Abstract
AIM: To investigate the regulation of mindin expression and the signaling pathway involved during inflammation.
METHODS: C57BL/6 mice were treated with 3% dextran sulfate sodium (DSS) in drinking water for 6 d to induce acute colitis, and then the colon was harvested for histological analysis or for RNA isolation. mRNA expression of mindin and nuclear factor (NF)-κB p65 was analyzed by quantitative real time polymerase chain reaction (RT-PCR) and mindin expression construct was confirmed by Western blotting. Mouse macrophage and intestinal epithelial lineage cells were stimulated with different cytokines and toll-like receptor (TLR) ligands, before pNF-κB-luciferase activity was assessed using the Dual-Luciferase reporter assay system.
RESULTS: mRNA expression of mindin was upregulated 4.7 ± 1.1 fold compared with the baseline during DSS-induced intestinal inflammation in the mice. Stimulation with CpG-ODN (a known TLR-9 ligand) induced 4.2 ± 0.3 fold upregulation of mindin expression in RAW 264.7 cells. Full-length of mindin was cloned from cDNA of mouse mesenteric lymph node, then the pCMV-Mindin-Flag expression vector was established and the protein expression level was confirmed. Transfection of the mindin construct and stimulation with CpG-ODN significantly increased the NF-κB-luciferase activity by 2.5 ± 0.3 and 4.5 ± 0.5 fold in RAW264.7 and CMT93 cells, respectively (P < 0.01).
CONCLUSION: Mindin expression is upregulated during intestinal inflammation and may induce NF-κB promoter activation in a TLR-9 mediated manner.
Keywords: Mindin, Nuclear factor-κB promoter activity, Toll-like receptor-9
INTRODUCTION
Mindin (also called spondin 2) is a member of the mindin-F-spondin family of secreted extracellular matrix proteins[1]. Mindin was initially identified in zebrafish and was found to selectively accumulate in the basal lamina. Subsequently, the genes encoding rat and human mindin were cloned[2,3]. Mouse Spon2 cDNA encodes an open reading frame of 330 amino acids with a calculated molecular mass of 36 kDa. Spon2 mRNA is abundantly expressed in the spleen and lymph nodes[4].
To investigate the involvement of mindin in the immune system, mindin-deficient mice have been generated[4]. Mice lacking mindin are resistant to lipopolysaccharide (LPS)-induced septic shock and exhibit an impaired ability to clear bacterial infections[4]. Macrophages and mast cells show defective responses to a broad spectrum of microbial stimuli. Mindin also functions as an opsonin for macrophage phagocytosis of bacteria[4]. Mice lacking mindin exhibit defective clearance of influenza virus, whereas mindin-deficient macrophages show impaired activation following influenza infection[5]. Thus, mindin is a pattern recognition molecule that is critical for initiating innate immune responses to both bacterial and viral pathogens[5,6].
Mindin-deficient mice display severely impaired recruitment of neutrophils and macrophages to inflammation sites[7]. These effects are mediated through the interaction of mindin with αMβ2 and α4β1 integrins on neutrophils. Mindin-integrin interactions also have a key function in T-cell priming by dendritic cells (DCs). DCs from mindin-deficient mice show an impaired capacity to prime CD4+ T cells due to inefficient engagement of T lymphocytes. Additionally, these DCs have reduced levels of the Rho guanosine triphosphatases Rac1 and Rac2, which control DC priming capability[8]. Mindin regulates Rac1 and Rac2 expression by signaling through α4β1 and α5β1 integrins on DCs[9].
Despite these previous studies indicating that mindin is important in both innate and adaptive immunity, information regarding its regulation is poorly understood and activation of signaling pathways are not well investigated.
The innate immune system uses pattern-recognition receptors (PRRs) to recognize pathogen-associated molecular patterns (PAMPs)[10,11]. Several cell surface PRRs such as toll-like receptors (TLRs) are essential in innate immune recognition and the microbial components recognized by TLRs have been identified in recent decades[12-16].
Our data demonstrate that mRNA expression of mindin is upregulated during dextran sulfate sodium (DSS)-induced acute intestinal inflammation and in vitro co-stimulating data suggest that mindin may induce nuclear factor (NF)-κB promoter activation in a TLR-9 mediated manner.
