Mouse models of inflammatory arthritis are strongly driven by excessive production of interleukin (IL)-1β. Much attention has focused on the generation of IL-1β by inflammasomes and caspase 1 in monocytes and macrophages. However, a number of other proteases can also process pro-IL-1β into the mature, bioactive cytokine. Despite that arthritis has complex etiologies based on genetic and environmental factors, global blockage of IL-1β signaling strongly ameliorates experimental models of arthritis. Yet, the relative contribution of the different IL-1β processing enzymes to disease initiation and perpetuation is elusive. Two papers in this issue of Arthritis & Rheumatism by Joosten et al. and Guma et al. shed more light on the complex proteolytic mechanisms behind excessive IL-1β maturation and release that perpetuates experimental models of inflammatory arthritis, which will have significant implications for designing future treatment strategies.
Rheumatoid arthritis (RA) is a complex chronic inflammatory disease, which presents as a symmetric polyarthritis of usually the small joints of hands and feet in approximately 1% of the population. RA is characterized by synovial inflammation, joint swelling, stiffness and pain, which leads to pannus formation and joint destruction, and approximately 25% of RA patients will require joint replacement. However, RA can also display systemic manifestations, including pulmonary and cardiovascular implications as well as a higher risk for cancer. The synovium is composed primarily of synovial fibroblasts (synoviocytes) and macrophages, the latter one are the main source for many inflammatory cytokines that are active in the joints of RA patients and are responsible for disease perpetuation, including TNFα, IL-1β, IL-18 and others, and are therefore significant for therapeutic intervention. The current standard treatment is administration of disease-modifying antirheumatic drugs (DMARDS), such as methotrexate and sulfasalazine, which causes remission (as defined by manageable disease) in ~20–30% of all patients, but also display significant toxicity. Those patients unresponsive to methotrexate, are usually treated by combination therapy of DMARDS with one of the TNFα blockers (etanercept, adalimumab, and infliximab), or the IL-6 blocker (tocilizumab), which also causes remission in ~20–30% of patients. However, 40–50% of all patients are still refractory to any of the current standard therapies. Therefore, efficacy of blocking other active cytokines in RA, including IL-15, LTβ, Light, BLyS, APRIL, and RANKL is currently under investigation in Phase II clinical trials (1). Anakinra, which is a non-glycosylated recombinant IL-1 receptor antagonist (Ra), and therefore interferes with binding of IL-1β to its receptor, is the only other approved anti-cytokine biological therapy. Although anakinra is inferior to TNFα blockers, it is moderately effective in RA, but proofed highly effective and safe for the treatment of adult-onset Still’s disease (AOSD) (2), systemic-onset juvenile idiopathic arthritis (SoJIA) (3), Schnitzler’s syndrome (4), gouty arthritis (5, 6), pseudogout (7), and hereditary periodic fever syndromes (8).
The significant role of IL-1β in the development of RA is long known, and directly demonstrated by the strong arthritogenic response in experimental mouse models resulting from either intra-articular injection of recombinant IL-1β or articular expression of IL-1β by local gene transfer (9, 10). Plasma levels of IL-1β correlate well with RA disease severity and joint destruction, and in all experimental models, bone and cartilage erosion is highly dependent on IL-1β (11, 12). This is consistent with IL-1β-dependent potent activation of synoviocytes, chondrocytes, osteoblasts and osteoclasts. TNFα induces IL-1β and vice versa, but TNFα-independent production of IL-1β is observed in experimental models of inflammatory arthritis induced by Streptococcal cell wall fragments (SCW). In addition there is increasing evidence that TNFα mediates its arthritogenic effects via production of IL-1β, and therefore TNFα deficient mice still develop CIA-induced arthritis, while anti-IL-1RI antibodies completely block joint inflammation in TNFα transgenic mice (13, 14). Arthritis induced by type II collagen (CIA) and by HTLV-1 is ameliorated in IL-1β deficient mice, and CIA-induced arthritis is also prevented by prophylactic treatment as well as treatment of established disease with the pharmacological caspase 1 inhibitor VE-13,045 (15, 16). Mice deficient in IL-1Ra spontaneously develop inflammatory arthritis that resembles RA and show a more severe CIA, while IL-1Ra transgenic mice show ameliorated CIA (17–19).
