Abstract
Inflammatory bowel diseases (IBD) represent important chronic conditions affecting the gastrointestinal tract in man. However, similar disorders are found in several animal species and the IBD affecting dogs are particularly important. These are encompassed by an umbrella of probably several different entities with common symptoms, some of which seem to share striking similarities with human conditions. This review will focus on the actual knowledge of IBD in dogs, and attempt to identify differences and similarities with human IBD conditions.
Keywords: Colitis, Dog, Inflammatory bowel diseases
INTRODUCTION
Inflammatory bowel diseases (IBD) are well known gastrointestinal disorders in humans; however, these disorders are also present and often investigated in animals[1]. IBD in man [Crohn’s disease (CD) and ulcerative colitis (UC)] can be defined as chronic idiopathic multifactorial diseases[2,3]. CD is typically a disease of ileum and colon, but can also affect other areas of the digestive tract[4], forms granulomas and involves the whole intestinal wall, while UC is an ulcerative and inflammatory disease usually limited to superficial layers (mucosa, superficial part of the submucosa) of the colon[5].
Dogs may also develop IBD[6], being part of the chronic enteropathies if lasting more than three weeks[7], and are properly defined when there is histological demonstration of mucosal inflammation and all other possible causes of enteritis/infiltrates have been investigated and excluded[7-10]. Enteritis is classified depending on which predominant cells infiltrate the intestinal wall and where this infiltration takes place[11].
Thus, chronic diseases of the small intestine that can be included among IBD are lymphocytic-plasmacytic enteritis (LPE), eosinophilic enteritis and eosinophilic gastro-enteritis (EGE)[10]. With regard to the large intestine, four main conditions are recognized as IBD in dogs: lymphocytic-plasmacytic colitis, eosinophilic colitis, histiocytic ulcerative colitis (HUC) (mainly PAS-positive macrophages), and regional granulomatous colitis (mainly PAS-negative macrophages)[10,12-16]. However, it is worth noting that such diseases may frequently involve both the small and the large intestine, or even include the stomach: lymphocytic-plasmacytic enterocolitis, eosinophilic enterocolitis (EEC) or gastroenterocolitis (EGEC) and granulomatous gastroenteritis[8,10,13,15].
In dogs, another important chronic enteritis, whose causes have not yet been clearly defined, but have been widely investigated and for which underlying allergic factors have been hypothesized, is the so-called food-responsive diarrhoea (FRD)[6]. This disease is especially important because it is often included in the differential diagnosis of IBD.
IBD is considered to have the same incidence in males and females, and middle-aged dogs seem to be more affected[10]. With regard to breed predisposition, this has been suggested for some specific forms of IBD such as immunoproliferative enteropathy in Basenjis, protein-losing enteropathy and associated protein-losing nephropathy in Soft-Coated Wheaten Terriers, and HUC in boxers[10,11]. However, although HUC displays a higher predisposition in boxers[17], it has also been described in other breeds of dogs such as French Bulldog, Doberman Pinscher, Mastiff and Alaskan Malamute[16,18].
PATHOGENESIS
Information in dogs
In dogs, the development of IBD is thought to originate as a consequence of a deregulation of mucosal immunity in predisposed animals[14]. The loss of tolerance to antigens (food, intestinal bacteria, etc.) is one of the most studied mechanisms that could justify the development of chronic intestinal inflammation[8,11,19]. The immune-mediated basis of the disease can be inferred by the response to the administration of immunomodulant drugs; the presence of increased IgE positive cells in diseased dogs compared to healthy dogs is a further aspect that also suggests the involvement of hypersensitivity reactions in the pathogenesis of canine IBD[20], as well as the increased concentration of eosinophils and mast cells in many dogs with EGE[8]. Interruption of the mucosal barrier, independently of the primary cause (bacterial, chemical, etc.), can also lead to further antigen exposure, allowing the process to become chronic[21], and is enforced by decreased apoptosis of lymphocytes, as demonstrated in dogs with IBD compared to control dogs[22].
Homeostasis inside the digestive tract is maintained by the equilibrium between the reactions to pathogens and to commensal bacteria or other inoffensive luminal antigens (tolerance) that are mediated by different molecules[23]. The presence of mucosal tolerance to harmless antigens is very important, because depending on its absence the subsequent inflammatory response can be exaggerated and even detrimental. Such tolerance is probably based on the fact that the antigen is presented or not, contextually to other danger signals[10,24]. The difference between tolerance and reaction is also based on pattern recognition receptors (PRRs)[25], which are able to recognize microflora according to their pathogen-associated molecular patterns or microbe-associated molecular patterns[26].
Similar to humans, the study of IBD in affected dogs has led to the hypothesis that genetic factors and enteric bacteria can play a pivotal role in the pathogenesis of these disorders, owing to the abnormal intestinal response to commensal microflora[26]. Once stimulated, PRRs such as Toll-like receptors (TLRs)[27] start their pro-inflammatory activity, and a recent study showed that three TLRs (2, 4, and 9) stimulated by bacteria are up-regulated in dogs with IBD[26]. These results are similar to those following the activation of TLR4 which has been demonstrated in humans suffering from IBD[28]. In these patients, both genetic predisposition and environmental factors are considered important elements in the development of the disease. Moreover, as already well known in humans[29,30], a recent study showed that in IBD dogs, small intestinal bacteria are different from those found in healthy dogs[31], strengthening the idea of a correlation between microflora and IBD.
