Abstract
Delayed donor Th2 cell infusion permits a graft-versus-tumor (GVT) effect to occur with subsequent amelioration of established graft-versus-host disease (GVHD). Relative to GVHD controls (B6-into-BALB/c model), recipients of delayed Th2 cells (day 14 post-BMT) had increased survival (3/3 experiments [exp]; each exp p<0.0001) and reduced GVHD by histology analysis five days post-Th2 infusion without increased tumor burden (3/3 exp; each exp p≤0.02). Th2 cell-mediated amelioration of GVHD was associated with greatly reduced allospecific IFN-γ secretion, in vivo augmentation of allospecific IL-4 and IL-10 secretion, and reduction in donor CD8+ T cell number post-BMT (3/3 exp; each comparison, p≤0.003). To better understand the molecular mechanism of this GVHD therapy, Th2 cells were generated from wild-type (WT), IL-4 deficient (KO), or IL-10 KO donors: remarkably, recipients of IL-4 or IL-10 KO Th2 cells had no survival advantage, no improvement in GVHD by histology, no reduction in CD8+ T cell expansion post-BMT, and no in vivo shift towards type II cytokines. We reasoned that IL-2 and alloantigen availability may be limiting factors for Th2 cell therapy, and as such, evaluated whether co-administration of IL-2 or co-infusion of host-type antigen presenting cells (APC) might intensify the anti-GVHD effect. However, contrary to these hypotheses, concomitant IL-2 therapy or APC administration fully abrogated the Th2 cell-mediated survival advantage and histology-defined GVHD reduction, reduced Th2 cell expansion in vivo while promoting CD8+ T cell expansion from cells originating from the initial allograft, and impaired type II polarization in vivo. In conclusion, Th2 cell therapy can rapidly ameliorate severe GVHD via IL-4 and IL-10 mediated mechanisms, and potentially, via IL-2 consumption and APC modulation mechanisms.
INTRODUCTION
The separation of graft-versus-leukemia (GVL) and graft-versus-tumor (GVT) effects from graft-versus-host disease (GVHD) can be difficult due to their shared biology(1). Experiments that evaluated the Th1/Th2 cellular balance in the allograft exemplify this conclusion: that is, CD4+Th2 and CD8+Tc2 cells mediated reduced GVHD relative to Th1/Tc1 cells or unmanipulated T cells(2, 3) but were also associated with reduced GVL(4, 5) and GVT(6) effects. To separate GVT effects from GVHD on a temporal basis, we developed an effective therapeutic strategy whereby an in vivo Th1/Tc1 response (generated by infusion of unmanipulated donor T cells) was followed by delayed administration of rapamycin-exposed donor Th2 cells(7). In the current project, our primary objectives were to: (1) perform mechanistic studies to better understand Th2 cell therapy of established GVHD; and (2) test therapeutic interventions that might enhance this therapy.
In our previous study(7), we found that prevention of GVHD was dependent upon Th2 cell production of the cytokine responsible for type II cytokine skewing, IL-4(8). In the current study, we evaluated whether Th2 cell therapy of 14-day established GVHD was also dependent upon IL-4. There is currently no data in the literature to address the role of IL-4 in the treatment of established GVHD. Previous in vitro data are somewhat disparate relative to the capacity of IL-4 to modulate established effector T cell responses. It has been shown that effector Th1 cells are relatively resistant to the polarizing effect of IL-4(9), and as such, it is possible that IL-4 may not play a role in the therapy of established GVHD. On the other hand, others found that IL-4 inhibited effector Th1/Tc1 cells(10), and as such, we reasoned that Th2 cell IL-4 secretion might indeed counteract established GVHD.
We also evaluated whether IL-10 may represent an effector mechanism for Th2 cell therapy of GVHD. High levels of IL-10 after clinical HLA-mismatched transplantation were associated with immune tolerance(11); furthermore, recipient IL-10 polymorphisms confer significant protection against the development of severe clinical acute GVHD(12). However, initial murine studies found that IL-10 administration did not reduce acute GVHD in models involving transplantation across minor(13) or major(14) histocompatibility barriers. The role of IL-10 in GVHD prevention was nonetheless confirmed in a subsequent murine study that found IL-10 deficient T cells to yield increased GVHD and identified low-dose, but not high-dose, IL-10 administration as an approach to prevent CD4- and CD8-mediated GVHD(15). IL-10 also contributes to the ability of adoptively transferred regulatory T cells(16, 17), donor antigen-presenting cells (APC)(18), or host APC(19) to prevent murine GVHD. Furthermore, IL-10 transduction of donor mesenchymal stem cells conferred protection against murine GVHD(20); and finally, inhibition of alloreactivity by type I regulatory T cells appears to involve IL-10 production, with no known contribution from IL-4(21). Although these multiple lines of experimentation clearly indicate that IL-10 plays a role in GVHD prevention, the role of IL-10 in the treatment of established GVHD has not been previously characterized. However, IL-10 contributes to the modulation of ongoing immune diseases such as autoimmune encephalomyelitis(22), and as such, we hypothesized that Th2 cell production of IL-10 would contribute to the therapy of established GVHD.
In addition, we evaluated two potential approaches to enhance the therapeutic efficacy of Th2 cell treatment of GVHD. First, we tested whether IL-2 administration after Th2 cell infusion would enhance the anti-GVHD effect. IL-2 administration is thought to enhance the efficacy of adoptive T cell therapy in the syngeneic setting(23), presumably in part because ex vivo expanded effector T cells are predominately IL-2 dependent(24, 25); this biology may be particularly relevant to Th2 cell therapy in light of this population’s requirement for IL-2/STAT5 signaling in the face of their negligible IL-2 production(26). Given this information, we hypothesized that IL-2 therapy post-Th2 cell infusion would improve the therapy of established GVHD. On the other hand, it is possible that IL-2 infusion may abrogate Th2 cell therapy: that is, IL-2 contributes to the initial afferent stages of murine GVHD(27, 28), and to some extent to the efferent stages of GVHD, as evidenced by the efficacy of anti-IL-2 therapy in the treatment of established clinical GVHD(29).
Second, we reasoned that Th2 cell therapy of established GVHD might be augmented by the infusion of host-type APC. That is, the Th2 cell product is generated by a co-stimulation method that yields a polyclonal T cell receptor repertoire(30), and as such, Th2 cell activation in vivo occurs by allosensitization(6). Host APC present at the time of conditioning induce donor T cell alloreactivity(31); however, such host APC are eventually replaced with donor APC post-transplant, and as such, delay in administration of donor lymphocytes results in reduced alloreactivity(32). As such, we hypothesized that delayed administration of host APC may enhance the in vivo activation of the Th2 cell product, thereby improving the anti-GVHD effect.
MATERIALS AND METHODS
Animals
BALB/c (H-2Kd) were utilized as hosts, whereas C57B1/6 (H-2Kb), Ly5.1 congenic (H-2Kb), and Thy1.1 congenic (CD90.1+; H-2Kb) mice were utilized as sources of donor cells; these mice were obtained from Frederick Cancer Research Facility (Frederick, MD). B6 mice genetically deficient in IL-4 or IL-10 production were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were 6–12 weeks old, maintained in a specific pathogen-free facility at the National Institutes of Health, and treated according to an approved animal protocol.
Ex Vivo Generation of Donor Th2 Cells and Donor and Host APC
Spleen cells from donor mice were harvested and RBC were lysed using ACK buffer (Quality Biological, Inc., Gaithersburg, MD). B cells were depleted using goat-anti-mouse conjugated magnetic bioparticles (Polysciences Inc., Warrington, PA), and CD8 cells were depleted (CD4 cell enrichment kit, Stem Cell Technologies, Vancouver, CA). To generate CD3, CD28 mAb coated beads: M450 Dynabeads (Dynal ASA, Oslo, Norway) were incubated with anti-murine CD3 and CD28 mAbs (BD PharMingen, San Diego, CA) in 0.1M-borate solution at 37°C overnight as described previously(33). CD3, CD28 mAb coated beads were then washed with PBS containing 3% FBS (Gemini Bio-Products, Woodland, CA), 5mM EDTA (Quality Biological, Inc.) and 0.1% sodium azide (Sigma, St. Louis, MO), and brought up in the same solution at a concentration of 40 × 106 beads/ml. Th2 cells were generated using CD3/CD28-coated beads in RPMI complete media with rhIL-2 (20 IU/mL), rhIL-7 (20 ng/mL), rmIL-4 (1000 IU/mL; all cytokines from Peprotech, Rocky Hill, NJ), rapamycin (10 µM; LC Laboratories, Woburn, MA), and 3.3 mM N-acetyl-cysteine (Bristol-Myers Squibb; Princeton, NJ). Specific cytokine-containing complete media was added to the Th2 cultures daily from days 2–6 to maintain a cell concentration between 0.2–1.0 × 106 cells/mL; Th2 cells were harvested for adoptive transfer experiments on day 6 of culture.
To generate APC for in vitro supernatant generation and for adoptive transfer experiments, donor-type B6 or host-type BALB/c bone marrow cells were cultured for 6 days in rmGM-CSF and rmIL-4 (each at 1000 IU/ml; PeproTech). Bacterial LPS (1 µg/ml; Calbiochem) was added to the final 24 h of culture.
Bone Marrow Transplantation (BMT) and Tumor Cell Inoculation
BALB/c mice were lethally irradiated (850cGy; 137Cs γ-irradiation source; Gamma Cell 40; Atomic Energy of Canada, Ottawa, Canada) and reconstituted by intravenous (i.v.) injection of B6 bone marrow (10 × 106 cells). To maintain continuity with our previous experiments(6, 7, 34) and to enable tumor burden as an endpoint for histology studies, all cohorts except for an engraftment control cohort received host-type mammary carcinoma cells [TS/A cell line(35); 0.1 × 106 cells by i.v. injection]. One treatment group received only BMT and TS/A cells (“tumor control cohort”). Another treatment group received BMT, TS/A cells, and additional donor splenic T cells enriched by B cell-depletion (i.v. injection of 5 or 20 × 106 T cells, dose specified in table footnotes; “GVHD control cohort”). As per our animal protocol, any transplant recipient that was pre-morbid (as defined by >20% loss in body weight, severe loss in activity, or pulmonary compromise) was euthanized. Lethality in the GVHD control cohort was approximately 50% by day 14 post-transplant; at this time point, surviving recipients were randomized to receive no further therapy (that is, maintained as a GVHD control) or further day 14 therapy with donor Th2 cells (10 × 106 cells by i.v. injection). For post-BMT IL-2 administration, mice received day 14 to day 18 therapy with an intermediate dose of IL-2 that we have previously utilized(36) (50,000 IU by i.p. injection, twice daily). For APC injection, mice received 10 × 106 cells by i.v. injection at day 14 post-BMT (just prior to Th2 cell infusion).
