Abstract
West Nile virus (WNV) is a mosquito-borne pathogen. During replication, WNV acquires different carbohydrates and lipid membranes, depending on its mosquito or vertebrate hosts. Consequently, WNV derived from mosquito and vertebrate cell lines differ in their infectivity for dendritic cells (DCs) and induction of type I interferon (IFN-α/β) in vitro. We evaluated the pathogenesis of WNV derived from mosquito (WNVC6/36) and vertebrate (WNVBHK) cell lines in mice. The tissue tropism, infectivity, clinical disease, and mortality did not differ for mice inoculated with WNVC6/36 or WNVBHK, and there were only minor differences in viral load and serum levels of IFN-α/β. The replication kinetics of WNVC6/36 and WNVBHK were equivalent in primary DCs and skin cells although primary DCs were more susceptible to WNVC6/36 infection than to WNVBHK infection, suggesting that less virus is produced per infected cell for WNVC6/36. In conclusion, viral source has minimal effect on WNV pathogenesis in vivo.
Keywords: West Nile virus, flavivirus, virus-host interaction, pathogenesis, Type I interferon, dendritic cells, skin, mosquito, mice
Introduction
West Nile virus (WNV), a member of the Flaviviridae family, is one of the most widespread arboviruses in the world. The virus is maintained in an enzootic cycle between mosquitoes and avian hosts, and many other animals, including humans and horses, can become infected with WNV and can develop disease (Briese and Bernard, 2005). The WNV genome is a positive-sense RNA, which forms a nucleocapsid with multiple copies of the viral capsid (C) protein. The outermost layer of the virion contains a lipid-bilayer envelope that is derived from the host membrane. Embedded within the envelope are two viral proteins, envelope (E) and membrane (prM/M) proteins, and both contain N-linked glycosylation sites (Adams et al., 1995; Hanna et al., 2005; Mukhopadhyay et al., 2003).
In nature, WNV is transmitted to its vertebrate hosts through the bite of an infected mosquito. A number of factors may influence this initial interaction of the virus with its target cell, including mosquito saliva, dose of virus, location in skin, and source of the virus. For example, the first round of infection is with virus derived from mosquito cells, and subsequent infections occur with vertebrate-derived virus. The initial target cell for WNV remains unknown; however, based on findings for other flaviviruses (Wu et al., 2000), it is thought that WNV first infects Langerhans cells, the resident dendritic cells (DCs) of the skin, at the site of inoculation, and the Langerhans cells then migrate to local draining lymph nodes (Johnston et al., 2000).
Differences in the source of virus, whether mosquito or vertebrate cell-derived, affects the interaction between the virus and its host cell. Carbohydrate processing in insect cells, particularly N-glycosylation, is markedly different from processing in vertebrate cells. Extensive studies have shown that N-glycans produced in arthropod cells are less complex than those produced in vertebrate cells (reviewed by Altmann et al., 1999). Consequently, viruses generated from these different sources differ in their carbohydrate content; viruses propagated in mosquito cells have high mannose glycans (Hsieh and Robbins, 1984; Lozach et al., 2005). In addition to the glycan structures, the lipid compositions of arthropod and mammalian cells are different (Brotherus and Renkonen, 1977; Mitsuhashi et al., 1983; Silberkang et al., 1983). Thus, the envelopes of viruses generated in different hosts vary in their carbohydrate and lipid compositions.
The impact of viral source on WNV pathogenesis has not been examined in vivo; however, in vitro studies showed that WNV generated in cells from different hosts influences infectivity and the innate immune response (Davis et al., 2006; Silva et al., 2007). Mosquito cell-derived WNV has greater infectivity for DCs than mammalian cell-derived WNV. This enhancement is mediated through interaction of the virus with DC-SIGNR, a C-type lectin (Davis et al., 2006). Similar results were observed in other arthropod-borne viruses such as the alphaviruses, Sindbis virus (Klimstra et al., 2003) and Ross River virus (Shabman et al., 2007). In addition, viruses derived from different hosts elicit different type I interferon (IFN-α/β) responses in vitro. Using primary cell cultures, Silva and coworkers (Silva et al., 2007) showed that mammalian cell-derived WNV induces more IFN-α than does mosquito cell-derived WNV in plasmacytoid DCs. These findings suggest that the source of virus plays an important role in pathogenesis since type I interferons are crucial for controlling WNV replication in vivo (Morrey et al., 2004; Samuel and Diamond, 2005).
In this study, we examined the effect of viral source on WNV pathogenesis using a mouse model. To our knowledge, this is the first study conducted in vivo to examine the effect of viral source for any arbovirus. We found that WNV derived from mosquito or mammalian cells did not differ substantially with respect to clinical disease, mortality rate, infectivity, tissue tropism, and replication kinetics in adult mice. In general, low levels of IFN-α/β were detected in the serum of mice after WNV inoculation. In contrast to the in vitro results by others (Silva et al., 2007), our results from mice suggest that mosquito cell-derived WNV elicits a faster IFN-α/β response than does mammalian cell-derived WNV at low viral doses (10 PFU). Similar to others (Davis et al., 2006), we showed that the mosquito cell-derived WNV infected greater numbers of DCs in vitro, confirming that our in vivo results were not unique to our virus preparation. The two viruses showed equivalent in vitro replication kinetics, suggesting that cells infected with mosquito cell-derived virus produce fewer viruses per infected cell. In summary, we conclude that although viral source differentially modulated WNV infectivity in vitro, viral source had minimal effect on WNV pathogenesis in mice. Knowledge obtained from our studies shed light on the early virus-host interaction for WNV and other arthropod-borne viruses.
Results
Mortality and infectivity of WNVC6/36 and WNVBHK are similar when assessed in adult mice
Viral stocks were derived from different host cells by transfection of in vitro transcribed WNV RNA into the mosquito cell line C6/36 (WNVC6/36) or the vertebrate cell line BHK (WNVBHK). These viruses were subsequently used to study the effect of viral source on WNV pathogenesis in vivo. We first determined the mortality rate and infectivity of WNVC6/36 and WNVBHK by inoculating mice with 1 or 10 PFU of virus subcutaneously (SC) in the left-rear footpad. The actual mortality rates (mortality for infected mice) of the two viruses were similar at the 1 PFU dose (WNVC6/36 and WNVBHK = 17%) and also the 10 PFU dose (WNVC6/36 = 25%; WNVBHK =30%) (Table 1). In addition, all infected mice had similar morbidity and day of disease onset (data not shown). Furthermore, the ID50 for both viruses was 1 PFU (Table 1). In conclusion, there were no significant differences in the clinical disease, mortality, and infectivity in mice between WNVC6/36 and WNVBHK.
Table 1.
Mortality and infectivity of WNVC6/36 and WNVBHK were indistinguishable in mice.
| Inoculum | Virus dose (PFU) | No. mice per group | Mortality (no. dead/total) | a Seroconversion (no. seropositive/survivors) | b Infection (no. infected/total) | Actual mortality (no. dead/no. infected) |
|---|---|---|---|---|---|---|
| WNVC6/36 | 1 | 12 | 1/12 (8%) | 5/11 (45%) | 6/12 (50%) | 1/6 (17%) |
| WNVC6/36 | 10 | 12 | 3/12 (25%) | 9/9 (100%) | 12/12 (100%) | 3/12 (25%) |
| WNVBHK | 1 | 11 | 1/11 (9%) | 5/10 (50%) | 6/11 (55%) | 1/6 (17%) |
| WNVBHK | 10 | 11 | 3/11 (27%) | 7/8 (88%) | 10/11 (91%) | 3/10 (30%) |
Adult, female C57BL/6 mice were inoculated SC with 1 or 10 PFU of WNVC6/36 or WNVBHK in the left rear footpad. They were observed for clinical signs and weighed daily.
Sera from mice that survived infection were tested for antibodies against WNV proteins by an ELISA.
Number of infected mice equals mice that died or were euthanized due to WN disease and surviving mice that seroconverted.