IL-1β is a pleiotropic cytokine with immune and inflammation-modulatory activities. To explain how IL-1β promotes inflammatory arthritis, one has to understand its principal mechanism of activation. It belongs to the IL-1 family, with includes also IL-1α, IL-1Ra, IL-18 and IL-33 besides others. Generation of biological active IL-1β is highly regulated at several levels. Inflammatory and infectious mediators, including IL-1β itself, induce NF-κB-dependent transcription of the IL1B gene. IL-1β is further regulated by RNA stability and translational control, and requires posttranslational processing to be released. Once in circulation, its effects are further controlled by multiple IL-1 receptors (IL-1R). IL-1β only binds with low affinity to IL-1RI, but recruitment of the IL-1R accessory protein (AcP) results in formation of a trimeric high-affinity complex. IL-1RII, which lacks the intracellular Toll/IL1R (TIR) signaling domain, acts as a decoy receptor and IL-1Ra, which is constitutively released from cells, competes with IL-1β for receptor binding. In addition, there are soluble forms of IL-1RI, IL-1RII, and AcP, which further allow fine-tuning of the IL-1 response. Once the IL-1RI is engaged, signal transduction is initiated by clustering of the intracellular TIR domain, recruitment of MyD88 and IRAKs, and activation of downstream transcription factors. Its potent pro-inflammatory effects are mediated by induction of additional inflammatory mediators, including TNFα, IL-1α, IL-1β, IL-6, IL-8, COX-2 and PGE2, which account for the pain, swelling and tenderness typically for RA joints. IL-1β mediates pannus formation leading to cartilage and bone destruction and impairs its repair, thus directly affecting the loss of patient functionality and causing disability of RA patients. For example, IL-1β activation of synoviocytes induces secretion of matrix degrading enzymes, such as matrix metalloproteases (MMPs), which drive cartilage breakdown. In chondrocytes, IL-1β potently inhibits proteoglycan synthesis and induces collagen degradation, thus impairing maintenance of cartilage, while long-term exposure to IL-1β causes apoptosis of chondrocytes via excessive production of nitric oxide. IL-1β is also responsible for bone resorption by increasing expression of RANKL in osteoblasts, which promotes osteoclast differentiation and enhancing their bone resorptive activity, while at the same time causing osteoblast apoptosis to prevent bone formation (20).
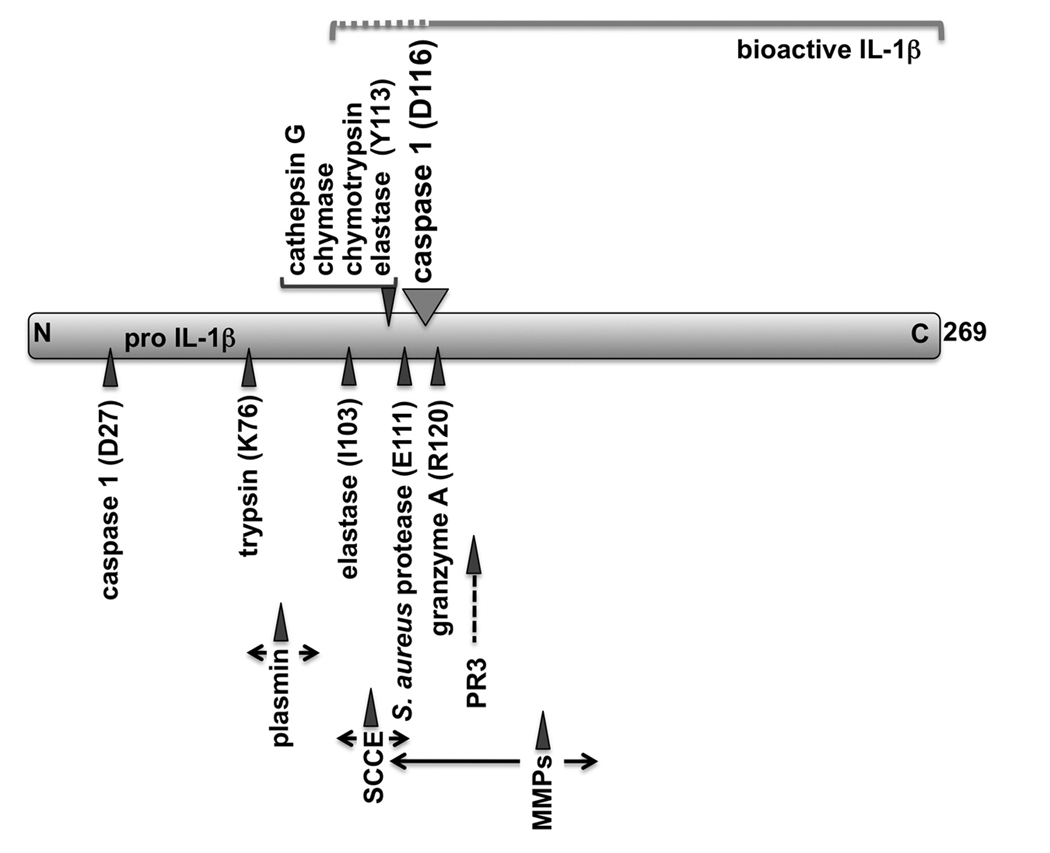
The critical step in the release of IL-1β from macrophages is the requirement for proteolytic processing to convert the 31 kDa precursor into the 17 kDa mature bioactive IL-1β, which is then released. The best characterized protease that processes IL-1β is caspase 1. Caspase 1 has been initially identified as the IL-1β converting enzyme (ICE), which is activated in inflammasomes (see below) and generates a 28 kDa inactive intermediate and the 17 kDa bioactive IL-1β (21, 22). Therefore, one would expect that preventing caspase 1 activity, should impact disease severity to a similar level as observed in IL-1β deficient mice. However, in contrast to deficiency in IL-1β or IL-1RI, which completely ameliorates inflammatory arthritis in many experimental models, inhibition of caspase 1 itself, surprisingly only partially inhibit inflammatory arthritis at approximately 50%. Caspase 1 is essential for the maturation of IL-1β by inflammasomes in macrophages, but perhaps the most interesting discovery has been that caspase 1 is not the sole IL-1β converting enzyme, but a number of serine proteases can proteolytically cleave the IL-1β precursor at distinct sites, some of which are giving raise to a mature peptide that closely matches caspase 1 processed IL-1β (Figure 1). Several of these enzymes are stored in azurophil granules in neutrophils (neutrophil elastase, proteinse-3, cathepsin