Abstract
Fibrocytes are bone marrow-derived cells. Fibrocytes can differentiate into adipocyte- and myofibroblast-like cells. Since fibrocytes can behave like mesenchymal progenitor cells, we hypothesized that fibrocytes have the potential to differentiate into other mesenchymal lineage cells, such as osteoblasts and chondrocytes. In this study, we found that fibrocytes differentiated into osteoblast-like cells when cultured in osteogenic media in a manner similar to osteoblast precursor cells. Under these conditions, fibrocytes and osteoblast precursor cells displayed increased calcium deposition, and increased expression of specific osteogenic genes. In addition, dephosphorylation of cAMP-responsive element binding protein was associated with the increased ratio of Receptor activator of the NF-κB Ligand/osteoprotegerin gene expression and enhanced gene expression of osterix in these cells under these conditions. Both events are important in promoting osteogenesis. In contrast, fibrocytes and mesenchymal stem cells cultured in chondrogenic media in the presence of transforming growth factor-β3 were found to differentiate to chondrocyte-like cells. Fibrocytes and mesenchymal stem cells under these conditions were found to express increased levels of aggrecan and type II collagen genes. Transcription factor genes associated with chondrogenesis were also found to be induced in fibrocytes and mesenchymal stem cells under these conditions. In contrast, β–catenin protein and the core binding factor alpha1 subunit protein transcription factor were decreased in expression under these conditions. These data indicate that human fibrocytes have the capability to differentiate into osteoblast- and chondrocyte-like cells. These findings suggest that such cells could be used in cell-based tissue-regenerative therapy.
Keywords: fibrocytes, osteoblasts, chondrocytes, progenitor cells, stem cells
1. Introduction
Mesenchymal stem cells (MSCs) are emerging as attractive candidates for therapeutic purposes, including gene therapy and tissue regeneration. MSCs are primarily derived from bone marrow, although stem cells can reside in other adult tissues, such as adipose tissue (Picinich et al., 2007). MSCs exhibit characteristics of self-renewal, which have been demonstrated in vitro by serial propagation of these cells (Colter et al., 2000). MSCs differentiate into multiple cell types of mesenchymal lineage including adipocytes, myocytes, osteoblasts, and chondrocytes (Bruder et al., 1997; Dennis et al., 1999; Ferrari et al., 1998; Galmiche et al., 1993; Prockop, 1997; Yoo et al., 1998). In addition, bone marrow-derived cells have recently been shown to differentiate into non-mesenchymal lineages such as hepatic, renal, cardiac, and neural cells (Alhadlaq and Mao, 2004; Marion and Mao, 2006). MSCs have been identified in an increasing number of species including humans (Alhadlaq and Mao, 2004). Osteoblasts and chondrocytes, which are derived from a common mesenchymal precursor cell, are involved in bone formation and in mediating the articular cartilage formation, respectively (Zou et al., 2006). Disorders in articular cartilage affect many people; and are one of the leading causes of invalidity and decreased quality of life in adults (Magne et al., 2005).
Fibrocytes are a distinct population of bone marrow-derived fibroblast-like cells found in peripheral blood (Bellini and Mattoli, 2007; Keeley et al., 2009a; Hong et al., 2005, 2007; Mehrad et al., 2009; Picinich et al., 2007; Strieter et al., 2009a). They express the CD34 cell surface antigen, which is believed to be expressed on hematopoietic stem cells (Gomperts and Strieter, 2007; Keeley et al., 2009a, 2009b; Quan et al., 2004; Strieter et al., 2009b). They also express the common leukocyte antigen CD45 and mesenchymal markers such as collagen I and fibronectin (Andersson-Sjoeland et al., 2008; Gomperts and Strieter, 2007; Keeley et al., 2009a, 2009b; Strieter et al., 2009b). They also express the chemokine receptor CXCR4, and patients with idiopathic pulmonary fibrosis and fibrotic non-specific interstitial pneumonia have recently been found to have increased numbers of circulating fibrocytes (Andersson-Sjoeland et al., 2008; Mehrad et al., 2007), and the elevated presence of these cells in circulation in these patients appears to be associated with worse prognosis (Moeller et al., 2009). Fibrocytes constitutively produce extracellular matrix (ECM) components as well as ECM-modifying enzymes. Human fibrocytes produce large amounts of ECM components by stimulation with pro-fibrotic cytokines and growth factors, and further differentiate into contractile myofibroblast-like cells (Hong et al., 2005; Hong et al., 2007; Mehrad et al., 2009). They can also function as antigen presenting cells, participate in normal or aberrant wound-healing, and activate pathologic fibrosis in response to local inflammation (Chesney et al., 1997; Grab et al., 1999; Phillips et al., 2004).
Our laboratory has discovered that circulating fibrocytes can behave as mesenchymal progenitor cells with the capacity to differentiate into adipocyte- or myofibroblast-like cells (Hong et al., 2005; Hong et al., 2007). Transforming growth factor-β1 (TGF-β1) induces differentiation of fibrocytes to myofibroblasts by activation of Smad2/3 and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathways (Hong et al., 2007). Conversely, peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist, troglitazone, promotes differentiation of fibrocytes to adipocytes with evidence of lipid accumulation and induction of adipocyte lipid-binding protein (aP2) (Hong et al., 2007). It has also been shown that circulating fibrocytes can differentiate into myofibroblast-like cells after recruitment into the bronchial tissue of asthmatic patients (Schmidt et al., 2003), in animal models of pulmonary fibrosis, and in patients with pulmonary fibrosis (Andersson-Sjoeland et al., 2008; Mehrad et al., 2009). These findings suggest that circulating fibrocytes have the ability to behave as progenitor cells and raise the notion that circulating fibrocytes can differentiate into other mesenchymal lineage cells, such as osteoblasts and chondrocytes.
To test this hypothesis, we isolated human fibrocytes and used commercially available osteoblast precursor cells (OBPC) as comparator cells. Fibrocytes and OBPC were exposed under the same conditions to osteogenic media supplemented with β-glycerophosphate, dexamethasone, and ascorbate. Both fibrocytes and OBPC that had undergone differentiation to osteoblasts displayed enhanced deposition of calcium. The increased deposition of calcium in these cells was also associated with enhanced expression of a number of osteogenic genes, which involved the signaling pathway cAMP/cAMP-dependent protein kinase A (PKA) through dephosphorylation of cAMP-responsive element binding (CREB) protein and an increase in the ratio of Receptor activator of the NF-κB Ligand (RANKL)/osteoprotegerin (OPG) gene expression. In contrast, fibrocytes and commercially available human MSC (e.g., used as a comparator cell) exposed to chondrogenic media in the presence of TGF-β3 underwent differentiation to chondrocytes. TGF-β3 in chondrogenic media promoted the accumulation of proteoglycans and glycosaminoglycans by staining, and induced the expression of aggrecan and col2A1 genes in both fibrocytes and MSC, compatible with these cells undergoing differentiation to chondrocytes. Moreover, fibrocytes and MSC undergoing differentiation to chondrocytes were found to have augmented expression of the chondrogenic transcriptional factor genes, SOX5, SOX6, and SOX9. The expression of these chondrogenic transcriptional genes, especially SOX9, was associated with downregulation of expression of β-catenin and cbfa1 mRNA as early as 3 days post-exposure TGF-β3 in chondrogenic media. Our findings support the notion that human fibrocytes have the capacity to differentiate into osteoblast- and chondrocyte-like cells, compatible with the concept that fibrocytes represent a circulating pool of mesenchymal progenitor cells.