Tissue tropism and replication kinetics do not differ substantially between WNVC6/36 and WNVBHK in mice inoculated at several doses of virus
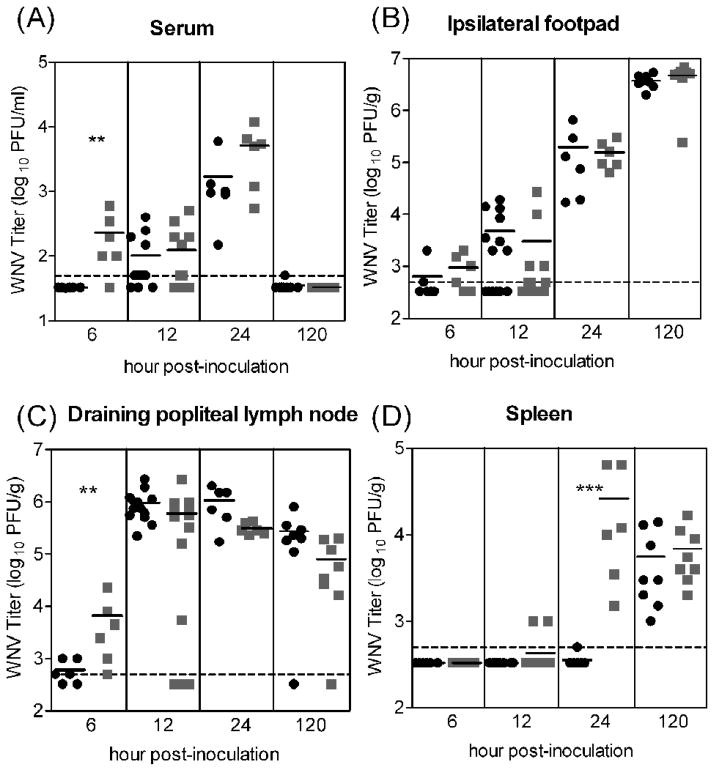
Others have shown that arboviruses derived from mosquito cells have greater infectivity for DCs in vitro than viruses derived from mammalian cells (Davis et al., 2006; Klimstra et al., 2003; Shabman et al., 2007); therefore, we predicted that mosquito cell-derived virus would result in higher viral loads than mammalian cell-derived virus in vivo. We compared the tissue tropism and viral load for these viruses in mice. Styer and coworkers previously showed that the median dose of WNV inoculated by Culex tarsalis mosquitoes is ~105 PFU (Styer et al., 2007). Accordingly, we inoculated mice with 105 PFU of either WNVC6/36 or WNVBHK and assessed WNV titers in two initial targets of WNV, the skin at the inoculation site (ipsilateral footpad) and the draining popliteal lymph node (Brown et al., 2007). We also assessed viremia and spread to secondary tissue targets, the spleen, brain and spinal cord. The viral loads in the skin were very similar for mice inoculated with either WNVC6/36 or WNVBHK at all time points (Fig 1B). In the other initial target tissue, the draining popliteal lymph node, the viral loads were similar for mice inoculated with either virus except at the earliest time point (Fig 1C). At 6 hour post-inoculation (hpi), significantly higher viral burden (P<0.05) was found in the draining popliteal lymph node of mice inoculated with WNVBHK (geometric mean of 103.8 PFU/g) than for mice inoculated with WNVC6/36 (geometric mean of 102.8 PFU/g). Also at 6 hpi, five of six mice inoculated with WNVBHK had detectable levels of viremia (geometric mean of 102.4 PFU/mL; p<0.05), whereas virus was not detected in the serum of mice inoculated with WNVC6/36 (Fig 1A). After 6 hpi, the viremic levels were not different for mice inoculated with either virus. Since progeny virus is produced after ~8 h for WNV (Shi et al., 2002), virus detected at 6 hpi is likely from the viral inoculum; therefore, higher viremia and viral load in the draining lymph node at 6 hpi are most likely due to more rapid spread of the WNVBHK inoculum into the blood and via lymphatics. The very early viremia at 6 hpi in mice inoculated with WNVBHK likely resulted in significantly higher viral load (P<0.005) in their spleens at 24 hpi (geometric mean 104.4 PFU/g) (Fig 1D). In comparison, only one of the six mice inoculated with WNVC6/36 had detectable virus in the spleen at this time point (102.7 PFU/g). By 120 hpi, similar amounts of virus were found in the spleen for both viruses (geometric mean of 103.8 PFU/g). Viral loads in brains and spinal cords were assessed at 120 hpi, and there were no significant differences between WNVC6/36- and WNVBHK-inoculated mice (data not shown). In addition, virus was detected in the brains of mice inoculated with either WNVC6/36 or WNVBHK at the same frequency (six of eight mice) (data not shown). Overall, these results suggest that WNVBHK spreads more rapidly from the inoculation site to the draining lymph node and into the blood, resulting in higher viral load in the spleen at 24 hpi. However, this difference in the spread of the virus did not affect the tissue tropism or viral load in other tissues, including the central nervous system.
Figure 1.
WNV titers did not differ substantially in mice inoculated with 105 PFU of WNVC6/36 or WNVBHK except at the earliest time points. Mice were inoculated subcutaneously with 105 PFU of WNVC6/36 (●) or WNVBHK ( ) in the left rear footpad. Tissues were harvested at various times post-inoculation, and plaque assays were performed to determine the viral load in (A) serum, (B) ipsilateral footpad (inoculation site), (C) draining popliteal lymph node, and (D) spleen. Data from two independent experiments are shown. Solid lines indicate the geometric mean for each group of 6–12 mice. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks denote significant difference between groups (Mann-Whitney test: ** p <0.05; ***p <0.005).
) in the left rear footpad. Tissues were harvested at various times post-inoculation, and plaque assays were performed to determine the viral load in (A) serum, (B) ipsilateral footpad (inoculation site), (C) draining popliteal lymph node, and (D) spleen. Data from two independent experiments are shown. Solid lines indicate the geometric mean for each group of 6–12 mice. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks denote significant difference between groups (Mann-Whitney test: ** p <0.05; ***p <0.005).
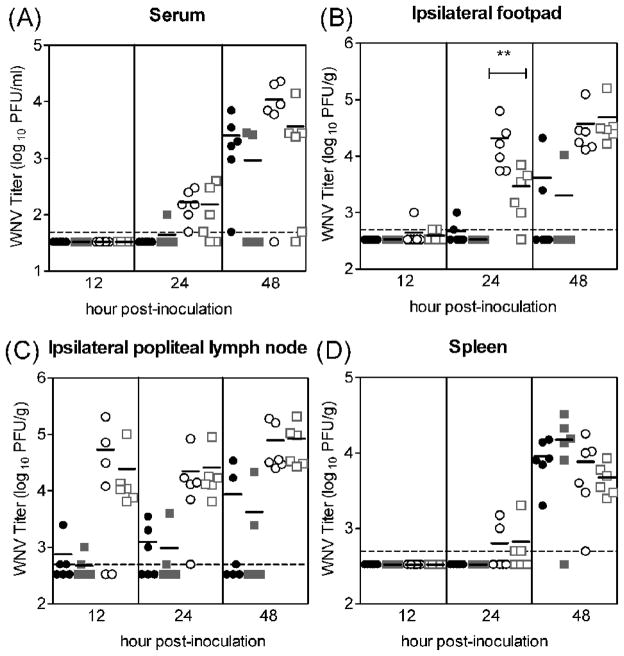
Our in vivo results are in contrast to our prediction based on published in vitro studies, in which higher infectivity of mosquito cell-derived WNV had been seen for DCs (Davis et al., 2006). One possible explanation for this discrepancy is that we saturated infection at 105 PFU, and thereby masked any subtle differences between the two viruses. Since mosquitoes inoculate a wide range of viral doses into hosts, ranging from 10 to 106 PFU for Cx. tarsalis mosquitoes (Styer et al., 2007), we determined whether differences between WNVC6/36 and WNVBHK in vivo could be detected when mice were inoculated with lower doses of virus. Mice were inoculated SC with 10 or 103 PFU of virus and sacrificed at various time points, and tissues were harvested. For the initial targets of infection (skin at inoculation site and draining popliteal lymph node), the viral loads were very similar between mice inoculated with either WNVC6/36 or WNVBHK at a given viral dose (Fig 2B and 2C) except for a single tissue at a single time point. In the skin at the inoculation site, levels of virus in mice inoculated with 103 PFU of WNVC6/36 (geometric mean of 104.3 PFU/g) were significantly (P<0.05) higher than mice inoculated with the same dose of WNVBHK (geometric mean of 103.5 PFU/g) at 24 hpi (Fig 2B). In addition, there were no significant differences in viremic levels or viral loads in the spleens between the two viruses for a given dose (Fig 2A and 2D). Although not significantly different from the corresponding WNVBHK-group, one of the WNVC6/36 groups had a higher percentage of mice with detectable viremia; viremia was detected in all mice inoculated with 10 PFU of WNVC6/36, but in only two of the six mice inoculated with WNVBHK at 48 hpi (Fig 2A). At the 10 and 103 PFU doses, the early spread of WNVBHK, which was observed at the 105 PFU dose, was not detected and is most likely due to the limit of detection for the assays. Although WNVBHK may spread from the inoculation site more quickly than WNVC6/36, the viremia and viral loads were not significantly affected after 24 hpi at any of the doses. In summary, viral loads in the initial and secondary targets of infection and viremic levels did not differ substantially between WNVC6/36 and WNVBHK when mice were inoculated with a high, medium, or low viral dose.