Histology
Mice from each cohort in each of the three transplant experiments were evaluated at day 19 post-BMT. Liver, stomach, small intestine, large intestine, cecum, and skin were harvested for GVHD score; lung was harvested for evaluation of tumor burden. Tissues were fixed in formaldehyde and stained with hematoxylin and eosin. GVHD severity score ranged from 0 to 4, where 0 was assigned as normal and 4 as maximal level of GVHD. Each organ was scored separately, yielding a maximum cumulative GVHD score of 24. Semi-quantitative determination of tumor burden was scored on a scale of 0 to 4: 0, no tumor cells identified; 1, <10% of lung involved with tumor; 2, 10–20% lung involvement; 3, 21–30% lung involvement; and 4, > 30% lung involvement. Scoring of GVHD and tumor burden was performed by a pathologist (M.A.E.) in a blinded fashion.
Flow Cytometry for Cell Surface Markers
Three-color flow cytometry was performed (FACSCalibur instrument and CellQuest software; BD Biosciences). Live events (5,000–10,000) were acquired, with propidium iodide exclusion of dead cells. To facilitate cell tracking post-BMT, Th2 cells were generated from Thy1.1 congenic mice, and unmanipulated donor splenic T cells were isolated from Ly5.1 congenic mice. Spleens were harvested at day 19 post-BMT, and single-cell suspensions were labeled with Abs against murine CD4, CD8, CD19, Ly5.1, or CD90.1 (anti-Thy1.1) conjugated with FITC, PE, or allophycocyanin (all obtained from BD PharMingen).
Determination of Post-BMT Cytokine Phenotype
Spleen cells were collected post-BMT and subjected to no stimulation, and B6 (syngeneic) and BALB/c (allogeneic) APC stimulation. Spleen cells were plated at 0.5 × 106 cells/ml; APC were utilized at a spleen cell to APC ratio of 10:1. After 24 h, supernatants were collected and analyzed for cytokine content (IL-2, IL-4, IL-10, and IFN-γ) by Bio-Plex multiplex sandwich immunoassay (Bio-Rad, Hercules, CA).
Statistics
Survival analysis was performed using Kaplan-Meier survival curves and log-rank test. For survival analyses involving day 14 post-BMT Th2 cell therapy, deaths through day 14 (prior to randomization) were censored out. For small sample size comparison, Student’s t test was used. Comparison values of p<0.05 were considered statistically significant.
RESULTS
IL-4 and IL-10 Contribute to Th2 Cell Therapy of Established GVHD: Survival
Using a model of a 14-day established acute GVHD, we first evaluated (experiment #1) the effect of wild-type (WT), IL-4 deficient (KO), or IL-10 KO Th2 cells on post-BMT survival (Table I). All engraftment control recipients (cohort 1) survived post-BMT. Tumor control recipients (cohort 2) that did not receive donor T cells in conjunction with the BMT died from systemic malignancy (as evidenced by pulmonary compromise) at a median of 21 days post-BMT (range, 21 to 23 days). Additional cohorts received tumor cells and BMT augmented with donor T cells; severe acute GVHD occurred in this cohort, as approximately 50% of recipients died by day 14 post-transplant. At day 14 post-BMT, surviving recipients in this cohort were randomized to receive no further therapy or further therapy with Th2 cells. Subjects randomized to no further therapy (cohort 3) had a median survival of 27 days (range, 25 to 29 days); death in this cohort was primarily attributable to GVHD because of an absence of pulmonary compromise, the presence of clinical GVHD (hunched posture, diarrhea), and evidence of severe GVHD by histology (see Fig. 1).
Table I.
Th2 Cell IL-4 and IL-10 Production Contributes to Enhanced Survival During GVHD
| Transplant Componentsb |
||||||
|---|---|---|---|---|---|---|
| Cohort # | Host Tumora | BM | T cells | Th2 Cells | Days of Post-BMT Survivalc | p-valuesd |
| 1 | − | + | − | − | 35(35–35) | |
| 2 | + | + | − | − | 21(21–23) | |
| 3 | + | + | + | − | 27(25–29) | |
| 4 | + | + | + | Th2 (WT) | 34(34–35) | p<0.0001 (#4 vs. #3) |
| 5 | + | + | + | Th2 (IL-4KO) | 29(28–30) | p=NS (#5 vs. #3) |
| 6 | + | + | + | Th2 (IL-10KO) | 23(21–24) | p=NS (#6 vs. #3) |
BALB/c hosts received 850 cGy XRT and host-type TS/A tumor cells (0.1×x106 cells).
C57BL/6-into-BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (20×106 cells);donor Th2 cells (WT, IL-4 KO, IL-10KO; 10×106 cells; day 14 post-BMT).
Median and range values for number of days of post-transplant survival; n=7/group.
Unpaired t test.
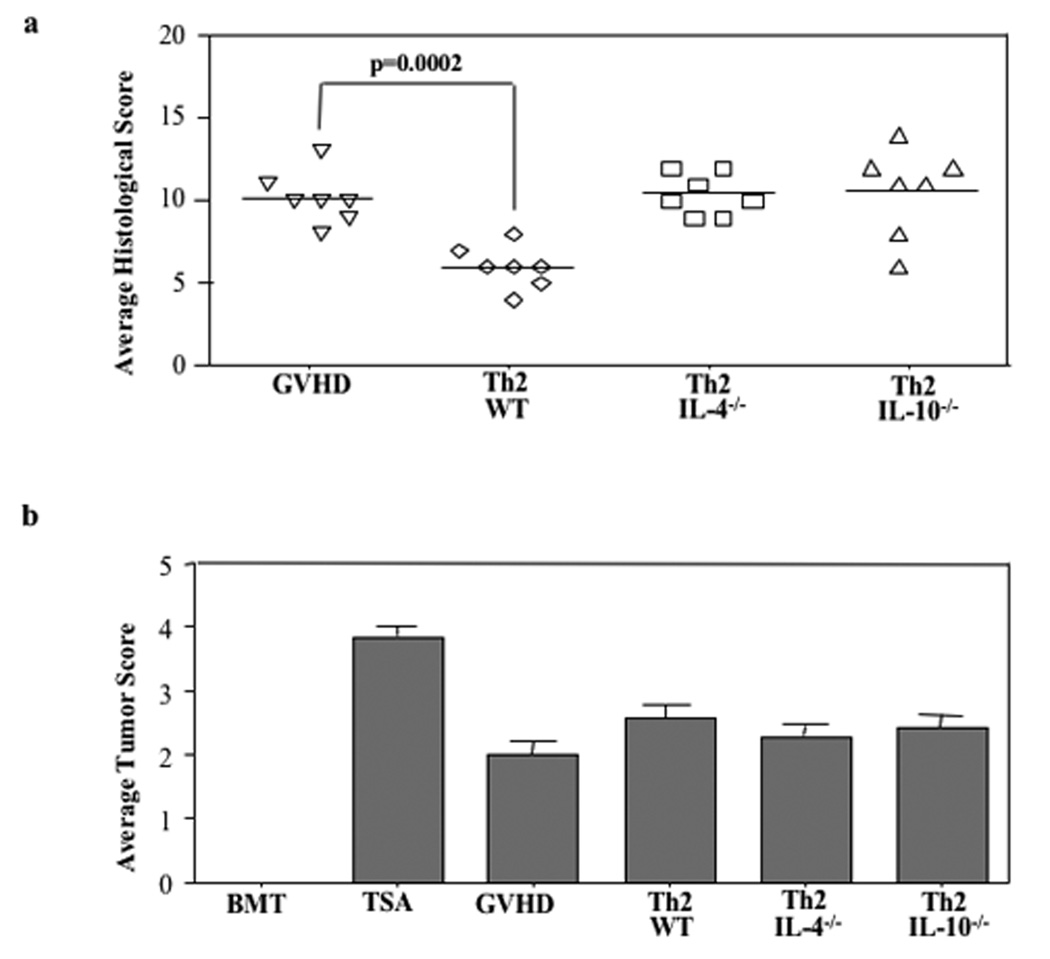
FIGURE 1. Delayed administration of Th2 cells improves balance of GVHD and GVT effects.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow alone ("BMT"); marrow and host-type TS/A tumor cells ("TSA"); or marrow, tumor cells, and additional donor T cells ("GVHD"). Other cohorts received marrow, tumor cells, donor T cells, and additional donor CD4+ Th2 cells generated ex vivo in high-dose rapamycin (10 µM; administered on day 14 post-BMT); Th2 cells were generated from either wild-type donors (“Th2”) or IL-4 deficient donors (“Th2 4−/−”) or IL-10 deficient donors (“Th2 10−/−”). On day 19 post-BMT, lungs from treatment cohorts were removed to evaluate tumor burden; liver, stomach, small intestine, large intestine, cecum, and skin were harvested to assess GVHD. (a) For GVHD, each organ was scored on a scale of 0 to 4, with cumulative GVHD severity score shown (maximum value, 24). (b) For tumor burden, a semi-quantitative determination of tumor cell infiltration was scored on a scale of 0 to 4. n = 7 subjects were evaluated in each cohort.
Recipients of T cell-containing allografts that were randomized to receive WT donor Th2 cells on day 14 post-BMT (cohort 4) had increased survival time relative to the GVHD control cohort (median survival of 34 days [range, 34 to 35 days]; p<0.0001); death in this cohort was primarily attributable to GVHD because pulmonary compromise was not apparent and clinical GVHD was apparent. The ameliorating effect of delayed Th2 cell infusion was abrogated if the Th2 cells were deficient in IL-4 production (cohort 5; median survival time of 29 days [range, 28 to 30 days]; not increased relative to GVHD controls) or deficient in IL-10 production (cohort 6; median survival time of 23 days [range, 21 to 24 days]; not increased relative to GVHD controls).