2. Materials and methods
2.1. Isolation, preparation, and purification of human fibrocytes
Fibrocytes were isolated, cultured and harvested from peripheral blood mononuclear cells (PBMCs) as previously described (Hong et al., 2005; Hong et al., 2007; Mehrad et al., 2007; Mehrad et al., 2009). Briefly, PBMCs were isolated from human leukophoresis packs by gradient centrifugation over Ficoll-Plaque (GE Healthcare) and stored at -150°C until used in the experiments. After thawing, PBMCs were cultured on fibronectin-coated flasks for 4 days in control media (Dulbecco's Modified Eagle's Medium with 20% fetal bovine serum, 10 mM HEPES, 1× Pen/Strep, and 4% L-glutamine; Invitrogen). After removal of non-adherent cells, the adherent cells were supplemented with new media and remained in culture for 7-10 days. For further isolation of fibrocytes, the cultured cells were rinsed with Hank's Balanced Salt Solution four times to remove any residual media, and then detached from flasks by incubation with Accutase (Innovative Cell Technologies) for 30 min at 37 °C. The fibrocytes were purified from contaminating monocytes, T cells, and B cells by negative selection using magnetic microbeads for CD14, CD2, and CD19 (Dynabeads, Invitrogen).
2.2. Osteogenic differentiation
Purified fibrocytes were seeded at the concentration of 1×105 cells/well of fibronectin-coated 12 well plate (Corning), and treated with osteogenic basal media, which was supplemented with dexamethasone, ascorbate, mesenchymal cell growth supplement (MCGS), L-glutamine, 1× Pen/Strep, and β-glycerophosphate (Lonza catalog number PT-3002; defined as “osteogenic media”). Dexamethasone is a glucocorticoid steroid capable of stimulating osteogenic differentiation of MSCs at its higher concentration (Marion and Mao, 2006). In addition to its stimulatory effects on cell proliferation, ascorbate further facilitates osteogenic differentiation, including collagen biosynthesis. β–glycerophosphate is critical to stimulate calcified matrix formation in combination with the effects of dexamethasone and ascorbate. Media was replaced every 3 days. Fibrocytes treated with control instead of osteogenic media were used for negative control comparison. OBPC, at 80-90% confluent (Lonza, Walkersville, MD, USA), were harvested, seeded, and treated with osteogenic media in the same manner as fibrocytes, and served the purpose as a positive comparator cell for osteogenesis. To exclude the potential of non-fibrocytes (e.g., CD45 negative circulating progenitor cells) contributing to fibrocyte osteogenic differentiation, in separate experiments isolated fibrocytes were further enriched to only a CD45-positive population using CD45 magnetic microbeads.
2.3. Chondrogenic differentiation
Purified fibrocytes were seeded at the concentration of 5×104 cells/tube in 15 ml sterile polypropylene tubes, followed by centrifugation at ∼300 ×g for 10 min to form micromass pellets using a modification as previously described for human MSC differentiation to chondrocytes (Mackay et al., 1998). Supernatant was carefully removed in order not to disrupt the fibrocyte micromass pellet, followed by the addition of chondrogenic differentiation (i.e., basal media supplemented with dexamethasone, ascorbate, L-glutamine, Pen/Strep, sodium pyruvate, proline, and ITS-supplement (Lonza catalog number PT-3003; defined as “chondrogenic media”). For further differentiation, chondrogenic media was supplemented with 10 ng/ml of TGF-β3 (PeproTech) as previously described (Mackay et al., 1998). Chondrogenic media with and without TGF-β3 was changed every 3 days. Human MSC, at 80-90% confluent (Lonza, Walkersville, MD, USA), were harvested, seeded, and treated with chondrogenic media with or without TGF-β3 in the same manner as fibrocytes, and served the purpose as a positive comparator cell for chondrogenesis. To exclude the potential of non-fibrocytes (e.g., CD45 negative circulating progenitor cells) contributing to fibrocyte chondrogenic differentiation, in separate experiments isolated fibrocytes were further enriched to only a CD45-positive population using CD45 magnetic microbeads.
2.4. Von Kossa staining
Von Kossa staining was used to detect calcium in fibrocytes and OBPC undergoing differentiation to osteoblasts, and was performed as previously described (Bonewald et al., 2003). Briefly, fibrocytes and OBPC that had undergone differentiation to osteoblasts, as compared to appropriate control cells, were washed twice with phosphate-buffered saline (PBS), and then fixed with 4% paraformaldehyde for 15 min at room temperature. After rinsing with distilled water, the cells were incubated with 1% silver nitrate solution, and placed under ultraviolet light for 20 min. The cells were rinsed in several changes of distilled water, and then incubated with 5% sodium thiosulfate for 5 min to remove unreacted silver. After rinsing in distilled water, the cells were dehydrated through successive treatment of 70%, 95% and 100% alcohol. Pictures of the cells were taken by Axiovert 40CFL microscope (Carl Zeiss, Germany).
2.5. Alizarin red S staining
Alizarin red S staining was used to detect calcium in fibrocytes and OBPC, and was performed as previously described (Hinoi et al., 2006). Briefly, cells were rinsed with PBS twice and fixed in 10%-buffered formalin for 30 min at room temperature. Fixed cells were washed twice with distilled water, and then stained with 2% alizarin Red S (Sigma) in water for 10 min. After rinsing in several changes of distilled water and air-drying, pictures of stained cells were taken by Axiovert 40CFL microscope (Carl Zeiss, Germany).
2.6. Safranin-O staining
Safranin-O staining was used to detect proteoglycans and glycosaminoglycans found in chondrocytes, and was performed as previously described (Rosemberg, 1971). Briefly the fibrocyte and MSC micromass pellets were gently disrupted in 1 ml of chondrogenic media by repetitive pipetting up-and-down. The cells were centrifuged onto microscopic slide (Cytospin3, Shandon), washed 2 times with PBS, and then fixed with 4% paraformaldehyde for 15 min at room temperature. After rinsing with distilled water, the cells were incubated with 1% Safranin-O solution (Sigma) for 1 h, washed in plenty of distilled water, and then dehydrated through successive treatment of 70%, 95% and 100% ethanol. Pictures of stained cells were taken by Axiovert 40CFL microscope (Carl Zeiss, Germany).
2.7. Alcian blue staining
Alcian blue staining, which stains acid mycosubstances and acetic mucins found in chondrocytes was performed as previously described (Rahman and Tsuchiya, 2001). Briefly, disrupted chondrogenic pellets were deposited into TissueTechs slide (BD Falcon) in the presence of 0.5 ml of chondrogenic media supplemented with 10 ng/ml TGF-β3 followed by incubation for 24 h at 37°C. Cells were gently washed twice with PBS and then fixed with 4% paraformaldehyde for 15 min at room temperature. After rinsing with distilled water cells were incubated with 1% alcian blue solution (Sigma) for 30 min at room temperature. After rinsing in several changes of distilled water and air-drying, pictures of stained cells were taken by Axiovert 40CFL microscope (Carl Zeiss, Germany).
2.8. Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was prepared from cells that were either undergoing differentiation or had differentiated to a mesenchymal phenotype using TRIzol (Invitrogen) reagent according to manufacturer's instructions, and then quantified with NanoDrop machine (NanoDrop Technologies). RT-PCR analyses were done with Access RT-PCR kit (Promega) according to manufacturer's instructions, using DNA Engine Thermal Cycler (Bio-Rad). Electrophoresis with 2% agarose gel was used for detection of amplified bands. Nucleotide sequences of RT-PCR primers used are shown in Table 1 (Frank et al., 2002; Yang et al., 2008).
Table 1.