Figure 2.
WNV titers were similar between WNVC6/36 and WNVBHK in mice inoculated with low or medium viral dose. Mice (n=6) were inoculated subcutaneously with 10 PFU of WNVC6/36 (●) or WNVBHK ( ) or 103 PFU of WNVC6/36 (○) or WNVBHK (□) in the left rear footpad. Tissues were harvested at various times post-inoculation, and plaque assays were performed to determine the viral load in (A) serum, (B) ipsilateral footpad (inoculation site), (C) draining popliteal lymph node, and (D) spleen. Data from one experiment is shown. Solid lines indicate the geometric mean for each group of 6 mice. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks show significant difference between groups (Mann-Whitney test: ** p <0.05).
) or 103 PFU of WNVC6/36 (○) or WNVBHK (□) in the left rear footpad. Tissues were harvested at various times post-inoculation, and plaque assays were performed to determine the viral load in (A) serum, (B) ipsilateral footpad (inoculation site), (C) draining popliteal lymph node, and (D) spleen. Data from one experiment is shown. Solid lines indicate the geometric mean for each group of 6 mice. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks show significant difference between groups (Mann-Whitney test: ** p <0.05).
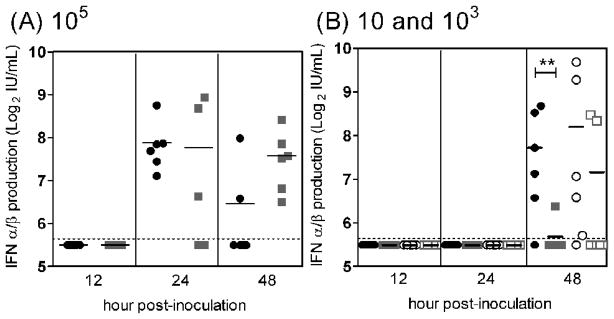
Mice inoculated with mosquito cell-derived WNV produce an earlier functional type I interferon response than mice inoculated with mammalian cell-derived WNV
Several laboratories have reported that mammalian cell-derived arboviruses induce higher levels of IFN-α/β than mosquito cell-derived arboviruses in vitro (Shabman et al., 2007; Silva et al., 2007); therefore, we predicted that WNVBHK would induce higher levels of type I interferon than WNVC6/36 in mice. Accordingly, we assessed the abilities of WNVC6/36 and WNVBHK to stimulate functional IFN-α/β in vivo at different viral doses. Contrary to our prediction, there was a trend toward earlier induction of IFN-α/β in mice inoculated with WNVC6/36 compared to mice inoculated with WNVBHK (Fig 3). A significant difference was only observed between mice inoculated with a single dose at a single time point; for the 10 PFU groups, IFN-α/β levels in sera were significantly higher in WNVC6/36-inoculated mice (mean of 211 IU/ml) than in WNVBHK-inoculated mice (mean of 51 IU/mL) (P<0.05) (Fig 3B). For the 103 PFU groups, IFN-α/β was more frequently detected at 48 hpi in sera of mice inoculated with WNVC6/36 than in mice inoculated with WNVBHK (Fig 3B). At 105 PFU of virus, IFN-α/β appeared to peak earlier in sera of mice inoculated with WNVC6/36 than in mice inoculated with WNVBHK; IFN-α/β was more frequently detected at 24 hpi and less frequently detected at 48 hpi in mice inoculated with WNVC6/36 than in mice inoculated with WNVBHK (Fig 3A). In summary, these results contradict our prediction and suggest that WNVC6/36 elicits an earlier IFN-α/β response in mice than WNVBHK.
Figure 3.
Mice inoculated with WNVC6/36 produced IFN-α/β earlier than mice inoculated with WNVBHK. Mice were inoculated subcutaneously with (A) 105 PFU of WNVC6/36 (●) or WNVBHK ( ), (B) 10 PFU of WNVC6/36 (●) or WNVBHK (
), (B) 10 PFU of WNVC6/36 (●) or WNVBHK ( ) or 103 PFU of WNVC6/36 (○) or WNVBHK (□) in the left rear footpad. Sera were harvested at different times post-inoculation, and IFN bioassays were performed on L929 cell monolayer. Data from two independent experiments are shown. Solid lines indicate the geometric mean of 6 mice per group. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks show significant difference between groups (Mann-Whitney test: ** p <0.05).
) or 103 PFU of WNVC6/36 (○) or WNVBHK (□) in the left rear footpad. Sera were harvested at different times post-inoculation, and IFN bioassays were performed on L929 cell monolayer. Data from two independent experiments are shown. Solid lines indicate the geometric mean of 6 mice per group. Dashed lines represent the limit of detection (LOD), and data points below the line are < LOD. Asterisks show significant difference between groups (Mann-Whitney test: ** p <0.05).
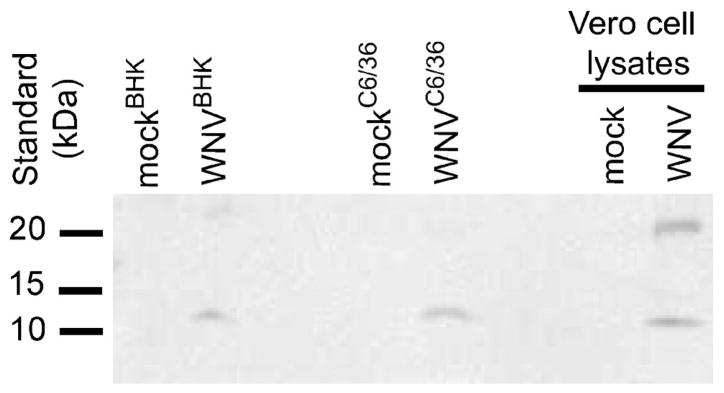
No immature particles were detected in virus prepared from mosquito and mammalian cells
The presence of non-infectious particles and/or immature particles could impact the induction of IFN-α/β in vivo and explain our unexpected results. Therefore, we examined the genome copy numbers and the presence of prM proteins in our virus preparations. WNV RNA was extracted from our virus stocks, and the genome copy numbers were determined using an established real-time reverse transcription PCR assay (Lanciotti et al., 2000). The stock of WNVBHK had a 2.7-fold higher genome copy-to-PFU ratio and, therefore, more non-infectious virus particles than the stock of WNVC6/36 (Table 2). In addition, equivalent genome copies of the viruses were analyzed by western blot using an antibody specific for prM/M to determine the presence of immature particles in the virus stocks. M protein was detected in WNVC6/36 and WNVBHK, but prM protein was not detected in the virus stocks (Fig 4), demonstrating that neither stock had detectable immature viral particles. These results suggest that the earlier induction of IFN-α/β in mice by WNVC6/36 was not due to more non-infectious or immature viral particles in the stock of WNVC6/36.
Table 2.
WNV prepared from mammalian cells contains more noninfectious particles than WNV prepared from mosquito cells.
| Virus | (genome copies)/mLa | PFU/mL | (genome copies)/PFU |
|---|---|---|---|
| WNVC6/36 | (5.7 ± 1.0) × 1010 | 1.1 × 109 | 51 |
| WNVBHK | (2.8 ± 0.3) × 1011 | 2.0 × 109 | 138 |
Viral RNA was extracted, and then real-time RT-PCR assays were performed. Average ± standard deviations from triplicates are shown
Figure 4.
Immature particles were not detected in virus preparations. Equivalent genome copies of WNVC6/36 and WNVBHK were electrophoresed on a 12% Tris-HCl gel under denaturing conditions. Following transfer to PVDF membrane, membranes were incubated with antibodies against M, followed by horseradish peroxidase conjugated-goat-anti-rabbit. Protein bands were visualized using chemiluminescent detection kit. Supernatants from mock-electroporated cells were negative controls, and a lysate from Vero cells infected with WNVBHK was a positive control for the detection of prM and M proteins. Three independent experiments were performed, and a representative western blot is shown.