Effect of WT, IL-4 or IL-10 KO Th2 Cells on GVHD and Tumor Burden: Histology
In experiment #1, in addition to the survival analysis, recipients from each cohort were evaluated for signs of GVHD and tumor by histology at day 19 post-BMT (five days post-Th2 cell infusion). Recipients of Th2 cells had significantly reduced GVHD relative to the GVHD control cohort (Fig. 1; median GVHD score reduced from 10.0 to 6.0, p=0.0002) without a significant increase in tumor burden. The anti-GVHD effect of the Th2 cell therapy was reflected across all tissues examined. For example, in this experiment, mean liver GVHD score was reduced from 2.0 (GVHD controls) to 1.1 (Th2 cell recipients; p=0.001), mean large intestine GVHD score was reduced from 2.3 to 1.0 (p=0.004), and mean skin GVHD score was reduced from 1.4 to 1.0 (p=0.07). In marked contrast, recipients of IL-4 KO and IL-10 KO Th2 cells did not have reduced GVHD by histology (median scores, 10.4 and 10.6, respectively).
Delayed Th2 Cell Infusion Inhibits Post-BMT Type I Cytokines in an IL-4 and IL-10 Dependent Manner
In experiment #1, the T cell cytokine phenotype was determined at day 19 post-BMT to further characterize the immune modulation effects and cytokine dependence of delayed Th2 cell infusion (Table II). As anticipated, T cells from engraftment and tumor controls (cohorts 1 and 2) had nominal post-BMT secretion of type I or type II cytokines in response to secondary in vitro allostimulation. Also as anticipated, T cells from GVHD controls (cohort 3) predominately secreted increased IFN-γ in response to alloantigen. As such, GVHD in this model was most consistent with a type I cytokine phenotype.
Table II.
Th2 Cells Polarize Post-BMT Immunity in an IL-4 and IL-10 Dependent Manner
| Transplant Componentsb |
Post-BMT Cytokine Productionc |
p-valuesd |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort # | Host Tumora | BM | T cells | Th2 Cells | IL-2 | IFN-γ | IL-4 | IL-10 | IL-2 | IFN-γ | IL-4 | IL-10 |
| 1 | − | + | − | − | BDL | BDL | 22±6 | 4±1 | ||||
| 2 | + | + | − | − | 8±7 | 57±8 | 65±5 | 3±1 | ||||
| 3 | + | + | + | − | 49±5 | 249±35 | 74±4 | 23±2 | ||||
| 4 | + | + | + | Th2 (WT) | 54±10 | 48±10 | 810±73 | 148±16 | p=NS (#4 vs. #3) |
p=0.0002 (#4 vs. #3) |
p<0.0001 (#4 vs. #3) |
p<0.0001 (#4 vs. #3) |
| 5 | + | + | + | Th2 (IL-4KO) | 37±6 | 227±51 | 182±16 | 49±6 | p=NS (#5 vs. #3) |
p=NS (#5 vs. #3) |
p<0.0001; p<0.0001 (#5 vs. #3); (#5 vs. #4) |
p=0.0026; p=0.0002 (#5 vs. #3); (#5 vs. #4) |
| 6 | + | + | + | Th2 (IL-10KO) | 42±5 | 277±90 | 224 ±16 | 74±20 | p=NS (#6 vs. #3) |
p=NS (#6 vs. #3) |
p p<0.000; p<0.0001 (#6 vs. #3); (#6 vs. #4) |
p=0.0322; p=0.0171 (#6 vs. #3); (#6 vs. #4) |
BALB/c host received 850 cGy XRT; host TS/A tumor cells 0.1× 106.
C57BL/6 into BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (20×106 cells); donor Th2 cells (WT, IL-4 KO, IL-10KO; 10×106 cells; day 14 post-BMT).
Cytokine production measured by Luminex assay at day19 post-BMT; IL-4, IL-10, IL-2, and IFN-γ (pg/ml); Mean ± SEM of n=7/group; BDL (below detection limit; <1 pg/ml).
Unpaired t test.
Infusion of Th2 cells at day 14 post-BMT resulted in an approximate 10-fold increase in the capacity of post-transplant T cells to produce the type II cytokines IL-4 and IL-10 upon alloantigen re-stimulation (cohort 4). Concomitantly, such Th2 recipients had significantly reduced secretion of IFN-γ relative to the GVHD cohort (reduction from a median value of 249 to 48 pg/ml, p=0.0002). Th2 cell deficiency of either IL-4 (cohort 5) or IL-10 (cohort 6) significantly reduced the capacity of Th2 cells to promote both IL-4 and IL-10 secretion post-BMT, and completely abrogated the ability of the delayed Th2 cell infusion to inhibit IFN-γ secretion post-BMT.
Delayed Th2 Cell Infusion Restricts the Number of Donor CD8+ T Cells Post-BMT
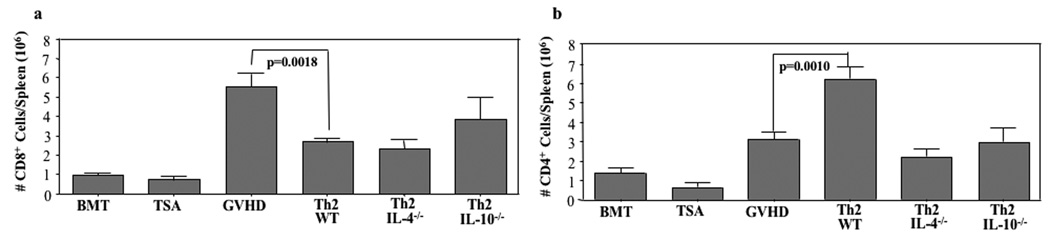
Utilizing these day 19 post-BMT cohorts, we characterized the effect of delayed Th2 cell infusion on the in vivo expansion of T cells contained in the initial transplant inoculum (Fig. 2). In experiment #1, the congenic donor strain was not available, and as such, we could not differentiate the post-BMT contribution of Th2 cells from CD4+ T cells contained in the initial allograft. Relative to GVHD controls, recipients of delayed WT Th2 cells had significantly increased post-BMT total CD4+ T cells (median value increased from 3.1 to 6.2 × 106 CD4 cells per spleen, p=0.0010); this CD4 cell increase was abrogated if the Th2 cell product was deficient in IL-4 or IL-10 (Fig. 2b). Relative to GVHD controls, recipients of delayed WT Th2 cells had significantly reduced numbers of donor CD8+ T cells post-BMT (median value reduced from 5.5 to 2.6 × 106 CD8 cells per spleen, p=0.0018). Interestingly, Th2 cells deficient in IL-4 or IL-10 were competent in their ability to prevent CD8+ T cell expansion post-BMT (Fig. 2a).
FIGURE 2. Donor Th2 cells inhibit donor CD8+ T cell expansion after allogeneic BMT.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow alone ("BMT"); marrow and TS/A tumor cells ("TSA"); or marrow, tumor cells, and additional donor T cells ("GVHD"). Other cohorts received marrow, tumor cells, donor T cells, and additional donor CD4+ Th2 cells generated from either wild-type donors (“Th2”) or IL-4 deficient donors (“Th2 4−/−”) or IL-10 deficient donors (“Th2 10−/−”). On day 19 post-BMT, spleen cells were isolated and the absolute number of donor CD8+ T cells (a) and CD4+ T cells (b) were calculated from flow cytometry data (cells per spleen, × 106). Data shown are mean ± SEM (n = 7 per cohort).
Th2 Cell Therapy of Established GVHD is Abrogated by IL-2 Administration
In experiment #2, we next tested our hypothesis that IL-2 administration would enhance the in vivo efficacy of Th2 cells. In this experiment (Table III), GVHD controls (cohort 3) had increased survival time relative to tumor controls (cohort 2), with an increase in survival time from a median of 23 days post-BMT (range, 22 to 26) to 33 days post-BMT (range, 31 to 35) until death that occurred in the setting of clinical GVHD. Relative to GVHD controls, delayed Th2 cell infusion resulted in a further extension of survival time (increase in median survival to 42 days post-BMT [range, 40 to 43]; p<0.0001). Contrary to our hypothesis, IL-2 administration completely abrogated the extension of survival time conferred by Th2 cells (cohort 6; median survival reduced from 42 days post-BMT to 33 days post-BMT [range, 31 to 36]; p<0.0001). A control group that received additional therapy with IL-2 without Th2 cells (cohort 4) had a trend towards modestly increased lethality relative to GVHD controls that did not receive IL-2 (median survival reduced from 33 days post-BMT to 31 days post-BMT [range, 27 to 32]; p=0.0684).
Table III.
Th2 Cell-Mediated Survival Advantage is Abrogated by IL-2 Therapy.
| Transplant Components b |
|||||||
|---|---|---|---|---|---|---|---|
| Cohort # | Host Tumora | BM | T cells | Th2 Cells | rhIL-2 Injectionc | Days of Post-BMT Survivald | p-valuese |
| 1 | − | + | − | − | − | 43(43–43) | |
| 2 | + | + | − | − | − | 23(22–26) | |
| 3 | + | + | + | − | − | 33(31–35) | |
| 4 | + | + | + | − | + | 31(27–32) | p=NS (#4 vs. #3) |
| 5 | + | + | + | Th2 (WT) | − | 42(40–43) | p<0.0001 (#5 vs. #3) |
| 6 | + | + | + | Th2 (WT) | + | 33(31–36) | p=NS (#6 vs. #3) |
BALB/c hosts received 850 cGy XRT and host-type TS/A tumor cells (0.1×106 cells).
C57BL/6-into-BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (20×106 cells); donor Th2 cells (WT; 10×106 cells; day 14 post-BMT).
rhIL-2 50,000 IU/mouse 2×/day I.P. for 5 days beginning at day 14 post BMT.
Median and range for number of days of post-transplant survival; n=7/group.
Unpaired t test.
