Abstract
AIM: To evaluate the feasibility and therapeutic effects of para-aortic nodal dissection (PAND) for advanced gastric cancer.
METHODS: Randomized controlled trials (RCTs) and non-randomized studies comparing D2 + PAND with D2 lymphadenectomy were identified using a pre-defined search strategy. Five-year overall survival rate, post-operative mortality, and wound degree of surgery between the two operations were compared by using the methods provided by the Cochrane Handbook for Systematic Reviews of Interventions.
RESULTS: Four RCTs (1120 patients) and 4 non-randomized studies (901 patients) were identified. Meta-analysis showed that there was no significant difference between these two groups in 5-year overall survival rate [risk ratio (RR) 1.04 (95% CI: 0.93-1.16) for RCTs and 0.96 (95% CI: 0.83-1.10) for non-randomized studies] and post-operative mortality [RR 0.99 (95% CI: 0.44-2.24) for RCTs and 2.06 (95% CI: 0.69-6.15) for non-randomized studies]. There was a significant difference between these two groups in wound degree of surgery, operation time was significantly longer [weighted mean difference (WMD) 195.32 min (95% CI: 114.59-276.05) for RCTs and 126.07 min (95% CI: 22.09-230.04) for non-randomized studies] and blood loss was significantly greater [WMD 301 mL (95% CI: 151.55-450.45) for RCTs and 302.86 mL (95% CI: 127.89-477.84) for non-randomized studies] in D2 + PAND.
CONCLUSION: D2 + PAND can be performed as safely as standard D2 resection without increasing post-operative mortality but fail to benefit overall survival in patients with advanced gastric cancer.
Keywords: Systematic review, Meta-analysis, Gastric cancer, D2 lymphadenectomy, Para-aortic nodal dissection
INTRODUCTION
Generally, either incidence or mortality of gastric cancer (GC) has fallen dramatically during the past decades in European and American countries. Despite its recent decline, GC is still the fourth most common carcinoma and the second leading cause of death by tumor worldwide[1-3]. GC can be divided into two groups, early GC and advanced GC, and advanced GC accounts for 92%-95% of cases in China, 40%-60% in Japan, and 80%-90% in Europe[4-6] presently. Up to now, treatments for GC include surgery, chemotherapy, radiotherapy and so on. Despite the poor prognosis, surgery is commonly accepted as the preferred treatment for advanced GC[7]. Five-year survival rate can achieve 45% or even more in Japan, 40% or so in China, only about 20% in Western countries and as low as 6% in sub-Saharan Africa[8-11] for advanced GC.
Although surgical operation remains the primary therapeutic modality, views over the optimal resection for patients with GC remain controversial[12]. In patients with GC, lymph node metastasis can occur during the early stages, and regional lymph node dissection (LND) is regarded as an important part of en bloc resection for GC. However, the extent of lymphadenectomy for the greatest result is controversial, and there is no consensus worldwide. Introduced by Japanese surgeons in 1960s, D2 lymphadenectomy is the recommended standard practice in patients undergoing an operation with a curative intent[13-16], which required the systematic dissection of lymph nodes in the first tier (perigastric) and the second tier (along the celiac artery and its branches). Compared with traditional D1 lymphadenectomy, D2 doesn’t increase the operation morbidity and mortality[17], and shows a higher 5-year survival rate[18]. Recently, it was reported that 18%-40% of patients with advanced GC had metastasis present in the para-aortic nodes[19-21], and more often if the tumor was located at the proximal third of the stomach[22]. Taking this into consideration, some researchers assumed that removing these lymph nodes could accomplish curative resection (R0) which might improve the clinical outcome of advanced GC patients[19,23,24]. However, this extended dissection not only can increase the post-operative morbidity and mortality but also can affect the function of near abdominal organs, and its survival benefit is still uncertain. Some studies considered that morbidity after para-aortic nodal dissection (PAND) such as pancreatic fistula and respiratory complications were significantly higher[25], and these complications were considered to be associated with the surgeons’ experience. However, some retrospective reports revealed that there was no significant difference in post-operative complications and mortality between D2 and D2 + PAND; D2 + PAND could be performed safely by well-trained surgeons[26,27]. With respective to long-term survival after the two operations, there was stormy dispute at the same time[27-29]. At the moment there is no consensus about whether patients with advanced GC should receive PAND or not, who should accept it, and how to ascertain the metastasis of PAN before operations.
Although a systematic review referring to the extent of lymphadenectomy for GC has been published, it only valued the short-term outcomes[30] and the assessment of 5-year survival after PAND in patients with GC was not presented. Five-year survival is considered to be an important outcome in evaluating the therapeutic effects of GC. Therefore a systemic review aiming to evaluate the feasibility and therapeutic effects of PAND is urgently needed. In this study, we assessed literature existing and conducted a meta-analysis for comparing the clinical effectiveness of these two operations.
MATERIALS AND METHODS
Search strategy
The following databases were searched systematically in our study: PUBMED, EMBASE, and the China Biological Medicine Database (CBM-disc), CNKI (China National Knowledge Infrastructure Whole Article Database) from January 1980 to February 2009, as well as the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library issue 1, 2009. All controlled trials comparing D2 lymphadenectomy and D2 + PAND in the surgical treatment of GC were identified. In this review, the language of search strategy was not limited, and the following search terms were used: stomach neoplasms, stomach cancer, stomach carcinoma, gastric cancer, gastric carcinoma, gastric neoplasms, D2+, D2 plus, D3, D4, R3, R4 (in some studies, the classification of gastric carcinoma are according to the new edition[31]; it is also called D3 or R3 in the new edition and called D4 or R4 according to the old edition[32]), superextended lymphadenectomy, para-aortic lymph nodes dissection, para-aortic nodal dissection. Both free text and MeSH search for keywords were employed.
To identify further potentially relevant studies, reference lists from selected studies through electronic searching were hand searched; furthermore in order to obtain any relevant unpublished materials, we contacted scholars in the field of gastroenterology.
Inclusion and exclusion criteria
Types of studies: Comparative trials with recorded 5-year overall survival rate or secondary outcomes (post-operative morbidity and mortality, wound degree of surgery) were included. Non-comparative studies, case series, case reports and studies using historical controls were excluded.
Types of patients: Trials in which patients were pathologically proved to have gastric adenocarcinoma and qualified for gastrectomy were included. Trials in which patients had distant metastasis, gastric stump cancer, disseminated cancer cells, synchronous malignancy in other organs, serious cardiovascular or respiratory disorders, hepatic or renal failure were excluded.