G), mast cell granules (chymase, cathepsin G), CTL granules (granzyme A), and matrix metalloproteinases (stromelysin-1, gelatinase A, gelatinase B) produced by a number of cells, as well as chymotrypsin, trypsin, plasmin, keratinocyte stratum corneum chymotryptic enzyme (SCCE), and Staphylococcus aureus protease. Whether all of these proteases are relevant in vivo is not known, but several are found in inflammatory lesions. Neutrophil elastase processing of pro-IL-1β occurs after I103, which only results in a 10-fold increase in activity (23). In addition, neutrophil elastase can also cleave after Y113, which is a hot spot for cleavage by several inflammatory fluid proteases to yield 17 and 18 kDa mature peptides with significant activity (24). Proteinase-3 has been identified as one of the main neutrophil IL-1β converting enzymes in a monocyte-neutrophil co-culture system using selective inhibitors and purified proteins (25). Proteinase-3 produces bioactive mature IL-1β as intracellular protease and not as secreted extracellular protease, indicating that this mechanism parallels the caspase 1-mediated IL-1β processing, where processing precedes release of IL-1β. A cell permeable peptide blocked activity, whereas α1-anti-trypsin, which specifically inhibits extracellular serine proteases, did not affect IL-1β maturation by neutrophils (26). Cathepsin G also cleaves pro-IL-1β after Y113, resulting in a mature peptide (17 kDa) identical to that produced by chymase, chymorypsin, and neutrophil elastase (24). Mast cells store chymase in their secretory granules, which also cleaves pro-IL-1β downstream of Y113, generating a mature IL-1β (17 kDa), which displays a similar activity as caspase 1-derived IL-1β (27). CTL-produced granzyme A converts pro-IL-1β into the mature, bioactive cytokine (17 kDa) by cleaving downstream of R120, which is just four amino acids downstream of the major caspase 1 cleavage site at D116 (28). Granzyme A-produced IL-1β is biologically active, though to a lesser extend (~30% activity) compared to caspase 1-derived IL-1β. Localized sites of inflammation, such as inflamed joints, also contain matrix metalloproteinases (MMPs), which are involved in tissue destruction, but stromelysin-1 (MMP-3), gelatinase A (MMP-2) and gelatinase B (MMP-9) can also function as an IL-1β converting enzyme (29, 30). Although, stromelysin-1 and gelatinase A are less potent, require higher enzyme concentrations and prolonged incubation times compared to gelatinase B, all process pro-IL-1β into a number of distinct peptides in the 14–17 kDa range, with larger intermediate products. Nevertheless, such MMP concentrations, even the high gelatinase A levels, can be found in serum or at sites of inflammation. Furthermore, prolonged stromelysin-1 incubation resulted in complete degradation of the mature peptide, suggesting that MMPs possess positive and negative regulation of IL-1β (29). This is reminiscent to observations with stratum corneum chymotryptic enzyme (SCCE), which can produce bioactive IL-1β slightly larger than caspase 1-derived IL-1β, whereas prolonged incubations resulted in degradation and loss of IL-1β activity (31). Staphylococcus aureus protease processing of pro-IL-1β causes a similar increase in activity (~300-fold), following cleavage after E111 (18 kDa) (23). Significantly, S. aureus infections can develop septic arthritis and is also delivered in experimental arthritis mouse models. Chymotrypsin also converts IL-1β by cleavage after Y113 (17 kDa) (23). Chymotrypsin-mediated maturation results in a 500-fold increase in activity, compared to pro-IL-1β. On the other hand, trypsin processing occurs further upstream after R75 or K76, which results in a poorly active cytokine of 25 kDa, with only a 7-fold increase in bioactivity, and treatment with plasmin can also generate a mature peptide of 23 kDa with only slightly increased activity (23, 30).