IL-2 Reverses Th2 Cell Therapy of Established GVHD: Histology
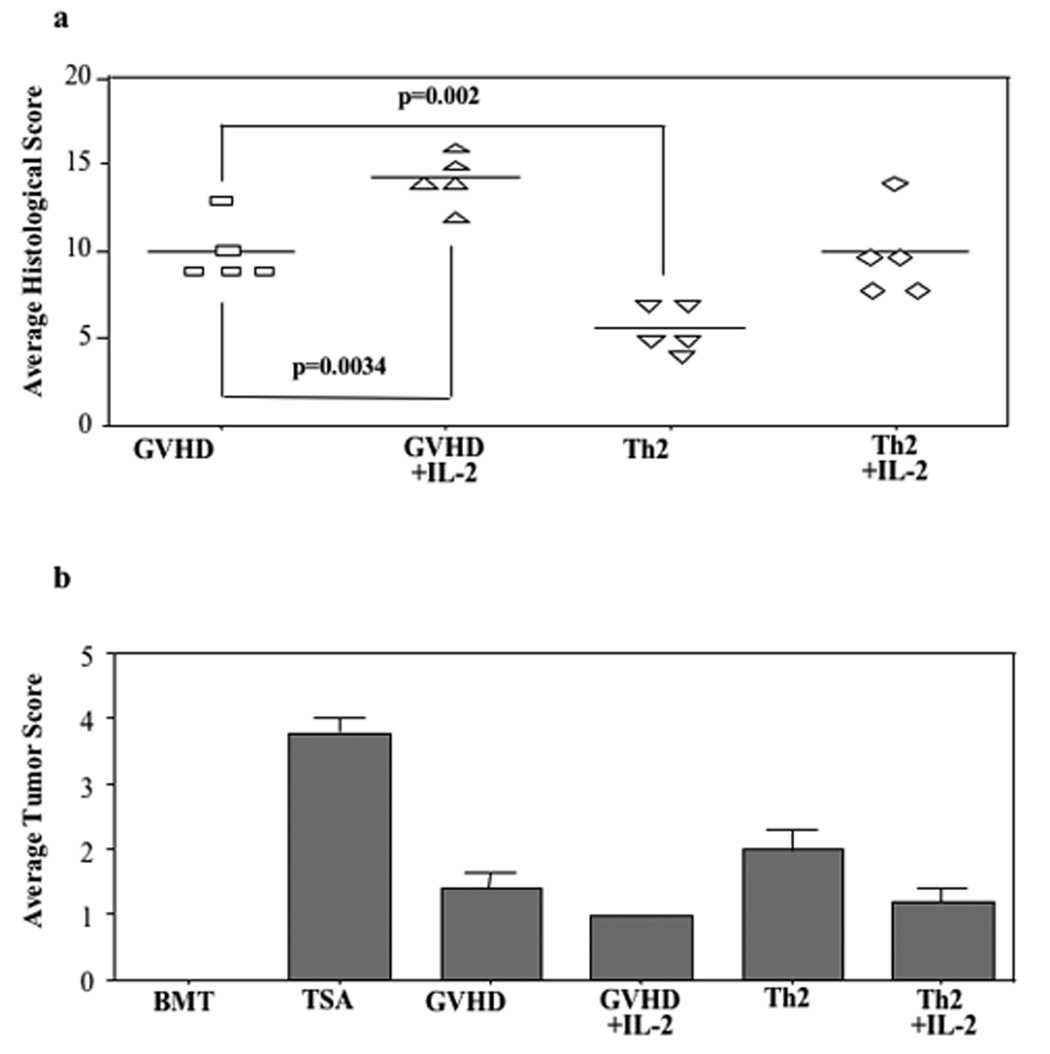
In experiment #2, mice from each cohort were evaluated at day 19 post-BMT to characterize the effect of day 14 Th2 cell infusion with or without IL-2 therapy on GVHD and tumor burden as defined by histology (Fig. 3). Consistent with the survival data, Th2 cell recipients had reduced GVHD relative to GVHD controls (average GVHD score reduced from 10.0 to 5.6, p=0.002). Similar to experiment #1, the anti-GVHD effect of Th2 cells was generally reflected across the different tissue sites evaluated: mean liver score was reduced from 2.2 to 0.8 (p=0.001), mean large intestine score was reduced form 1.4 to 0.6 (p=0.049), and mean skin score was reduced from 1.2 to 1.0 (p=NS). Remarkably, the Th2 cell-mediated improvement in GVHD was completely reversed by IL-2 therapy (average GVHD score in this cohort, 10.0). IL-2 administration also increased GVHD in the control cohort (average GVHD score, 14.2; increased relative to GVHD controls that did not receive IL-2, p=0.0034). Each of the treatment cohorts that received T cell-replete BMT with or without Th2 cells and with or without IL-2 had reduced tumor burden relative to tumor controls (Fig. 3b).
FIGURE 3. In vivo IL-2 administration abrogates Th2 cell therapy: histology analysis.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow alone ("BMT"); marrow and TS/A tumor cells ("TSA"); marrow, tumor cells, and additional donor T cells ("GVHD"); or marrow, tumor cells, donor T cells, and IL-2 therapy (“GVHD+IL-2”; 50,000 I.U., B.I.D. from day 14 to day 18 post-BMT). Other cohorts received marrow, tumor cells, donor T cells, and donor Th2 cells on day 14 post- BMT either alone ("Th2”) or with IL-2 infusion (“Th2+IL-2”). On day 19 post-BMT, lungs from treatment cohorts were removed to evaluate tumor burden; liver, stomach, small intestine, large intestine, cecum, and skin were harvested to assess GVHD. (a) For GVHD, each organ was scored on a scale of 0 to 4, with cumulative GVHD score shown (maximum value, 24). (b) For tumor burden, a semi-quantitative determination of tumor cell infiltration was scored on a scale of 0 to 4. n = 5 subjects were evaluated per cohort.
IL-2 Reverses Th2 Cell Therapy of GVHD: Post-BMT Cytokine Phenotype
In addition to histology studies, day 19 post-BMT cohorts from experiment #2 were evaluated for T cell cytokine phenotype (Table IV). Post-BMT T cells from GVHD controls had a high level of allospecific IFN-γ secretion (cohort 3; median value of 2196 pg/ml) with nominal secretion of IL-4 or IL-10. Consistent with our initial results, day 14 infusion of Th2 cells resulted in a near complete down-regulation of IFN-γ secretion (median value reduced from 2196 to 81 pg/ml, p<0.0001) and an approximate one- to two-log increase in IL-4 and IL-10 secretion (to median values of 3501 and 707 pg/ml, respectively). Remarkably, administration of IL-2 after Th2 cell therapy tempered both the observed down-regulation in IFN-γ(median value, 320 pg/ml, p=0.042) and the observed up-regulation of IL-4 (median value, 1117 pg/ml, p<0.0001) and IL-10 (median value, 288 pg/ml, p=0.0002). In marked contrast to these relatively dramatic effects of IL-2 therapy in the setting of Th2 cell infusion, IL-2 therapy did not greatly modulate the post-BMT cytokine phenotype in GVHD controls (cohort 4).
Table IV.
IL-2 Therapy Reduces Th2 Cell Capacity to Polarize Immunity Post-BMT.
| Transplant Componentsb |
Post-BMT Cytokine Productiond |
p-valuese |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort # | HostTumora | BM | T cells | Th2 Cells | rh IL-2 Injectionc | IL-2 | IFN-γ | IL-4 | IL-10 | IL-2 | IFN-γ | IL-4 | IL-10 |
| 1 | − | + | − | − | 3±1 | 5±1 | 9±2 | 2±1 | |||||
| 2 | + | + | − | − | 7±3 | 78±17 | 110±28 | 19±3 | |||||
| 3 | + | + | + | − | 7±2 | 2196±37 | 60±14 | 29±10 | |||||
| 4 | + | + | + | − | + | 9±1 | 2170±85 | 205±45 | 56±12 | ||||
| 5 | + | + | + | Th2 (WT) | − | 3±1 | 81±22 | 3501±179 | 707±58 | p=NS (#5 vs. #3) |
p<0.0001 (#5 vs. #3) |
p<0.0001 (#5 vs. #3) |
p<0.0001 (#5 vs. #3) |
| 6 | + | + | + | Th2 (WT) | + | 5±1 | 320±97 | 1117±260 | 288±37 | p=NS (#6 vs. #5) |
p=0.0420 (#6 vs. #5) |
p<0.0001 (#6 vs. #5) |
p=0.0002 (#6 vs. #5) |
BALB/c host received 850 cGy XRT; 0.1×106 host TS/A tumor.
C57BL/6 into BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (20×106 cells); donor Th2 cells (10×106 cells; day 14 post-BMT).
rhIL-2 50,000 IU/mouse 2×/day I.P. for 5 days beginning day14 post BMT.
Cytokine production measured by Luminex assay at day19 post-BMT; IL-4, IL-10, IL-2, and IFN-γ (pg/ml); Mean ± SEM of n=5/group.
Unpaired t test.