Types of interventions: Trials comparing the results of D2 and D2 + PAND were included. Trials comparing the results of these two operations but also with pre-operative or post-operative chemotherapy were excluded.
Types of outcome measures: Primary outcome: 5-year overall survival rate; secondary outcome: post-operative morbidity, mortality and potential risk factors for post-operative morbidity, wound degree of surgery (operation time and blood loss during operation).
Data collection and analysis
Method of agreeing inclusion of studies: This course was performed by two authors (Wang Z and Chen JQ) independently according to preformed inclusion criteria. Titles and abstracts were scanned first to make a list of possibly related literature, and then full texts were obtained for those articles identified as either relevant or not clear, only trials coincident with pre-determined criteria of this review were included. Disagreements were settled by consensus.
Quality assessment: Since there was no consensus about quality assessment of non-randomized studies, in our review, the Cochrane Handbook for Systematic Reviews of Interventions in which criteria for non-randomized studies were the same as randomized controlled trials (RCTs) was used to assess the methodological quality of included studies[33]. The criteria included six items as following: (1) Adequate sequence generation? (2) Allocation concealment? (3) Blinding? In our review, if result measurements (only wound degree of surgery and post-operative morbidity were included, not considering 5-year survival rate and post-operative mortality) were masked, it would be regarded as low risk of both performance and detection bias; (4) Incomplete outcome data addressed? In our review, we considered the missing outcome data which were less than 10% to be low risk of bias, 10%-15% to be moderate risk of bias, and more than 15% to be high risk of bias; (5) Free of selective reporting? and (6) Free of other bias?
Options for the results of quality assessment: Yes (low risk of bias), probably yes (moderate risk of bias), No (high risk of bias). No rating of the studies was performed; each was accepted or rejected based on the six items noted above. The guidelines in the QUOROM Statement[34] were consulted by us from the literature search to the presentation of the results and discussion.
Data extraction: Using a pre-defined data extraction form, two reviewers (Wang Z and Chen JQ) extracted data about characteristics of included studies and baseline characteristics of patients independently, which included following items: author, publication date, number of patients, age and sex of patients, serosa and lymph node states, 5-year overall survival rate, post-operative morbidity and mortality, operation time and blood loss during operation. If necessary, the authors of the original articles were contacted for available data. Final agreement was achieved through discussion.
Data synthesis: In our study, the individual and pooled statistics were calculated using the fixed effect model, but a random effect model was used if P value of heterogeneity test was less than 0.1. The results were expressed with risk ratios (RR) for dichotomous data and weighted mean differences (WMD) for continuous data[33], and 95% confidence intervals (CI) were also calculated. Heterogeneity between included studies was tested using χ2 test. If heterogeneity was present, we would try to check the cause out from aspects of study design and quality, differences in intervention and baseline characteristics of included patients, by using methods of subgroup and sensitivity analysis. Funnel plots were drawn to assess publication bias. The RCTs and non-randomized studies were analyzed separately, and trials with continuous data recorded as the form of median and range were excluded from meta-analysis. Statistical analysis was performed using RevMan 5.0.18, which was provided by the Cochrane Collaboration[35].
RESULTS
Selection of included studies
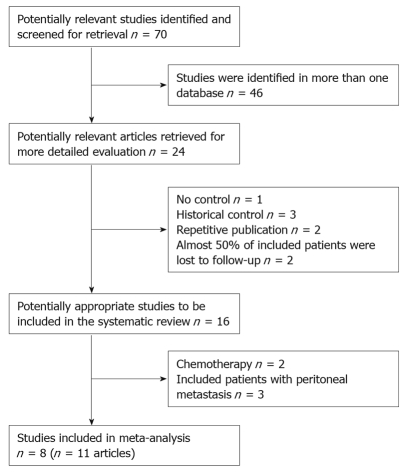
According to the search strategy referred to above, a total of 70 studies were yielded: 23 in PubMed, 16 in EMBASE, 11 in CENTRAL, 20 in CBM-disc and CNKI. Many literatures were searched in more than one database; finally 4 RCTs and 4 non-randomized studies (11 articles) were considered eligible for inclusion. The trial selection process was summarised in Figure 1. The reasons for excluding studies were as following: study type, baseline characteristics of included patients, repetitive publication, chemotherapy, distant metastasis, confounding allocation and high rate of loss of follow-up.
Figure 1.
QUORUM flow chart for studies.
Description and risk of bias of included studies
Since both RCTs and non-randomized studies were included in our study, we described them both respectively. Additionally, although there was no significant difference in each study, we did not summarize the surgical details as a table in either RCTs or non-randomized included studies since there were different descriptions in different studies. Characteristics of included studies, baseline characteristics of patients and potential bias of included studies were listed in Tables 1, 2, 3, respectively.
Table 1.
Characteristics of included studies (ITT: intention-to-treat)
| Study ID (author, yr) | Design | Methods | Patients | Interventions | Outcomes | Notes |
| Jiang et al 2000[36] | RCT | Single center, parallel group, not clear about the method of randomization | Histologically proved curable advanced gastric cancer | D2 vs D2+ | Post-operative morbidity and mortality; Operation time | Regional lymph nodes were classified according to 1th English edition 1995[32] |
| Sano et al 2004[37] and Sasako et al 2008[39] Kodera et al 2005[38] | RCT | Multicenter, parallel group, computer stratified randomization | Histologically proved advanced and curable gastric cancer | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Blood loss during operation; Five-year survival rate | ITT was used in result analysis; Regional lymph nodes were classified according to 1th English edition 1995[32] |
| Yonemura et al 2006 and 2008[40,41] | RCT | Multicenter, parallel group, computer randomization | Younger than 75-yr old, histologically proved advanced and curable gastric cancer | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Blood loss during operation; Five-year survival rate | Sample size was calculated before trials |
| Kulig et al 2007[42] | RCT | Multicenter, parallel group, simple computer randomization. | Histologically proved curable gastric adenocarcinoma | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Blood loss during operation | Regional lymph nodes were classified according to 2nd English edition 1998[31]; ITT was used in result analysis |
| Maeta et al 1999[43] | non-RCT | Single center, parallel group, without randomization | Histologically proved advanced gastric cancer, younger than 75-yr old | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Blood loss during operation | In D4 group, lymph nodes (a1, a2, b1 and b2) were all removed; Sample size was smaller than that calculated before study |
| Bostanci et al 2004[44] | non-RCT | Single center, parallel group, prospective study without randomization | Histologically proved gastric cancer | D2 vs D2+ | Post-operative morbidity and mortality; Operation time | Surgical procedure was not very clearly described in the article |
| Kunisaki et al 2006[45] | non-RCT | Multicenter, parallel group, retrospective analysis | Curable advanced gastric cancer proved by histology | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Blood loss during operation; Five-year survival rate | Data about length of post-operative hospital stay were obtained from the author |
| Hu et al 2009[46] | non-RCT | Single center, parallel group, retrospective analysis | Histologically proved curable gastric cancer | D2 vs D2+ | Post-operative morbidity and mortality; Operation time; Five-year survival rate | In D4 group, lymph nodes (a1, a2 and b1) were dissected |
RCT: Randomized controlled trial.