Thus, even in small animals, and similar to that in man, intestinal lymphocyte subset distribution and major histocompatibility complex class II antigens, as well as cytokine gene expression and other markers, have yielded interesting and sometimes overlapping results[9,32-36]. For instance, one study showed that dogs with IBD display a larger number of IgE positive cells than healthy dogs, in a manner similar to that for interleukin 4 (IL-4) expression in man with IBD[20]. In addition, the modulation of the expression of intestinal lamina propria lymphocytes P glycoprotein (P-gp) seems to play a similar role in both human and dog IBD. In fact, in IBD patients scarcely responsive to steroid treatment, P-gp is highly expressed, and in dogs showing a good response to treatment this protein is modestly represented[37].
As previously documented in humans, in IBD dogs the investigation of specific subsets of cell populations led to the demonstration of decreased numbers of mast cells (MCs) and of an increase in both CD3+ cells and IgG+ plasma cells[38].
In veterinary medicine, encouraging results also originate from the study of nuclear receptors (NRs, presumably also involved in the genesis of IBD in man), such as peroxisome proliferator-associated receptor α (PPARα) and especially of NR target genes [such as multi-drug-resistance gene-1 (MDR1)], multiple drug resistance-associated proteins (MRP2), cytochrome P450 (CYP3A12), and phenol-sulfating phenolsulfotransferase (SULT1A1)[39,40]. In a recent study it was highlighted that MRP2, CYP3A12, SULT1A1, and PPARα are more expressed in FRD and/or IBD dogs and that MDR1 can also differentiate between them[21]. Moreover, in a study on German shepherd dogs with IBD, the mRNA expression of many cytokines such as IL-2, IL-5, IL-12p40, interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α), and transforming growth factor-β1 (TGF-β1) has been shown to be higher in diseased animals compared to controls[34]; however, it must also be kept in mind that other studies reconsidered the increased expression of some cytokines in dogs with IBD[35,36].
Information in humans
IBD in man, particularly CD, probably recognise a genetic predisposition and many genes such as NOD2/CARD15, HLA and IL-1-Ra genes have been investigated and are thought to be implicated in CD development[41-43]. However, environmental factors[44,45] and gut microbiota can also be involved in IBD[46-49], as shown by the fact that antibacterial drugs may help in controlling symptoms[50-52]. An important point for the perpetuation of damage in human IBD is that once stimulated, lymphocytes tend to accumulate in the lamina propria because they seem to be more resistant to apoptosis[53]. Moreover, an imbalance between proinflammatory and anti-inflammatory cytokines may also play a role[54].
In man, nuclear factor-κB (NF-κB) has also been associated with IBD[55], and it has been shown that IL-23 can lead to the differentiation of Th17 lymphocytes producing IL-17 with subsequent activation of NF-κB pro-inflammatory signals[56]. In a recent study, the link between IBD and NF-κB has also been hypothesized in dogs, where biopsy samples from dogs with IBD showed that the presence of NF-κB activation in lamina propria macrophages was higher than in the control group[23].
Differences between species
An important development in human medicine has shown the different involvement of lymphocyte subtypes (Th1 and Th2) in IBD, with Th1 being mainly implicated in CD while Th2 predominate in UC[57], with subsequent different cytokine activation[23]. In dogs, different to man, there is mixed activation of Th1 and Th2 in IBD[6,23,38], leading to different expression of some cytokines, even though it has recently been hypothesized that different Th cells can be involved in different IBD types[8]. Some authors have shown that MC can be increased or decreased, depending on the predominant cellular infiltrate, and that these are predominantly reduced in LPE, and increased in EGE, thus hypothesizing a possible different genesis for the two types of IBD (Th1 for LPE and Th2 for EGE)[8].
Even though the above studies signify an active interest in IBD pathogenesis, it is also evident that the mechanisms behind the disease are far from understood, both in man and dogs. HUC, a peculiar condition, in which a central role for bacteria has been proposed due to the presence of intralesional microbes and PAS + macrophages, similar to Whipple’s disease in man[7].
CLINICAL AND DIAGNOSTIC ASPECTS
Clinical manifestations of IBD in dogs are numerous and nonspecific; the most common clinical signs are weight loss, persistent or recurrent vomiting and/or diarrhoea[8], frequently associated with symptoms that are an expression of eventual complications, such as ascites (if hypoalbuminemia is present) or pallor of mucous membranes (in the case of chronic gastrointestinal bleeding)[11,58].
Before reaching a diagnosis of IBD it is important to exclude all other possible causes of chronic enteritis[6,59] by complete clinical examination, laboratory tests and instrumental investigations, including biopsy samples for histological assessment. Diet correction is also an important tool to exclude or eventually confirm FRD[23].