Nucleotide sequences of RT-PCR primers.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Osteocalcin | 5′-TGA GAG CCC TCA CAC TCC TC-3′ | 5′-CGC CTG GGT CTC TTC ACT AC-3′ |
| Osteonectin | 5′-GAG GGC CTG GAT CTT CTT TC-3′ | 5′-TCC ACC TGG ACA GGA TTA GC-3′ |
| Osteopontin | 5′-ATG ATG GCC GAG GTG ATA GT-3′ | 5′-GAT GGC CTT GTA TGC ACC AT-3′ |
| RANKL | 5′-AAT AGA ATA TCA GAA GAT GGC ACT C-3′ | 5′-TAA GGA GGG GTT GGA GAC CTC G-3′ |
| OPG | 5′-GCT AAC CTC ACC TTC GAG-3′ | 5′-TGA TTG GAC CTG GTT ACC-3′ |
| COL2A1 | 5′-TGG TGA ACC TGG TGT CTC TG-3′ | 5′-GTT CCT GGG AAA CCA CGA G-3′ |
| Aggrecan | 5′-CCA GGT GTG TGG GAC TGA A-3′ | 5′-ACA CTC AGC GAG TTG TCA TGG-3′ |
| SOX5 | 5′-AGC CAG AGT TAG CAC AAT AGG-3′ | 5′-CAT GAT TGC CTT GTA TTC-3′ |
| SOX6 | 5′-ACT GTG GCT GAA GCA CGA GTC-3′ | 5′-TCC GCC ATC TGT CTT CAT ACC-3′ |
| SOX9 | 5′-GAA CGC ACA TCA AGA CGG AG-3′ | 5′-TCT CGT TGA TTT CGC TGC TC-3′ |
| GAPDH | 5′-AGC CAC ATC GCT CAG AAC AC-3′ | 5′-GAG GCA TTG CTG ATG ATC TTG-3′ |
2.9. Western blotting
Total cellular protein was prepared using whole cell extraction buffer [20 mM HEPES, pH 7.9, 25% glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 1% NP-40, 1× protease inhibitor cocktail (Roche) and 1× phosphatase inhibitor cocktail 2 (Sigma)]. Total protein was quantified with BCA Protein Assay Kit (Pierce). The appropriate amount of protein was diluted with SDS-PAGE sample buffer, boiled at 100°C for 5 min and clarified by centrifugation. Equal amounts of protein (30 μg) were then loaded onto 10% Tris-Glycine gels and electrophoresed at 100 V for 90 min. The gels were transferred to PVDF membrane using the manufacturer's recommended protocol. The membranes are blocked using 5% (w/v) non-fat dried milk in Tris-buffered saline containing 0.001% (v/v) Tween 20. The membranes were incubated with primary antibody overnight using antibodies against the targets listed: osteonectin, aggrecan (R&D Systems), CREB, phosphor-CREB (Cell Signaling Technology), SOX9 (Abcam), or β-tubulin (Novus Biologicals). After removing unbound primary antibody by washing, anti-mouse horseradish peroxidase-conjugated secondary antibody (Bio-Rad) was used at 1:4,000 dilution. Final chemiluminescence detection is based on the ECL+ kit (GE Healthcare) per the manufacturer's protocol.
2.10. Immunocytochemistry
Immunostaining experiments were performed using a modification of a previously described method (Hong et al., 2007). Briefly, fibrocytes that had undergone differentiation to osteoblasts were washed twice with PBS. The cells were fixed in 10%-buffered formalin for 30 min at room temperature, and then blocked with 1× PowerBlock (Biogenex) for 1 h. After washing twice with PBS, the anti-human osteonectin antibody (R&D Systems) was incubated with the cells (1:200 dilution, overnight at 4°C). Unbound antibodies were removed by washing with PBS. The cells were then incubated with Alexa Fluor 594 anti-mouse IgG (Invitrogen; 1:2,000) for 1 h at room temperature. After washing with PBS to remove unbound secondary antibodies, the cells were visualized with a Zeiss fluorescence microscope equipped with the Axiovert 40CFL imaging system (Carl Zeiss, Germany).
2.11. Statistical analysis
Comparisons were evaluated by Student's unpaired t-test. Data were considered statistically significant if p < 0.05.
3. Results
3.1. Fibrocytes display the ability to differentiate into osteoblasts
For initial studies to determine whether cultured fibrocytes and OBPC could differentiate to osteoblasts, we isolated fibrocytes from PBMCs and exposed the cells to osteogenic media for up to 21 days. For negative control, isolated fibrocytes were treated in parallel with only conventional media. Von Kossa staining showed that at day 7 after initiation of osteogenesis of fibrocytes, the cells revealed detectable calcium deposition (Fig. 1A). At day 14 there was a significant increase of calcium deposition in differentiated fibrocytes as compared to day 7, and the increased staining of calcium was maintained up to 21 days (Fig. 1A). Fibrocytes undergoing differentiation to osteoblasts showed detectable calcium deposition by von Kossa staining after exposure to osteogenic media for 21 days (Fig. 1B). OBPC were used as a positive control for osteogenic differentiation. OBPC, which were seeded 1 × 105 cells/well in 12-well plates and exposed to osteogenic media for 21 days also showed calcium deposition by von Kossa staining (Fig. 1B). In contrast, neither fibrocytes nor OBPC exposed to control media for 21 days showed any calcium deposition (Fig. 1B). The deposition of calcium by fibrocytes undergoing differentiation to osteoblasts in osteogenic media was further evaluated using alizarin red S staining. Both fibrocytes and OBPC that had undergone differentiation to osteoblasts in osteogenic media for 21 days demonstrated positive alizarin red S staining which represented deposition of calcium (Fig. 1C). Neither fibrocytes nor OBPC exposed to control media for 21 days showed any detectable calcium deposition by alizarin red S staining (Fig. 1C). These results suggest that fibrocytes exposed to osteogenic media differentiated into cells that induce calcium deposition similar to OBPC, which is compatible with differentiation to osteoblasts.
Fig. 1.
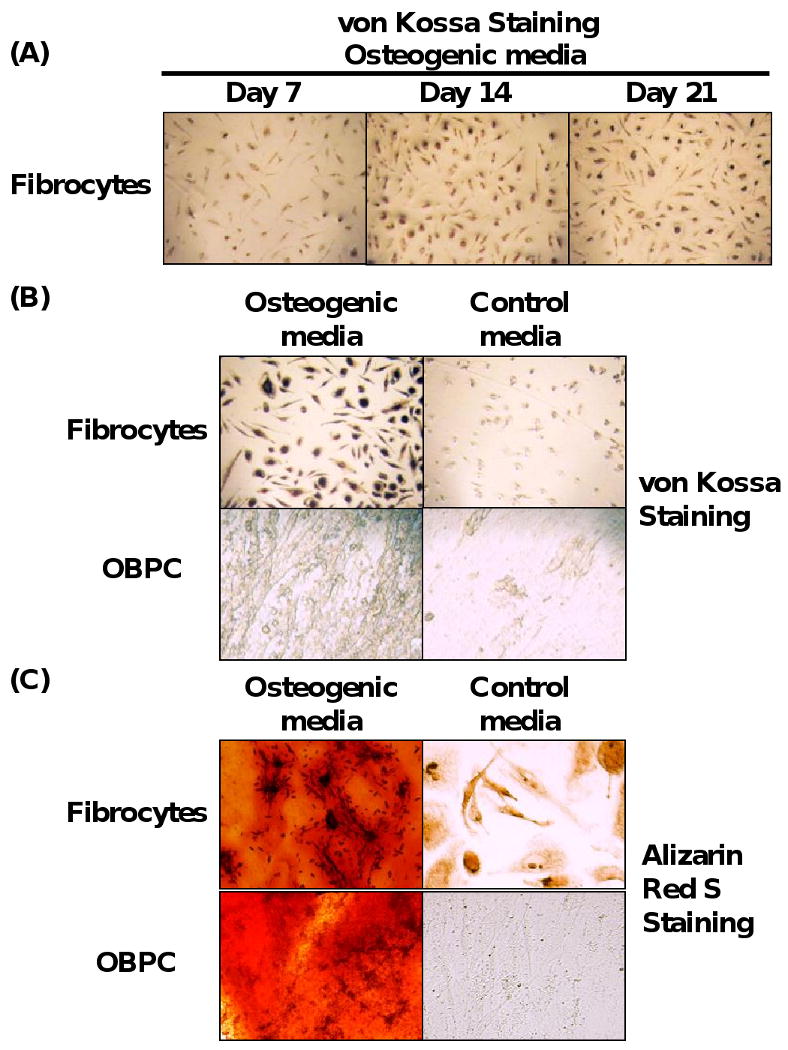
Fibrocytes display the ability to differentiate into osteoblasts. (A) Time course of calcium deposition from fibrocytes undergoing differentiation to osteoblast-like cells in the presence of osteogenic media, as assessed by von Kossa staining. (B) von Kossa staining and (C), Alizarin Red S staining of fibrocytes and OBPC exposed to osteogenic media for 21 days. Magnification 400×. Representative picture from at least 5 experiments for each panel is shown.