MATERIALS AND METHODS
Mice and induction of acute colitis
C57BL/6 mice were purchased from the laboratory animal center, Shanghai, China. All procedures involving experimental animals were performed in accordance with protocols approved by the Committee for Animal Research of the University of Xiamen and complied with the Guide for the Care and Use of Laboratory Animals (NIH publication No. 86-23, revised 1985). Mice were housed in a specific pathogen-free animal facility and at 8 to 12 wk of age were treated with 3% (wt/vol) DSS (MP Biomedicals, Irvine, CA) for 6 d, then the colon was harvested, two tissue pieces 0.5 cm in length were dissected out, 2 cm away from the anus of each study mouse. One piece of colon tissue was fixed in 4% neutral buffered formalin, cut into 6 μm thick sections and then stained with hematoxylin and eosin (HE) for light microscopic examination and another piece was prepared for RNA isolation. All experiments were repeated at least 3 times.
Cell culture and stimulation
RAW 264.7 (purchased from Sigma-Aldrich), CMT93 and HEK293 cells (both purchased from ATCC, USA) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum and a 0.5% penicillin G/streptomycin mixture at 37°C in a 5% CO2 atmosphere. About 1 × 105 of RAW 264.7 and CMT93 cells were cultured in 12-well plates and stimulated with the cytokines; 10 ng/mL of mouse recombinant tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) (both from R&D Systems); and different TLR ligands consisting of 0.05 μg/mL of Pam3-CSK4, 5 μg/mL of peptidoglycans (PGN), 0.1 μg/mL of LPS, 0.5 μg/mL of flagellin (S. typhimurium) and 5 μg/mL of CpG-ODN 1585 (all of the ligands were purchased from Invivogen), for 8 h, then cells were harvested for RNA isolation.
Quantitative real time polymerase chain reaction
The total RNA from mice colon tissue or RAW 264.7 cells stimulated with different cytokines and TLR ligands was extracted using Trizol reagent (InvitroGen). The cDNA templates were synthesized using the iScript™ cDNA synthesis kit and quantitative polymerase chain reaction (PCR) reactions were performed with iQ™ SYBR green PCR supermix (both from Bio-Rad). Quantitative real time (RT)-PCR analysis was performed using the Bio-Rad iQ5 system and gene expression levels for each individual sample were normalized to GAPDH. Mean relative gene expression was determined and differences were calculated using the 2-∆C(t) method as described previously[17]. The following oligonucleotides were used as primers: mindin forward, 5'-CAATGGGCTGAGGGACTTTG and reverse, 5'-TCTCTCCTGCAGCTTCGATCTC, NF-κB p65 forward, 5'-AGGCTTCTGGGCCTTATGTG and reverse, 5'-TGCTTCTCTCGCCAGGAATAC and GAPDH forward, 5'-TGGCAAAGTGGAGATTGTTGCC and reverse, 5'-AAGATGGTGATGGGCTTCCCG.
For the RT-PCR analysis of mindin expression in different organs, the following primers were employed: mindin forward, 5'-CAGCCCTGACTGGTTTGTGGGC and reverse, 5'-CCCTGGGACTCTGCTGTAGCCGCACG and hypoxanthine phosphoribosyltransferase (HPRT) forward, 5'-GTTGGATACAGGCCAGACTTTGTTG and reverse, 5'-GATTCAACTTGCGCTCATCTTAGGC.
Establishment of mindin expression vector
Full-length of mindin was amplified by PCR from a cDNA template from mouse mesenteric lymph node (MLN) using upstream (containing BamHI site) of 5'-CGGGATCCCGTGATGGAAAACGTGAGTCTTG and downstream (containing EcoRI site) of 5'-CGGAATTCCGACGCAGTTATCTGGGGCACACT primers. Then the fragment was inserted into the BamHI and EcoRI site of the pCMV-Flag-tag 4C (Stratagen) vector, which resulted in the construction of a pCMV-Mindin-Flag expression vector, confirmed by sequencing.