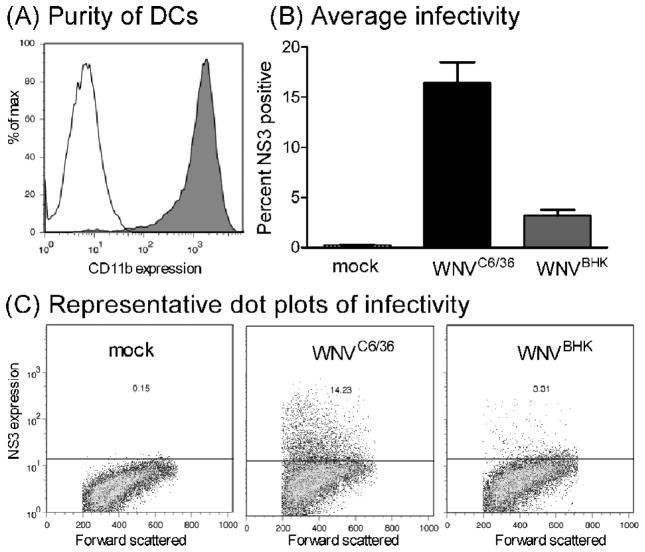
Primary myeloid dendritic cells are more susceptible to mosquito cell-derived virus than to mammalian cell-derived virus
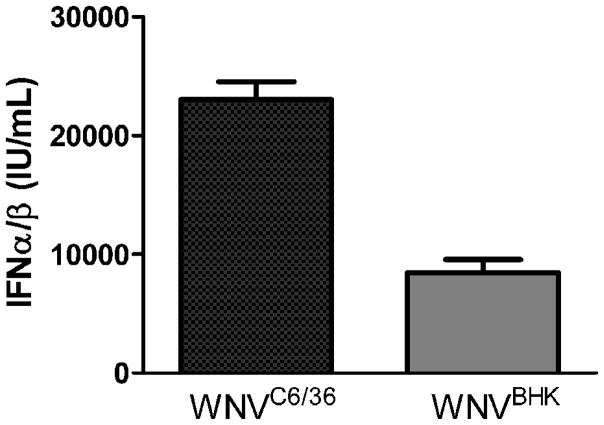
Mosquito cell-derived arboviruses are more infectious than mammalian cell-derived arboviruses for myeloid DCs (mDCs) in vitro (Davis et al., 2006; Klimstra et al., 2003; Shabman et al., 2007); however, we showed that there were minimal differences in the replication kinetics between WNVC6/36 and WNVBHK in vivo. We conducted studies to ensure that the disparities between our results and the results of published reports were not due to differences in our virus preparations by examining the infectivity on DCs. We generated immature mDCs from the bone marrow of mice. Approximately 96% of these cells expressed CD11b, a cell surface marker for mDCs, after 7 days in culture (Fig 5A). We subsequently inoculated these cells with WNVC6/36 or WNVBHK, and the presence of WNV nonstructural protein 3 (NS3) was measured as an indicator of infection at 48 hpi. As shown in Figure 5B and 5C, WNVC6/36 infected approximately five-fold more mDCs than did WNVBHK (16 ± 2% vs. 3 ± 1%), which is consistent with results in published reports for WNV (Davis et al., 2006). In addition, WNVC6/36 stimulated approximately three-fold more IFN-α/β (23,000±1,000 IU/mL) than did WNVBHK (8,000±1,000 IU/mL) in DCs (Fig 6), which is consistent with the findings of Silva and co-workers for mDCs (Silva et al., 2007) and supports our in vivo results (Fig 3). Furthermore, these results confirm that the viral stocks that were used in our in vivo studies generated similar data in primary mDCs as published studies.
Figure 5.
The percentage of primary dendritic cells infected by WNVC6/36 was greater than the percentage infected by WNVBHK. (A) After 7 days in culture, primary immature DCs were incubated with PE-conjugated anti-CD11b (grey, filled) or isotype control (black, unfilled) prior to infection with WNV. (B and C) Primary DCs were inoculated with diluent (mock) or with WNVC6/36 or WNVBHK at MOI of 50. Cells were harvested at 48 hpi, and incubated with Alexa fluor 647-conjugated antibodies against NS3. All samples were fixed overnight at 4°C and then analyzed by flow cytometry. (B) Average ± standard error from 2 independent experiments and (C) representative dot plots are shown.
Figure 6.
WNVC6/36 induced greater IFN α/β production in primary myeloid dendritic cells than WNVBHK. Primary DCs were inoculated with diluent (mock) or with WNV at MOI of 50. Culture supernatants were harvested at 48 hpi, and IFN bioassays were performed as described in legend of figure 3. Two independent experiments were performed, and the averages ± standard deviations from one experiment, performed in triplicates, are shown.
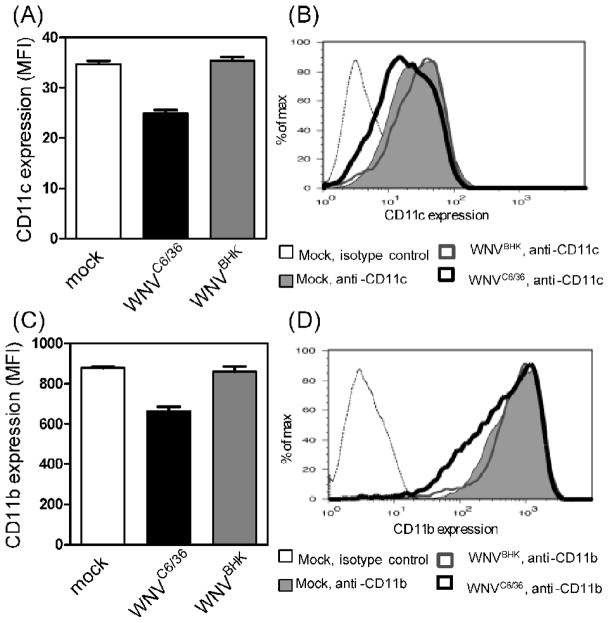
During our flow cytometric analysis, we also examined the expression of CD11b and CD11c, surface markers for mDCs. The expression of both markers was decreased following inoculation with WNVC6/36, but not after inoculation with WNVBHK or mock-inoculation (Fig 7). For the DCs inoculated with WNVC6/36, the surface expression of CD11c decreased from a mean fluorescent intensity (MFI) of 34.7±1.2 (mock) to 24.8±1.3 (Fig 7A and 7B), and the surface expression of CD11b decreased from a MFI of 878±14 (mock) to 662±39 (Fig 7C and 7D). These results suggest that viruses generated from different host cells differentially affect the phenotype of mDCs. This effect was not due to more non-infectious or immature particles in our stock of WNVC6/36 (Table 2 and Fig 4).
Figure 7.
WNVC6/36, but not WNVBHK, down-regulated expression of CD11c and CD11b on primary dendritic cells. Primary DCs were inoculated with diluent (mock) or with WNVC6/36 or WNVBHK at MOI of 50. Cells were harvested at 48 hpi and incubated with (A and B) FITC-conjugated anti-CD11c and (C and D) PE-conjugated anti-CD11b. Samples were fixed overnight at 4°C and then analyzed by flow cytometry. Mean fluorescent intensities (MFI) ± standard deviations from triplicates are reported in (A and C). Two independent experiments were conducted, and representative histograms are shown in B and D.
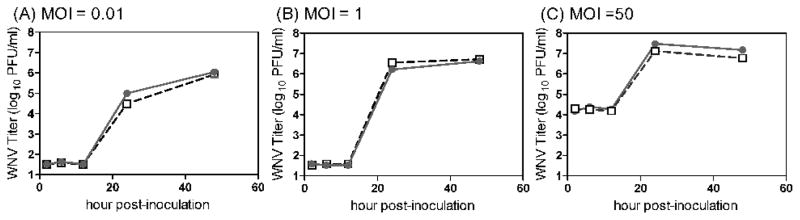
Replication kinetics of WNVC6/36 and WNVBHK are indistinguishable in primary mDCs and primary skin cells
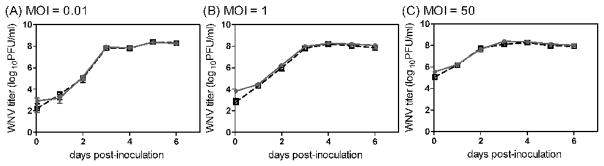
The DCs of the skin, Langerhans cells, are postulated to be an initial cell target for WNV, which subsequently migrate to the draining lymph node upon infection (Johnston et al., 2000). Since WNVC6/36 infected more DCs than did WNVBHK (Fig 5), we predicted higher viral loads in the draining lymph nodes for WNVC6/36 during early viral replication in mice; however, we did not observe any significant differences after 6 hpi (Fig 1 and 2). Thus, we examined viral replication kinetics in primary DCs, using several multiplicities of infection (MOI), to address these discrepancies between our in vitro and in vivo studies. Growth curves for WNVC6/36 and WNVBHK were very similar in primary DCs at all MOIs (Fig 8). These data, together with the data on infectivity for DCs (Fig 5), indicate that DCs infected with WNVC6/36 produced two- to five-fold fewer viruses per infected cell than did DCs infected with WNVBHK (i.e., 200–700 PFU/cell and 1,000–1500 PFU/cell for WNVC6/36 and WNVBHK, respectively). These results may explain why there were no significant differences in viral load due to replication (after 6 hpi) in draining lymph nodes of mice inoculated with WNVC6/36 or WNVBHK. In addition to Langerhans cells, other possible initial cell targets reside in the skin (Brown et al., 2007). Thus, we also investigated the growth of WNVC6/36 and WNVBHK in primary skin cells. As shown in figure 9, titers of WNVC6/36 in primary skin cells were identical to the titer of WNVBHK at all MOIs and were consistent with the similar results that we observed for the two viruses in the skin of mice (Fig 1B and 2B). In conclusion, replication kinetics of WNVC6/36 and WNVBHK were identical in primary DCs and primary skin cells, supporting our in vivo results.