3.2. Fibrocytes undergoing osteogenesis express genes for osteogenic markers
In order to confirm whether the fibrocytes exposed to osteogenic media were differentiated into osteoblasts, we next evaluated whether these cells expressed specific osteogenic gene markers during osteogenesis. Osteogenic differentiation of progenitor cells in vitro is characterized by formation of mineralized bone-like nodules containing osteonectin, osteopontin, and osteocalcin protein (Frank et al., 2002). On this basis, osteonectin, osteopontin, and osteocalcin mRNA were chosen as gene markers for evidence of osteogenesis as assessed by RT-PCR. As shown in Fig. 2A, all three osteogenic genes were upregulated in both fibrocytes or OBPC exposed to osteogenic media as early as day 7. Each osteogenic gene marker showed a slightly different expression profile during the time-course of osteogenic differentiation of fibrocytes. Fibrocytes exposed to osteogenic media showed that osteonectin mRNA was highly expressed as early as day 7, with persistence of osteonectin expression at days 14 and 21. In contrast, fibrocytes undergoing osteogenic differentiation showed that osteopontin mRNA expression was minimally expressed by day 7, but was maximal by day 21. Osteocalcin gene expression in fibrocytes undergoing osteogenic differentiation was induced as early as day 7 with persistence of maximal expression at days 14 and 21 similar to osteonectin (Fig. 2A). The kinetics of osteonectin, osteopontin, and osteocalcin gene expression by OBPC were similar to fibrocytes under the same osteogenic culture conditions, with the exception that osteopontin gene expression appeared to peak earlier in OBPC at day 14 (Fig. 2A).
Fig. 2.
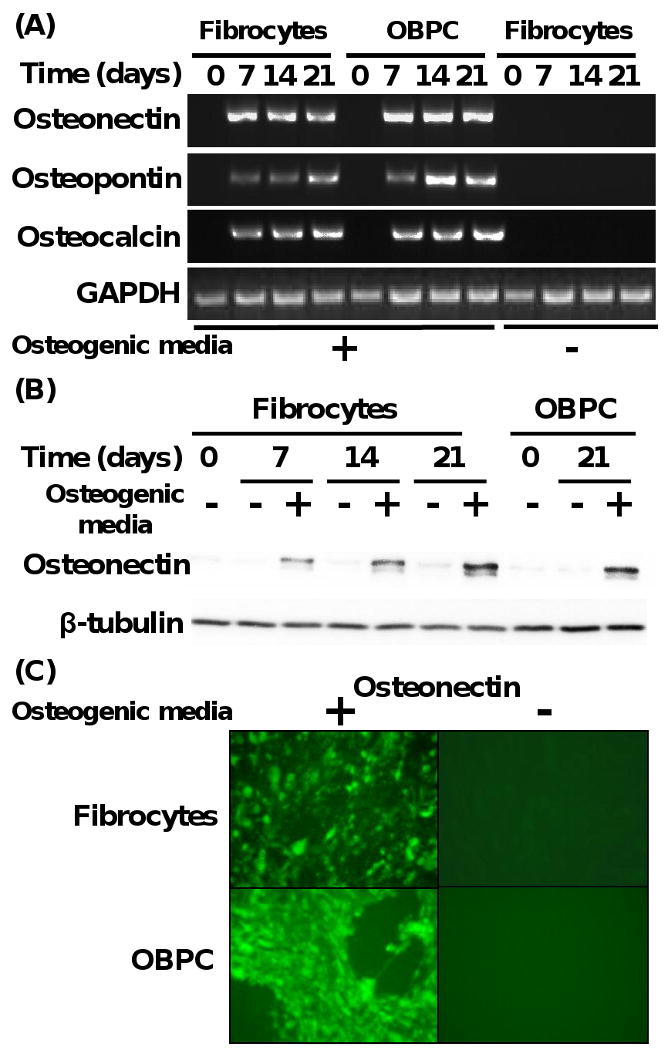
Fibrocytes undergoing osteogenesis express genes for osteogenic markers. (A) Time course of gene expression of osteonectin, osteopontin, and osteocalcin from fibrocytes and OBPC undergoing differentiation to osteoblast-like cells in the presence or absence of osteogenic media. GAPDH served as a control housekeeping gene. (B) Time course of osteonectin protein expression from fibrocytes and OBPC exposed to the presence or absence of osteogenic media. β-tubulin was used as a control for loading equivalent amounts of protein per well. Results are representative of 3 experiments. (C) Immunofluorescent image of osteonectin protein from fibrocytes and OBPC exposed to the presence or absence of osteogenic media. Magnification 400×. Results are representative of 2 experiments.
To exclude the hypothesis that non-fibrocytes (e.g., CD45-negative circulating progenitor cells) were contributing to osteogenesis, in separate experiments isolated fibrocytes were further enriched to only a CD45-positive population using CD45 magnetic microbeads. CD45 positive fibrocytes initially expressed collagen type I, which is a fibrocyte gene marker; however, CD45 positive fibrocytes that were exposed to osteogenic media undergoing differentiation to osteoblasts demonstrated loss of collagen type I and concomitant gain of osteonectin gene expression at day 21 (Supplemental Fig. 1B). These findings support the notion that fibrocytes, and not the potential contamination of non-fibrocytes, are the cells that are undergoing osteogenic differentiation when exposed to osteogenic media.
We next determined whether fibrocytes and OBPC undergoing osteogenic differentiation not only expressed mRNA for the osteogenic genes as above, but also the relevant osteogenic protein. Total cellular protein was processed from both fibrocytes and OBPC undergoing osteogenic differentiation at days 7, 14, and 21 in response to osteogenic media, as compared to cells that were exposed to control media alone. Western blot analysis demonstrated that both fibrocytes and OBPC exposed to osteogenic media displayed markedly elevated levels of osteonectin as early as 7 days (e.g., for fibrocytes) (Fig. 2B). In contrast, both fibrocytes and OBPC exposed to control media failed to express significant levels of osteonectin protein (Fig. 2B). We next assessed whether osteonectin protein was cellularly associated with fibrocytes undergoing osteogenesis using immunofluorescence microscopy. As shown in Fig. 2C, both fibrocytes and OBPC that had been exposed to osteogenic media for 21 days demonstrated positive immunofluorescence for osteonectin protein, as compared to absence of immunofluorescence for osteonectin protein in cells exposed to control media alone. These results indicated that there was increased expression of osteogenic gene markers in the fibrocytes and OBPC undergoing osteogenic differentiation.
3.3. Fibrocytes undergoing osteogenic differentiation demonstrate dephosphorylation of CREB protein, enhanced ratio of RANKL/OPG gene expression, and induction of osterix gene expression
Since it was apparent that fibrocytes similar to OBPC had the ability to undergo osteogenic differentiation, we next assessed whether signaling pathway(s) known to be involved in osteogenic differentiation were found in fibrocytes under these conditions. Recently, it has been shown that the cAMP/PKA signaling pathway is important in regulating bone homeostasis via controlling cyto-differentiation of MSCs and RANKL/OPG gene expression (Yang et al., 2008). Cyclic AMP is an important intracellular second messenger molecule, and its main role is to activate the PKA. We evaluated whether the initiation of osteogenic differentiation of fibrocytes altered the activated (e.g., phosphorylated CREB) cellular expression of CREB protein, which is a key regulator of cAMP signaling pathway in cells. As shown in Fig. 3A, the magnitude of total CREB protein expression did not significantly differ in fibrocytes and OBPC exposed to osteogenic media for 21 days; however, activated CREB (e.g., phosphorylated CREB) was markedly reduced under these same conditions. These findings imply that the cAMP/PKA signaling pathway is involved in the regulation of osteogenesis of fibrocytes and OBPC.