Western blotting analysis
HEK293 cells were transfected with pCMV-Flag, pCMV-Mindin-Flag clone 1 and clone 2 for 24 h, then Western blotting analysis was performed as described previously[18]. Briefly, 2 × 105 of transfected HEK293 cells were lysed in RIPA buffer (50 mmol/L Tris pH 8, 0.1% SDS, 0.5% deoxycholate, 1% NP-40, 150 mmol/L NaCl, 1 tablet complete mini protease inhibitor/10 mL, Roche Diagnostics GmbH, Germany). Next, equal amounts of total cell lysates were electrophoresed and transferred to Pall Fluoro Trans W membrane (WAKO, Japan). The membranes were incubated with anti-Flag antibody. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody and visualized using an enhanced chemiluminescence detection system (ECL Advance, Amersham Bioscience, UK).
Dual-luciferase assay
About 5 × 104 of the RAW 264.7 and CMT93 cells were cultured in 24-well plates and transfected with 0.01 μg of pNF-κB-Luc (firefly luciferase) (Clontech), 0.001 μg of pRL-0 vector (Promega) and 0.25 μg of pCMV-mindin-Flag or pCMV-Flag vectors with Lipofectamine 2000 (Invitrogen, Carlsbad, CA). After 12 h of transfection, the cells were additionally stimulated with 0.05 μg/mL of Pam3-CSK4, 5 μg/mL of PGN, 0.1 μg/mL of LPS, 0.5 μg/mL of flagellin and 5 μg/mL of CpG-ODN 1585 for 12 h. Then, luciferase assays were performed using the Dual-Luciferase reporter assay system (Promega, Madison, WI) according to the manufacturer’s instructions. Renilla luciferase activity was used as an internal control. All experiments were carried out at least 3 times.
Statistical analysis
The data were expressed as the mean ± SD. Groups were compared using Student’s t-test. P < 0.05 was considered statistically significant.
RESULTS
Mindin mRNA expression is upregulated during acute intestinal inflammation
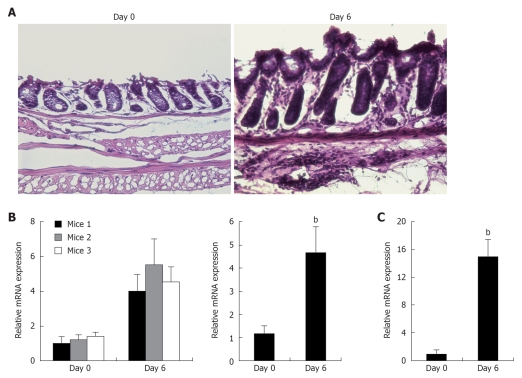
To investigate the regulation of the mindin gene during acute colitis, we used a colitis model in which 3% of DSS is added to drinking water for six days, which results in epithelial damage and acute inflammation. HE staining of the colon tissue (Figure 1A) revealed that DSS treatment induced severe colitis compared with normal tissue, characterized by epithelial and muscle hypertrophy with patchy lymphocytic infiltrates extending into the muscle layers. Quantitative mRNA expression of NF-κB p65 (Figure 1C) showed over 15 ± 2.5 fold upregulation compared with control group, supporting the inflammation at the mRNA level. Then we used the same cDNA samples to analyze the mindin expression, and, surprisingly, mRNA expression of mindin was upregulated in all of the colitis mice (Figure 1B, left graph, n = 3, each group). When quantitatively analyzed from triplicate samples of the study mice (Figure 1B, right graph), mindin expression was upregulated 4.7 ± 1.1 fold compared with the baseline during intestinal inflammation.
Figure 1.
Mindin mRNA expression is upregulated during acute intestinal inflammation. A: Histological analysis of normal tissue and of acute inflammation specimen after 6 d of DSS. HE staining of colonic sections (magnification 40 ×); B: Quantitative mRNA expression of mindin in study mice (left graph, n = 3 of each group, triplicate samples from each mouse, right graph combines the data from each group); C: Relative mRNA expression of NF-κB p65 in study groups. Bars represent mean ± SD; bP < 0.01 vs day 0.