Figure 8.
The replication kinetics for WNVC6/36 in primary dendritic cells was similar to the kinetics for WNVBHK. Primary DCs were inoculated with WNVC6/36 ( ) or WNVBHK (□) at MOI of (A) 0.01, (B) 1, or (C) 50. At various times post-inoculation, medium was harvested and stored at −70°C. WNV titer in the medium was determined by plaque assays on Vero cell monolayers. The averages ± standard deviations from triplicate wells are shown. The experiment was repeated at least three times at each MOI, and representative results are shown.
) or WNVBHK (□) at MOI of (A) 0.01, (B) 1, or (C) 50. At various times post-inoculation, medium was harvested and stored at −70°C. WNV titer in the medium was determined by plaque assays on Vero cell monolayers. The averages ± standard deviations from triplicate wells are shown. The experiment was repeated at least three times at each MOI, and representative results are shown.
Figure 9.
The replication kinetics for WNVC6/36 and WNVBHK was nearly identical in primary skin cells. Primary skin cells were inoculated with WNVC6/36 ( ) or WNVBHK (□) at MOI of (A) 0.01, (B) 1, or (C) 50. At various times post-inoculation, medium was harvested and stored at −70°C. WNV titer in the medium was determined by plaque assays on Vero cell monolayers. The averages ± standard deviations from triplicate wells are shown.
) or WNVBHK (□) at MOI of (A) 0.01, (B) 1, or (C) 50. At various times post-inoculation, medium was harvested and stored at −70°C. WNV titer in the medium was determined by plaque assays on Vero cell monolayers. The averages ± standard deviations from triplicate wells are shown.
Discussion
The effect of viral source, whether the virus is derived from mammalian or mosquito cells, on arbovirus-host interactions has been studied by several investigators using in vitro models (Davis et al., 2006; Klimstra et al., 2003; Shabman et al., 2007). In general, mosquito cell-derived virus results in more infected dendritic cells and lower levels of type I interferon than mammalian cell-derived virus. The assumption in the arbovirus field is that viral source would similarly affect the pathogenesis in vivo.
The goal of the present study was to test this assumption for WNV, and we demonstrated that the viral source has minimal effect on WNV pathogenesis in mice. These results are very robust since three doses, 10, 103 and 105 PFU, were studied, covering the lowest to median dose inoculated by Cx. tarsalis mosquitoes (Styer et al., 2007). The clinical disease, mortality rates, ID50, tissue tropism, and neuroinvasion were equivalent for mice inoculated with mosquito cell-derived WNV and mammalian cell-derived WNV. In addition, the viral loads in initial and secondary target tissues and viremic levels were similar after the initiation of viral replication (>6 hpi) with only two exceptions, and after 24 hpi, there were no significant differences in viremia or viral load of any tissue tested for a particular viral dose. There was a trend for an earlier induction of IFN-α/β in mice inoculated with WNVC6/36 compared to mice inoculated with WNVBHK, but a significant difference in serum levels of IFN-α/β was only observed at low virus dose at 48 hpi. In conclusion, although the source of WNV had minor effects on viral loads and IFN-α/β production in mice at early time points, it did not significantly affect WNV pathogenesis in vivo.
Our results contradict the current thinking in the arbovirus field that mosquito cell-derived virus induces less type I interferon in vivo as it does in vitro (Shabman et al., 2007; Silva et al., 2007). Plasmacytoid DCs (pDCs) inoculated with mammalian cell-derived WNV produce over 1,000-fold higher levels of IFN-α than cells inoculated with mosquito cell-derived WNV (Shabman et al., 2007; Silva et al., 2007). Thus, we expected higher levels of IFN-α/β in the mice inoculated with WNVBHK, but we actually observed a trend toward earlier and in one instance higher IFN-α/β in the mice inoculated with WNVC6/36. A possible explanation is that pDCs are not initial targets of WNV infection in vivo. pDCs are rare in normal, non-inflamed skin and are present in low numbers in quiescent lymph nodes, migrating into these tissues during inflammation (reviewed in Villadangos and Young, 2008). Thus, pDCs may not play a role until after initiation of WNV infection when the cells are exposed to viral progeny of vertebrate origin. On the other hand, conventional mDCs, present in relatively high numbers in skin and lymph nodes, are exposed to the viral inoculum and are assumed to participate in the first round of infection (Johnston et al., 2000). In our in vitro studies, mDCs that were infected with WNVC6/36 produced three-fold more IFN-α/β than cells infected with WNVBHK, and these results are consistent with those of Silva and co-workers (Silva et al., 2007). Furthermore, our results with primary mDCs infected with WNV in culture are consistent with our in vivo results; WNVC6/36 tended to induce an earlier type I interferon response in mice than WNVBHK.
Several other factors may contribute to the apparent contradiction of our in vivo results with published in vitro results, including the particle-to-PFU ratios of the viruses, the infected cell type, and sensitivity of our methods. UV-inactivated WNV triggers IFN-α release by pDCs (Silva et al., 2007), and defective interfering particles of Sindbis virus stimulate type I IFN production in primary cells (Fuller and Marcus, 1980). These studies suggest that the particle-to-PFU ratio of the virus is a determinant of IFN-α/β production. Thus, we determined the genome copy-to-PFU ratios for our stocks of WNVC6/36 and WNVBHK, and the latter had approximately three-fold higher ratio. These results indicate that our stock of WNVBHK contains slightly more non-infectious particles, arguing against the possibility that WNVC6/36 has more non-infectious particles, inducing a greater IFN-α/β response in vivo. In addition, we did not detect immature viral particles in either viral stock, providing additional support that our data was not due to an artifact of our virus preparation. Another explanation is that cells that do not belong to the DC lineage may be infected initially. Cells in the skin support WNV replication at the inoculation site and distal sites (Brown et al., 2007), suggesting that non-migrating cells in the skin are infected. These cells may produce similar amounts of type I interferon in response to WNV derived from mosquito and mammalian cells. An additional possibility is that we measured IFN-α/β in the serum, which reflects production from all infected tissues. This method may not be sensitive enough to pick up any differences early during infection.
The similar viremic titers and viral loads in mice inoculated with WNVC6/36 or WNVBHK after the initiation of viral replication (> 6 hpi) were unexpected since previous reports demonstrated that mosquito cell-derived WNV is more infectious for DCs than mammalian cell-derived virus (Davis et al., 2006). We confirmed that our viral stocks were not unique and had similar infectivity as reported by Davis and co-workers; WNVC6/36 was approximately five-fold more infectious for primary mDCs than WNVBHK. We examined why this increase in infectivity did not result in greater viral loads for WNVC6/36 by conducting growth curves. The multi-step and one-step growth curves on primary mDCs were equivalent for the two different viruses at a specific MOI. Assuming that all infected cells produced progeny virions, we estimate that WNVC6/36-infected DCs produced two- to five-fold fewer viruses per cell than did WNVBHK-infected DCs. This may explain the lower than expected viral loads for WNVC6/36-inoculated mice. Another explanation is that DCs may not be the only initial target cell, and again, cells in the skin are likely initial targets, contributing to early viral production in the animal. Furthermore, multi-step and one-step growth curves on primary skin cultures were equivalent for the two different viruses at a specific MOI.