Table 2.
Baseline characteristics of patients in included studies
| Study ID (author, yr, ND2/ND2+) |
Age (yr) (mean/SD or median/range) |
Sex (male/female) |
Pathological T stage (serosa negative/positive) |
Pathological node status (negative/positive) |
||||||||
| D2 | D2+ | P | D2 | D2+ | P | D2 | D2+ | P | D2 | D2+ | P | |
| Sano et al 2004[37], Sasako et al 2008[39] (263/260) | 60 (25-75) | 61(27-75) | > 0.05 | 176/87 | 183/77 | > 0.05 | 134/129 | 146/114 | > 0.05 | 79/184 | 96/164 | > 0.05 |
| Kulig et al 2007[42] (141/134) | 56 (31-81) | 54 (34-77) | > 0.05 | 85/56 | 83/51 | > 0.05 | 64/77 | 54/84 | > 0.05 | 50/91 | 56/78 | > 0.05 |
| Yonemura et al 2006 and 2008[40,41] (135/134) | 63.8 (9.7) | 62.5 (10.2) | > 0.05 | 90/45 | 91/43 | > 0.05 | 63/72 | 61/73 | > 0.05 | 37/98 | 35/99 | > 0.05 |
| Jiang et al 2000[36] (32/21) | 61 (46-83) | 65 (34-84) | NR | 19/13 | 11/10 | NR | NR | NR | NR | NR | NR | NR |
| Maeta et al 1999[43] (35/35) | 60 (11) | 59 (9) | > 0.05 | 20/15 | 21/14 | > 0.05 | 2/33 | 6/29 | > 0.05 | 15/20 | 12/23 | > 0.05 |
| Hu et al 2009[46] (55/62) | 58.8 (11.4) | 54.3 (11.4) | < 0.05 | 42/13 | 48/14 | > 0.05 | 13/42 | 15/47 | > 0.05 | 16/39 | 23/39 | > 0.05 |
| Kunisaki et al 2006[45] (430/150) | 62.2 (12.5) | 59.3 (10.7) | > 0.05 | 286/144 | 109/41 | > 0.05 | 197/233 | 71/79 | > 0.05 | 126/304 | 34/116 | < 0.05 |
| Bostanci et al 2004[44] (100/34) | 58.5 (13) | 59 (12.6) | > 0.05 | 63/37 | 21/13 | > 0.05 | 98/2 | 29/35 | NR | NR | NR | NR |
NR: Not reporting.
Table 3.
Potential bias of included studies
| Study ID (author, yr) | Adequate sequence generation | Allocation concealment | Blinding | Incomplete outcome data addressed | Free of selective reporting | Free of other bias |
| Jiang et al 2000[36] | Probably yes | Probably yes | Probably yes | Yes | Probably yes | Probably yes |
| Sano et al 2004[37], Sasako et al 2008[39] | Yes | Yes | Probably yes | Yes | Probably yes | Probably yes |
| Yonemura et al 2006 and 2008[40,41] | Yes | Probably yes | Probably yes | Yes | Probably yes | Probably yes |
| Kulig et al 2007[42] | Yes | Probably yes | Probably yes | Yes | Probably yes | Probably yes |
| Maeta et al 1999[43] | No | No | Probably yes | Yes | Probably yes | Probably yes |
| Bostanci et al 2004[44] | No | No | Probably yes | Yes | Probably yes | Probably yes |
| Kunisaki et al 2006[45] | No | No | Probably yes | Yes | Probably yes | Probably yes |
| Hu et al 2009[46] | No | No | Probably yes | Probably yes | Probably yes | Probably yes |
Description and risk of bias of RCTs
In total 1120 patients in 4 RCTs (7 articles)[36-42] were eligible for inclusion, 571 patients in the D2 group and 549 patients in the D2 + PAND group, and the sample size of studies varied from 53 to 523. All included RCTs except one[36] used computer randomization, but only one trial used a central telephone registration system[37]; the concealment of allocation was unclear in three trials. Blinding referred to result measurements being blinded in our study, and wasn’t clearly described in all trials. Completeness of data was good in 4 included trials. There was no significant difference in baseline characteristics of patients (age, sex, T stage and lymph node states) between D2 and D2 + PAND groups in included trials except the trial reported by Jiang et al[36], in which T stage and lymph node state were not clearly reported. Three articles showed that only surgeons skilled in D2 and D2 + PAND operation participated in their studies[37,40,42]. Selecting reports and other bias were not clearly recorded in their published reports.
Description and risk bias of non-randomized studies
In total 901 patients in 4 non-randomized studies[43-46] were included, 620 patients in the D2 group and 281 patients in the D2 + PAND group, and sample size of studies ranged from 70 to 580. No randomization and concealment of allocation were performed. According to the definition of blinding in our review, blinding was unclear in 4 included studies. Completeness of data was good; the rate of loss of follow-up was less than 15% for all included studies. The description of selective reporting and other bias was not described in detail. Baseline characteristics were similar between D2 and D2 + PAND groups in all included studies.
Effects of interventions (Effectiveness in RCTs)
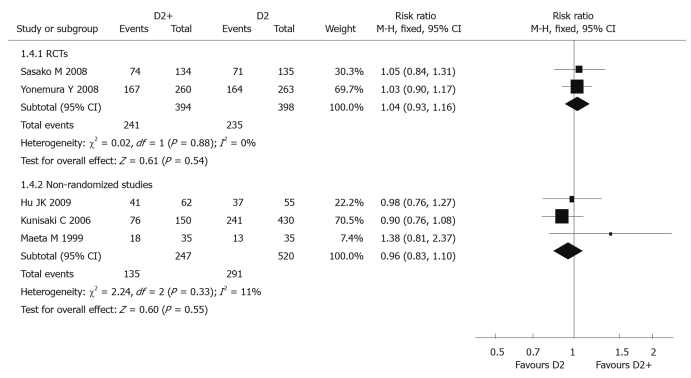
Five-year overall survival rate: Two RCTs[39,41] (792 patients) reported 5-year overall survival rate, and the pooled RR was 1.04 (95% CI: 0.93-1.16). There was no significant difference between D2 + PAND and D2 groups. The P value of heterogeneity test was 0.88 (Figure 2).