Mindin mRNA expression is upregulated by CpG-ODN stimulation
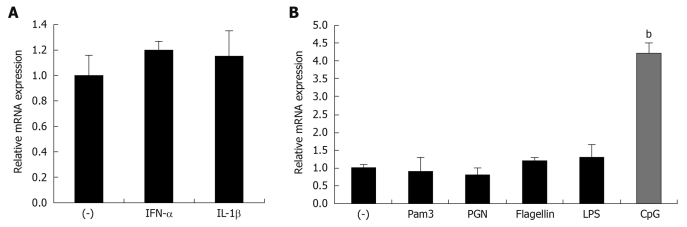
To further define the specific regulators of mindin expression in vitro, we employed the mouse leukemic monocyte macrophage cell line, RAW 264.7, and stimulated the cells with different cytokines and TLR-ligands. The results show that cytokines of TNF-α and IL-1β, and the TLR-2, -6, -5 ligands of Pam3-CSK4, PGN and flagellin do not induce upregulation of mindin expression (Figure 2A and B). Of interest, regarding the TLR-9 ligand of CpG-ODN, this ligand induced 4.2 ± 0.3 fold upregulation of mindin mRNA expression. This result suggests that a TLR-9 mediated signaling pathway is involved in this regulation.
Figure 2.
Mindin mRNA expression is upregulated by CpG-ODN stimulation. About 1 × 105 RAW 264.7 cells were cultured in 12-well plates and stimulated with cytokines of mouse recombinant tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β), toll-like receptor (TLR) ligands of Pam3-CSK4, peptidoglycans (PGN), lipopolysaccharide (LPS), flagellin and CpG-ODN 1585 (B) for 8 h, then cells were harvested for RNA isolation and relative mRNA expression of mindin was analyzed using quantitative real time polymerase chain reaction (RT-PCR). In each group, bars represent mean ± SD; bP < 0.01 vs unstimulated cells.
Mindin induces NF-κB promoter activation in a TLR-9 mediated manner
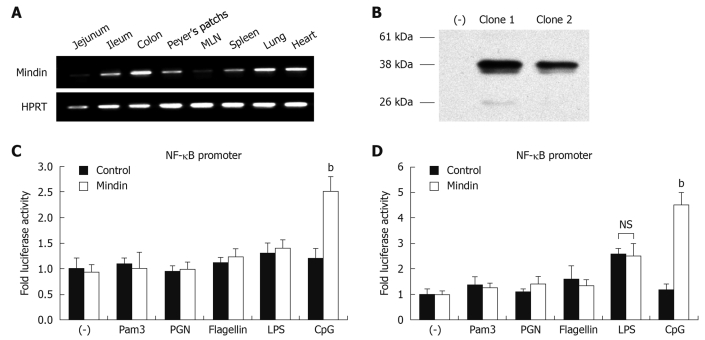
We analyzed mindin expression in multiple mice organs and it was abundantly expressed in intestine, Peyer’s patches, spleen, MLN, lung and heart tissue (Figure 3A), in agreement with previous documentation[4].
Figure 3.
Mindin induces nuclear factor (NF)-κB promoter activation in a TLR-9 mediated manner. A: Mindin mRNA expression in mouse multiple organs, HPRT expression as a total RNA control; B: HEK293 cells were transfected with pCMV-Flag (lane at left side), pCMV-Mindin-Flag clone 1 and clone 2 for 24 h and Western blotting analysis was performed. RAW 264.7 (C) and CMT93 (D) cells were transfected with pNF-κB-Luc (firefly luciferase), pRL-0 vector and pCMV-mindin-Flag or pCMV-Flag control vectors. Twelve hours after transfection, cells were stimulated with the different TLR ligands of Pam3-CSK4, PGN, LPS, flagellin and CpG-ODN 1585 for an additional 12 h, then luciferase assays were performed. NS: Not significant. bP < 0.01 vs control.
To precisely investigate whether the TLRs are involved in a mindin-mediated signaling pathway, we cloned the full-length of mindin from cDNA of mouse MLN and established the pCMV-Mindin-Flag expression vector. HEK293 cells were transfected with pCMV-Flag, pCMV-Mindin-Flag clone 1 and clone 2 for 24 h, followed by Western blotting analysis. As shown in Figure 3B, around 37 kDa of mindin-Flag fusion protein was detected in both clone 1 and 2 lanes and clone 1 was employed in a further experiment, with no detection of protein on control lane at left side.