Since in dogs the diagnosis of IBD is by exclusion, it is obvious that many tests performed during the diagnostic iter (for example blood, urine and faeces examinations) are necessary to exclude other causes of inflammation, and are rarely specific for IBD, thus, not overestimating the incidence of such a diagnosis[10]. This aspect is very important, because if the cause of the chronic enteritis is misdiagnosed and it is treated as an IBD, it is unlikely to resolve[58].
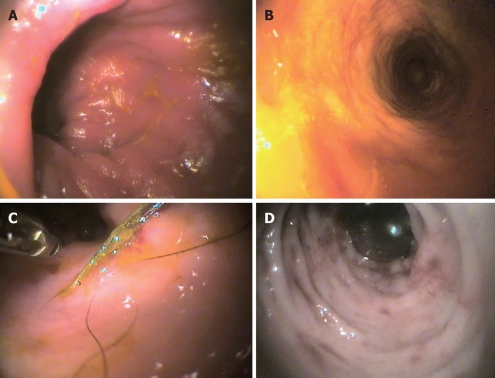
In diagnosing IBD, instrumental diagnostics are important and often of paramount importance, even though of the three most used techniques, i.e. radiology (X-R), ultrasonography (US) and endoscopy, only the latter yields more specific information for the diagnosis of IBD (Figure 1), especially as it allows biopsy samples, which are indispensable to distinguish the various subtypes of mucosal infiltration[60]. X-R and US (which give important information on gut layering and wall thickness) seem to be more helpful for the exclusion of other possible causes[61], and because the importance of intestinal wall thickening in dogs with IBD has recently been revaluated[10,62,63].
Figure 1.
Representative endoscopic images in the dog. A: Lymphocytic-plasmacytic colitis; B: Macrofollicular and diffuse interstitial lymphocytic colitis; C: Histiocytic colitis; D: Neutrophilic-eosinophilic colitis.
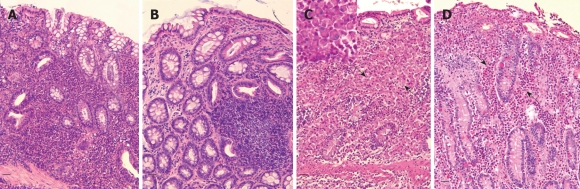
After having excluded the most common causes of chronic enteropathies, intestinal biopsies, obtained surgically or endoscopically depending on circumstances (endoscopy is less invasive, but sampling is limited in terms of site of execution and/or of dimension), can allow the diagnosis of IBD[10] (Figure 2). However, it is important to stress that biopsy samples are not unequivocally interpretable[6,10,64], even though recent work helped in clarifying such interpretation by providing a histopathological score for mucosal changes in dogs[65].
Figure 2.
Representative histologic images in the dog (HE, bar = 50 μm). A: Lymphocytic-plasmacytic colitis. Note the interstitial diffuse pattern of infiltrate represented by a large amount of lymphocytes mixed with plasma cells and some macrophages; B: Lymphocytic-plasmacytic colitis (follicular variant); C: Histiocytic colitis. Severe mucosal abnormalities with loss of crypts and diffuse infiltration by large macrophages (arrows) that in the insert (PAS stain) are shown as the main cells infiltrating the lamina propria; D: Eosinophilic colitis. Note the presence of a large number of eosinophils (arrows).
In humans, to diagnose and/or to monitor IBD, several serological markers such as anti-neutrophil cytoplasmic antibody (p-ANCA), anti-Saccharomyces cerevisiae (ASCA), outer membrane porin C antibody (anti-OmpC), anti-laminaribioside antibody, anti-mannobioside antibody, and anti-chitobioside antibody, anti-pancreas Ab and anti-caliciform intestinal cells Ab, faecal calprotectin, lactoferrin and polymorphonuclear neutrophil elastase are available and have been tested, although not all of these are employed in clinical practice[66-68].
Together with the clinicopathological diagnosis/monitoring of IBD, in veterinary medicine several markers such as p-ANCA and ASCA, have also been investigated for this purpose. However, even if these were indicative of IBD[69], no correlation with symptoms or pathological aspects was documented for p-ANCA[19]. Conversely, in dogs with IBD mucosal permeability measured through the administration of lactulose and rhamnose has been correlated to the histological gravity of the lesions[70]. The serum concentrations of folate and cobalamin have also been investigated, and although they represent nonspecific findings[71] these are important for supplementation during treatment[10]. Moreover, IgG and nitrite concentrations in colonic lavage fluid can be higher in dogs with IBD than in controls, with important implications in the diagnosis and monitoring of the disease[72]. Other markers, such as microalbuminuria and C-reactive protein, yielded less encouraging results, since they showed no correlation with symptoms or pathological assessment[73]. As stated above, IBD in dogs can be accompanied by protein loss, which is easily verified by measuring serum albumins; the presence of a new test to dose α1-proteinase inhibitor (α1-PI) in faeces may allow the earlier identification of protein loss, because this protein has a molecular mass similar to that of albumin, but has the advantage of persisting unaltered in stool[71].