Figure 2.
Meta-analysis of 5-year overall survival rate comparing D2 + PAND with D2 gastrectomy.
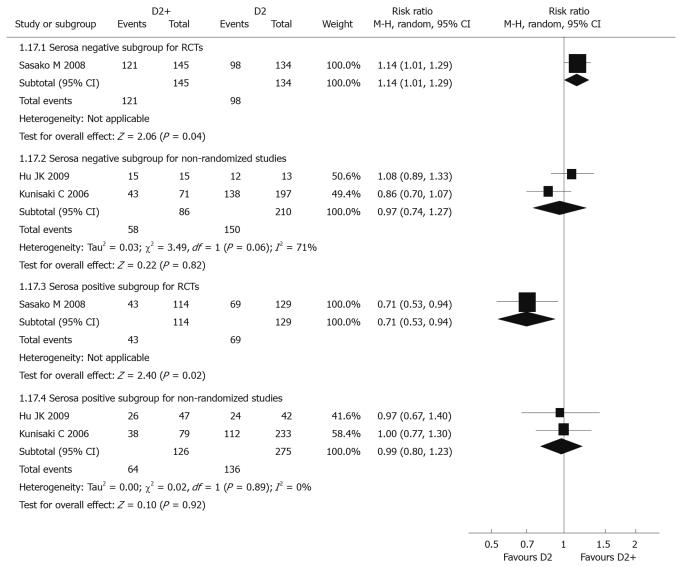
Data from only 1 RCT[39] could be analyzed, with individual RR 1.14 (95% CI: 1.01-1.29) for the serosa negative subgroup and 0.71 (95% CI: 0.53-0.94) for the serosa positive subgroup. There was a significant difference between these two subgroups. Compared with D2 dissection, five-year overall survival rate for D2 + PAND in the serosa negative subgroup was significantly higher than that in the serosa positive subgroup, but tests for heterogeneity were not applicable in these two subgroups (Figure 3).
Figure 3.
Meta-analysis of 5-year overall survival rate for serosa negative and positive subgroups comparing D2 + PAND with D2 gastrectomy.
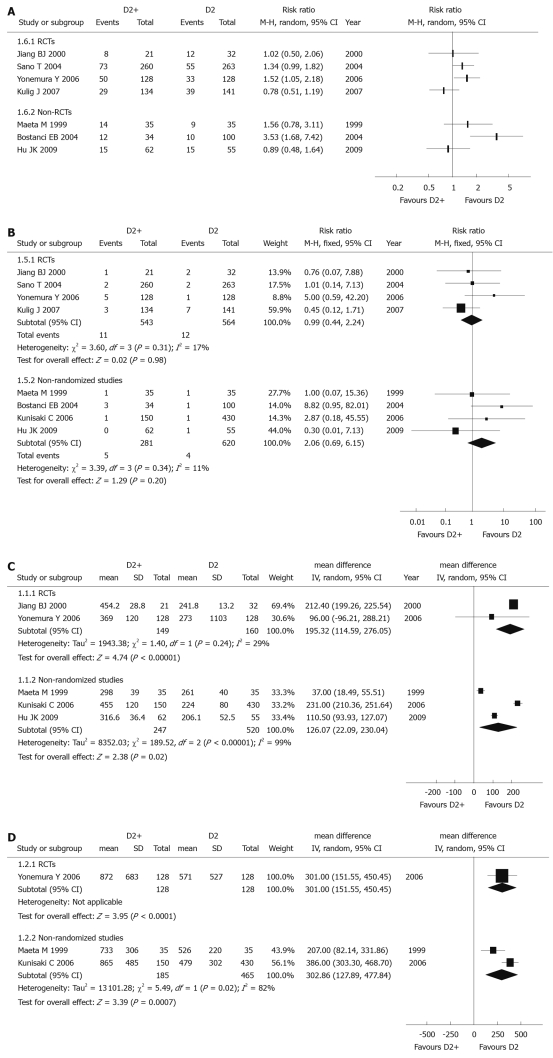
Post-operative morbidity and mortality: Since there were different definitions and contents in each study, post-operative morbidity data couldn’t be used in a meta-analysis. All RCTs (1107 patients) reported data on post-operative morbidity, but the results in four included studies were not consistent. Apart from the study reported by Yonemura et al[40] [individual RR 1.52 (95% CI: 1.05-2.18)], three other trials reported there was no significant difference between D2 and D2 + PAND groups (Figure 4A).
Figure 4.
Meta-analysis of post-operative morbidity comparing (A), post-operative mortality (B), operation time (C), blood loss during operation (D) D2 + PAND with D2 gastrectomy.
The post-operative mortality data of all included RCTs (1107 patients) could be used, and the combined RR was 0.99 (95% CI: 0.44-2.24), which revealed that there was no significant difference between D2 + PAND and D2 groups. The test for heterogeneity was negative with a P value of 0.31 (Figure 4B).
Operation time: Data of two RCTs[36,40] (309 patients) were applicable; the pooled WMD was 195.32 min (95% CI: 114.59-276.05), suggesting there was a significant difference between these two groups. Operation time in the D2 + PAND group was significantly longer than that in the D2 group. The P value of the heterogeneity test was 0.24 (Figure 4C).
Blood loss during operation: Only one RCT[40] (256 patients) was useful for analysis, and individual WMD was 301 mL (95% CI: 151.55-450.45). There was a significant difference between these two groups. Blood loss in the D2 + PAND group was significantly more than that in the D2 group, and the test for heterogeneity was not applicable (Figure 4D).
Effects of interventions (Effects in non-randomized studies)
Five-year survival rate: Three non-randomized studies (767 patients)[43,45,46] reported 5-year overall survival rate, and the pooled RR was 0.96 (95% CI: 0.83-1.10), suggesting there was no statistical significance between the two groups; the P value of the heterogeneity test was 0.33 (Figure 2).
Two non-randomized studies[45,46] were included in the analysis. Pooled RR for serosa negative and positive subgroups were 0.97 (95% CI: 0.74-1.27) and 0.99 (95% CI: 0.80-1.23), respectively, revealing there was no significant difference between these two subgroups. Comparing with D2, D2 + PAND didn’t improve 5-year overall survival whether in serosa negative or positive subgroups. The P values of heterogeneity tests were 0.06 and 0.89, respectively (Figure 3).