To define whether NF-κB pathway activation is involved in this process, RAW 264.7 and CMT93 cells were transfected with pNF-κB-Luc (firefly luciferase), pRL-0 vector and pCMV-mindin-Flag or pCMV-Flag control vectors. Twelve hours after transfection, stimulation took place with different TLR ligands of Pam3-CSK4, PGN, LPS, flagellin and CpG-ODN 1585 for an additional 12 h, then luciferase assays were performed.
As shown in Figure 3C and D, luciferase activity of NF-κB promoter was significantly increased by 2.5 ± 0.3 fold and 4.5 ± 0.5 fold, in cells transfected with mindin and stimulated with CpG-ODN, for RAW264.7 and CMT93 cells respectively, compared with the control. LPS induced 2.6 ± 0.2 fold activity in CMT93 cells, but there was no significant difference between mindin and control group. No significant induction of NF-κB luciferase activation was noted in mindin-transfected cells which underwent Pam3-CSK4, PGN, or flagellin stimulation. This result suggests that mindin induces NF-κB promoter activation in a TLR-9 mediated manner.
DISCUSSION
Our data demonstrated that mRNA expression of mindin is upregulated during DSS-induced acute intestinal inflammation and in vitro co-stimulating data suggested that mindin may induce NF-κB promoter activation in a TLR-9 mediated manner.
The immune system uses many sensors to detect and report microbial invaders. Most of these sensors are associated with immune cells, but the extracellular matrix also seems to be essential for this sentinel duty[19]. Investigators generated mice deficient in mindin; these mice have severe defects in many of their immune responses. The mindin-deficient cells are hyporesponsive to many PAMPs, including lipotechoic acid, peptidoglycan, zymosan, mannan and CpG DNA[7]. Other results have indicated that the F-spondin domain mediates integrin binding and identified the binding site by mutagenesis, suggesting that mindin-integrin interactions are critical for inflammatory cell recruitment in vivo[20,21].
These studies indicate the essential role of mindin in both innate and adaptive immunity, but information regarding its regulation is poorly understood. Also, the role of other cytokines and the interaction of DCs with T cells need to be further investigated, and different organ-specific inflammation models need to be established. DSS is thought to induce colitis by inducing mucosal erosion which is observed 5 d after DSS administration, allowing commensal bacteria to infiltrate into the lamina propria. Our data showed that DSS-induced colitis is associated with an increased activation of NF-κB. Hall et al[22] demonstrate that commensal bacterial DNA can suppress Treg cell conversion via TLR9-mediated activation of lamina propria dendritic cells and thus potentially disrupt intestinal homeostasis, and also show a relationship with mindin expression[22,23]. Our data demonstrated that mRNA expression of mindin is upregulated during DSS-induced acute intestinal inflammation and that CpG-ODN stimulation induced 4.2 ± 0.3 fold upregulation of mindin expression in RAW 264.7 cells, suggesting that mindin has indispensable diverse roles in mucosal immunity.
The activation of known signaling pathways in response to LPS is largely unaffected in mindin-deficient cells, implicating that mindin may bind to a distinct receptor and activate other TLRs or different signal transduction pathways. Production of TNF and IL-6 induced by PAMPs is reduced in mindin-deficient macrophages indicating that mindin-induced signaling is as important as those well-defined signaling pathways that are largely transduced through TLRs[7,10]. However, the exact mechanism of mindin-mediated TLR recognition of microbial components remains unclear. Recent findings suggest that NF-κB has not only proinflammatory but also tissue-protective functions in inflammatory diseases. NF-κB has multiple, often opposing functions in the intestine[24]. Antiapoptotic actions of NF-κB in intestinal epithelial cells dominate tissue responses to many acute inflammatory and injurious challenges, whereas proinflammatory and cell survival functions of NF-κB in macrophages and T cells govern chronic intestinal inflammation. Our data showed that luciferase activity of the NF-κB promoter is significantly increased in mice macrophage and intestinal epithelial cell lineages stimulated with exogenous mindin and CpG-ODN, but not in the cells stimulated with other TLR ligands, suggesting that mindin induces NF-κB promoter activation through a TLR-9 mediated pathway. While basolateral TLR stimulation mobilizes an inflammatory cascade, apical TLR-9 stimulation delivers negative signals that curtail inflammatory responses to basolateral stimulation via different TLRs[25]. Mindin was initially identified in zebrafish and was found to selectively accumulate in the basal lamina[1]. Taken together, our data suggest that while basolateral TLR-9 signaling is fully capable of inducing a NF-κB-mediated pro-inflammatory response, apical TLR-9 signaling does not induce an inflammatory response due to a defect in NF-κB activation.