Fig. 3.
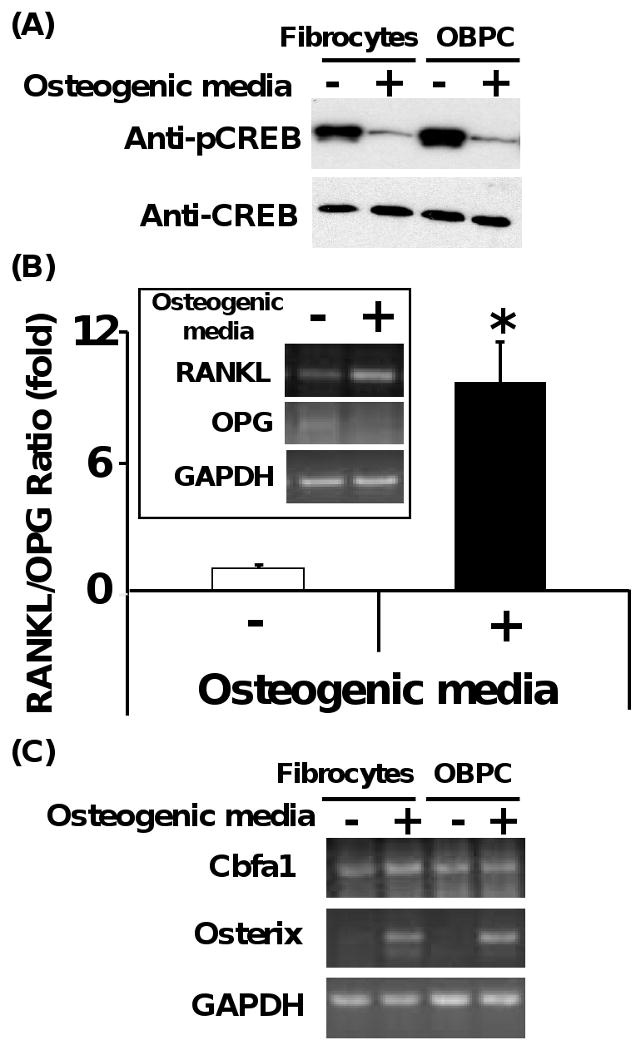
Fibrocytes undergoing osteogenic differentiation demonstrate dephosphorylation of CREB protein, enhanced ratio of RANKL/OPG gene expression, and induction of osterix gene expression. (A) Dephosphorylation of CREB is seen in fibrocytes and OBPC undergoing differentiation to osteoblast-like cell in the presence of osteogenic media for 21 days. (B) Ratio of RANKL/OPG gene expression from fibrocytes exposed to the presence or absence of osteogenic media for 21 days. Intensity of the bands from RT-PCR with the corresponding genes was quantified by densitometer (Quantity-1 software, Bio-Rad). Representative image from 3 different experiments of gel electrophoresis is shown. N=6 for each data for quantification of band intensity using densitometer software (*p<0.01). (C) Osterix and Cbfa1 gene expression from fibrocytes and OBPC exposed to the presence or absence of osteogenic media for 21 days. Representative image from 3 different experiments of gel electrophoresis was shown.
The ratio of RANKL/OPG gene expression was evaluated in the fibrocytes exposed to osteogenic or control media for 21 days (Fig. 3B). Fibrocytes exposed to osteogenic media for 21 days demonstrated increased RANKL gene expression, while OPG gene expression remained at steady levels, as compared to fibrocytes exposed to control media (Fig. 3B). When the ratio of RANKL/OPG was quantified by densitometer, fibrocytes exposed to osteogenic media for 21 days showed a 9-fold increase of RANKL/OPG gene expression, as compared to fibrocytes exposed to control media (Fig. 3B). These data indicated that the cAMP/PKA signaling pathway may contribute to the osteogenesis of cultured fibrocytes through dephosphorylation of CREB and enhanced ratio of RANKL/OPG gene expression. In further support of this notion, fibrocytes exposed to osteogenic or control media were cultured in the presence or absence of H89 (10 μM), a PKA inhibitor for 21 days (Kato et al., 2006). The presence of H89 in the culture markedly reduced the ratio of RANKL/OPG gene expression in fibrocytes exposed to osteogenic media to a ratio comparable to control media conditions (Supplemental Fig. 2). These results support the importance of the cAMP/PKA signaling pathway during osteogenic differentiation of fibrocytes.
Runx2/core binding factor alpha 1 (Cbfa1) and osterix are osteoblast-specific transcription factors essential for the development of mature osteoblasts, and these genes are thought to activate osteoblast gene markers in vivo to produce a bone-specific matrix (Igarashi et al., 2004). Cbfa1 is necessary for an early step in the osteoblasts/chondrocyte differentiation pathway, whereas osterix (e.g, a zinc finger-containing protein) is required for the differentiation of pre-osteoblasts into fully functioning osteoblasts. Osterix appears to act downstream of Cbfa1 during osteogenic differentiation (Tai et al., 2004). As shown in Fig. 3C, osterix gene expression was markedly elevated in both fibrocytes and OBPC exposed to osteogenic media for 21 days, as compared with cells exposed to control media alone. There was no significant change in expression of Cbfa1 in fibrocytes or OBPC under these conditions (Fig. 3C). These results showed that osterix transcription factor may be related to the osteogenesis of cultured fibrocytes and OBPC downstream of Cbfa1.
3.4. Fibrocytes display the ability to differentiate into chondrocytes
Since we found that fibrocytes could differentiate into osteoblasts, we next determined whether fibrocytes could differentiate into chondrocytes. Previously it has been shown that TGF-β3 in combination with dexamethasone in defined media can induce MSC chondrogenesis when MSC are in micromass pellets (Mackay et al., 1998). We determined that both fibrocytes and human MSC in the presence TGF-β3 required culture in a micromass pellet in order to undergo chondrogenesis, whereas culture of these cells as a monolayer failed to induce chondrogenesis (data not shown). In terms of number of fibrocytes placed into a micromass pellet, we found that 5×104 cells/pellet was the lower limit of the number of fibrocytes that could yield successful detection of fibrocytes undergoing chondrogenesis. On this basis, we cultured fibrocytes and human MSC in the form of micromass pellets and in the presence of chondrogenic media in the presence or absence of TGF-β3 in a kinetic manner for 28 days. Fibrocytes and MSC exposed to chondrogenic media alone without TGF-β3 were used as negative controls. At specific time points, fibrocytes and MSC were isolated and subjected to alcian blue staining, which stains acid mycosubstances and acetic mucins in chondrocytes. As shown in Fig. 4A, fibrocytes that had been exposed to chondrogenic media in the presence of TGF-β3 stained positive for alcian blue as early as 9 days, with an increase in alcian blue staining out to 28 days, as compared to the absence of staining of cells exposed to control media. Human MSC cultured under the same conditions demonstrated similar kinetics for alcian blue staining as fibrocytes (data not shown). These findings suggest that TGF-β3 is essential to induce chondrogenesis of fibrocytes under these conditions. To further confirm that fibrocytes can undergo differentiation to chondrocytes, we performed staining with safranin-O. Safranin-O staining detects proteoglycans and glycosaminoglycans, which are deposited in the differentiated chondrocytes (Rosenberg, 1971). As shown in Fig. 4B, we found that fibrocytes cultured in chondrogenic media in the presence of TGF-β3 for 28 days were positive for safranin-O staining, as compared to fibrocytes cultured in chondrogenic media alone. These findings demonstrate fibrocytes exposed to both chondrogenic media in the presence of TGF-β3 develop a chondrocyte phenotype with increased ability to express proteoglycans and glycosaminoglycans within the cells. In addition fibrocytes undergoing chondrogenesis also showed a more compact morphology as compared to fibrocytes exposed to chondrogenic media alone.