Post-operative morbidity and mortality: For similar reasons as listed for RCTs, the post-operative morbidity data couldn’t be combined. Post-operative morbidity was reported in three articles[43,44,46] (321 patients), but results were not coincident. Higher post-operative morbidity was reported by Bostanci et al[44] (individual RR 3.53 (95% CI: 1.68-7.42); however, two other articles[43,46] reported there was no significant difference between D2 and D2 + PAND groups (Figure 4A).
For post-operative mortality, all included non-randomized studies (901 patients) could be analyzed, and the combined RR was 2.06 (95% CI: 0.69-6.15), suggesting there was no significant difference between the two groups. This indicated D2 + PAND could be performed without increasing post-operative mortality; the test for heterogeneity was negative with a P value of 0.34 (Figure 4B).
Operation time: Data of three non-randomized studies[43,45,46] (767 patients) were useful for meta-analysis, and the pooled WMD was 126.07 min (95% CI: 22.09-230.04), suggesting that there was a significant difference between the two groups. The operation time for D2 + PAND was significantly longer than that for D2. The P value of the heterogeneity test was less than 0.1 (Figure 4C), and a sensitivity analysis identified that the study reported by Kunisaki et al[45] was the main source of heterogeneity.
Blood loss during operation: Two non-randomized studies[43,45] (650 patients) could be used for meta-analysis, and the pooled WMD was 302.86 mL (95% CI: 127.89-477.84), revealing there was a significant difference between the two groups. This suggested that there was more blood loss in D2 + PAND. The test for heterogeneity was positive (P = 0.02, Figure 4D).
Potential risk factors for post-operative morbidity
Univariate and multivariate analysis were used to identify the potential risk factors for post-operative morbidity in only one RCT[38]; multivariate analysis revealed that pancreatectomy [RR 5.62 (95% CI: 1.94-16.27)] was the most important risk factor for overall complications, but meta-analysis wasn’t practicable.
Testing for publication bias
Because there were too few included studies, we didn’t draw funnel plots in our review.
DISCUSSION
The choice for the extent of lymphadenectomy in GC has been controversial[18,30]. To perform a comparison of the effectiveness and safety between standard D2 and D2 + PAND, we conducted a systemic review based on current studies using the method provided by Cochrane Collaboration[33]. Four RCTs (total 1120 patients) and four non-randomized studies (total 901 patients) were included in our review. The results of meta-analysis showed that the 5-year overall survival rate was similar between the two operations. Compared with that in D2 resection, post-operative morbidity and mortality didn’t increase in D2 + PAND, but the operation time was significantly longer and the blood loss was significantly greater in D2 + PAND in RCTs and non-randomized studies.
Minimal heterogeneity was found between the RCTs for all the measured outcomes, which ensured the quality of the meta-analysis; however, this systematic review was not faultless. First, baseline characteristics were statistically similar in included studies, but were not exactly the same among each RCT; for example, T1 patients were included by Kulig et al[42] but not by Sano et al[37], which suggested a potential selection risk. Second, regional lymph nodes were classified by different classifications[31,32] in included studies, thus the station of lymphadenectomy was confounding in D2 and D2 + PAND groups, which led to the mixture of interventions between included studies. Additionally, surgical outcomes were related to experience of the surgeon[47]; considering classification standards of lymph node and learning curves of surgeons performance bias might be introduced in our study. Third, from Table 3, blinding could be seen to be unclear in included studies, so measurement bias might exist. Although this was a general phenomenon in surgical clinical trials, it might weaken the reliability of our results. Fourth, completeness of data could affect the statistical results; according to reports of primary literature, the loss rate of data in all included studies was less than 15%, however this does not eliminate the possibility of potential bias. Fifth, stage migration, as it was known, might improve pathological stage- and N-stage-specific survival in GC[48,49]. However, this factor wasn’t taken into consideration in our included studies; this might affect the stage-specific survival after D2 and D2 + PAND. Finally, it was impossible for us to identify all relevant literature, even though we made great efforts; moreover, publication bias might exist due to the low number of included studies.
Because there were few RCTs, non-randomized studies were included in our review to obtain as much comprehensive information as possible, and this problem is common in surgical systemic reviews. Considering the potential heterogeneity induced by methodology, we didn’t combine the data of RCTs and non-randomized studies together. Additionally, although combined results of the non-randomized studies were affected by considerable bias in view of differences in methodology and baseline characteristics of included patients, the aim of meta-analysis of non-randomized studies was just to offer an overview of the general trend of outcomes in these studies. In addition, results of non-randomized studies were found to be similar to that of RCTs, which increased the reliability of our review to some extent.
Comparing with that of D2 resection, the survival advantage of D2 + PAND was controversial[27-29]. It was found that patients with positive PAN and stage IIIb patients got survival benefit from D2 + PAND by some researchers[20,23,50]. In our review, we failed to discover an overall survival benefit for D2 + PAND [RCTs: RR 1.04 (95% CI: 0.93-1.16); non-randomized studies: RR 0.96 (95% CI: 0.83-1.10)]. In order to identify possible survival discrepancy between D2 and D2 + PAND groups, we conducted a subgroup analysis which used serosa invasion as the only factor due to shortage of primary data. Although it was considered to be an important prognostic factor for GC, we could not analyze TNM stages. In RCTs, there was a survival benefit for serosa negative patients [RR 1.14 (95% CI: 1.01-1.29)], but in non-randomized studies, there was no significant difference in 5-year survival rate between the serosa negative subgroup [RR 0.97 (95% CI: 0.74-1.27)] and the serosa positive subgroup [RR 0.99 (95% CI: 0.80-1.23)]. Theoretically, a larger range of surgery options should produce survival benefit in more advanced carcinoma, so the finding in our meta-analysis seems unbelievable, and is the opposite to some studies[51]. Although definitive survival data of a study by Kulig et al[42] are still to come, there is little reason to expect dramatic changes. Therefore, potential survival benefit of D2 + PAND compared with D2 gastrectomy for patients in specific tumor stages, as well as a method to identify para-aortic lymph node metastasis pre-operatively, should be urgently researched in future.