Figure 1.
From these data it seems obvious that processing of pro-IL-1β has to occur close to the caspase 1 processing site in order to produce bioactive IL-1β, while conversion into larger peptides generally correlate with a severely reduced biological activity. Recombinant IL-1β purified from E. coli containing additional 46 amino acids of the pro-domain (IL-1β71-269) shows some pyrogenic activity in rabbits, but removal of all but 5 amino acids of the pro-domain (IL-1β112-269) increases the specific activity by 50-fold (32). In another study, trypsin produced IL-1β77-269 was 10,000-fold less active than the elastase-produced IL-1β114-269, clearly emphasizing the significance of the specific processing site for bioactivity (24). Whether it is essential to have the proper NH2 region, or whether the correct size of the mature peptide is critical is currently not known. Many inflammatory reactions are not systemic, but rather localized, such as in RA joints, and proteases present in these extracellular fluids could be highly significant for determining these localized inflammatory reactions. For example, incubation of recombinant pro-IL-1β with synovial fluids from arthritic joints results in the generation of several IL-β peptides with distinct molecular weight and pI, in addition to the caspase 1-derived mature IL-1β, and inflamed synovial fluids are known to contain a number of these proteolytic enzymes (24). However, many of the original experiments have been performed in vitro, thus not all IL-1β converting enzymes might actually be relevant in vivo (33).
In this regard, the present two studies reported in this issue are highly significant, because they demonstrate this proteolytic activity in an experimental disease model in vivo, demonstrating that indeed different cell types with a distinct repertoire on IL-1β converting enzymes producing distinct bioactive IL-1β, contribute to inflammatory arthritis. Considering that most of these proteases are actually released from cells, it is noteworthy that several cell types exist, such as fibroblasts, smooth muscle cells, and endothelial cells, which produce pro-IL-1β, but lack a caspase 1 maturation mechanism. Although, release of pro-IL-1β has even been described in activated monocytes. In addition, sterile inflammatory conditions are frequently associated with neutrophil infiltration, which are short-lived cells and might be a source of pro-IL-1β following cell death. These IL-1β precursors could be used by proteases present in inflammatory fluids to produce bioactive IL-1β. Nevertheless, activated macrophages also rapidly release active caspase 1 together with other inflammasome components and IL-1β, and it is feasibly that this extracellular caspase 1 has activity towards pro-IL-1β as well. The precise mechanism by which IL-1β is released is still controversial and several models suggest either cell rupture, ATP-induced blebbing of microvesicles, or exocytosis of secretory lysosomes (34).
The molecular mechanism by which IL-1β converting enzymes are activated is distinct for caspase 1 and serine proteases. Activation of caspase 1 depends on the formation of inflammasomes. Inflammasomes are protein platforms that link recognition of damage-associated molecular patterns (DAMPs) by members of the Nod-like receptor (NLR) family of cytosolic pattern recognition receptors (PRRs) to the activation of caspase 1-dependent processing and release of IL-1β and IL-18 in macrophages (35). In response to the recognition of DAMPs from either pathogens (pathogen associated molecular patterns; PAMPs) or cellular stress (stress-associated molecular patterns; SAMPs or danger signals), NLRs undergo NTP-dependent oligomerization and recruit the adaptor protein ASC. Caspase 1 is then recruited by ASC into the NLR-ASC complex, which results in activation of caspase 1 by induced proximity. Although little is known on the stimuli that activate inflammasomes, it appears that each NLR is selectively activated. For example, NLRP1, one of the 22 human NLRs, in concert with Nod2 is involved in the recognition of peptidoglycan (PGN) and B. anthraces lethal toxin, while NLRC2 and NAIP are required for recognition of intracellular flagellin in conjunction with a bacterial type III or IV secretion system. NLRP3 (cryopyrin), perhaps the best studies NLR, is activated in response to a diverse set of infectious and stress signals, including PGN, bacterial and viral RNA, reactive oxygen species (ROS), asbestos and silica particles, skin irritants, and in concert with P2X7 receptors extracellular ATP (36). Furthermore, NLRP3-containing inflammasomes are relevant for rheumatic disease and are activated in response to uric acid and calcium pyrophosphate crystals that cause the painful symptoms in gout and pseudogout patients (37).