There are several possible mechanisms for the lower viral yield in mDCs infected with WNVC6/36 compared to cells infected with WNVBHK. One possible explanation is that infection by WNVC6/36 was aborted in a subset of DCs through some unknown mechanism(s). Alternatively, the greater IFN-α/β response elicited by WNVC6/36 may subsequently lead to lower amounts of progeny virus production. A third possibility is an unknown effect of the virus on DC function, resulting in lower viral yield or abortive infection. Several viruses inhibit DC function upon infection, including herpes simplex virus, hepatitis C virus, and lymphocytic choriomeningitis virus (Eksioglu et al., 2009; Kruse et al., 2000; Pollara et al., 2003; Sevilla et al., 2004). Our results suggest that WNV generated from mosquito cells alters the function of mDCs; incubation of DCs with mosquito cell-derived WNV, but not mammalian cell-derived WNV, resulted in down-regulation of CD11b and CD11c on mDCs. This finding was previously reported for human cytomegalovirus-infected macrophages (Gafa et al., 2005). To our knowledge, we are the first to report lower expression of these integrins on DCs due to viral infection. In addition, down-modulation of CD11c and CD11b may not require infection by WNV because cells that were negative for WNV antigen also had lower levels of CD11c and CD11b following WNV inoculation (data not shown). CD11c and CD11b are alpha integrin chains, which form heterodimers with the beta integrin chain, CD18. These complexes have many functions and play a role in phagocytosis, opsonization, cell migration, and antigen presentation. One ligand of CD11b/CD18 and CD11c/CD18 is iC3b, an opsonin produced during activation of the complement system (reviewed by (Bajtay et al., 2006). Although a direct role has not been documented for these receptor-ligand interactions during WNV infection, complement activation is crucial for controlling the virus, given that mice lacking the third complement component are more susceptible to lethal WNV infection (Mehlhop et al., 2005). Our results suggest that WNV derived from mosquito cells potentially inhibits the functions of DCs; however, any effect in vivo would be limited to the first round of infection, which most likely does not have any lasting effects on viral immunity since viral source did not affect infectivity, morbidity, or mortality in mice.
Although our mouse studies revealed similar viremic titers and viral loads in mice inoculated with WNVC6/36 or WNVBHK after the initiation of viral replication, there were differences between mice inoculated with either of the viruses at the earliest time point (6 hpi) at the 105 PFU dose. Virus detected at 6 hpi represents extracellular virus from the initial viral inocula, rather than progeny viruses, which are not detected until at least 8 hpi (Shi et al., 2002). We propose the following model to explain differences in spread of the WNVC6/36 or WNVBHK inocula and subsequent viral replication. WNV derived from mosquito cells binds cells and/or the extracellular matrix in the skin more tightly than does WNV derived from mammalian cells. Thus, upon subcutaneous inoculation, mosquito cell-derived WNV from the initial viral inoculum is more likely to be trapped at the site, resulting in minimal spread of the inoculum to the blood and draining lymph node at 6 hpi and potentially higher levels of infectious virus in the skin at the inoculation site at 24 hpi. In contrast, WNV generated from mammalian cells may have weaker binding to cells and/or the extracellular matrix in the skin, allowing more rapid dissemination of the viral inoculum to other tissues through the blood and/or lymphatics. This model is supported by the higher viremia and viral burden in the draining lymph node at 6 hpi for WNVBHK-inoculated mice. In addition, we propose that the more rapid spread of WNVBHK resulted in higher viral load in the spleen at 24 hpi. It is important to note that these observations were dose dependent. Higher viral load in the skin of WNVC6/36-inoculated mice was only observed at the medium dose of 103 PFU, and difference in spread of the viral inocula was only observed at the high dose of 105 PFU. Despite this early difference in spread, inoculation of the very low doses (1 PFU and 10 PFU) resulted in equivalent ID50 for both WNVC6/36 and WNVBHK, indicating that both viruses were equally infectious for mice. Furthermore, these differences at early time points did not affect the viral burdens after 24 hpi, neuroinvasion, or clinical outcome.
Our model that viral source affects binding in vivo is consistent with the results of in vitro studies. WNVC6/36 infects a greater number of DCs in culture, as shown by our present results and in a previous report (Davis et al., 2006). The presence of high mannose glycans on mosquito cell-derived viruses (Hsieh and Robbins, 1984; Hsieh et al., 1983) could facilitate virus attachment to cell surface receptors on skin cells. Interactions between C-type lectins and mosquito cell-derived viruses contribute to enhanced infectivity in vitro for several mosquito-borne viruses, such as WNV (Davis et al., 2006), dengue virus (Navarro-Sanchez et al., 2003), and alphaviruses (Klimstra et al., 2003; Shabman et al., 2007).
To our knowledge, this is the first study to examine the effect of viral source on viral pathogenesis in vivo. Contrary to the accepted assumption of the arbovirus field, we demonstrated that the source of virus did not significantly impact WNV pathogenesis, using a mouse model that closely resembles WN disease in humans (Brown et al., 2007; Petersen et al., 2002), and our results can likely be extended to other flaviviruses. The outcome for alphaviruses, however, may differ since there is a more pronounced effect of viral source on type I interferon induction in conventional mDCs for alphaviruses (Klimstra et al., 2003; Shabman et al., 2007) than is observed for WNV. Thus, further studies are warranted to understand the effect of mosquito cell- and mammalian cell-derived viruses for other arbovirus systems. In addition, viral source is only one factor in the early arbovirus-host interaction. In nature, WNV is transmitted via the bite of WNV-infected mosquitoes, and several studies have shown that mosquito transmission enhances WNV infection in hosts (Schneider et al., 2006; Styer et al., 2006). Future studies will be conducted to determine how mosquito saliva impacts the early virus-host interaction and affects the pathogenesis of arboviruses.
Materials and methods
Cells
African green monkey kidney cells (Vero; ATCC #CCL-81) and baby hamster kidney cells (BHK-21; ATCC #CCL-10) were maintained in minimal essential medium (MEM, Gibco Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum (HI-FBS), 2 mM L-glutamine, 1.5 g/L sodium bicarbonate, 100 U/mL of penicillin, and 100 μg/mL of streptomycin]. Mouse fibroblast cells (L929; ATCC #CCL-1) were maintained in MEM supplemented with 10% HI-FBS, 2 mM L-glutamine, and 0.6 g/L sodium bicarbonate (L929 medium). Aedes albopictus mosquito cells (C6/36; ATCC # CRL-1660) were maintained in MEM supplemented with 10% HI-FBS, 1.1 g/L sodium bicarbonate, 2 mM L-glutamine, 0.1 mM nonessential amino acids, 100 U/mL of penicillin, and 100 μg/mL of streptomycin. Cells were incubated in 5% CO2 at 37°C (Vero, BHK-21, and L929) or 28°C (C6/36).
Viruses
WNV was produced from a full-length cDNA clone of a 2000 New York strain as previously described (Shi et al., 2002). Briefly, in vitro transcribed RNA from the full length cDNA clone was electroporated into BHK-21 cells and into C6/36 cells, to produce WNVBHK and WNVC6/36, respectively. For virus stocks, supernatants were harvested on day 3 (BHK-21) or day 4 (C6/36) after electroporation when cytopathic effect was observed. Supernatants were clarified by centrifugation at 10,000 x g for 20 min at 4°C and stored in aliquots at −80°C. Viral titers were determined by plaque assays on Vero cell monolayers. Encephalomyocarditis virus (EMCV) was a gift from Dr. Laura White at University of North Carolina at Chapel Hill. EMCV was produced in BHK-21 cells, and viral titers were determined by plaque assays on L929 cells.
Viral RNA was isolated in triplicate from virus stocks using Qiagen RNeasy Mini kit (Qiagen, Valencia, CA). A real-time RT-PCR assay for WNV RNA was performed as described previously (Lanciotti et al., 2000).
Mice
We purchased five-week-old female C57BL/6 mice from Taconic (Germantown, NY). Mice were acclimated in the biosafety-level-3 animal facility for at least 1 week and were provided with food and water ad libitum. All studies were approved by Institutional Animal Care and Use Committee of the Wadsworth Center and followed criteria established by the National Institutes of Health.
Mortality and infectivity of WNV-inoculated mice
Six-week-old mice were inoculated SC in the left rear footpad with 10 μL of WNVC6/36 or WNVBHK at 1 or 10 plaque forming unit (PFU) (n=11 to 12 mice per group) as described previously (Brown et al., 2007). Virus was diluted in low endotoxin PBS (tissue culture grade; Invitrogen) with 1% HI-FBS. All mice were observed for clinical signs, which included ruffled fur, ataxia, weakness, and weight loss, at least once a day during the entire study. They were weighed daily for at least 14 days post-inoculation (p.i.) and three times weekly during the rest of the study. Mice with severe disease were euthanized. On day 28, all surviving mice were bled, and virus infection was confirmed in WNV-inoculated mice by the presence of WNV-specific antibodies using an ELISA as previously described (Ebel et al., 2002).
