plaques is presently unclear. We conclude that bioavailable Cu has beneficial and specific effects in an AD mouse model and suggest that our observation should be regarded as a proof-of-concept for a prophylactic approach to address a CNS Cu deficiency in AD.
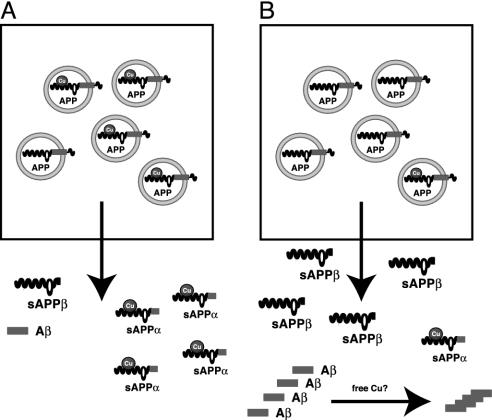
Fig. 6.
Model for the metallobiology of APP. APP is involved in Cu homeostasis in brain with implications for AD. (A) Normal interaction of APP with Cu. Cu binds to the N-terminal domain of APP that is in the lumen of intracellular compartments. After fusion with the plasma membrane, Cu is released into the extracellular space as α-secretory APP–Cu complexes. In this case, APP-Cu is cleaved by the α-secretase close to the plasma membrane and then released. APP molecules without Cu are cleaved by β- and γ-secretase activities within intracellular compartments, which is probably due to a different conformation as compared with APP charged with Cu. Low levels of Aβ peptides are secreted from the cell. (B) Depletion of Cu, as observed in brains of AD patients and APP transgenic mice, enhances amyloidogenic processing of APP. Our data show reduced SOD-1 activity and Cu levels in brain tissue of APP transgenic mice that could both be rescued by Cu treatment. This is a coherent finding, because intracellular Cu availability recently turned out to be a critical determinant of SOD-1 activity (45). An enhanced release of Aβ peptides might act as a trap for Cu, although we did not find evidence for this notion in our in vivo studies.
Acknowledgments
We thank Esther Barth and Oliver Wirths for expert technical assistance and Hermann Dieter for continuous advice. This work was supported by grants from the Deutsche Forschungsgemeinschaft (G.M. and T.A.B.) and the International Copper Association.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APP, amyloid precursor protein; AD, Alzheimer's disease; CuBD, Cu-binding domain; SOD, superoxide dismutase; ICP-MS, inductively coupled plasma MS.
References
- 1.Selkoe, D. J. (2001) Physiol. Rev. 81, 741–766. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi, R. E., Gusella, J. F., Watkins, P. C., Bruns, G. A., St. George-Hyslop, P., Van Keuren, M. L., Patterson, D., Pagan, S., Kurnit, D. M. & Neve, R. L. (1987) Science 235, 880–884. [DOI] [PubMed] [Google Scholar]
- 3.Kang, J., Lemaire, H. G., Unterbeck, A., Salbaum, J. M., Masters, C. L., Grzeschik, K. H., Multhaup, G., Beyreuther, K. & Muller-Hill, B. (1987) Nature 325, 733–736. [DOI] [PubMed] [Google Scholar]
- 4.Dodart, J. C., Mathis, C. & Ungerer, A. (2000) Rev. Neurosci. 11, 75–93. [DOI] [PubMed] [Google Scholar]
- 5.Hesse, L., Beher, D., Masters, C. L. & Multhaup, G. (1994) FEBS Lett. 349, 109–116. [DOI] [PubMed] [Google Scholar]
- 6.Atwood, C. S., Scarpa, R. C., Huang, X., Moir, R. D., Jones, W. D., Fairlie, D. P., Tanzi, R. E. & Bush, A. I. (2000) J. Neurochem. 75, 1219–1233. [DOI] [PubMed] [Google Scholar]
- 7.Multhaup, G., Schlicksupp, A., Hesse, L., Beher, D., Ruppert, T., Masters, C. L. & Beyreuther, K. (1996) Science 271, 1406–1409. [DOI] [PubMed] [Google Scholar]
- 8.Simons, A., Ruppert, T., Schmidt, C., Schlicksupp, A., Pipkorn, R., Reed, J., Masters, C. L., White, A. R., Cappai, R., Beyreuther, K., et al. (2002) Biochemistry 41, 9310–9320. [DOI] [PubMed] [Google Scholar]