In contrast, activation of serine proteases is uncoupled from the PRR system and thus independent from direct recognition of infections and cellular stress. Serine proteases present in vesicles are activated by the lysosomal exocystein peptidase dipetidyl peptidase I (DPPI or cathepsin C), which removes the NH2-terminal leader peptide in cathepsin G, neutrophil elastase, proteinase-3, granzyme A, MMP-9, and chymase, thereby promoting their maturation. DPPI itself also requires autocatalytic removal of its prodomain for activation, which is enhanced at low pH (38). Many MMPs can be activated by plasmin, including MMP-3 and MMP-9 and also by other MMPs. Plasmin itself is activated by conversion of plasminogen into plasmin by urokinase plasminogen activator (uPA), tissue plasminogen activator (tPA), and factor XII, and subsequently auto activates itself as well as chymotrypsin.
The reason why different cell types have acquired several alternative mechanisms that can convert IL-1β into the bioactive form is not fully understood. One simple explanation could be that having redundant mechanisms for releasing this potent cytokine essential for the inflammatory host response, might ensure that the bioactive cytokine is available, even if one system fails. It is well established that besides macrophages, also neutrophils and mast cells significantly contribute to inflammatory arthritis (39–42). Perhaps, this might explain the observations that caspase 1 deficient mice are not fully protected form IL-1β-dependent inflammatory diseases, including arthritis. Because most of the potential IL-1β converting serine proteases have the DPPI activating process in common, it is not surprisingly that DPPI deficient mice are largely resistant to CIA-induced arthritis in mice (43). However, consistent with multiple IL-1β converting enzymes, a number of mice still developed inflammation and bone erosion, likely resulting from inflammasome-dependent IL-1β generation. Although the existence of distinct IL-1β converting enzymes is known for some time, their relative contribution to the development of inflammatory arthritis has not been investigated, and the two studies reported in this issue are the first to address this.
As suggested by Joosten et al. and Guma et al. in this issue, serine proteases play a dominant role during the acute phase of arthritis, which is characterized by strong neutrophil infiltration, while caspase 1 could play a major role during the chronic phase of arthritis, which is highly dependent on macrophages (Figure 2). Joosten and colleagues tested an acute K/BxN serum transfer and acute and chronic SCW arthritis model and show that arthritis progresses similarly in caspase 1 deficient- and wild type mice during the first 4 days, while caspase deficient mice showed partial protection of joint inflammation and chondrocyte proteoglycan synthesis inhibition. In contrast, IL-1β deficient mice were fully protected. In a chronic arthritis model of repeated SCW injections, caspase 1 deficiency ameliorated disease, concomitant with a significant reduced neutrophil infiltration, although not as efficient as IL-1β deficiency. Dual blockage of caspase 1 and DPPI-dependent serine proteases using pharmacological caspase 1 inhibition by pralnacasan in a DPPI deficient background resulted in significant ameliorated disease, comparable to IL-1β deficiency. Based on two additional mouse models that are deficient in MMP-9 or lacking neutrophil elastase and cathepsin G (beige/beige), this study concluded that proteinase-3 is the main serine protease working in concert with caspase 1 in the development of inflammatory arthritis. Guma and colleagues also tested the K/BxN model and the uric acid crystal-induced peritonitis model to demonstrate the significant contribution of serine proteases in inflammatory arthritis, but concluded that mast cell chymase and neutrophil elastase might be the alternative IL-1β converting enzymes in arthritis. These investigators also observed only partial protection in caspase 1 deficient mice, and based on the previously described contribution of mast cells and neutrophils to experimental arthritis, they delivered selective pharmacological inhibitors to delineate the contribution of specific serine proteases in a caspase 1 deficient background. Pharmacological inhibition of mast cell chymase and neutrophil elastase resulted in ameliorated K/BxN-induced arthritis and also MSU-induced peritonitis was significantly attenuated following blockage of neutrophil elastase, but significantly less potent than in IL-1β deficient mice. Neutrophil elastase inhibition alone resulted in a similar reduction as caspase 1 deficiency.