WNV replication in vivo
Six-week-old mice were inoculated with virus as described above, at a dose of 10, 103, or 105 PFU of WNV. At various times after inoculation, mice (n=6–8) were euthanized, and the following tissues were harvested: blood, ipsilateral footpad (inoculation site), draining popliteal lymph node, spleen, spinal cord, and brain. For the 24 and 48 hour time points, mice were sacrificed and perfused with PBS with 1% HI-FBS at 6 mL per min for 10 minutes prior to harvesting tissues. Blood was processed to collect serum, which was frozen at −80°C. All other tissues were processed as previously described (Brown et al., 2007). Briefly, buffer was added to make a 20% homogenate for central nervous system tissues and a 10% homogenate for peripheral tissues, and samples were stored at −80°C until testing by plaque assays on Vero cells.
Interferon α/β bioassay
The presence of functional type I interferon in the serum was determined as described previously (Trgovcich et al., 1996). Briefly, L929 cells were seeded in 96-well plates (4×104 cells/well) and incubated at 37°C overnight. Serum samples and recombinant mouse interferon β (standards; Chemicon International, Temecula, CA) were diluted in L929 medium, acidified to pH 2 with 2 N HCl, and incubated at 4°C overnight. Samples were neutralized to pH 7 with 2 N NaOH. Samples and standards were added to triplicate wells of 96-well plates of L929 cells and serially diluted two-fold. Plates were incubated at 37°C overnight. EMCV (2×105 PFU) was added to all wells containing standards and samples. After 24 hour at 37°C, cell viability was assessed using CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (Promega, Madison, WI), as per manufacturer’s instruction. The amounts of IFN-α/β in the serum were interpolated from the standard curve. The values reported are averages from triplicate wells.
Western blot analysis
Virus was mixed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and then incubated at 95°C for 10 min. Equivalent genome copies of the virus were electrophoresed on a 12% Tris-HCl gel (Bio-Rad Laboratories, Hercules, CA). Supernatants from mock-electroporated cells were used as negative controls, and lysates of Vero cells inoculated with WNVBHK or mock-inoculated were used as controls for the detection of prM. Following transfer to PVDF membranes (Bio-Rad Laboratories, Hercules, CA), prM and M proteins were detected with antibodies against WNV M protein (1:200 dilution; Imgenex, San Diego, CA), followed by incubation with horseradish peroxidase conjugated goat-anti-rabbit (1:1000 dilution; Vector, Burlingame, CA). Protein bands were visualized using Immun-Star WesternC Chemiluminescent Kit (Bio-Rad Laboratories, Hercules, CA).
Isolation of dendritic cells
Primary mDCs were isolated from bone marrow of adult C57BL/6 mice and cultured as previously described (Serody et al., 2000; Shabman et al., 2007). Briefly, cells were isolated from bone marrow by flushing the femur and tibia with RPMI 1640 medium (Invitrogen; Carlsbad, CA), supplemented with 10% HI-FBS, 2 mM L-glutamine, 50 μM 2-mercaptoethanol, 100 U/mL Penicillin, and 100 μg/mL streptomycin sulfate (R10F medium). Red blood cells were lysed using ACK lysis buffer (0.15 M NH4Cl, 1 mM KHCO3, 0.1 mM Na2EDTA, pH 7.4), and cells were cultured in ultra-low binding plates (Corning, Lowell, MA) in the presence of 20 ng/mL of recombinant granulocyte-macrophage colony stimulating factor (GM-CSF; PeproTech Inc, Rocky Hill, NJ). Cells were grown in the presence of GM-CSF and recombinant interleukin-4 (IL-4; PeproTech Inc, Rocky Hill, NJ) at 10 ng/mL (days 3–4) and then at 5 ng/mL (days 5–7) to generate immature mDCs. After 7 days in culture, cells were used for flow cytometric analysis or were infected with virus.
WNV infection of immature dendritic cells
On day 7, immature mDCs were harvested and infected with WNV prepared in RPMI-1649, supplemented with 1% HI-FBS and 10 mM HEPES (RPMI-1H) in triplicate for 2 h at 37°C. Cells were washed three times to remove extracellular viruses, then cultured in RPMI-1H, supplemented with 5 ng/mL of GM-CSF and IL-4 at 37°C. At various times post-inoculation, an aliquot of the supernatant was harvested and stored at −80°C for plaque assay analysis.
Cell surface and intracellular staining for flow cytometry
Flow cytometric analysis was performed to characterize the immature mDCs and to determine the infectivity of WNV in mDCs. Cells were harvested, washed with cold staining buffer (PBS supplemented with 1% HI-FBS) by centrifugation at 400xg for 5 min, and resuspended in staining buffer. Samples were incubated with Fc block (BD Pharmingen) at room temperature for 10 min. FITC-conjugated antibodies against CD11c (1:100 dilution; clone N418; eBioscience, San Diego, CA) and PE-conjugated antibodies against CD11b (1:100 dilution; clone M1/70; BD Pharmingen) were added to the cells and incubated for 20 min at 4°C. Samples were washed 3 times with staining buffer and were then fixed with 2% paraformaldehyde (PFA) for 20 min at 4°C. Cells were washed once with permeabilization buffer (PBS containing 0.1% (w/v) saponin, 0.09% sodium azide, and 0.5% bovine serum albumin), incubated in permeabilization buffer for 10 min at 4°C, and washed. Cells were resuspended with Alexa674-conjugated monoclonal antibodies against WNV NS3 protein (provided by Dr. David Lawrence, Wadsworth Center, Albany, NY) and Fc block, and incubated at 4°C for 20 min. Samples were washed twice with permeabilization buffer and once with staining buffer. Finally, cells were resuspended in 2% PFA and stored at 4°C overnight. Data were collected using a FACScalibur flow cytometer (BD Pharmingen) and were analyzed using FLOWJO flow cytometric analysis software (Tree Star, Inc). Cells were gated based on forward and side scatter plot, and 10,000 cells were collected for each sample. During flow cytometry analysis, isotype controls were used to set the background for expression of CD11c and CD11b, and mock-inoculated cells that had been incubated with antibodies against NS3 were used to set the background for WNV infection. Expression of CD11c was presented as mean fluorescent intensity (MFI).
Isolation and infection of primary skin cells
We adapted a previously described method to isolate primary skin cells from ears of adult mice (Johnston et al., 2000). Mice were euthanized, and ears were removed and placed in PBS with 10% HI-FBS. Ears were rinsed with PBS, split into dorsal and ventral halves, and placed in PBS containing 0.03% trypsin (Invitrogen; Carlsbad, CA), 0.01% DNase I (Promega, Madison, WI),100 U/mL of penicillin, 100 μg/mL of streptomycin, and 4 μg/mL of amphotericin B at 4°C overnight. On the next day, the epidermal layer was separated from the dermal layer, and both layers were incubated in PBS with 0.03% trypsin at 37°C for 20 min. Following incubation, tissues were cut into smaller pieces, DMEM supplemented with 10% HI-FBS, 25 mM HEPES, 100 U/mL of penicillin, 100 μg/mL of streptomycin, and 4 μg/mL of amphotericin B (DMEM) was added, and tissues were pipetted vigorously for 10 min. Cell suspensions were strained through a 100 μm cell strainer and centrifuged at 460 x g for 10 min at 4°C. Cells were washed and cultured in DMEM at 37°C overnight to allow for the recovery of cell surface protein. On the next day, cells were inoculated with virus for 1 h at 37°C, washed three times, and cultured in DMEM at 37°C, 5% CO2. At various times post-inoculation, an aliquot of the medium was removed from the cells and stored at −80°C for later titration.
Statistical analysis
The Mann-Whitney test (GraphPad, San Diego, CA) was used to assess the difference between the two viruses for all the data. A P-value of <0.05 was considered statistically significant.
Acknowledgments
We would like to thank Drs. Laura White and Mark Heise for providing protocols and virus and for their helpful discussions. In addition, we acknowledge the Wadsworth Center Tissue Culture Core for cell preparation and Immunology Core for monoclonal antibody services and flow cytometry analysis.