- 9.White, A. R., Multhaup, G., Galatis, D., McKinstry, W. J., Parker, M. W., Pipkorn, R., Beyreuther, K., Masters, C. L. & Cappai, R. (2002) J. Neurosci. 22, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnham, K. J., McKinstry, W. J., Multhaup, G., Galatis, D., Morton, C. J., Curtain, C. C., Williamson, N. W., White, A. R., Hinds, M. G., Norton, R. S., et al. (2003) J. Biol. Chem., in press. [DOI] [PubMed]
- 11.White, A. R., Bush, A. I., Beyreuther, K., Masters, C. L. & Cappai, R. (1999) J. Neurochem. 72, 2092–2098. [DOI] [PubMed] [Google Scholar]
- 12.Multhaup, G., Ruppert, T., Schlicksupp, A., Hesse, L., Bill, E., Pipkorn, R., Masters, C. L. & Beyreuther, K. (1998) Biochemistry 37, 7224–7230. [DOI] [PubMed] [Google Scholar]
- 13.Multhaup, G. & Masters, C. L. (1999) Met. Ions Biol. Syst. 36, 365–387. [PubMed] [Google Scholar]
- 14.Atwood, C. S., Moir, R. D., Huang, X., Scarpa, R. C., Bacarra, N. M., Romano, D. M., Hartshorn, M. A., Tanzi, R. E. & Bush, A. I. (1998) J. Biol. Chem. 273, 12817–12826. [DOI] [PubMed] [Google Scholar]
- 15.Huang, X., Cuajungco, M. P., Atwood, C. S., Hartshorn, M. A., Tyndall, J. D., Hanson, G. R., Stokes, K. C., Leopold, M., Multhaup, G., Goldstein, L. E., et al. (1999) J. Biol. Chem. 274, 37111–37116. [DOI] [PubMed] [Google Scholar]
- 16.Huang, X., Atwood, C. S., Hartshorn, M. A., Multhaup, G., Goldstein, L. E., Scarpa, R. C., Cuajungco, M. P., Gray, D. N., Lim, J., Moir, R. D., et al. (1999) Biochemistry 38, 7609–7616. [DOI] [PubMed] [Google Scholar]
- 17.White, A. R., Zheng, H., Galatis, D., Maher, F., Hesse, L., Multhaup, G., Beyreuther, K., Masters, C. L. & Cappai, R. (1998) J. Neurosci. 18, 6207–6217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsiao, K., Chapman, P., Nilsen, S., Eckman, C., Harigaya, Y., Younkin, S., Yang, F. & Cole, G. (1996) Science 274, 99–102. [DOI] [PubMed] [Google Scholar]
- 19.Maynard, C. J., Cappai, R., Volitakis, I., Cherny, R. A., White, A. R., Beyreuther, K., Masters, C. L., Bush, A. I. & Li, Q. X. (2002) J. Biol. Chem. 277, 44670–44676. [DOI] [PubMed] [Google Scholar]
- 20.Borchardt, T., Camakaris, J., Cappai, R., Masters, C. L., Beyreuther, K. & Multhaup, G. (1999) Biochem. J. 344, 461–467. [PMC free article] [PubMed] [Google Scholar]
- 21.Borchardt, T., Schmidt, C., Camarkis, J., Cappai, R., Masters, C. L., Beyreuther, K. & Multhaup, G. (2000) Cell. Mol. Biol. (Noisy-le-grand) 46, 785–795. [PubMed] [Google Scholar]
- 22.Scheuermann, S., Hambsch, B., Hesse, L., Stumm, J., Schmidt, C., Beher, D., Bayer, T. A., Beyreuther, K. & Multhaup, G. (2001) J. Biol. Chem. 276, 33923–33929. [DOI] [PubMed] [Google Scholar]
- 23.Sturchler-Pierrat, C., Abramowski, D., Duke, M., Wiederhold, K. H., Mistl, C., Rothacher, S., Ledermann, B., Burki, K., Frey, P., Paganetti, P. A., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 13287–13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nebot, C., Moutet, M., Huet, P., Xu, J. Z., Yadan, J. C. & Chaudiere, J. (1993) Anal. Biochem. 214, 442–451. [DOI] [PubMed] [Google Scholar]