IL-2 Reverses Th2 Cell Therapy of GVHD: Donor T Cell Tracking
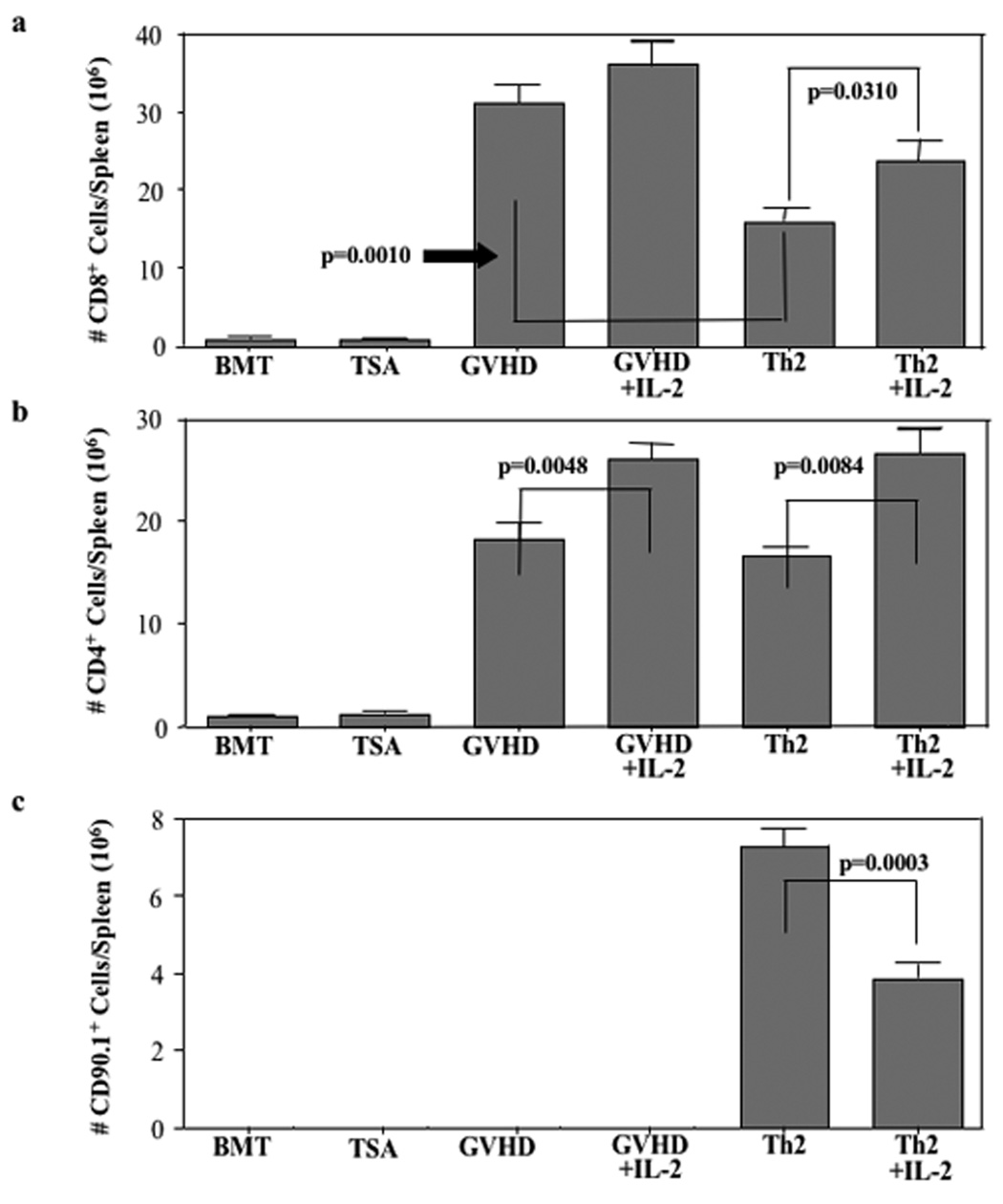
In experiment #2, donor Th2 cells were generated from mice expressing the CD90.1 congenic marker. As such, we were able to track the Th2 cell product and evaluate the effect of Th2 cells on donor CD4+ and CD8+ T cells in the allograft. First, and perhaps most importantly, we found that IL-2 therapy reduced the number of Th2 cells present at day 19 post-BMT (Fig. 4c; median Th2 cell number reduced from 7.3 to 3.9 × 106 cells per spleen, p=0.0003).
FIGURE 4. Th2 cell inhibition of CD8+ T cell expansion is abrogated by IL-2.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow alone ("BMT"); marrow and TS/A tumor cells ("TSA"); marrow, tumor cells, and additional donor T cells ("GVHD"); or marrow, tumor cells, donor T cells, and IL-2 therapy (“GVHD+IL-2”; 50,000 I.U., B.I.D. from day 14 to day 18 post-BMT). Other cohorts received marrow, tumor cells, donor T cells, and donor Th2 cells on day 14 post- BMT either alone ("Th2”) or with IL-2 infusion (“Th2+IL-2”). On day 19 post-BMT, spleen cells were isolated and the absolute number of donor CD8+ T cells (a) and CD4+ T cells (b) were calculated from flow cytometry data (cells per spleen, × 106). Data shown are mean ± SEM (n = 5 per cohort).
Relative to the GVHD control cohort, delayed administration of donor Th2 cells did not reduce the number of post-BMT CD4+ T cells emanating from the initial allograft (Fig. 4b; median values of 16.6 and 18.1 × 106 cells/spleen; p=NS). Interestingly, in marked contrast to the inhibitory effect of IL-2 on in vivo Th2 cell expansion, IL-2 therapy increased the number CD4 cells emanating from the initial allograft whether such T cells were administered with Th2 cells (Fig. 4b; increase in median CD4 number from 16.6 to 26.6 × 106 cells/spleen, p=0.0048) or without Th2 cells (Fig. 4b; increase in median CD4 number from 18.1 to 26.1 × 106 cells/spleen, p=0.0084).
Similar to our previous results (Fig. 2), Th2 cell infusion significantly reduced the post-BMT number of donor CD8+ T cells relative to GVHD controls (Fig. 4a; median values reduced from 31.1 to 15.9 × 106 cells/spleen, p=0.0010). Th2 cell-mediated restriction of CD8+ T cell expansion was partially reversed by IL-2 administration (increase in median CD8 number from 15.9 to 23.9 × 106 cells/spleen, p=0.0310). IL-2 administration did not significantly increase the number of post-transplant CD8 cells in GVHD controls (increase from 31.1 to 36.2 × 106 cells/spleen, p=NS).
Th2 Cell Therapy of GVHD is Abrogated by Adoptive Transfer of APC
In experiment #3, we next tested our hypothesis that a relative paucity of host APC may represent a limiting factor to Th2 cell therapy of established GVHD. In this experiment (Table V), GVHD controls (cohort 3) had an extension of survival time relative to tumor controls (cohort 2; median + (range) survival increased from 21(20–21) to 33(31–35) days, p<0.0001). Similar to our previous experiments, infusion of Th2 cells at day 14 post-BMT resulted in an extension of survival time relative to GVHD controls (median + (range) survival increased to 45(42–46) days, p=0.0001).
Table V.
APC Adoptive Transfer Abrogates Th2 Cell Therapy of GVHD.
| Transplant Componentsb |
||||||||
|---|---|---|---|---|---|---|---|---|
| Cohort # | Host Tumora | BM | T cells | Th2 Cells | Donor DCc | Host DCd | Days of Post-BMT Survivale | p-valuesf |
| 1 | − | + | − | − | 46(46–46) | |||
| 2 | + | + | − | − | 21(20–21) | |||
| 3 | + | + | + | − | 33(31–35) | p<0.0001 (#3 vs. #2) |
||
| 4 | + | + | + | − | + | 32(27–32) | p=NS (#4 vs. #3) |
|
| 5 | + | + | + | − | − | + | 32(27–34) | p=NS (#5 vs. #3) |
| 6 | + | + | + | Th2 | − | − | 45(42–46) | p=0.0001 (#6 vs. #3) |
| 7 | + | + | + | Th2 | + | − | 34(21–36) | p=NS (#7 vs. #3) |
| 8 | + | + | + | Th2 | − | + | 25(18–29) | p=0.02 (#8 vs. #3) |
BALB/c host received 850 cGy XRT; 0.1×106 host TS/A tumor.
C57BL/6 into BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (5×106 cells); donor Th2 cells (10×106 cells; day 14 post-BMT).
Fresh DC cells generated from donor C57BL/6 (10×106 cells; day14 post-BMT).
Fresh DC cells generated from host BALB/c (10×106 cells; day14 post-BMT.
Median and range of number of days of post-transplant survival; n=5/group.
Unpaired t test.
In GVHD controls, adoptive transfer of donor APC (cohort 4) or host APC (cohort 5) did not greatly alter post-transplant survival time (median + (range) survival times of 33(31–35) days [without APC infusion], 32(27–32) days [donor APC infusion], and 32(27–34) days [host APC infusion]). In marked contrast, in the setting of delayed Th2 cell therapy, infusion of donor APC (cohort 7) or host APC (cohort 8) completely abrogated the extension of survival time conferred by the Th2 cell population (median + (range) survival times reduced to 34(21–36) days [donor APC infusion] and 25(18–29) days [host APC infusion]).
APC Transfer Abrogates Th2 Cell Therapy of Established GVHD: Histology
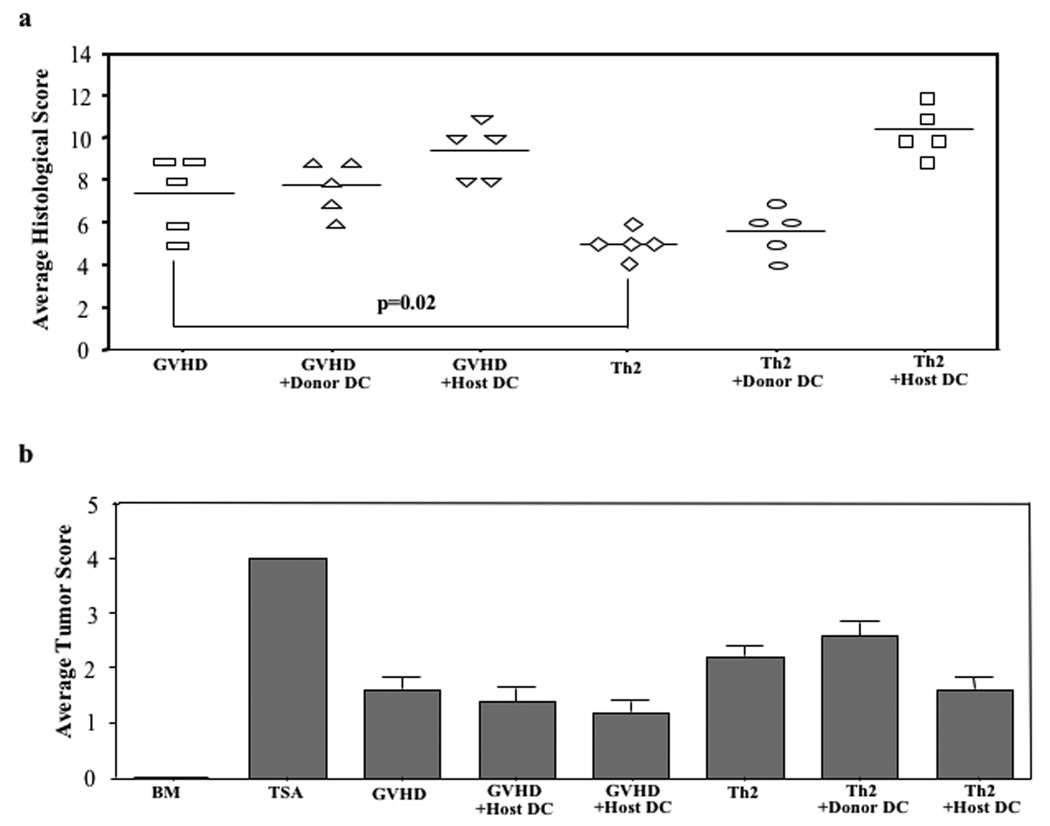
In experiment #3, similar to our prior results, Th2 cell recipients had reduced GVHD by histology relative to the GVHD control cohort (Fig. 5a; average GVHD score reduced from 7.4 to 5.0, p=0.02). Similar to experiments #1 and #2, Th2 cell modulation of GVHD was generally apparent across tissue sites evaluated. Specifically, mean liver score was reduced from 0.6 to 0.4 (p=NS), mean large intestine score was reduced from 2.2 to 1.0 (p=0.03), and mean skin GVHD score was reduced from 1.6 to 0.8 (p=0.01). Remarkably, co-infusion of host-type APC and Th2 cells completely abrogated the Th2 cell anti-GVHD effect (average GVHD score, 10.4); in the absence of Th2 cells, host APC infusion also increased GVHD (average GVHD score increased from 7.4 to 9.4). Infusion of donor-type APC did not significantly alter GVHD scores either in the presence or absence of Th2 cell infusion. Each of the cohorts that received the T cell-replete allograft had reduced tumor burden relative to the tumor control cohort (Fig. 5b).