If diagnosis of the disease necessitates a long and complete diagnostic plan, important tools for the clinical evaluation of IBD are the canine chronic enteropathy activity index and the canine IBD activity index (CIBDAI) (similar to the Crohn’s disease activity index in man[74]) that also allows follow-up of the patient during treatment[75-77], even though the CIBDAI does not seem to correlate with histopathologic grade[73]. One important aspect in managing IBD is that it is very difficult to foresee the outcome of the patient; fortunately, many important markers have recently been studied as prognostic indicators, such as canine pancreatic lipase immunoreactivity (cPLI), cobalamin and albumin concentration[78-80].
TREATMENT
As in human medicine, the main difficulties in treating dogs with IBD originate from an incomplete understanding of the pathophysiological basis of these diseases; thus, it is not surprising that in many cases therapeutic protocols are partially borrowed from those adopted in man[23,78]. Usually, the therapeutic protocols of choice for IBD in dogs depend on the seriousness of the disease and, eventually, on the resistance to some drugs. Some patients, such as those with FRD, may improve or even resolve their condition following the simple administration of a specific diet, such as those based on hydrolysed proteins[58].
In general, the first approach in dogs with IBD usually involves some dietary modification, the use of prebiotics-probiotics[10,11], antimicrobials and, eventually, corticosteroids (prednisolone or, more recently, budesonide)[58]. Metronidazole represents an important therapeutic agent, because of its simultaneous antimicrobial and immunomodulatory action[10,58]. Other immunomodulatory or anti-inflammatory drugs, such as azathioprine, cyclosporin, chlorambucil, cyclophosphamide and 5-aminosalicylates can be administered as an alternative or in association with the steroids, depending on the molecule[10,12,58,78]. When treating a dog with IBD it is also important to add symptomatic therapeutic measures such as gastroprotectors, antiemetics, motility modulators, etc., and to correct any possible imbalance (for example, by means of cobalamin or electrolyte supplementation)[10,58].
Considering that the knowledge of the cytokine patterns involved in IBD human patients represents an important tool in the development of new therapeutic strategies[61], this could also supply new therapeutic tools in veterinary medicine[10,58]; the results of a recent study on intestinal biopsies from dogs with IBD, assessing growth hormone receptor and insulin-like growth factor (IGF-1/-2) mRNA expression, gave further perspectives for IBD treatment[82].
CONCLUSION
To date, the relationship between IBD in humans and dogs has not been completely defined, especially with regard to pathophysiology and therapeutic protocols; thus, of the aspects investigated in man only a few could probably be successfully translated to dogs. In fact, IBD in the latter displays several and some strikingly different forms with respect to humans, in whom these diseases display more standardized clinical, endoscopic, and pathologic aspects.
However, as suggested by some authors[83,84], the parallel study of IBD in animals could also lead to important information in man[85], and hopefully develop better therapeutic measures to alleviate these troubles in our canine friends and companions.
Footnotes
Peer reviewer: Dr. Pingchang Yang, MD, PhD, Department of Pahtology & Molecular Medicine, McMaster University, BBI-T3330, 50 Charlton Ave East, Hamilton, L8N 4A6, Canada
S- Editor Tian L L- Editor Webster JR E- Editor Lin YP
References
- 1.Ostanin DV, Bao J, Koboziev I, Gray L, Robinson-Jackson SA, Kosloski-Davidson M, Price VH, Grisham MB. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–G146. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 3.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn's disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- 4.Stange EF, Travis SP, Vermeire S, Beglinger C, Kupcinkas L, Geboes K, Barakauskiene A, Villanacci V, Von Herbay A, Warren BF, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. Gut. 2006;55 Suppl 1:i1–i15. doi: 10.1136/gut.2005.081950a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stange EF, Travis SPL,Vermeire S, Reinisch W, Geboes K, Barakauskiene A, Feakins R, Fléjou JF, Herfarth H, Hommes DW, Kupcinskas L, Lakatos PL, Mantzaris GJ, Schreiber S, Villanacci V, Warren BF; European Crohn's and Colitis Organisation (ECCO) European evidence-based Consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohn’s Colitis. 2008;2:1–23. doi: 10.1016/j.crohns.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Cave NJ. Chronic inflammatory disorders of the gastrointestinal tract of companion animals. N Z Vet J. 2003;51:262–274. doi: 10.1080/00480169.2003.36380. [DOI] [PubMed] [Google Scholar]