However, while mindin binds to some kinds of bacteria and integrins, it is still unclear whether mindin functions as a coreceptor for several PPRs or other novel receptors and we urge further broad studies to address these questions. Our results raise the importance of the TLR-9 mediated pathway and provide a clue to help define more precisely the function and signaling pathways of the mindin protein.
COMMENTS
Background
Mindin is a member of the mindin-F-spondin family of secreted extracellular matrix proteins. Mindin mRNA is abundantly expressed in the spleen and lymph nodes. Mindin-deficient mice are resistant to lipopolysaccharide-induced septic shock and exhibit an impaired ability to clear bacterial infections. Mindin-deficient macrophages and mast cells show defective responses to a broad spectrum of microbial stimuli. Mindin also functions as an opsonin for macrophage phagocytosis of bacteria. Despite previous studies indicating that mindin is important in both innate and adaptive immunity, information regarding its regulation is poorly understood and activation of signaling pathways are not well investigated.
Research frontiers
Mindin has an indispensable role in both innate and adaptive immunity. This study investigated regulation of mindin expression and the signaling pathway involved.
Innovations and breakthroughs
Despite previous studies indicating that mindin is important in both innate and adaptive immunity, information regarding its regulation is poorly understood and activation of signaling pathways are not well investigated. The data of this study demonstrated that mRNA expression of mindin is upregulated during dextran sulfate sodium-induced acute intestinal inflammation and in vitro co-stimulating data suggested that mindin may induce nuclear factor (NF)-κB promoter activation in a toll-like receptor (TLR)-9 mediated manner.
Applications
While mindin binds to some kinds of bacteria and integrins, it is still unclear whether mindin functions as a coreceptor for several PPRs or other novel receptors and we urge further broad studies to address these questions. The results of this study raise the importance of the TLR-9 mediated pathway and provide a clue to help define more precisely the function and signaling pathways of the mindin protein.
Terminology
Mindin is an extracellular matrix protein. NF-κB has not only proinflammatory but also tissue-protective functions in inflammatory diseases. Basolateral TLR-9 signaling is fully capable of inducing a NF-κB-mediated pro-inflammatory response.
Peer review
In this work the authors propose that mindin expression is upregulated during intestinal inflammation and may induce NF-κB promoter activation through a TLR-9 mediated manner. The initial observation is interesting, but the authors should include the biological and clinical significance of the mindin upregulation in colitis in the discussion section.
Footnotes
Supported by National Natural Science Foundation of China, No. 30750013
Peer reviewer: María IT López, Professor, Experimental Biology, University of Jaen, araje de las Lagunillas s/n, Jaén 23071, Spain
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
References
- 1.Higashijima S, Nose A, Eguchi G, Hotta Y, Okamoto H. Mindin/F-spondin family: novel ECM proteins expressed in the zebrafish embryonic axis. Dev Biol. 1997;192:211–227. doi: 10.1006/dbio.1997.8760. [DOI] [PubMed] [Google Scholar]
- 2.Feinstein Y, Borrell V, Garcia C, Burstyn-Cohen T, Tzarfaty V, Frumkin A, Nose A, Okamoto H, Higashijima S, Soriano E, et al. F-spondin and mindin: two structurally and functionally related genes expressed in the hippocampus that promote outgrowth of embryonic hippocampal neurons. Development. 1999;126:3637–3648. doi: 10.1242/dev.126.16.3637. [DOI] [PubMed] [Google Scholar]
- 3.Adams JC, Tucker RP. The thrombospondin type 1 repeat (TSR) superfamily: diverse proteins with related roles in neuronal development. Dev Dyn. 2000;218:280–299. doi: 10.1002/(SICI)1097-0177(200006)218:2<280::AID-DVDY4>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 4.He YW, Li H, Zhang J, Hsu CL, Lin E, Zhang N, Guo J, Forbush KA, Bevan MJ. The extracellular matrix protein mindin is a pattern-recognition molecule for microbial pathogens. Nat Immunol. 2004;5:88–97. doi: 10.1038/ni1021. [DOI] [PubMed] [Google Scholar]