Fig. 4.
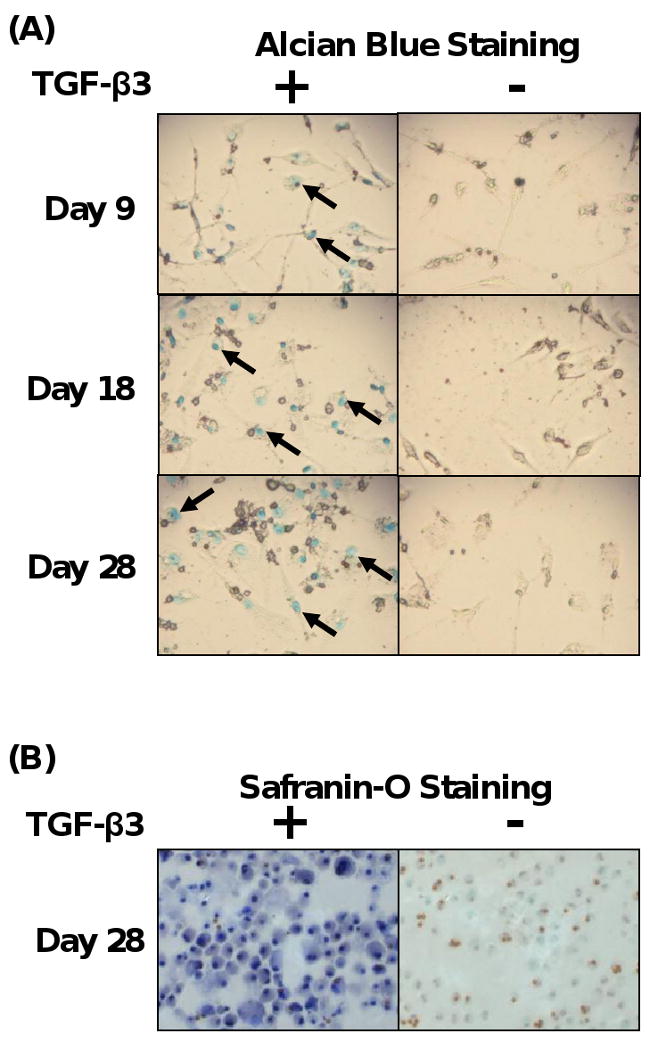
Fibrocytes display the ability to differentiate into chondrocytes. (A) Time course of alcian blue staining of fibrocytes exposed to chondrogenic media in the presence or absence of TGF-β3 (10 ng/ml). (B) Safranin-O staining of fibrocytes exposed to chondrogenic media with or without TGF-β3. Magnification 400×. Representative images from at least 3 experiments.
3.5. Fibrocytes undergoing chondrogenesis express specific chondrogenic genes
Since fibrocytes undergoing differentiation to chondrocytes were found to produce more chondrocyte-specific molecules, we next assessed the expression profile of chondrogenic gene markers [e.g., aggrecan and type II collagen (Col2A1) genes (Marion and Mao, 2006)] in fibrocytes and MSC undergoing differentiation to chondrocytes. Fibrocytes and MSC were first cultured in a kinetic manner up to 28 days in the presence of chondrogenic media with or without TGF-β3 and assessed for the expression of aggrecan and Col2A1. As shown in Fig. 5A, fibrocytes were found to express aggrecan and Col2A1 mRNA as early as 9 days, and with maximal expression by 28 days in response to chondrogenic media and TGF-β3. MSC were found to express similar levels of aggrecan and Col2A1 mRNA at 28 days (Fig. 5A). Western blot analysis of total cellular proteins from fibrocytes and MSC under these conditions demonstrated that fibrocytes and MSC exposed to chondrogenic media supplemented with TGF-β3 expressed aggrecan protein, as compared to lack of expression of aggrecan when the cells were cultured in chondrogenic media alone (Fig. 5B). To exclude that CD45-negative progenitor cells were contributing to the above findings, we specifically isolated only CD45 positive fibrocytes and subjected them to similar conditions to induce chondrogenesis. We found that CD45-positive fibrocytes exposed to chondrogenic media in the presence of TGF-β3, as compared to chondrogenic media alone, demonstrated gain in expression of aggrecan and loss of expression of collagen type I gene expression (Supplemental Fig. 1C). These findings suggest that fibrocytes exposed to chondrogenic media in the presence of TGF-β3 undergo chondrogenesis with associated expression of specific chondrogenic genes.
Fig. 5.
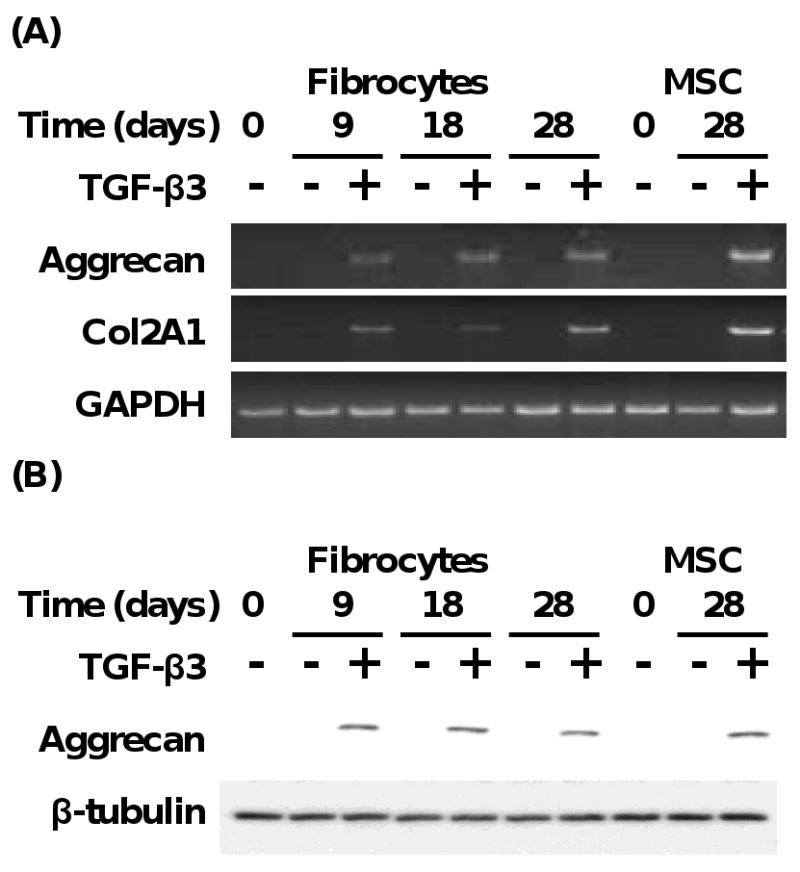
Fibrocytes undergoing chondrogenesis express specific chondrogenic genes. (A) Time course of gene expression of aggrecan and Col2A1 from fibrocytes and MSC exposed to chondrogenic media with or without TGF-β3 (10 ng/ml). (B) Time course of aggrecan protein expression from fibrocytes and MSC exposed to chondrogenic media with or without TGF-β3 (10 ng/ml). Results are representative of 3 experiments.