- 7.Washabau RJ, Holt DE. Diseases of the large intestine. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis: Elsevier-Saunders; 2005. pp. 1378–1407. [Google Scholar]
- 8.Kleinschmidt S, Meneses F, Nolte I, Hewicker-Trautwein M. Characterization of mast cell numbers and subtypes in biopsies from the gastrointestinal tract of dogs with lymphocytic-plasmacytic or eosinophilic gastroenterocolitis. Vet Immunol Immunopathol. 2007;120:80–92. doi: 10.1016/j.vetimm.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Schreiner NM, Gaschen F, Gröne A, Sauter SN, Allenspach K. Clinical signs, histology, and CD3-positive cells before and after treatment of dogs with chronic enteropathies. J Vet Intern Med. 2008;22:1079–1083. doi: 10.1111/j.1939-1676.2008.0153.x. [DOI] [PubMed] [Google Scholar]
- 10.Hall EJ, German AJ. Malattia infiammatoria intestinale. In: Steiner JM, editor. Gastroenterologia del cane e del gatto. Milano: Elsevier; 2009. pp. 296–311. [Google Scholar]
- 11.German AJ, Hall EJ, Day MJ. Chronic intestinal inflammation and intestinal disease in dogs. J Vet Intern Med. 2003;17:8–20. doi: 10.1892/0891-6640(2003)017<0008:ciiaid>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Zoran DL. Fisiopatologia e trattamento delle colopatie nel cane. Suppl Veterinaria. 2001;15:19–30. [Google Scholar]
- 13.Scherding RG. Diseases of the large intestine. In: Tams TR, editor. Handbook of Small Animal Gastroenterology. 2nd ed. St. Louis: Saunders; 2003. pp. 251–285. [Google Scholar]
- 14.Jergens AE, Zoran DL. Diseases of the colon and rectum. In: Hall EJ, Simpson JW, Williams DA, editors. BSAVA Manual of Canine and Feline Gastroenterology, 2nd edition. Gloucester: British Small Animal Veterinary Association; 2005. pp. 203–212. [Google Scholar]
- 15.Willard MD. Patologie dell’apparato digerente. In: Nelson RW, Couto CG, editors. Medicina interna del cane e del gatto. 3rd ed. Milano: Elsevier Masson; 2007. pp. 255–482. [Google Scholar]
- 16.Leib MS. Il grosso intestino. In: Steiner JM, editor. Gastroenterologia del cane e del gatto. Milano: Elsevier; 2009. pp. 205–212. [Google Scholar]
- 17.Davies DR, O'Hara AJ, Irwin PJ, Guilford WG. Successful management of histiocytic ulcerative colitis with enrofloxacin in two Boxer dogs. Aust Vet J. 2004;82:58–61. doi: 10.1111/j.1751-0813.2004.tb14643.x. [DOI] [PubMed] [Google Scholar]
- 18.Stokes JE, Kruger JM, Mullaney T, Holan K, Schall W. Histiocytic ulcerative colitis in three non-boxer dogs. J Am Anim Hosp Assoc. 2001;37:461–465. doi: 10.5326/15473317-37-5-461. [DOI] [PubMed] [Google Scholar]
- 19.Luckschander N, Allenspach K, Hall J, Seibold F, Gröne A, Doherr MG, Gaschen F. Perinuclear antineutrophilic cytoplasmic antibody and response to treatment in diarrheic dogs with food responsive disease or inflammatory bowel disease. J Vet Intern Med. 2006;20:221–227. doi: 10.1892/0891-6640(2006)20[221:pacaar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Locher C, Tipold A, Welle M, Busato A, Zurbriggen A, Griot-Wenk ME. Quantitative assessment of mast cells and expression of IgE protein and mRNA for IgE and interleukin 4 in the gastrointestinal tract of healthy dogs and dogs with inflammatory bowel disease. Am J Vet Res. 2001;62:211–216. doi: 10.2460/ajvr.2001.62.211. [DOI] [PubMed] [Google Scholar]
- 21.Greger DL, Gropp F, Morel C, Sauter S, Blum JW. Nuclear receptor and target gene mRNA abundance in duodenum and colon of dogs with chronic enteropathies. Domest Anim Endocrinol. 2006;31:327–339. doi: 10.1016/j.domaniend.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Dandrieux JR, Bornand VF, Doherr MG, Kano R, Zurbriggen A, Burgener IA. Evaluation of lymphocyte apoptosis in dogs with inflammatory bowel disease. Am J Vet Res. 2008;69:1279–1285. doi: 10.2460/ajvr.69.10.1279. [DOI] [PubMed] [Google Scholar]
- 23.Luckschander N, Hall JA, Gaschen F, Forster U, Wenzlow N, Hermann P, Allenspach K, Dobbelaere D, Burgener IA, Welle M. Activation of nuclear factor-kappaB in dogs with chronic enteropathies. Vet Immunol Immunopathol. 2010;133:228–236. doi: 10.1016/j.vetimm.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 24.Hall EJ, German AJ. Diseases of the small intestine. In: Ettinger SJ, Feldman EC, editors. Textbook of Veterinary Internal Medicine. 6th ed. St. Louis: Elsevier Saunders; 2005. pp. 1332–1378. [Google Scholar]