Figure 2.
Collectively, both studies convincingly demonstrate that, besides caspase 1, several IL-1β converting enzymes from mast cells and neutrophils play a considerable role in the development of inflammatory arthritis, and provide more insight into the disease mechanisms, which can be expected to have significant impact on future therapies. Nonetheless, it should be noted that IL-1β is not the sole substrate of caspase 1, which also cleaves two other IL-1 family cytokines, IL-18 and IL-1F7, and at least IL-18 is also processed by proteinase-3 and contributes to arthritis (44, 45). IL-33, yet another IL-1 family cytokine relevant for RA, has been proposed to require caspase 1 for maturation, but recent data suggest a predominantly intracellular function similar to IL-1α, which is only released during damage of cells, and that caspase 1 cleavage in fact inactivates IL-33 (46). Thus, the role of these proteases in arthritis could be far more complex than being limited to the maturation of IL-1β.
One of the problems of anakinra is its very short half live of only a few hours, and novel anti-IL-1β therapies are designed to improve the limitation of the required daily administration of anakinra. Much emphasis has therefore been on the development of improved anti-IL-1β therapies, including caspase 1 inhibitors. Two other drugs that target IL-1β are currently approved or are in clinical trials for the treatment of cryopyrinopathies, but could benefit also other IL-1β-dependent disease patients. Rilonacept is based on the Trap technology, which is a fusion of the IL-1RI and IL-1AcP with the Fc region of human IgG1, and canakinumab is a fully humanized monoclonal anti-IL-1β antibody. For global inhibition of IL-1β by anakinra and other cytokine traps, potentially considerable side effects due to the severe immune compromised patients, similar to global TNFα inhibition, need to be considered. As such, partial inhibition of the IL-1β pathway by selectively targeting a particular IL-1β converting protease could be a promising and superior approach. Such strategies would also allow for personalized therapy customized to the patients need. That this is a viable approach has just been very recently demonstrated by the identification of an NLRP3-specific inhibitor, which abrogates caspase 1 activation only downstream of NLRP3, but not other NLRs, which might have implication in gouty arthritis without the side effects of global caspase 1 inhibition. It is currently not understood, if there is a particular NLR defect in RA that causes excessive caspase 1 activation, but NLRP 1 and NLRP3 have been linked to rheumatic diseases. Genetic variants of NLRP1 are associated with a number of autoimmune diseases including RA, while NLRP3 has been linked to gout, pseudogout, and elevated expression is detected in synovial fluids of RA patients (47, 48). Whether specific defects promote the increased activation of serine proteases in inflammatory arthritis, or this merely result from the enhanced infiltration of neutrophils and mast cells, will need further investigations. In any case, several specific serine protease inhibitory drugs are approved that are targeting neutrophil elastase, MMPs, and others, which could be evaluated for efficacy in inflammatory arthritis in light of the two articles in this issue.
Acknowledgments
Supported by the NIH (grants 5R01GM071723 and 1R21AI082406) and the John P. Gallagher Research Professorship.