FIGURE 5. Host-type APC infusion abrogates Th2 cell anti-GVHD effect: histology.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow ("BMT"); marrow and TS/A tumor cells ("TSA"); marrow, tumor cells, and donor T cells ("GVHD"); marrow, tumor cells, donor T cells, and donor-type APC (“GVHD+Donor DC”); or marrow, tumor cells, donor T cells and host-type APC (“GVHD+Host DC”). Other cohorts received marrow, tumor cells, donor T cells, and donor Th2 cells either alone ("Th2”; day 14 post-BMT), with donor-type APC (“Th2+Donor DC”), or with host-type APC (“Th2+Host DC”). On day 19 post-BMT, lungs from treatment cohorts were removed to evaluate tumor burden; liver, stomach, small intestine, large intestine, cecum, and skin were harvested to assess GVHD. (a) For GVHD, each organ was scored (scale, 0 to 4), with cumulative GVHD score shown (maximum value, 24). (b) For tumor burden, a semi-quantitative determination of tumor cell infiltration was scored (scale, 0 to 4). n = 5 subjects were evaluated per cohort.
APC Transfer Abrogates Th2 Cell Therapy of GVHD: Cytokine Phenotype
Similar to our previous results, post-BMT T cells from GVHD controls in experiment #3 primarily secreted IFN-γ in an allospecific manner (Table VI, cohort 3; median value, 388 pg/ml). In GVHD controls, the additional infusion of donor-type APC at day 14 post-transplant did not significantly alter IFN-γ secretion (cohort 4, median value, 328 pg/ml); in contrast, additional infusion of host-type APC significantly increased IFN-γ secretion (cohort 5, median value, 812 pg/ml, p=0.03).
Also consistent with our prior results, delayed therapy with Th2 cells greatly reduced post-BMT IFN-γ secretion (median value decreased from 388 to 83 pg/ml, p=0.0005) and greatly increased both IL-4 secretion (median value increased from 67 to 497 pg/ml, p<0.0001) and IL-10 secretion (median value increased from 26 to 160 pg/ml, p<0.0001). Co-infusion of donor-type APC in addition to Th2 cells tended to increase IFN-γ secretion (median value increased from 83 to 115 pg/ml, p=0.07); by comparison, coinfusion of host-type APC in addition to Th2 cells significantly increased post-BMT IFN-γ secretion (median value increased from 83 to 279 pg/ml, p<0.0004). Furthermore, coinfusion of donor- or host-type APC tended to also reduce the Th2 cell-mediated promotion of post-BMT IL-4 and IL-10 secretion.
APC Transfer Abrogates Th2 cell therapy of GVHD: Donor T Cell Tracking
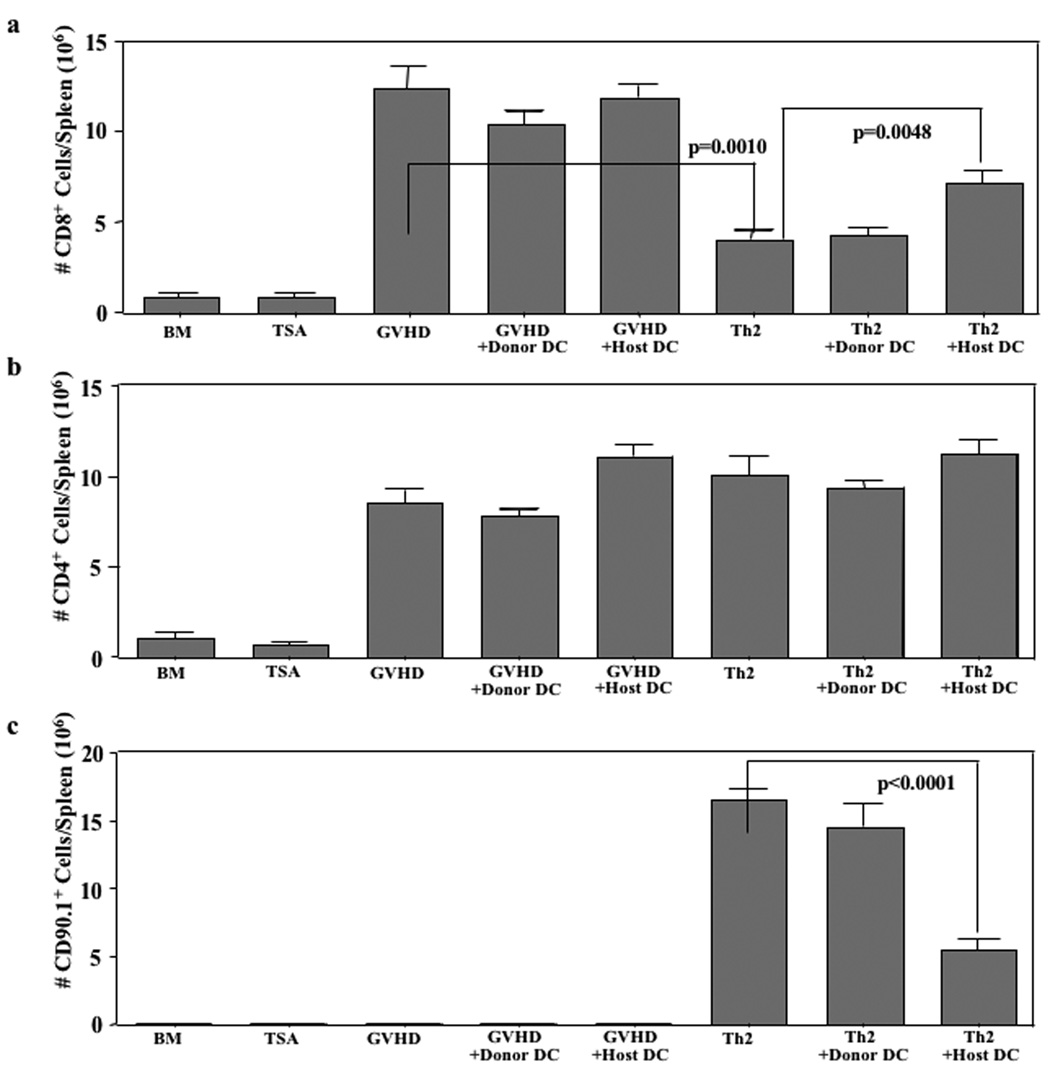
In experiment #3, we tracked the Th2 cell product in vivo using the CD90.1 marker and evaluated the effect of Th2 cells with or without co-infusion of donor or host APC on donor CD4+ and CD8+ T cells emanating from the allograft. First, we found that coinfusion of host-type APC, but not donor-type APC, significantly reduced the number of Th2 cells present in vivo at day 19 post-BMT (Fig. 6c; median Th2 cell number reduced from 16.6 to 5.4 × 106 cells per spleen, p<0.0001). Second, similar to our prior results, we found that Th2 cell therapy did not significantly reduce the number of post-BMT CD4+ T cells emanating from the initial allograft (Fig. 6b); co-infusion of donor- or host-type APC did not greatly alter CD4 cell counts post-BMT.
FIGURE 6. Host-type APC transfer abrogates Th2 cell therapy: donor T cell tracking.
B6-into-BALB/c BMT was performed (850 cGy host irradiation). Cohorts received donor marrow alone ("BMT"); marrow and TS/A tumor cells ("TSA"); marrow, tumor cells, and additional donor T cells ("GVHD"); marrow, tumor cells, donor T cells, and donortype APC (“GVHD+Donor DC”); or marrow, tumor cells, donor T cells and host-type APC (“GVHD+Host DC”). Other cohorts received marrow, tumor cells, donor T cells, and donor Th2 cells either alone ("Th2”; day 14 post-BMT), with donor-type APC (“Th2+Donor DC”), or with host-type APC (“Th2+Host DC”). On day 19 post-BMT, spleen cells were isolated and the absolute number of donor CD8+ T cells (a) and CD4+ T cells (b) were calculated from flow cytometry data (cells per spleen, × 106). Data shown are mean ± SEM (n = 5 per cohort).
And third, consistent with our previous results, we observed that Th2 cell therapy significantly reduced the post-BMT number of donor CD8+ T cells relative to GVHD controls (Fig. 6a; median values reduced from 12.4 to 4.1 × 106 cells/spleen, p=0.0010). Th2 cell-mediated restriction of CD8+ T cell expansion was partially reversed by host-type, but not donor-type, APC administration (Fig. 6a; increase in median CD8 number from 4.1 to 7.2 × 106 cells/spleen, p=0.0048). Host- or donor-type APC infusion did not significantly alter post-BMT CD8+ T cells numbers in the absence of Th2 cell infusion.