3.6. Chondrogenic transcription factor gene expression are upregulated during fibrocyte differentiation to chondrocytes
SOX, SOX6, and SOX9 are transcription factors that promote the biosynthesis of Col2A1and aggrecan protein during chondrogenesis (Marion and Mao, 2006). Previous studies have supported that SOX9 is activated at an early stage of chondrogenesis during mouse embryonic development (Lefebvre and de Crombrugghe, 1998). SOX5 and SOX6 have a high degree of sequence identity with each other, and are coexpressed with SOX9 during chondrogenic differentiation (Lefebvre et al., 2001). On this basis, we assessed the SOX5, SOX6, and SOX9 gene expression during fibrocyte differentiation to chondrocytes. As shown in Fig. 6A, both fibrocytes and MSC exposed to chondrogenic media in the presence of TGF-β3 for 3 days expressed significant levels of SOX9>SOX6>SOX5 mRNA, as compared to the absence of gene expression in cells exposed to chondrogenic media alone. These findings support the notion that SOX5, SOX6, and SOX9 mRNA expression is initiated during the early stage of fibrocyte and MSC chondrogenesis. To extend these studies, we determined the kinetics of SOX9 gene expression in fibrocytes undergoing chondrocyte differentiation out to 28 days. As shown in Fig. 6B, SOX6 gene expression was markedly upregulated at days 9, 18, and 28 in fibrocytes exposed to chondrogenic media with TGF-β3, as compared to chondrogenic media alone. Similar findings for SOX gene expression were shown from MSC cultured under the same conditions for 28 days (Fig. 6B).
Fig. 6.
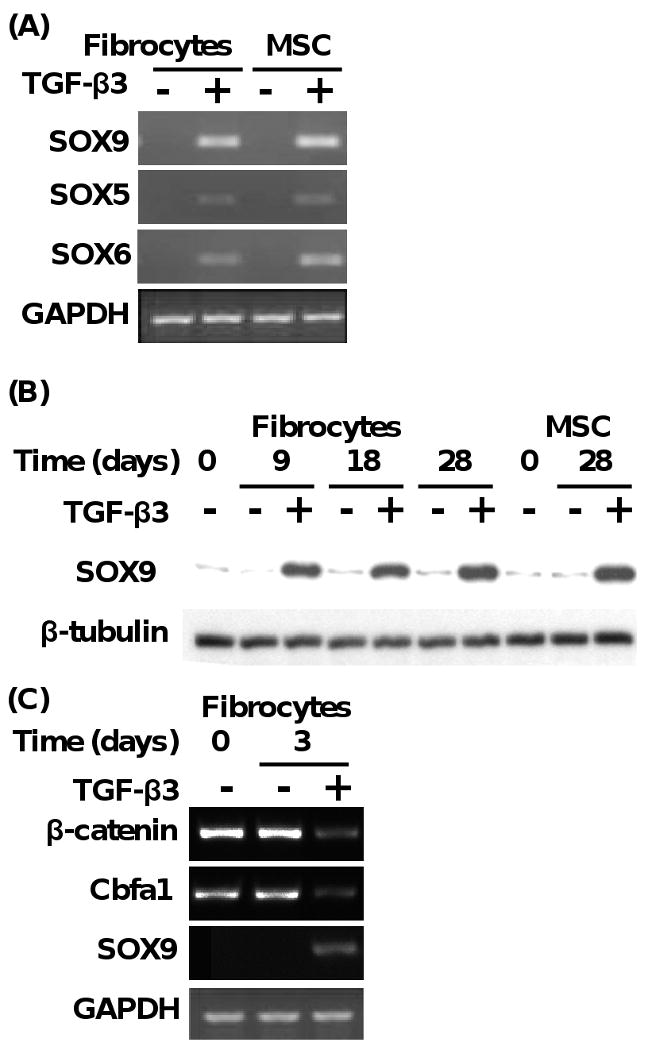
Chondrogenic transcription factor gene expression (i.e., SOX5, SOX6, and SOX9) are upregulated during fibrocyte differentiation to chondrocytes. (A) Expression of SOX9, SOX5, and SOX6 transcription factor genes from fibrocytes and MSC exposed to chondrogenic media with or without TGF-β3 (10 ng/ml) for 3 days. (B) Time course of the expression of SOX9 protein from fibrocytes and MSC exposed to chondrogenic media in the presence or absence of TGF-β3 (10 ng/ml). Results are representative of 3 experiments. (C) Expression of β-catenin, Cbfa1, and SOX9 genes from fibrocytes exposed to chondrogenic media for 3 days in the presence or absence of TGF-β3 (10 ng/ml). Results are representative of 3 experiments.
Beta-catenin has been shown to be an important molecular switch that appears to be involved in modulating differentiation of MSC to chondrocytes (Zou et al., 2006). During chondrogenesis low levels of β-catenin have been found to suppress cbfa1 expression and at the same time induce the gene expression of SOX9, which leads to increased chondrogenic gene expression, such as aggrecan and Co12A1 (Zou et al., 2006). To determine whether these mechanisms may be operative in fibrocytes undergoing differentiation to chondrocytes, we assessed β-catenin, Cbfa1, and SOX9 gene expression at day 3. As shown in Fig. 6C, β-catenin and Cbfa1 mRNA was found to be elevated in fibrocytes in the presence of chondrogenic media alone, but was found to be markedly reduced in fibrocytes that were exposed to chondrogenic media and TGF-β3. In conjunction with the reduction in the expression of β-catenin and Cbfa1 mRNA, SOX9 gene expression was found to be induced when fibrocytes were undergoing chondrogenesis (Fig. 6C). These findings suggest that β-catenin-Cbfa1 molecular axis may inversely regulate SOX9 signaling, and may contribute to the initiation of differentiation of circulating fibrocytes into chondrocytes. Future studies will access this link.
4. Discussion
Fibrocytes have previously been shown to differentiate to other mesenchymal lineage cells, such as adipocyte- and myofibroblast-like cells (Hong et al., 2005; Hong et al., 2007). Differentiation of fibrocytes to myofibroblasts is induced by TGF-β1. TGF-β1 induces the activation of Smad2/3 and SAPK/JNK signaling pathways that leads to fibrocyte differentiation to myofibroblast-like cells (Hong et al., 2007). The differentiation of fibrocytes to adipocytes has been found to be induced by PPAR-γ that leads to lipid accumulation and induction of aP2 gene expression (Hong et al., 2007). Troglitazone, a PPAR-γ agonist, was found to inhibit fibrocyte differentiation to myofibroblast-like cells via decreased Smad 2/3 transactivation, which was a result of disruption of the SAPK/JNK signaling pathway (Hong et al., 2007). In contrast, TGF-β1 was found to inhibit PPAR-γ-dependent transactivation and induction of aP2 through SAPK/JNK pathway activation (Hong et al., 2007). These findings suggest that circulating fibrocytes have the ability to behave as a circulating multipotent mesenchymal progenitor cell.
In this study we showed that fibrocytes from human peripheral blood are circulating progenitor cells that possess the ability to differentiate into osteoblast- and chondrocyte-like cells. We showed that fibrocytes exposed to osteogenic media can differentiate into osteoblast-like cells. Differentiation of fibrocytes to osteoblast-like cells was confirmed by histochemical staining of calcium deposition by Alizarin Red S and von Kossa staining, and by upregulated gene expression of osteonectin, osteopontin and osteocalcin, which are markers of osteogenesis. In addition, we showed that the cAMP/PKA signaling pathway was involved in regulation of osteogenesis of circulating fibrocytes by finding that dephosphorylation of CREB protein and enhanced ratio of RANKL/OPG gene expression occurred in both fibrocytes and OBPC undergoing osteogenesis. The osteogenic transcription factor gene expression, osterix, was also shown to be upregulated during fibrocyte and OBPC osteogenesis. In contrast, we found that fibrocytes exposed to chondrogenic media in the presence of TGF-β3 resulted in fibrocyte differentiation to chondrocyte-like cells in a similar manner as MSC. The presence of TGF-β3 in chondrogenic media led to enhanced production of proteoglycans and glycosaminoglycans. TGF-β3 also induced upregulation of col2A1 and aggrecan gene expression in fibrocytes undergoing differentiation to chondrocyte-like cells. These findings are compatible with the development of a chondrocyte-like cell.