- 25.Phinney, A. L., Drisaldi, B., Lugowski, S., Schmidt, S. D., Coronado, V., Liang, Y., Horne, P., Yang, J., Sekoulidis, J., Coomaraswamy, J., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 14193–14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong, P. C., Waggoner, D., Subramaniam, J. R., Tessarollo, L., Bartnikas, T. B., Culotta, V. C., Price, D. L., Rothstein, J. & Gitlin, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 2886–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fields, M., Ferretti, R. J., Smith, J. C., Jr., & Reiser, S. (1983) J. Nutr. 113, 1335–1345. [DOI] [PubMed] [Google Scholar]
- 28.Carlson, G. A., Borchelt, D. R., Dake, A., Turner, S., Danielson, V., Coffin, J. D., Eckman, C., Meiners, J., Nilsen, S. P., Younkin, S. G. & Hsiao, K. K. (1997) Hum. Mol. Genet. 6, 1951–1959. [DOI] [PubMed] [Google Scholar]
- 29.Hsiao, K. K., Borchelt, D. R., Olson, K., Johannsdottir, R., Kitt, C., Yunis, W., Xu, S., Eckman, C., Younkin, S., Price, D., et al. (1995) Neuron 15, 1203–1218. [DOI] [PubMed] [Google Scholar]
- 30.Iadecola, C., Zhang, F., Niwa, K., Eckman, C., Turner, S. K., Fischer, E., Younkin, S., Borchelt, D. R., Hsiao, K. K. & Carlson, G. A. (1999) Nat. Neurosci. 2, 157–161. [DOI] [PubMed] [Google Scholar]
- 31.White, A. R., Reyes, R., Mercer, J. F., Camakaris, J., Zheng, H., Bush, A. I., Multhaup, G., Beyreuther, K., Masters, C. L. & Cappai, R. (1999) Brain Res. 842, 439–444. [DOI] [PubMed] [Google Scholar]
- 32.Huffman, D. L. & O'Halloran, T. V. (2001) Annu. Rev. Biochem. 70, 677–701. [DOI] [PubMed] [Google Scholar]
- 33.Rae, T. D., Torres, A. S., Pufahl, R. A. & O'Halloran, T. V. (2001) J. Biol. Chem. 276, 5166–5176. [DOI] [PubMed] [Google Scholar]
- 34.McLoughlin, D. M., Standen, C. L., Lau, K. F., Ackerley, S., Bartnikas, T. P., Gitlin, J. D. & Miller, C. C. (2001) J. Biol. Chem. 276, 9303–9307. [DOI] [PubMed] [Google Scholar]
- 35.Culotta, V. C., Klomp, L. W., Strain, J., Casareno, R. L., Krems, B. & Gitlin, J. D. (1997) J. Biol. Chem. 272, 23469–23472. [DOI] [PubMed] [Google Scholar]
- 36.Casareno, R. L., Waggoner, D. & Gitlin, J. D. (1998) J. Biol. Chem. 273, 23625–23628. [DOI] [PubMed] [Google Scholar]
- 37.Cherny, R. A., Atwood, C. S., Xilinas, M. E., Gray, D. N., Jones, W. D., McLean, C. A., Barnham, K. J., Volitakis, I., Fraser, F. W., Kim, Y., et al. (2001) Neuron 30, 665–676. [DOI] [PubMed] [Google Scholar]
- 38.Bush, A. I. (2002) Neurobiol. Aging 23, 1031–1038. [DOI] [PubMed] [Google Scholar]
- 39.Sugawara, N., Sugawara, C. & Katakura, M. (1991) Res. Commun. Chem. Pathol. Pharmacol. 72, 97–103. [PubMed] [Google Scholar]
- 40.Rae, T. D., Schmidt, P. J., Pufahl, R. A., Culotta, V. C. & O'Halloran, T. V. (1999) Science 284, 805–808. [DOI] [PubMed] [Google Scholar]
- 41.Wei, J. P., Srinivasan, C., Han, H., Valentine, J. S. & Gralla, E. B. (2001) J. Biol. Chem. 276, 44798–44803. [DOI] [PubMed] [Google Scholar]
- 42.De Freitas, J. M., Liba, A., Meneghini, R., Valentine, J. S. & Gralla, E. B. (2000) J. Biol. Chem. 275, 11645–11649. [DOI] [PubMed] [Google Scholar]
- 43.Torsdottir, G., Kristinsson, J., Hreidarsson, S., Snaedal, J. & Johannesson, T. (2001) Pharmacol. Toxicol. (Copenhagen) 89, 320–325. [DOI] [PubMed] [Google Scholar]
- 44.Squitti, R., Lupoi, D., Pasqualetti, P., Dal Forno, G., Vernieri, F., Chiovenda, P., Rossi, L., Cortesi, M., Cassetta, E. & Rossini, P. M. (2002) Neurology 59, 1153–1161. [DOI] [PubMed] [Google Scholar]
- 45.Bartnikas, T. B. & Gitlin, J. D. (2003) J. Biol. Chem. 278, 33602–33608. [DOI] [PubMed] [Google Scholar]