DISCUSSION
We have previously determined that delayed infusion of donor Th2 cells represents one approach to improve the problematic dissection of GVT effects from GVHD(7). This sequential allogeneic immunotherapy strategy first allows for a Th1/Tc1-mediated GVT effect, with subsequent down-regulation of GVHD by Th2 cell infusion. In this report, we have confirmed that Th2 cells can rapidly down-regulate severe, established GVHD in the setting of fully MHC-disparate transplantation; the pace of Th2 cell amelioration of GVHD was relatively rapid, as significant reversal of histologically-defined GVHD was observed within 5 days of Th2 cell infusion. Of note, the model that we have utilized is relatively stringent, as Th2 cell therapy was administered at day 14 post-transplant during GVHD-related cohort lethality. It is important to note that Th2 cells only resulted in a survival prolongation without a survival advantage; in an attempt to demonstrate a Th2 cell survival advantage, subsequent experiments will utilize lower donor T cell doses relative to the doses used in the current experiments. Prior investigations into the role of cell therapy for treatment of established GVHD have been limited, and in such cases, have utilized a relatively early cell infusion, such as day 2 or day 10 post-BMT infusion of regulatory T cells in models of GVHD and GVL effects involving transplantation into major or minor histocompatibility disparate hosts, respectively(37). Furthermore, we have extended our prior observations by elucidating cellular and molecular mechanisms associated with this therapy, thereby adding to the body of knowledge regarding the role of Th2 cells in the modulation of GVHD and identifying IL-4 and IL-10 as molecular mechanisms that contribute to Th2 cell therapy of GVHD.
Th2 cell infusion during established GVHD resulted in a relatively dramatic alteration in the Th1/Th2 cytokine balance post-BMT that was characterized by a marked reduction in allospecific IFN-γ secretion and a concomitant increase in allospecific IL-4 and IL-10 secretion. The experiment we performed using Th2 cells from cytokine-deficient donors demonstrated that type II cytokines were indeed the drivers for the protective effect: that is, recipients of Th2 cells deficient in either IL-4 or IL-10 had a post-BMT increase in allospecific IFN-γ, an increase in GVHD by histology analysis, and reduced post-BMT survival. We have previously shown that IL-4 secretion from Th2 cells is operative in the prevention of GVHD(7); the current results are the first to demonstrate that Th2 cell IL-4 secretion is also involved in the therapy of established GVHD. Similarly, IL-10 has previously been linked to GVHD prevention through cytokine therapy(15), regulatory T cell(16) or mesenchymal stem cell(20) therapy, or through other donor(17, 18) or host(19, 38) variables. The current results are the first to link IL-10 mechanistically to the therapy of established GVHD in general and to Th2 cell therapy in specific. It is interesting to note that Th2 cell deficiency in either IL-4 or IL-10 was sufficient to prevent the Th2 cell anti-GVHD effect. Of note, IL-4- or IL-10-deficient Th2 cells each led to reductions in both IL-4 and IL-10 secretion post-BMT and an increase in IFN-γ secretion post-BMT. As such, it appears that Th2 cell IL-4 and IL-10 may operate in an inter-dependent manner. Further studies will be required to clarify this biology, which we hypothesize may involve other cell populations, for example IL-4 polarization of unmanipulated donor T cells and IL-10 modulation of host- or donor-type APC. Interestingly, Th2 cell prevention of donor CD8+ T cell expansion occurred in a relatively IL-4 and IL-10 independent manner, thereby suggesting that Th2 cell regulation of CD8 cell expansion occurs by an alternative mechanism.
Contrary to our hypothesis, we found that IL-2 administration completely inhibited the biological and clinical effect of Th2 cells in the therapy of established GVHD. It is important to note that IL-2 infusion in the setting of the GVHD control cohort increased GVHD by histology and increased the number of post-BMT donor CD4+ T cells but did not reduce survival duration, did not increase the number of post-BMT CD8+ T cells, and did not alter the post-BMT Th1 cytokine profile; in sum, these data suggest that IL-2 availability was only partially limiting within the GVHD control cohort. By comparison, IL-2 administration reduced Th2 cell expansion post-BMT, increased expansion of CD4+ and CD8+ T cells originating from the initial allograft, increased allospecific IFN-secretion while limiting IL-4 and IL-10 secretion, and increased GVHD by histology and survival time analysis. These findings suggest that in the model we utilized, IL-2 is not a limiting cytokine for Th2 cell survival and/or expansion at day 14 post-transplant. Indeed, our findings are more consistent with a scenario whereby donor Th2 cells act in part as a “sink” for IL-2 that otherwise promotes effector T cell expansion; this biology would be consistent with findings that consumption of IL-2(39) or other common-γ chain signaling cytokines such as IL-7 and IL-15(40) by regulatory T cell populations can limit effector T cell expansion. Previous murine experiments have primarily associated IL-2 as a cytokine operational in the initial afferent stages of GVHD(27, 41); our murine findings are consistent with the clinical conclusion that IL-2 also appears to play a role in the pathogenesis of established acute GVHD(29). Further studies will be required to evaluate whether alternative cytokinebased strategies might enhance Th2 cell therapy through preferential expansion of Th2 cells relative to initial allograft T cells, such as low-dose IL-2 or administration of IL-7. Taken together, these data suggest that Th2 cell therapy in this model may operate not only through specific molecular pathways (IL-4 and IL-10 secretion) but also through additional, more complex mechanisms such as limitation of cytokine availability.
In addition, we found that infusion of host-type APC completely inhibited the biological and clinical effect of Th2 cells in the therapy of established GVHD. Importantly, infusion of host-type APC in the setting of the GVHD control cohorts had a relatively modest effect because only post-BMT IFN-γ secretion was increased (no increase in donor T cell numbers post-BMT, no increase in GVHD by histology, and no reduction in survival time); infusion of donor-type APC in the setting of the GVHD control cohort did not modulate any of these four experimental endpoints. These findings suggest that APC number and/or function were not particularly limiting factors for GVHD in the control cohort. In marked contrast, in the setting of Th2 cell therapy, day 14 post-BMT administration of host-type APC modulated each of the four experimental endpoints relative to control Th2 cell recipients. That is, host APC: [1] reduced Th2 cell expansion and increased expansion of CD4+ and CD8+ T cells originating from the initial allograft; [2] increased allospecific IFN-γ secretion while limiting IL-4 and IL-10 secretion; [3] increased GVHD by histology; and [4] reduced post-BMT survival time. By comparison to host APC infusion, donor-type APC were less robust in terms of their capacity to modulate Th2 cell therapy, as only two out of the four experimental endpoints were significantly modulated (survival time was reduced and Th2 polarization was blunted but GVHD by histology and donor T cell numbers were not significantly modulated). These finding are consistent with the conclusion reached by other investigators that both direct and indirect pathways of antigen-presentation contribute to established murine GVHD(42, 43); in our model using Th2 cell therapy, host-type APC appear to have played a dominant role. As such, contrary to our hypothesis, Th2 cell therapy was not optimized through the provision of additional alloantigen during established GVHD. Rather, our results are consistent with the notion that Th2 cells might ameliorate established GVHD through APC modulation, in particular, host-type APC modulation. Because IL-10 was required for Th2 cell efficacy, we speculate that Th2 cells may have inhibited established GVHD through the well-characterized ability of IL-10 to inhibit APC function [reviewed in(44)], with subsequent reduction in the expansion and activation of allograft T cells.
In conclusion, these experiments provide further support to the characterization of acute GVHD as predominately a Th1/Tc1-type process that is amenable to regulation by cell therapy with ex vivo generated donor Th2 cells. To our knowledge, Th2 cell donor lymphocyte infusion is the only form of adoptive immunotherapy identified as being effective in ameliorating established GVHD at a phase of severe tissue damage and cohort lethality. This therapeutic effect required cell product secretion of IL-4 and IL-10, thereby providing a mechanistic signature that is classical for a Th2-type response. Our attempts to enhance Th2 cell therapy with IL-2 administration or APC infusion proved counter-productive; indeed, these failed therapeutic attempts suggest that the mechanism of Th2 cell amelioration of established GVHD likely involve additional components, including cytokine consumption and APC modulation. As such, further research will seek to identify alternative strategies to achieve more profound type I → type II cytokine polarization in vivo for potential consolidation of Th2 cell-mediated anti-GVHD effects.
Table VI.
APC Adoptive Transfer Reduces Th2 Cell Capacity to Polarize Immunity Post-BMT.
| Transplant Componentsb |
Post-BMT Cytokine Productione |
p-valuesf |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cohort # | Host Tumora | BM | T Cells | Th2 Cells | Donor DCc | Host DCd | IL-2 | IFN-γ | IL-4 | IL-10 | IL-2 | IFN-γ | IL-4 | IL-10 |
| 1 | − | + | − | − | 2±1 | 2±1 | 4±1 | 2±1 | ||||||
| 2 | + | + | − | − | 1±1 | 1±1 | 9±1 | 3±1 | ||||||
| 3 | + | + | + | − | 11±4 | 388±54 | 67±11 | 26±7 | ||||||
| 4 | + | + | + | − | − | 10±3 | 328±128 | 27±5 | 22±4 | p=NS (#4 vs. #3) |
p=NS (#4 vs. #3) |
p=0.006 (#4 vs. #3) |
p=NS (#4 vs. #3) |
|
| 5 | + | + | + | − | − | + | 21±6 | 812±151 | 35±17 | 34±14 | p=NS (#5 vs. #3) |
p=0.03 (#5 vs. #3) |
p=NS (#5 vs. #3) |
p=NS (#5 vs. #3) |
| 6 | + | + | + | Th2 | − | − | 12±1 | 83±5 | 497±47 | 160±32 | p=NS (#6 vs. #3) |
p=0.0005 (#6 vs. #3) |
p<0.0001 (#6 vs. #3) |
p=0.003 (#6 vs. #3) |
| 7 | + | + | + | Th2 | − | − | 10±1 | 115±16 | 291±59 | 79±15 | p=NS; p=NS (#7 vs. #3); (#7 vs. #6) |
p=0.001; p=NS (#7 vs. #3); (#7 vs. #6) |
p=0.01;p=0.03 (#7 vs. #3); (#7 vs. #6) |
p=0.01;p=0.053 (#7 vs. #3); (#7 vs. #6) |
| 8 | + | + | + | Th2 | − | + | 35±17 | 279±33 | 217±96 | 51±22 | p=NS ; p=NS (#8 vs. #3); (#8 vs. #6) |
p=NS ; p=0.0004 (#8 vs. #3); (#8 vs. #6) |
p=NS; p=0.03 (#8 vs. #3); (#8 vs. #6) |
p=NS; p=NS (#8 vs. #3); (#8 vs. #6) |
BALB/c host received 850 cGy XRT; host TS/A tumor cells 0.1× 106.