References
- 1.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118(11):3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgerald AA, Leclercq SA, Yan A, Homik JE, Dinarello CA. Rapid responses to anakinra in patients with refractory adult-onset Still's disease. Arthritis Rheum. 2005;52(6):1794–1803. doi: 10.1002/art.21061. [DOI] [PubMed] [Google Scholar]
- 3.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–1486. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Koning HD, Bodar EJ, van der Meer JW, Simon A. Schnitzler syndrome: beyond the case reports: review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin Arthritis Rheum. 2007;37(3):137–148. doi: 10.1016/j.semarthrit.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 5.So A, De Smedt T, Revaz S, Tschopp J. A pilot study of IL-1 inhibition by anakinra in acute gout. Arthritis Res Ther. 2007;9(2):R28. doi: 10.1186/ar2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGonagle D, Tan AL, Shankaranarayana S, Madden J, Emery P, McDermott MF. Management of treatment resistant inflammation of acute on chronic tophaceous gout with anakinra. Ann Rheum Dis. 2007;66(12):1683–1684. doi: 10.1136/ard.2007.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGonagle D. Successful treatment of resistant pseudogout with anakinra. Arthritis Rheum. 2008;58(2):631–633. doi: 10.1002/art.23119. [DOI] [PubMed] [Google Scholar]
- 8.Stojanov S, Kastner DL. Familial autoinflammatory diseases: genetics, pathogenesis and treatment. Curr Opin Rheumatol. 2005;17(5):586–599. doi: 10.1097/bor.0000174210.78449.6b. [DOI] [PubMed] [Google Scholar]
- 9.van de Loo AA, van den Berg WB. Effects of murine recombinant interleukin 1 on synovial joints in mice: measurement of patellar cartilage metabolism and joint inflammation. Ann Rheum Dis. 1990;49(4):238–245. doi: 10.1136/ard.49.4.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pettipher ER, Higgs GA, Henderson B. Interleukin 1 induces leukocyte infiltration and cartilage proteoglycan degradation in the synovial joint. Proc Natl Acad Sci U S A. 1986;83(22):8749–8753. doi: 10.1073/pnas.83.22.8749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet. 1988;2(8613):706–709. doi: 10.1016/s0140-6736(88)90185-7. [DOI] [PubMed] [Google Scholar]
- 12.Fong KY, Boey ML, Koh WH, Feng PH. Cytokine concentrations in the synovial fluid and plasma of rheumatoid arthritis patients: correlation with bony erosions. Clin Exp Rheumatol. 1994;12(1):55–58. [PubMed] [Google Scholar]
- 13.van den Berg WB. Arguments for interleukin 1 as a target in chronic arthritis. Ann Rheum Dis. 2000;59 Suppl 1:i81–i84. doi: 10.1136/ard.59.suppl_1.i81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell IK, O'Donnell K, Lawlor KE, Wicks IP. Severe inflammatory arthritis and lymphadenopathy in the absence of TNF. J Clin Invest. 2001;107(12):1519–1527. doi: 10.1172/JCI12724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saijo S, Asano M, Horai R, Yamamoto H, Iwakura Y. Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 2002;46(2):533–544. doi: 10.1002/art.10172. [DOI] [PubMed] [Google Scholar]
- 16.Ku G, Faust T, Lauffer LL, Livingston DJ, Harding MW. Interleukin-1 beta converting enzyme inhibition blocks progression of type II collagen-induced arthritis in mice. Cytokine. 1996;8(5):377–386. doi: 10.1006/cyto.1996.0052. [DOI] [PubMed] [Google Scholar]
- 17.Ma Y, Thornton S, Boivin GP, Hirsh D, Hirsch R, Hirsch E. Altered susceptibility to collagen-induced arthritis in transgenic mice with aberrant expression of interleukin-1 receptor antagonist. Arthritis Rheum. 1998;41(10):1798–1805. doi: 10.1002/1529-0131(199810)41:10<1798::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 18.Horai R, Saijo S, Tanioka H, Nakae S, Sudo K, Okahara A, et al. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J Exp Med. 2000;191(2):313–320. doi: 10.1084/jem.191.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicklin MJ, Hughes DE, Barton JL, Ure JM, Duff GW. Arterial inflammation in mice lacking the interleukin 1 receptor antagonist gene. J Exp Med. 2000;191(2):303–312. doi: 10.1084/jem.191.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strand V, Kavanaugh AF. The role of interleukin-1 in bone resorption in rheumatoid arthritis. Rheumatology (Oxford) 2004;43 Suppl 3:iii10–iii6. doi: 10.1093/rheumatology/keh202. [DOI] [PubMed] [Google Scholar]
- 21.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 22.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256(5053):97–100. doi: 10.1126/science.1373520. [DOI] [PubMed] [Google Scholar]
- 23.Black RA, Kronheim SR, Cantrell M, Deeley MC, March CJ, Prickett KS, et al. Generation of biologically active interleukin-1 beta by proteolytic cleavage of the inactive precursor. J Biol Chem. 1988;263(19):9437–9442. [PubMed] [Google Scholar]