Pancreatic fistula and respiratory complications after PAND were significantly more common in a report by Kunisaki et al[25]; however, some retrospective reports revealed that there was no significant difference in post-operative complications and mortality between D2 and D2 + PAND, and D2 + PAND can be performed safely by well-trained surgeons[26-27]. Post-operative morbidity data were not combined by us due to different definitions and contents in each included study, but controversy also appeared in different studies. Generally speaking, the extent of lymphadenectomy could influence the morbidity rates of GC patients, and it was reported that the greater the resection of lymph nodes, the greater the probability of complications[40,44]. But on the contrary, some researchers[36,37,42,43,46] revealed that there was no significant difference in the postoperative morbidity between D2 and D2 + PAND patients. In our review, post-operative mortality data were consistent in RCTs and non-randomized studies, with pooled RR 0.99 (95% CI: 0.44-2.24) and 2.06 (95% CI: 0.69-6.15), respectively, suggesting that D2 + PAND might be performed as safely as standard D2. To identify potential risk factors for post-operative morbidity, we searched all relevant literature, and found only one RCT data could be used[38]. Results of multivariate analysis from this only fit RCT showed that pancreatectomy was the most important risk factor for overall complications, with RR of 5.62 (95% CI: 1.94-16.27). This finding was concurrent with some other studies[52], and demonstrated that the resection of combined organs should be prudent.
In this review, we found that PAND, whether in RCTs or in non-randomized studies, was correlated with longer operation time [RCTs: WMD 195.32 min (95% CI: 114.59-276.05); non-randomized studies: WMD 126.07 min (95% CI: 22.09-230.04)] and more blood loss [RCTs: WMD 301 mL (95% CI: 151.55-450.45); non-randomized studies: WMD 302.86 mL (95% CI: 127.89-477.84)], which were different from that of a related meta-analysis published recently[30], in which blood loss during operation wasn’t analyzed, but operation time was similar with a WMD - 112.60 min (95% CI: 286.22-61.02) between the two operations; clinically, the operative procedure to eradicate para-aortic lymph nodes is more complicated, so the wound degree of surgery should be heavier in D2 + PAND, thus our results were more credible to some extent. As we know, wound degree of surgery was intensively affected by skills of surgeons, so if we want to reduce the wound degree of surgery for PAND patients, pre-operative training of surgeon is necessary. Furthermore, in order to reduce the publication bias from different authors in different nations, standard operating procedures or rules for PAND should be achieved by consensus in time; otherwise, in the future, it will be also difficult to judge which RCT is more reliable.
Quality of life after surgery, as we know, is an important index to evaluate treatment effectiveness for patients with malignant tumors. Unfortunately, very few studies reported this clinical outcome of patients with advanced GC after PAND; so quality of life after PAND should be taken into consideration in future clinical studies.
In conclusion, D2 + PAND can be performed as safely as standard D2. Compared with standard D2, D2 + PAND doesn’t have any overall survival benefit, but its’ wound degree of surgery is significantly higher, demonstrated as a longer operation time and greater blood loss. Consequently we feel D2 + PAND should be performed prudently. For reducing wound degree during D2 + PAND, pre-operative training of the surgeon is necessary and standard operating procedures or rules for PAND should be achieved by consensus. Additionally, research on the relationship between prognosis of GC patients and combined organ resection, and potential survival benefit of D2 + PAND for some specific stages of advanced GC are urgently needed in the future.
COMMENTS
Background
Although radical gastrectomy is thought to be an important treatment measure, prognosis of advanced gastric cancer (GC) is still poor. A reasonable operation procedure for advanced GC is an issue worthy of investigating nowadays.
Research frontiers
It is reported that 18%-40% of patients with advanced GC had metastasis present in the para-aortic nodes and additional dissection of para-aortic nodes is considered to be a necessary procedure as well as standard D2 gastrectomy in order to achieve R0 resection. However, the effectiveness of D2 + para-aortic nodal dissection (PAND) is still controversial.
Innovations and breakthroughs
Some researchers assumed that PAND might achieve R0 resection and benefit the survival of patients with advanced GC, despite its high post-operative morbidity and mortality. The present systematic review found that D2 + PAND failed to benefit the overall survival of patients with advanced GC, but it could be performed as safely as standard D2 resection without increasing post-operative mortality.
Applications
D2 + PAND should be performed prudently and its potential survival benefit for patients with specific tumor stages of advanced GC should be urgently researched in the future.
Terminology
Standard D2 lymphadenectomy refers to systematic dissection of lymph nodes in the first tier (perigastric) and the second tier (along the celiac artery and its branches); and D2 + PAND refers to additional dissection of para-aortic lymph nodes.
Peer review
This manuscript is well prepared, study design is reasonable, statistical methods are appropriate, and conclusions are based on the convincing statistical analysis. The results and information conveyed in the article might contribute to the knowledge of gastric cancer management.
Footnotes
Supported by The National Natural Science Foundation of China, Grant No. 30560151
Peer reviewer: Hayrullah Derici, MD, Associate Professor, Department of General Surgery, Balıkesir University Medical Faculty, Balıkesir 10145, Turkey
S- Editor Tian L L- Editor O’Neill M E- Editor Ma WH
References