- 25.Himmel ME, Hardenberg G, Piccirillo CA, Steiner TS, Levings MK. The role of T-regulatory cells and Toll-like receptors in the pathogenesis of human inflammatory bowel disease. Immunology. 2008;125:145–153. doi: 10.1111/j.1365-2567.2008.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgener IA, König A, Allenspach K, Sauter SN, Boisclair J, Doherr MG, Jungi TW. Upregulation of toll-like receptors in chronic enteropathies in dogs. J Vet Intern Med. 2008;22:553–560. doi: 10.1111/j.1939-1676.2008.0093.x. [DOI] [PubMed] [Google Scholar]
- 27.Bauer S, Müller T, Hamm S. Pattern recognition by Toll-like receptors. Adv Exp Med Biol. 2009;653:15–34. doi: 10.1007/978-1-4419-0901-5_2. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka K. Expression of Toll-like receptors in the intestinal mucosa of patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2008;2:193–196. doi: 10.1586/17474124.2.2.193. [DOI] [PubMed] [Google Scholar]
- 29.Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685–693. doi: 10.1136/gut.2003.025403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takaishi H, Matsuki T, Nakazawa A, Takada T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 31.Xenoulis PG, Palculict B, Allenspach K, Steiner JM, Van House AM, Suchodolski JS. Molecular-phylogenetic characterization of microbial communities imbalances in the small intestine of dogs with inflammatory bowel disease. FEMS Microbiol Ecol. 2008;66:579–589. doi: 10.1111/j.1574-6941.2008.00556.x. [DOI] [PubMed] [Google Scholar]
- 32.German AJ, Bland PW, Hall EJ, Day MJ. Expression of major histocompatibility complex class II antigens in the canine intestine. Vet Immunol Immunopathol. 1998;61:171–180. doi: 10.1016/s0165-2427(97)00144-x. [DOI] [PubMed] [Google Scholar]
- 33.German AJ, Hall EJ, Day MJ. Analysis of leucocyte subsets in the canine intestine. J Comp Pathol. 1999;120:129–145. doi: 10.1053/jcpa.1998.0262. [DOI] [PubMed] [Google Scholar]
- 34.German AJ, Helps CR, Hall EJ, Day MJ. Cytokine mRNA expression in mucosal biopsies from German shepherd dogs with small intestinal enteropathies. Dig Dis Sci. 2000;45:7–17. doi: 10.1023/a:1005436721798. [DOI] [PubMed] [Google Scholar]
- 35.Peters IR, Helps CR, Calvert EL, Hall EJ, Day MJ. Cytokine mRNA quantification in duodenal mucosa from dogs with chronic enteropathies by real-time reverse transcriptase polymerase chain reaction. J Vet Intern Med. 2005;19:644–653. doi: 10.1892/0891-6640(2005)19[644:cmqidm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 36.Jergens AE, Sonea IM, O'Connor AM, Kauffman LK, Grozdanic SD, Ackermann MR, Evans RB. Intestinal cytokine mRNA expression in canine inflammatory bowel disease: a meta-analysis with critical appraisal. Comp Med. 2009;59:153–162. [PMC free article] [PubMed] [Google Scholar]
- 37.Allenspach K, Bergman PJ, Sauter S, Gröne A, Doherr MG, Gaschen F. P-glycoprotein expression in lamina propria lymphocytes of duodenal biopsy samples in dogs with chronic idiopathic enteropathies. J Comp Pathol. 2006;134:1–7. doi: 10.1016/j.jcpa.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 38.German AJ, Hall EJ, Day MJ. Immune cell populations within the duodenal mucosa of dogs with enteropathies. J Vet Intern Med. 2001;15:14–25. doi: 10.1892/0891-6640(2001)015<0014:icpwtd>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Wu GD. Is there a role for PPAR gamma in IBD? Yes, no, maybe. Gastroenterology. 2003;124:1538–1542. doi: 10.1016/s0016-5085(03)00345-7. [DOI] [PubMed] [Google Scholar]
- 40.Dubuquoy L, Rousseaux C, Thuru X, Peyrin-Biroulet L, Romano O, Chavatte P, Chamaillard M, Desreumaux P. PPARgamma as a new therapeutic target in inflammatory bowel diseases. Gut. 2006;55:1341–1349. doi: 10.1136/gut.2006.093484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noomen CG, Hommes DW, Fidder HH. Update on genetics in inflammatory disease. Best Pract Res Clin Gastroenterol. 2009;23:233–243. doi: 10.1016/j.bpg.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Achkar JP, Fiocchi C. Gene-gene interactions in inflammatory bowel disease: biological and clinical implications. Am J Gastroenterol. 2009;104:1734–1736. doi: 10.1038/ajg.2009.179. [DOI] [PubMed] [Google Scholar]
- 43.Ishihara S, Aziz MM, Yuki T, Kazumori H, Kinoshita Y. Inflammatory bowel disease: review from the aspect of genetics. J Gastroenterol. 2009;44:1097–1108. doi: 10.1007/s00535-009-0141-8. [DOI] [PubMed] [Google Scholar]
- 44.Mendoza JL, Lana R, Diaz-Rubio M, de la Concha EG, Urcelay E. Pharmacogenetics of therapy in inflammatory bowel disease patients. Curr Pharmacogenomics. 2007;5:235–247. [Google Scholar]