- 5.Jia W, Li H, He YW. Pattern recognition molecule mindin promotes intranasal clearance of influenza viruses. J Immunol. 2008;180:6255–6261. doi: 10.4049/jimmunol.180.9.6255. [DOI] [PubMed] [Google Scholar]
- 6.McDonald C, Nuñez G. Mindin the fort. Nat Immunol. 2004;5:16–18. doi: 10.1038/ni0104-16. [DOI] [PubMed] [Google Scholar]
- 7.Jia W, Li H, He YW. The extracellular matrix protein mindin serves as an integrin ligand and is critical for inflammatory cell recruitment. Blood. 2005;106:3854–3859. doi: 10.1182/blood-2005-04-1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, Popoff M, Tybulewicz VL, Amigorena S. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–1153. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 9.Li H, Oliver T, Jia W, He YW. Efficient dendritic cell priming of T lymphocytes depends on the extracellular matrix protein mindin. EMBO J. 2006;25:4097–4107. doi: 10.1038/sj.emboj.7601289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann JA, Kafatos FC, Janeway CA, Ezekowitz RA. Phylogenetic perspectives in innate immunity. Science. 1999;284:1313–1318. doi: 10.1126/science.284.5418.1313. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 13.Hoshino K, Takeuchi O, Kawai T, Sanjo H, Ogawa T, Takeda Y, Takeda K, Akira S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J Immunol. 1999;162:3749–3752. [PubMed] [Google Scholar]
- 14.Takeuchi O, Kawai T, Sanjo H, Copeland NG, Gilbert DJ, Jenkins NA, Takeda K, Akira S. TLR6: A novel member of an expanding toll-like receptor family. Gene. 1999;231:59–65. doi: 10.1016/s0378-1119(99)00098-0. [DOI] [PubMed] [Google Scholar]
- 15.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000;408:740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 16.Tai LH, Goulet ML, Belanger S, Toyama-Sorimachi N, Fodil-Cornu N, Vidal SM, Troke AD, McVicar DW, Makrigiannis AP. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J Exp Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guleng B, Tateishi K, Ohta M, Kanai F, Jazag A, Ijichi H, Tanaka Y, Washida M, Morikane K, Fukushima Y, et al. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 18.Guleng B, Tateishi K, Kanai F, Jazag A, Ohta M, Asaoka Y, Ijichi H, Tanaka Y, Imamura J, Ikenoue T, et al. Cancer-derived VEGF plays no role in malignant ascites formation in the mouse. World J Gastroenterol. 2005;11:5455–5459. doi: 10.3748/wjg.v11.i35.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patti JM, Allen BL, McGavin MJ, Höök M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Cao C, Jia W, Yu L, Mo M, Wang Q, Huang Y, Lim JM, Ishihara M, Wells L, et al. Structure of the F-spondin domain of mindin, an integrin ligand and pattern recognition molecule. EMBO J. 2009;28:286–297. doi: 10.1038/emboj.2008.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Z, Garantziotis S, Jia W, Potts EN, Lalani S, Liu Z, He YW, Foster WM, Hollingsworth JW. The extracellular matrix protein mindin regulates trafficking of murine eosinophils into the airspace. J Leukoc Biol. 2009;85:124–131. doi: 10.1189/jlb.0208135. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 22.Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mills KH. TLR9 turns the tide on Treg cells. Immunity. 2008;29:518–520. doi: 10.1016/j.immuni.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 24.Spehlmann ME, Eckmann L. Nuclear factor-kappa B in intestinal protection and destruction. Curr Opin Gastroenterol. 2009;25:92–99. doi: 10.1097/MOG.0b013e328324f857. [DOI] [PubMed] [Google Scholar]
- 25.Lee J, Mo JH, Katakura K, Alkalay I, Rucker AN, Liu YT, Lee HK, Shen C, Cojocaru G, Shenouda S, et al. Maintenance of colonic homeostasis by distinctive apical TLR9 signalling in intestinal epithelial cells. Nat Cell Biol. 2006;8:1327–1336. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]