- 1.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parkin DM, Whelan SL, Ferlay J. Cancer Incidence in Five Continents, vol VII. Lyon, France: International Agency for Research on Cancer; 1997. pp. 822–823. [Google Scholar]
- 3.Yamamoto S. Stomach cancer incidence in the world. Jpn J Clin Oncol. 2001;31:471. doi: 10.1093/jjco/31.9.471. [DOI] [PubMed] [Google Scholar]
- 4.Wang ZN, Lu C, Xu HM. Lymph node metastasis of upper gastric cancer and its significance in surgical treatment. Zhongguo Shiyong Waike Zazhi. 2002;22:611–612. [Google Scholar]
- 5.Tsubono Y, Hisamichi S. Screening for gastric cancer in Japan. Gastric Cancer. 2000;3:9–18. doi: 10.1007/pl00011692. [DOI] [PubMed] [Google Scholar]
- 6.Roukos DH, Kappas AM. Perspectives in the treatment of gastric cancer. Nat Clin Pract Oncol. 2005;2:98–107. doi: 10.1038/ncponc0099. [DOI] [PubMed] [Google Scholar]
- 7.Alberts SR, Cervantes A, van de Velde CJ. Gastric cancer: epidemiology, pathology and treatment. Ann Oncol. 2003;14 Suppl 2:ii31–ii36. doi: 10.1093/annonc/mdg726. [DOI] [PubMed] [Google Scholar]
- 8.Ito Y, Ohno Y, Rachet B, Coleman MP, Tsukuma H, Oshima A. Cancer survival trends in Osaka, Japan: the influence of age and stage at diagnosis. Jpn J Clin Oncol. 2007;37:452–458. doi: 10.1093/jjco/hym047. [DOI] [PubMed] [Google Scholar]
- 9.Verdecchia A, Francisci S, Brenner H, Gatta G, Micheli A, Mangone L, Kunkler I. Recent cancer survival in Europe: a 2000-02 period analysis of EUROCARE-4 data. Lancet Oncol. 2007;8:784–796. doi: 10.1016/S1470-2045(07)70246-2. [DOI] [PubMed] [Google Scholar]
- 10.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 11.Chen L, Zhang Y, Wei B, Zhao XY, Li T. [Surgical treatment for patients with gastric cancer: report of 2335 cases] Zhonghua Weichang Waike Zazhi. 2007;10:421–424. [PubMed] [Google Scholar]
- 12.Lewis WG, Edwards P, Barry JD, Khan S, Dhariwal D, Hodzovic I, Allison MC, Shute K. D2 or not D2? The gastrectomy question. Gastric Cancer. 2002;5:29–34. doi: 10.1007/s101200200004. [DOI] [PubMed] [Google Scholar]
- 13.Degiuli M, Sasako M, Ponzetto A, Allone T, Soldati T, Calgaro M, Balcet F, Bussone R, Olivieri F, Scaglione D, et al. Extended lymph node dissection for gastric cancer: results of a prospective, multi-centre analysis of morbidity and mortality in 118 consecutive cases. Eur J Surg Oncol. 1997;23:310–314. doi: 10.1016/s0748-7983(97)90696-x. [DOI] [PubMed] [Google Scholar]
- 14.Volpe CM, Driscoll DL, Miloro SM, Douglass HO Jr. Survival benefit of extended D2 resection for proximal gastric cancer. J Surg Oncol. 1997;64:231–236. doi: 10.1002/(sici)1096-9098(199703)64:3<231::aid-jso10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama K, Sasako M, Kinoshita T, Sano T, Katai H, Hada M, Schmidt-Matthiesen A, Dahl O. Should systematic lymph node dissection be recommended for gastric cancer? Eur J Cancer. 1998;34:1480–1489. doi: 10.1016/s0959-8049(98)00201-9. [DOI] [PubMed] [Google Scholar]
- 16.Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, Saragoni L, Tomezzoli A, Kurihara H. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: a longitudinal multicenter study. Ann Surg Oncol. 2002;9:894–900. doi: 10.1007/BF02557527. [DOI] [PubMed] [Google Scholar]
- 17.Zilberstein B, da Costa Martins B, Jacob CE, Bresciani C, Lopasso FP, de Cleva R, Pinto Junior PE, Junior UR, Perez RO, Gama-Rodrigues J. Complications of gastrectomy with lymphadenectomy in gastric cancer. Gastric Cancer. 2004;7:254–259. doi: 10.1007/s10120-004-0301-8. [DOI] [PubMed] [Google Scholar]
- 18.McCulloch P, Nita ME, Kazi H, Gama-Rodrigues J. Extended versus limited lymph nodes dissection technique for adenocarcinoma of the stomach. Cochrane Database Syst Rev. 2004:CD001964. doi: 10.1002/14651858.CD001964.pub2. [DOI] [PubMed] [Google Scholar]
- 19.Nakane Y, Okamura S, Masuya Y, Okumura S, Akehira K, Hioki K. Incidence and prognosis of para-aortic lymph node metastasis in gastric cancer. Hepatogastroenterology. 1998;45:1901–1906. [PubMed] [Google Scholar]
- 20.Isozaki H, Okajima K, Fujii K, Nomura E, Izumi N, Mabuchi H, Nakamura M, Hara H. Effectiveness of paraaortic lymph node dissection for advanced gastric cancer. Hepatogastroenterology. 1999;46:549–554. [PubMed] [Google Scholar]
- 21.Takashima S, Kosaka T. Results and controversial issues regarding a para-aortic lymph node dissection for advanced gastric cancer. Surg Today. 2005;35:425–431. doi: 10.1007/s00595-004-2976-1. [DOI] [PubMed] [Google Scholar]
- 22.De Manzoni G, Di Leo A, Borzellino G, Bonfiglio M, Pedrazzani C, Tasselli S, Castelli A, Zerman G, Fersini A. [Para-aortic lymph node involvement in gastric adenocarcinoma] Ann Chir. 2001;126:302–306; discussion 306-307. doi: 10.1016/s0003-3944(01)00521-1. [DOI] [PubMed] [Google Scholar]
- 23.Yonemura Y, Katayama K, Kamata T, Fushida S, Segawa M, Ooyama S, Miwa K, Miyazaki I. Surgical treatment of advanced gastric cancer with metastasis in para-aortic lymph node. Int Surg. 1991;76:222–225. [PubMed] [Google Scholar]
- 24.Baba M, Hokita S, Natsugoe S, Miyazono T, Shimada M, Nakano S, Takao S, Aikou T. Paraaortic lymphadenectomy in patients with advanced carcinoma of the upper-third of the stomach. Hepatogastroenterology. 2000;47:893–896. [PubMed] [Google Scholar]
- 25.Kunisaki C, Shimada H, Yamaoka H, Wakasugi J, Takahashi M, Akiyama H, Nomura M, Moriwaki Y. Significance of para-aortic lymph node dissection in advanced gastric cancer. Hepatogastroenterology. 1999;46:2635–2642. [PubMed] [Google Scholar]
- 26.Günther K, Horbach T, Merkel S, Meyer M, Schnell U, Klein P, Hohenberger W. D3 lymph node dissection in gastric cancer: evaluation of postoperative mortality and complications. Surg Today. 2000;30:700–705. doi: 10.1007/s005950070080. [DOI] [PubMed] [Google Scholar]
- 27.Zhan WH, Han FH, He YL, Li YM, Zheng ZQ, Peng JS, Cai SR, Ma JP. [Disciplinarian of lymph node metastasis and effect of paraaortic lymph nodes dissection on clinical outcomes in advanced gastric carcinoma] Zhonghua Weichang Waike Zazhi. 2006;9:17–22. [PubMed] [Google Scholar]
- 28.Wan Y, Pan Y, Liu Y, Wang Z, Ye J, Huang S. [Lymph node metastasis and the extent of lymph node dissection for gastric cancer: report of 326 cases] Zhonghua Waike Zazhi. 2000;38:752–755. [PubMed] [Google Scholar]