- 45.Lakatos PL. Environmental factors affecting inflammatory bowel disease: have we made progress? Dig Dis. 2009;27:215–225. doi: 10.1159/000228553. [DOI] [PubMed] [Google Scholar]
- 46.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 47.Chichlowski M, Hale LP. Bacterial-mucosal interactions in inflammatory bowel disease: an alliance gone bad. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1139–G1149. doi: 10.1152/ajpgi.90516.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Macfarlane GT, Blackett KL, Nakayama T, Steed H, Macfarlane S. The gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:1528–1536. doi: 10.2174/138161209788168146. [DOI] [PubMed] [Google Scholar]
- 49.Andoh A, Benno Y, Kanauchi O, Fujiyama Y. Recent advances in molecular approaches to gut microbiota in inflammatory bowel disease. Curr Pharm Des. 2009;15:2066–2073. doi: 10.2174/138161209788489186. [DOI] [PubMed] [Google Scholar]
- 50.Perencevich M, Burakoff R. Use of antibiotics in the treatment of inflammatory bowel disease. Inflamm Bowel Dis. 2006;12:651–664. doi: 10.1097/01.MIB.0000225330.38119.c7. [DOI] [PubMed] [Google Scholar]
- 51.Rubin DT, Kornblunth A. Role of antibiotics in the management of inflammatory bowel disease: a review. Rev Gastroenterol Disord. 2005;5 Suppl 3:S10–S15. [PubMed] [Google Scholar]
- 52.Sartor RB, Muehlbauer M. Microbial host interactions in IBD: implications for pathogenesis and therapy. Curr Gastroenterol Rep. 2007;9:497–507. doi: 10.1007/s11894-007-0066-4. [DOI] [PubMed] [Google Scholar]
- 53.Neurath MF, Finotto S, Fuss I, Boirivant M, Galle PR, Strober W. Regulation of T-cell apoptosis in inflammatory bowel disease: to die or not to die, that is the mucosal question. Trends Immunol. 2001;22:21–26. doi: 10.1016/s1471-4906(00)01798-1. [DOI] [PubMed] [Google Scholar]
- 54.Nikoopour E, Schwartz JA, Singh B. Therapeutic benefits of regulating inflammation in autoimmunity. Inflamm Allergy Drug Targets. 2008;7:203–210. doi: 10.2174/187152808785748155. [DOI] [PubMed] [Google Scholar]
- 55.Atreya I, Atreya R, Neurath MF. NF-kappaB in inflammatory bowel disease. J Intern Med. 2008;263:591–596. doi: 10.1111/j.1365-2796.2008.01953.x. [DOI] [PubMed] [Google Scholar]
- 56.Abraham C, Cho J. Interleukin-23/Th17 pathways and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:1090–1100. doi: 10.1002/ibd.20894. [DOI] [PubMed] [Google Scholar]
- 57.José León A, Garrote JA, Arranz E. [Cytokines in the pathogenesis of inflammatory bowel diseases] Med Clin (Barc) 2006;127:145–152. doi: 10.1157/13090382. [DOI] [PubMed] [Google Scholar]
- 58.Sturgess K. Diagnosis and management of idiopathic inflammatory bowel disease in dogs and cats. In Practice. 2005;27:291–301. [Google Scholar]
- 59.Tams TR. Chronic diseases of the small intestine. In: Tams TR, editor. Handbook of Small Animal Gastroenterology. 2nd ed. St. Louis: Saunders; 2003. pp. 211–250. [Google Scholar]
- 60.Rychlik A, Nieradka R, Kander M, Depta A, Nowicki M, Sarti K. Usefulness of endoscopic examination for the diagnosis of inflammatory bowel disease in the dog. Pol J Vet Sci. 2007;10:113–118. [PubMed] [Google Scholar]
- 61.Penninck D, Smyers B, Webster CR, Rand W, Moore AS. Diagnostic value of ultrasonography in differentiating enteritis from intestinal neoplasia in dogs. Vet Radiol Ultrasound. 2003;44:570–575. doi: 10.1111/j.1740-8261.2003.tb00509.x. [DOI] [PubMed] [Google Scholar]
- 62.Gaschen L. The role of imaging in dogs and cats with vomiting and chronic diarrhoea. 11th FECAVA Congress. The Netherlands: EJCAP; 2005. pp. 15197–15203. [Google Scholar]
- 63.Gaschen L, Kircher P. Two-dimensional grayscale ultrasound and spectral Doppler waveform evaluation of dogs with chronic enteropathies. Clin Tech Small Anim Pract. 2007;22:122–127. doi: 10.1053/j.ctsap.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 64.Schreiner NM, Gaschen F, Gröne A, Sauter SN, Allenspach K. Clinical signs, histology, and CD3-positive cells before and after treatment of dogs with chronic enteropathies. J Vet Intern Med. 2008;22:1079–1083. doi: 10.1111/j.1939-1676.2008.0153.x. [DOI] [PubMed] [Google Scholar]
- 65.Day MJ, Bilzer T, Mansell J, Wilcock B, Hall EJ, Jergens A, Minami T, Willard M, Washabau R. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: a report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol. 2008;138 Suppl 1:S1–S43. doi: 10.1016/j.jcpa.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 66.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 67.Li X, Conklin L, Alex P. New serological biomarkers of inflammatory bowel disease. World J Gastroenterol. 2008;14:5115–5124. doi: 10.3748/wjg.14.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barahona-Garrido J, Sarti HM, Barahona-Garrido MK, Hernández-Calleros J, Coss-Adame E, Garcia-Saenz SM, Yamamoto-Furusho JK. Serological markers in inflammatory bowel disease: a review of their clinical utility. Rev Gastroenterol Mex. 2009;74:230–237. [PubMed] [Google Scholar]