- 24.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990;265(11):6318–6322. [PubMed] [Google Scholar]
- 25.Coeshott C, Ohnemus C, Pilyavskaya A, Ross S, Wieczorek M, Kroona H, et al. Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A. 1999;96(11):6261–6266. doi: 10.1073/pnas.96.11.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greten FR, Arkan MC, Bollrath J, Hsu LC, Goode J, Miething C, et al. NF-kappaB is a negative regulator of IL-1beta secretion as revealed by genetic and pharmacological inhibition of IKKbeta. Cell. 2007;130(5):918–931. doi: 10.1016/j.cell.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizutani H, Schechter N, Lazarus G, Black RA, Kupper TS. Rapid and specific conversion of precursor interleukin 1 beta (IL-1 beta) to an active IL-1 species by human mast cell chymase. J Exp Med. 1991;174(4):821–825. doi: 10.1084/jem.174.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irmler M, Hertig S, MacDonald HR, Sadoul R, Becherer JD, Proudfoot A, et al. Granzyme A is an interleukin 1 beta-converting enzyme. J Exp Med. 1995;181(5):1917–1922. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schonbeck U, Mach F, Libby P. Generation of biologically active IL-1 beta by matrix metalloproteinases: a novel caspase-1-independent pathway of IL-1 beta processing. J Immunol. 1998;161(7):3340–3346. [PubMed] [Google Scholar]
- 30.Matsushima K, Taguchi M, Kovacs EJ, Young HA, Oppenheim JJ. Intracellular localization of human monocyte associated interleukin 1 (IL 1) activity and release of biologically active IL 1 from monocytes by trypsin and plasmin. J Immunol. 1986;136(8):2883–2891. [PubMed] [Google Scholar]
- 31.Nylander-Lundqvist E, Egelrud T. Formation of active IL-1 beta from pro-IL-1 beta catalyzed by stratum corneum chymotryptic enzyme in vitro. Acta Derm Venereol. 1997;77(3):203–206. doi: 10.2340/0001555577203206. [DOI] [PubMed] [Google Scholar]
- 32.Dinarello CA, Cannon JG, Mier JW, Bernheim HA, LoPreste G, Lynn DL, et al. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cameron P, Limjuco G, Rodkey J, Bennett C, Schmidt JA. Amino acid sequence analysis of human interleukin 1 (IL-1). Evidence for biochemically distinct forms of IL-1. J Exp Med. 1985;162(3):790–801. doi: 10.1084/jem.162.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wewers MD. IL-1beta: an endosomal exit. Proc Natl Acad Sci U S A. 2004;101(28):10241–10242. doi: 10.1073/pnas.0403971101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinon F, Burns K, Tschopp J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-1b. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 36.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annu Rev Immunol. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 37.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 38.Zavasnik-Bergant T, Turk B. Cysteine proteases: destruction ability versus immunomodulation capacity in immune cells. Biol Chem. 2007;388(11):1141–1149. doi: 10.1515/BC.2007.144. [DOI] [PubMed] [Google Scholar]
- 39.Wipke BT, Allen PM. Essential role of neutrophils in the initiation and progression of a murine model of rheumatoid arthritis. J Immunol. 2001;167(3):1601–1608. doi: 10.4049/jimmunol.167.3.1601. [DOI] [PubMed] [Google Scholar]
- 40.Nigrovic PA, Binstadt BA, Monach PA, Johnsen A, Gurish M, Iwakura Y, et al. Mast cells contribute to initiation of autoantibody-mediated arthritis via IL-1. Proc Natl Acad Sci U S A. 2007;104(7):2325–2330. doi: 10.1073/pnas.0610852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297(5587):1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 42.Corr M, Crain B. The role of FcgammaR signaling in the K/B x N serum transfer model of arthritis. J Immunol. 2002;169(11):6604–6609. doi: 10.4049/jimmunol.169.11.6604. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, Pham CT. Dipeptidyl peptidase I regulates the development of collagen-induced arthritis. Arthritis Rheum. 2005;52(8):2553–2558. doi: 10.1002/art.21192. [DOI] [PubMed] [Google Scholar]
- 44.Sugawara S, Uehara A, Nochi T, Yamaguchi T, Ueda H, Sugiyama A, et al. Neutrophil proteinase 3-mediated induction of bioactive IL-18 secretion by human oral epithelial cells. J Immunol. 2001;167(11):6568–6575. doi: 10.4049/jimmunol.167.11.6568. [DOI] [PubMed] [Google Scholar]
- 45.Dai SM, Shan ZZ, Xu H, Nishioka K. Cellular targets of interleukin-18 in rheumatoid arthritis. Ann Rheum Dis. 2007;66(11):1411–1418. doi: 10.1136/ard.2006.067793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci U S A. 2009;106(22):9021–9026. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosengren S, Hoffman HM, Bugbee W, Boyle DL. Expression and regulation of cryopyrin and related proteins in rheumatoid arthritis synovium. Ann Rheum Dis. 2005;64(5):708–714. doi: 10.1136/ard.2004.025577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356(12):1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]