The work was supported in part by funds from the National Institute of Allergy and Infectious Disease, National Institutes of Health, under contract N01-AI25490 and from the Centers for Disease Control and Prevention grant R01-CI000232-01. The BSL-3 vivarium at the Wadsworth Center was used, which is funded in part as a core facility by NIH/NIAID U54-AI057158 (Northeast Biodefense Center).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams SC, Broom AK, Sammels LM, Hartnett AC, Howard MJ, Coelen RJ, MacKenzie JS, Hall RA. Glycosylation and antigenic variation among Kunjin virus isolates. Virology. 1995;206:49–56. doi: 10.1016/s0042-6822(95)80018-2. [DOI] [PubMed] [Google Scholar]
- Altmann F, Staudacher E, Wilson IB, Marz L. Insect cells as hosts for the expression of recombinant glycoproteins. Glycoconj J. 1999;16:109–123. doi: 10.1023/a:1026488408951. [DOI] [PubMed] [Google Scholar]
- Bajtay Z, Csomor E, Sandor N, Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- Briese T, Bernard KA. West Nile virus--an old virus learning new tricks? J Neurovirol. 2005;11:469–475. doi: 10.1080/13550280500187617. [DOI] [PubMed] [Google Scholar]
- Brotherus J, Renkonen O. Subcellular distributions of lipids in cultured BHK cells: evidence for the enrichment of lysobisphosphatidic acid and neutral lipids in lysosomes. J Lipid Res. 1977;18:191–202. [PubMed] [Google Scholar]
- Brown AN, Kent KA, Bennett CJ, Bernard KA. Tissue tropism and neuroinvasion of West Nile virus do not differ for two mouse strains with different survival rates. Virology. 2007;368:422–430. doi: 10.1016/j.virol.2007.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis CW, Nguyen HY, Hanna SL, Sanchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290–1301. doi: 10.1128/JVI.80.3.1290-1301.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel GD, Dupuis AP, Nicholas D, Young D, Maffei J, Kramer LD. Detection by enzyme-Linked immunosorbent assay of antibodies to West Nile virus in birds. Emerg Infect Dis. 2002;8:979–982. doi: 10.3201/eid0809.020152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksioglu EA, Bess JR, Zhu H, Xu Y, Dong HJ, Elyar J, Nelson DR, Liu C. Hepatitis C virus modulates human monocyte-derived dendritic cells. J Viral Hepat. 2009 doi: 10.1111/j.1365-2893.2009.01231.x. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller FJ, Marcus PI. Interferon induction by viruses. Sindbis virus: defective-interfering particles temperature-sensitive for interferon induction. J Gen Virol. 1980;48:391–394. doi: 10.1099/0022-1317-48-2-391. [DOI] [PubMed] [Google Scholar]
- Gafa V, Manches O, Pastor A, Drouet E, Ambroise-Thomas P, Grillot R, Aldebert D. Human cytomegalovirus downregulates complement receptors (CR3, CR4) and decreases phagocytosis by macrophages. J Med Virol. 2005;76:361–366. doi: 10.1002/jmv.20358. [DOI] [PubMed] [Google Scholar]
- Hanna SL, Pierson TC, Sanchez MD, Ahmed AA, Murtadha MM, Doms RW. N-linked glycosylation of west nile virus envelope proteins influences particle assembly and infectivity. J Virol. 2005;79:13262–13274. doi: 10.1128/JVI.79.21.13262-13274.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P, Robbins PW. Regulation of asparagine-linked oligosaccharide processing. Oligosaccharide processing in Aedes albopictus mosquito cells. J Biol Chem. 1984;259:2375–2382. [PubMed] [Google Scholar]
- Hsieh P, Rosner MR, Robbins PW. Host-dependent variation of asparagine-linked oligosaccharides at individual glycosylation sites of Sindbis virus glycoproteins. J Biol Chem. 1983;258:2548–2554. [PubMed] [Google Scholar]
- Johnston LJ, Halliday GM, King NJ. Langerhans cells migrate to local lymph nodes following cutaneous infection with an arbovirus. J Invest Dermatol. 2000;114:560–568. doi: 10.1046/j.1523-1747.2000.00904.x. [DOI] [PubMed] [Google Scholar]
- Klimstra WB, Nangle EM, Smith MS, Yurochko AD, Ryman KD. DC-SIGN and L-SIGN can act as attachment receptors for alphaviruses and distinguish between mosquito cell- and mammalian cell-derived viruses. J Virol. 2003;77:12022–12032. doi: 10.1128/JVI.77.22.12022-12032.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse M, Rosorius O, Kratzer F, Stelz G, Kuhnt C, Schuler G, Hauber J, Steinkasserer A. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J Virol. 2000;74:7127–7136. doi: 10.1128/jvi.74.15.7127-7136.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Kerst AJ, Nasci RS, Godsey MS, Mitchell CJ, Savage HM, Komar N, Panella NA, Allen BC, Volpe KE, Davis BS, Roehrig JT. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach PY, Burleigh L, Staropoli I, Navarro-Sanchez E, Harriague J, Virelizier JL, Rey FA, Despres P, Arenzana-Seisdedos F, Amara A. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J Biol Chem. 2005;280:23698–23708. doi: 10.1074/jbc.M504337200. [DOI] [PubMed] [Google Scholar]
- Mehlhop E, Whitby K, Oliphant T, Marri A, Engle M, Diamond MS. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuhashi J, Nakasone S, Horie Y. Sterol-free eukaryotic cells from continuous cell lines of insects. Cell Biol Int Rep. 1983;7:1057–1062. doi: 10.1016/0309-1651(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Morrey JD, Day CW, Julander JG, Blatt LM, Smee DF, Sidwell RW. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. doi: 10.1126/science.1089316. [DOI] [PubMed] [Google Scholar]
- Navarro-Sanchez E, Altmeyer R, Amara A, Schwartz O, Fieschi F, Virelizier JL, Arenzana-Seisdedos F, Despres P. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell- derived dengue viruses. EMBO Reports. 2003;4:723–728. doi: 10.1038/sj.embor.embor866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LR, Roehrig JT, Hughes JM. West Nile virus encephalitis. N Engl J Med. 2002;347:1225–1226. doi: 10.1056/NEJMo020128. [DOI] [PubMed] [Google Scholar]
- Pollara G, Speidel K, Samady L, Rajpopat M, McGrath Y, Ledermann J, Coffin RS, Katz DR, Chain B. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J Infect Dis. 2003;187:165–178. doi: 10.1086/367675. [DOI] [PubMed] [Google Scholar]
- Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Soong L, Girard YA, Campbell G, Mason P, Higgs S. Potentiation of West Nile encephalitis by mosquito feeding. Viral Immunol. 2006;19:74–82. doi: 10.1089/vim.2006.19.74. [DOI] [PubMed] [Google Scholar]
- Serody JS, Collins EJ, Tisch RM, Kuhns JJ, Frelinger JA. T cell activity after dendritic cell vaccination is dependent on both the type of antigen and the mode of delivery. J Immunol. 2000;164:4961–4967. doi: 10.4049/jimmunol.164.9.4961. [DOI] [PubMed] [Google Scholar]
- Sevilla N, McGavern DB, Teng C, Kunz S, Oldstone MB. Viral targeting of hematopoietic progenitors and inhibition of DC maturation as a dual strategy for immune subversion. J Clin Invest. 2004;113:737–745. doi: 10.1172/JCI20243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabman RS, Morrison TE, Moore C, White L, Suthar MS, Hueston L, Rulli N, Lidbury B, Ting JP, Mahalingam S, Heise MT. Differential induction of type I interferon responses in myeloid dendritic cells by mosquito and mammalian-cell-derived alphaviruses. J Virol. 2007;81:237–247. doi: 10.1128/JVI.01590-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi PY, Tilgner M, Lo MK, Kent KA, Bernard KA. Infectious cDNA clone of the epidemic West Nile virus from New York City. J Virol. 2002;76:5847–5856. doi: 10.1128/JVI.76.12.5847-5856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberkang M, Havel CM, Friend DS, McCarthy BJ, Watson JA. Isoprene synthesis in isolated embryonic Drosophila cells. I Sterol-deficient eukaryotic cells. J Biol Chem. 1983;258:8503–8511. [PubMed] [Google Scholar]
- Silva MC, Guerrero-Plata A, Gilfoy FD, Garofalo RP, Mason PW. Differential activation of human monocyte-derived and plasmacytoid dendritic cells by West Nile virus generated in different host cells. J Virol. 2007;81:13640–13648. doi: 10.1128/JVI.00857-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styer LM, Bernard KA, Kramer LD. Enhanced early West Nile virus infection in young chickens infected by mosquito bite: effect of viral dose. Am J Trop Med Hyg. 2006;75:337–345. [PubMed] [Google Scholar]
- Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:e132. doi: 10.1371/journal.ppat.0030132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trgovcich J, Aronson JF, Johnston RE. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology. 1996;224:73–83. doi: 10.1006/viro.1996.0508. [DOI] [PubMed] [Google Scholar]
- Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Wu SJ, Grouard-Vogel G, Sun W, Mascola JR, Brachtel E, Putvatana R, Louder MK, Filgueira L, Marovich MA, Wong HK, Blauvelt A, Murphy GS, Robb ML, Innes BL, Birx DL, Hayes CG, Frankel SS. Human skin Langerhans cells are targets of dengue virus infection. Nat Med. 2000;6:816–820. doi: 10.1038/77553. [DOI] [PubMed] [Google Scholar]