- 69.Allenspach K, Luckschander N, Styner M, Seibold F, Doherr M, Aeschbach D, Gaschen F. Evaluation of assays for perinuclear antineutrophilic cytoplasmic antibodies and antibodies to Saccharomyces cerevisiae in dogs with inflammatory bowel disease. Am J Vet Res. 2004;65:1279–1283. doi: 10.2460/ajvr.2004.65.1279. [DOI] [PubMed] [Google Scholar]
- 70.Kobayashi S, Ohno K, Uetsuka K, Nakashima K, Setoguchi A, Fujino Y, Tsujimoto H. Measurement of intestinal mucosal permeability in dogs with lymphocytic-plasmacytic enteritis. J Vet Med Sci. 2007;69:745–749. doi: 10.1292/jvms.69.745. [DOI] [PubMed] [Google Scholar]
- 71.Suchodolski JS, Steiner JM. Laboratory assessment of gastrointestinal function. Clin Tech Small Anim Pract. 2003;18:203–210. doi: 10.1016/S1096-2867(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 72.Gunawardana SC, Jergens AE, Ahrens FA, Niyo Y. Colonic nitrite and immunoglobulin G concentrations in dogs with inflammatory bowel disease. J Am Vet Med Assoc. 1997;211:318–321. [PubMed] [Google Scholar]
- 73.McCann TM, Ridyard AE, Else RW, Simpson JW. Evaluation of disease activity markers in dogs with idiopathic inflammatory bowel disease. J Small Anim Pract. 2007;48:620–625. doi: 10.1111/j.1748-5827.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 74.Loftus EV Jr. Clinical perspectives in Crohn's disease. Objective measures of disease activity: alternatives to symptom indices. Rev Gastroenterol Disord. 2007;7 Suppl 2:S8–S16. [PubMed] [Google Scholar]
- 75.Jergens AE, Schreiner CA, Frank DE, Niyo Y, Ahrens FE, Eckersall PD, Benson TJ, Evans R. A scoring index for disease activity in canine inflammatory bowel disease. J Vet Intern Med. 2003;17:291–297. doi: 10.1111/j.1939-1676.2003.tb02450.x. [DOI] [PubMed] [Google Scholar]
- 76.Jergens AE. Clinical assessment of disease activity for canine inflammatory bowel disease. J Am Anim Hosp Assoc. 2004;40:437–445. doi: 10.5326/0400437. [DOI] [PubMed] [Google Scholar]
- 77.Allenspach K, Wieland B, Gröne A, Gaschen F. Chronic enteropathies in dogs: evaluation of risk factors for negative outcome. J Vet Intern Med. 2007;21:700–708. doi: 10.1892/0891-6640(2007)21[700:ceideo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 78.Craven M, Simpson JW, Ridyard AE, Chandler ML. Canine inflammatory bowel disease: retrospective analysis of diagnosis and outcome in 80 cases (1995-2002) J Small Anim Pract. 2004;45:336–342. doi: 10.1111/j.1748-5827.2004.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 79.Allenspach K. Tests to investigate gastrointestinal diseases in dogs--which markers are actually useful for the practitioner? J Small Anim Pract. 2007;48:607–608. doi: 10.1111/j.1748-5827.2007.00486.x. [DOI] [PubMed] [Google Scholar]
- 80.Kathrani A, Steiner JM, Suchodolski J, Eastwood J, Syme H, Garden OA, Allenspach K. Elevated canine pancreatic lipase immunoreactivity concentration in dogs with inflammatory bowel disease is associated with a negative outcome. J Small Anim Pract. 2009;50:126–132. doi: 10.1111/j.1748-5827.2008.00693.x. [DOI] [PubMed] [Google Scholar]
- 81.Bosani M, Ardizzone S, Porro GB. Biologic targeting in the treatment of inflammatory bowel diseases. Biologics. 2009;3:77–97. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82.Spichiger AC, Allenspach K, Ontsouka E, Gaschen F, Morel C, Blum JW, Sauter SN. Abundance of mRNA of growth hormone receptor and insulin-like growth factors-1 and -2 in duodenal and colonic biopsies of dogs with chronic enteropathies*. J Vet Med A Physiol Pathol Clin Med. 2005;52:491–497. doi: 10.1111/j.1439-0442.2005.00770.x. [DOI] [PubMed] [Google Scholar]
- 83.Cerquetella M, Spaterna A. Malattia infiammatoria cronica intestinale nel cane possibile modello di studio comparativo nell’uomo? Il Gastroenterologo. 2007;4:88–92. [Google Scholar]
- 84.Qin X. What is human inflammatory bowel disease (IBD) more like: Johne's disease in cattle or IBD in dogs and cats? Inflamm Bowel Dis. 2008;14:138. doi: 10.1002/ibd.20240. [DOI] [PubMed] [Google Scholar]
- 85.Wehkamp J, Stange EF. A new look at Crohn's disease: breakdown of the mucosal antibacterial defense. Ann N Y Acad Sci. 2006;1072:321–331. doi: 10.1196/annals.1326.030. [DOI] [PubMed] [Google Scholar]