- 29.Bittorf BR, Günther F, Merkel S, Horbach T, Hohenberger W, Günther K. [D3 versus D2 dissection in stomach carcinoma. A case-control study of postoperative morbidity, survival and early oncologic outcome] Chirurg. 2002;73:336–347. doi: 10.1007/s00104-002-0457-x. [DOI] [PubMed] [Google Scholar]
- 30.Yang SH, Zhang YC, Yang KH, Li YP, He XD, Tian JH, Lv TH, Hui YH, Sharma N. An evidence-based medicine review of lymphadenectomy extent for gastric cancer. Am J Surg. 2009;197:246–251. doi: 10.1016/j.amjsurg.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer. 1998;1:10–24. doi: 10.1007/s101209800016. [DOI] [PubMed] [Google Scholar]
- 32.Japanese Research Society for Gastric Cancer. Japanese Classification of Gastric Carcinoma. Tokyo: Kanehara; 1995. [Google Scholar]
- 33.Higgins JPT, Green S, editors . Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated september 2008]. The Cochrane Collaboration, 2008. Available from: http:// www.cochrane-handbook.org. [Google Scholar]
- 34.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of Reporting of Meta-analyses. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 35. http://www.cc-ims.net/RevMan/current.htm. [Google Scholar]
- 36.Jiang BJ, Gao YF, Sun RX, Shen H, Lu M, Tu CL. Clinical study on the dissection of lymph nodes around abdominal aortic artery in advanced gastric cancer. Zhongguo Putong Waike Zazhi. 2000;9:292–295. [Google Scholar]
- 37.Sano T, Sasako M, Yamamoto S, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y, et al. Gastric cancer surgery: morbidity and mortality results from a prospective randomized controlled trial comparing D2 and extended para-aortic lymphadenectomy--Japan Clinical Oncology Group study 9501. J Clin Oncol. 2004;22:2767–2773. doi: 10.1200/JCO.2004.10.184. [DOI] [PubMed] [Google Scholar]
- 38.Kodera Y, Sasako M, Yamamoto S, Sano T, Nashimoto A, Kurita A. Identification of risk factors for the development of complications following extended and superextended lymphadenectomies for gastric cancer. Br J Surg. 2005;92:1103–1109. doi: 10.1002/bjs.4979. [DOI] [PubMed] [Google Scholar]
- 39.Sasako M, Sano T, Yamamoto S, Kurokawa Y, Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, et al. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med. 2008;359:453–462. doi: 10.1056/NEJMoa0707035. [DOI] [PubMed] [Google Scholar]
- 40.Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata S, Yamamoto H, Kim BS, Matsuki N, et al. Operative morbidity and mortality after D2 and D4 extended dissection for advanced gastric cancer: a prospective randomized trial conducted by Asian surgeons. Hepatogastroenterology. 2006;53:389–394. [PubMed] [Google Scholar]
- 41.Yonemura Y, Wu CC, Fukushima N, Honda I, Bandou E, Kawamura T, Kamata T, Kim BS, Matsuki N, Sawa T, et al. Randomized clinical trial of D2 and extended paraaortic lymphadenectomy in patients with gastric cancer. Int J Clin Oncol. 2008;13:132–137. doi: 10.1007/s10147-007-0727-1. [DOI] [PubMed] [Google Scholar]
- 42.Kulig J, Popiela T, Kolodziejczyk P, Sierzega M, Szczepanik A. Standard D2 versus extended D2 (D2+) lymphadenectomy for gastric cancer: an interim safety analysis of a multicenter, randomized, clinical trial. Am J Surg. 2007;193:10–15. doi: 10.1016/j.amjsurg.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 43.Maeta M, Yamashiro H, Saito H, Katano K, Kondo A, Tsujitani S, Ikeguchi M, Kaibara N. A prospective pilot study of extended (D3) and superextended para-aortic lymphadenectomy (D4) in patients with T3 or T4 gastric cancer managed by total gastrectomy. Surgery. 1999;125:325–331. [PubMed] [Google Scholar]
- 44.Bostanci EB, Kayaalp C, Ozogul Y, Aydin C, Atalay F, Akoglu M. Comparison of complications after D2 and D3 dissection for gastric cancer. Eur J Surg Oncol. 2004;30:20–25. doi: 10.1016/j.ejso.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 45.Kunisaki C, Akiyama H, Nomura M, Matsuda G, Otsuka Y, Ono H, Nagahori Y, Hosoi H, Takahashi M, Kito F, et al. Comparison of surgical results of D2 versus D3 gastrectomy (para-aortic lymph node dissection) for advanced gastric carcinoma: a multi-institutional study. Ann Surg Oncol. 2006;13:659–667. doi: 10.1245/ASO.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 46.Hu JK, Yang K, Zhang B, Chen XZ, Chen ZX, Chen JP. D2 plus para-aortic lymphadenectomy versus standardized D2 lymphadenectomy in gastric cancer surgery. Surg Today. 2009;39:207–213. doi: 10.1007/s00595-008-3856-x. [DOI] [PubMed] [Google Scholar]
- 47.Lee JH, Ryu KW, Lee JH, Park SR, Kim CG, Kook MC, Nam BH, Kim YW, Bae JM. Learning curve for total gastrectomy with D2 lymph node dissection: cumulative sum analysis for qualified surgery. Ann Surg Oncol. 2006;13:1175–1181. doi: 10.1245/s10434-006-9050-8. [DOI] [PubMed] [Google Scholar]
- 48.Kunisaki C, Takahashi M, Fukushima T, Nagahori Y, Akiyama H, Makino H, Otsuka Y, Ono HA, Kosaka T, Takagawa R, et al. The influence of stage migration on the comparison of surgical outcomes between D2 gastrectomy and D3 gastrectomy (para-aortic lymph node dissection): a multi-institutional retrospective study. Am J Surg. 2008;196:358–363. doi: 10.1016/j.amjsurg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19–25. doi: 10.1200/JCO.1995.13.1.19. [DOI] [PubMed] [Google Scholar]
- 50.Gunji Y, Suzuki T, Kobayashi S, Hori S, Hayashi H, Shimada H, Matsubara H, Nabeya Y, Ochiai T. Evaluation of D3/D4 lymph node dissection for patients with grossly N2 positive advanced gastric cancer. Hepatogastroenterology. 2003;50:1178–1182. [PubMed] [Google Scholar]
- 51.Kunisaki C, Shimada H, Yamaoka H, Takahashi M, Ookubo K, Akiyama H, Nomura M, Moriwaki Y. Indications for paraaortic lymph node dissection in gastric cancer patients with paraaortic lymph node involvement. Hepatogastroenterology. 2000;47:586–589. [PubMed] [Google Scholar]
- 52.Yu W, Choi GS, Chung HY. Randomized clinical trial of splenectomy versus splenic preservation in patients with proximal gastric cancer. Br J Surg. 2006;93:559–563. doi: 10.1002/bjs.5353. [DOI] [PubMed] [Google Scholar]