C57BL/6 into BALB/c (day 0); BM (C57BL/6; 10×106 cells); donor T cells (5×106 cells); donor Th2 cells (10×106 cells; day 14 post-BMT).
Fresh DC cells generated from donor C57BL/6 (10×106 cells; day14 post-BMT).
Fresh DC cells generated from host BALB/c (10×106 cells; day14 post-BMT).
Cytokine production measured by Luminex assay at day19 post-BMT; IL-4, IL-10, IL-2, and IFN-γ (pg/ml); Mean ± SEM of n=5/group.
Unpaired t test.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57:225–244. doi: 10.1016/j.critrevonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Fowler DH, Kurasawa K, Husebekk A, Cohen PA, Gress RE. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft-versus-host reaction. Regulation of cytokines and CD8+ lymphoid engraftment. Journal of Immunology. 1994;152:1004–1013. [PubMed] [Google Scholar]
- 3.Krenger W, Snyder KM, Byon JC, Falzarano G, Ferrara JL. Polarized type 2 alloreactive CD4+ and CD8+ donor T cells fail to induce experimental acute graft-versus-host disease. Journal of Immunology. 1995;155:585–593. [PubMed] [Google Scholar]
- 4.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. Journal of Immunology. 1996;157:4811–4821. [PubMed] [Google Scholar]
- 5.Fowler DH, Breglio J, Nagel G, Hirose C, Gress RE. Allospecific CD4+, Th1/Th2 and CD8+, Tc1/Tc2 populations in murine GVL: type I cells generate GVL and type II cells abrogate GVL. Biology of Blood & Marrow Transplantation. 1996;2:118–125. [PubMed] [Google Scholar]
- 6.Jung U, Foley JE, Erdmann AA, Eckhaus MA, Fowler DH. CD3/CD28-costimulated T1 and T2 subsets: differential in vivo allosensitization generates distinct GVT and GVHD effects. Blood. 2003;102:3439–3446. doi: 10.1182/blood-2002-12-3936. [DOI] [PubMed] [Google Scholar]
- 7.Foley JE, Jung U, Miera A, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005;175:5732–5743. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 8.Le Gros G, Ben-Sasson SZ, Seder R, Finkelman FD, Paul WE. Generation of interleukin 4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing cells. Journal of Experimental Medicine. 1990;172:921–929. doi: 10.1084/jem.172.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang H, Paul WE. Impaired interleukin 4 signaling in T helper type 1 cells. Journal of Experimental Medicine. 1998;187:1305–1313. doi: 10.1084/jem.187.8.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sad S, Mosmann TR. Interleukin (IL) 4, in the absence of antigen stimulation, induces an anergy-like state in differentiated CD8+ TC1 cells: loss of IL-2 synthesis and autonomous proliferation but retention of cytotoxicity and synthesis of other cytokines. J Exp Med. 1995;182:1505–1515. doi: 10.1084/jem.182.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacchetta R, Bigler M, Touraine JL, et al. High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. Journal of Experimental Medicine. 1994;179:493–502. doi: 10.1084/jem.179.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 13.Krenger W, Snyder K, Smith S, Ferrara JL. Effects of exogenous interleukin-10 in a murine model of graft-versus-host disease to minor histocompatibility antigens. Transplantation. 1994;58:1251–1257. [PubMed] [Google Scholar]
- 14.Blazar BR, Taylor PA, Smith S, Vallera DA. Interleukin-10 administration decreases survival in murine recipients of major histocompatibility complex disparate donor bone marrow grafts. Blood. 1995;85:842–851. [PubMed] [Google Scholar]
- 15.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, et al. Interleukin-10 dose-dependent regulation of CD4+ and CD8+ T cell-mediated graft-versus-host disease. Transplantation. 1998;66:1220–1229. doi: 10.1097/00007890-199811150-00018. [DOI] [PubMed] [Google Scholar]
- 16.Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389–399. doi: 10.1084/jem.20020399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald KP, Rowe V, Clouston AD, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–1850. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 18.Morris ES, MacDonald KP, Rowe V, et al. Donor treatment with pegylated GCSF augments the generation of IL-10-producing regulatory T cells and promotes transplantation tolerance. Blood. 2004;103:3573–3581. doi: 10.1182/blood-2003-08-2864. [DOI] [PubMed] [Google Scholar]
- 19.Rowe V, Banovic T, MacDonald KP, et al. Host B cells produce IL-10 following TBI and attenuate acute GVHD after allogeneic bone marrow transplantation. Blood. 2006;108:2485–2492. doi: 10.1182/blood-2006-04-016063. [DOI] [PubMed] [Google Scholar]
- 20.Min CK, Kim BG, Park G, Cho B, Oh IH. IL-10-transduced bone marrow mesenchymal stem cells can attenuate the severity of acute graft-versus-host mesenchymal stem cells can attenuate the severity of acute graft-versus-host disease after experimental allogeneic stem cell transplantation. Bone Marrow Transplant. 2007;39:637–645. doi: 10.1038/sj.bmt.1705644. [DOI] [PubMed] [Google Scholar]
- 21.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 22.Mekala DJ, Alli RS, Geiger TL. IL-10-dependent suppression of experimental allergic encephalomyelitis by Th2-differentiated, anti-TCR redirected T lymphocytes. J Immunol. 2005;174:3789–3797. doi: 10.4049/jimmunol.174.6.3789. [DOI] [PubMed] [Google Scholar]
- 23.Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson BH, Lord JD, Greenberg PD. Cytoplasmic domains of the interleukin-2 receptor beta and gamma chains mediate the signal for T-cell proliferation. Nature. 1994;369:333–336. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Rosenberg SA. Interleukin-2-independent proliferation of human melanoma-reactive T lymphocytes transduced with an exogenous IL-2 gene is stimulation dependent. J Immunother. 2003;26:190–201. doi: 10.1097/00002371-200305000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cote-Sierra J, Foucras G, Guo L, et al. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara JL, Marion A, McIntyre JF, Murphy GF, Burakoff SJ. Amelioration of acute graft vs host disease due to minor histocompatibility antigens by in vivo administration of anti-interleukin 2 receptor antibody. J Immunol. 1986;137:1874–1877. [PubMed] [Google Scholar]
- 28.Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graft-versus-host disease. Int Immunol. 1993;5:565–572. doi: 10.1093/intimm/5.6.565. [DOI] [PubMed] [Google Scholar]
- 29.Przepiorka D, Kernan NA, Ippoliti C, et al. Daclizumab, a humanized anti-interleukin-2 receptor alpha chain antibody, for treatment of acute graft-versus-host disease. Blood. 2000;95:83–89. [PubMed] [Google Scholar]
- 30.Levine BL, Bernstein WB, Connors M, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–5930. [PubMed] [Google Scholar]
- 31.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of graft versus host disease by inactivation of host antigen-presenting cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 32.Xia G, Truitt RL, Johnson BD. Graft-versus-leukemia and graft-versus-host reactions after donor lymphocyte infusion are initiated by host-type antigen-presenting cells and regulated by regulatory T cells in early and long-term chimeras. Biol Blood Marrow Transplant. 2006;12:397–407. doi: 10.1016/j.bbmt.2005.11.519. [DOI] [PubMed] [Google Scholar]
- 33.Levine BL, Ueda Y, Craighead N, Huang ML, June CH. CD28 ligands CD80 B7-1) and CD86 (B7-2) induce long-term autocrine growth of CD4+ T cells and induce similar patterns of cytokine secretion in vitro. Int Immunol. 1995;7:891–904. doi: 10.1093/intimm/7.6.891. [DOI] [PubMed] [Google Scholar]
- 34.Jung U, Foley JE, Erdmann AA, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006;12:905–918. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 35.Kummar S, Ishii A, Yang HK, Venzon DJ, Kim SJ, Gress RE. Modulation of graft-versus-tumor effects in a murine allogeneic bone marrow transplantation model by tumor-derived transforming growth factor-betaI. Biol Blood Marrow Transplant. 2001;7:25–30. doi: 10.1053/bbmt.2001.v7.pm11215695. [DOI] [PubMed] [Google Scholar]
- 36.Erdmann AA, Gao ZG, Jung U, et al. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SC, Murphy GF, Korngold R. Post-hematopoietic cell transplantation control of graft-versus-host disease by donor CD425 T cells to allow an effective graft-versus-leukemia response. Biol Blood Marrow Transplant. 2003;9:243–256. doi: 10.1053/bbmt.2003.50027. [DOI] [PubMed] [Google Scholar]
- 38.Lin MT, Storer B, Martin PJ, et al. Relation of an interleukin-10 promoter polymorphism to graft-versus-host disease and survival after hematopoietic-cell transplantation. N Engl J Med. 2003;349:2201–2210. doi: 10.1056/NEJMoa022060. [DOI] [PubMed] [Google Scholar]
- 39.Thornton AM, Donovan EE, Piccirillo CA, Shevach EM. Cutting edge: IL-2 is critically required for the in vitro activation of CD4+CD25+ T cell suppressor function. J Immunol. 2004;172:6519–6523. doi: 10.4049/jimmunol.172.11.6519. [DOI] [PubMed] [Google Scholar]
- 40.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Via CS, Finkelman FD. Critical role of interleukin-2 in the development of acute graft-versus-host disease. International Immunology. 1993;5:565–572. doi: 10.1093/intimm/5.6.565. [DOI] [PubMed] [Google Scholar]
- 42.Anderson BE, McNiff JM, Jain D, Blazar BR, Shlomchik WD, Shlomchik MJ. Distinct roles for donor- and host-derived antigen-presenting cells and costimulatory molecules in murine chronic graft-versus-host disease: requirements depend on target organ. Blood. 2005;105:2227–2234. doi: 10.1182/blood-2004-08-3032. [DOI] [PubMed] [Google Scholar]
- 43.Chakraverty R, Sykes M. The role of antigen-presenting cells in triggering graft-versus-host disease and graft-versus-leukemia. Blood. 2007;110:9–17. doi: 10.1182/blood-2006-12-022038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton BE. STAT3: a potential therapeutic target in dendritic cells for the induction of transplant tolerance. Expert Opin Ther Targets. 2006;10:459–470. doi: 10.1517/14728222.10.3.459. [DOI] [PubMed] [Google Scholar]