Previous studies have found that TGF-β3 can induce the expression of chondrocyte specific genes in MSC, and that these cells can form cartilaginous matrix in vitro in a number of species (Hao et al., 2008; Moioli and Mao, 2006; Park et al., 2008; Mrugala et al., 2008; Jin et al., 2006). The differentiation of both fibrocytes and MSC were found to be dependent on culture as micromass pellets in the presence of TGF-β3. These findings are compatible with previous studies that determined that MSC cultured in monolayers in the presence of TGF-β3 expressed undetectable or very low levels of chondrocyte specific genes, as compared to these cells expressing high levels of these genes when cultured as micromass pellets with TGF-β3 (Wang et al., 2005). These findings suggest that a 3-dimensional environment is more conducive for fibrocytes and MSC to undergo differentiation to chondrocyte-like cells in vitro that may simulate their microenvironment in vivo.
The finding of co-expression of SOX5, SOX6, and SOX9 in both fibrocytes and MSC undergoing chondrogenesis that correlated with their ability to differentiate into chondrocyte-like cells is consistent with previous studies that have determined that these specific transcriptional factors are required for specific chrondrocyte gene expression (Lefebvre and de Crombrugghe, 1998; Lefebvre et al., 2001). The SOX-series gene family consists of transcription factors, which are characterized by a high-mobility-group-box DNA binding domain, whose sequence is at least 50% identical with that of the sex determining factor SRY (Ikeda et al., 2005; Smits et al., 2001). The SOX family is composed of several subgroups based on sequence homologies both inside and outside of HMG-box domain (Ikeda et al., 2005). SOX9 is expressed in all chondrocyte progenitor cells undergoing chondrogenesis and in differentiated chondrocytes (Ng et al., 1997). Studies have shown that chondrocyte-specific promoters for col2a1, col11a2, and CDRAP genes contain SOX9 binding sites (Ikeda et al., 2005).
Our findings that fibrocytes have the capacity to undergo differentiation to osteoblast- and chondrocyte-like cells raises the possibility that isolated fibrocytes could be used for regenerative therapy related to bone or articular cartilage repair. Previous studies have demonstrated that MSC can induce bone formation in a transgenic mouse model of Osteogenesis Imperfecta (OI) (Picinich et al., 2007). Infusion of wild-type MSCs into OI mice resulted in a significant increase in collagen and mineral content in their bone (Pereira et al., 1998). Based on findings of this preclinical study, a clinical trial was performed to test the efficacy of allogeneic bone marrow transplantation for the treatment of children with severe OI (Horwitz et al., 2001). Patients that received this intervention demonstrated improvement in growth, bone structure and function (Horwitz et al., 2001). However, the concern from this study was that they used allogeneic bone marrow transplantation, and not treatment with isolated allogeneic MSC that may have had less immunological impact on this patient population. Thus, one could speculate that isolated fibrocytes could also serve as a source of alternative progenitor cells for regeneration of bone in this type of patient.
The repair of articular cartilage remains an unresolved and challenging problem (Mierisch et al., 2003). Limited potential exists for the repair of critical-sized defects in mature articular cartilage, related to the relative avascular nature of the cartilage and absence of local stem cells (Mierisch et al., 2003). Partial-thickness defects show only minimal healing response (Mierisch et al., 2003). Both partial- and full-thickness defects of articular cartilage in the knee may progress to osteoarthritis (Brittberg et al., 1994). Studies have had only limited success when they have transplanted autologous fully differentiated chondrocytes into the knee to repair articular defects (Brittberg et al., 1994; Horas et al., 2003). However, a recent preclinical study has demonstrated in full-thickness articular defects, treatment with MSC can induce reconstitution of the articular cartilage surface (Mierisch et al., 2003). Based on our experimental results in vitro, fibrocytes could be considered as another attractive candidate for a source of autologous progenitor cells, which could be enriched in vitro, followed by use as therapy for articular cartilage defects.
In summary, our experimental results support the notion that human circulating fibrocytes can differentiate into osteoblast- or chondrocyte-like cells when exposed to specific defined media and cytokines in a similar manner as OBPC and MSC, respectively. We and others have shown that fibrocytes have the capacity to differentiate into other mesenchymal lineage cells, such as adipocyte- and myofibroblast-like cells (Hong et al., 2005; Hong et al., 2007). Taken together, human circulating fibrocytes have the capability to differentiate into diverse mesenchymal lineage cells. These findings also suggest that human circulating fibrocytes could potentially be used in cell-based tissue-regenerative therapy. This could open new possibilities for the novel repair of bone and articular cartilage damage. Circulating fibrocytes could be isolated from the patient's own blood, processed for differentiation into osteoblasts or chondrocytes, followed by transplantation into the damaged tissue. This notion can be assessed with future studies that will directly focus on the role of fibrocytes in regenerative therapy in pre-clinical models.
Supplementary Material
CD45-positive fibrocytes undergoing osteogenesis and chondrogenesis. (A) Removal of CD45-negative progenitor cells from purified CD45-postive fibrocytes. Fibrocytes were first isolated from PBMC and cultured as described in Materials and Methods section. After culture, fibrocytes were harvested, then incubated with CD45 magnetic microbeads (Miltenyi Biotec) followed by selection. Total cell proteins from harvested fibrocytes (S), CD45-positive cells (+) or CD45-negative cells (-) were subject to SDS-PAGE followed by immunodetection using anti-human CD45 antibody (BD Pharmingen). (B) Time course of the gene expression of osteonectin and collagen I (fibrocytic marker) from CD45-postive fibrocytes treated with osteogenic media (+) or with (-) control media. Results are representative of 2 experiments. (C) Time course of the gene expression of aggrecan and collagen I from CD45-postive fibrocytes treated with chondrogenic media supplemented with (+) or without (-)TGF-β3 (10 ng/ml). Results are representative of 2 experiments. Nucleotide sequences of collagen I RT-PCR primers are 5′-GAT CTG CGT CTG CGA CAA CG-3′ (forward) and 5′-AAA TTC CTC CGG TTG ATT TC-3′ (reverse).
Inhibition of PKA (i.e., by H89) attenuates the ability of fibrocytes to undergo differentiation to osteoblast-like cells and is associated with a change in the RANKL/OPG ratio to levels similar to control media at day 21. Intensity of the bands from RT-PCR was quantified by densitometer (Quantity-1 software, Bio-Rad). N=6 for each data for quantification of band intensity using densitometer software (*p<0.01).
Acknowledgments
This work was supported by National Institutes of Health Grants HL66027 and CA87879 (RMS).
Abbreviations
- aP2
adipocyte lipid-binding protein
- Cbfa1
core binding factor alpha1 subunit protein
- CREB
cAMP-responsive element binding
- ECM
extracellular matrix
- JNK
c-Jun N-terminal kinase
- MCGS
mesenchymal cell growth supplement
- MSC
mesenchymal stem cells
- OBPC
osteoblast precursor cell
- OI
Osteogenesis imperfecta
- OPG
osteoprotegerin
- PBMC
peripheral blood mononuclear cell
- PBS
phosphate-buffered saline
- PKA
cAMP-dependent protein kinase A
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- RANKL
Receptor activator of the NF-κB Ligand
- RT-PCR
Reverse transcriptase-polymerase chain reaction
- SAPK
stress-activated protein kinase
- SOX
SRY (sex determining region Y)–box
- TGF
transforming growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004;13:436–48. doi: 10.1089/scd.2004.13.436. [DOI] [PubMed] [Google Scholar]
- Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–40. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–70. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, Boyan B, Boskey A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–47. doi: 10.1007/s00223-002-1057-y. [DOI] [PubMed] [Google Scholar]
- Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–95. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- Bruder SP, Jaiswal N, Haynesworth SE. Growth kinetics, self-renewal, and the osteogenic potential of purified human mesenchymal stem cells during extensive subcultivation and following cryopreservation. J Cell Biochem. 1997;64:278–94. doi: 10.1002/(sici)1097-4644(199702)64:2<278::aid-jcb11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–12. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213–8. doi: 10.1073/pnas.070034097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JE, Merriam A, Awadallah A, Yoo JU, Johnstone B, Caplan AI. A quadripotential mesenchymal progenitor cell isolated from the marrow of an adult mouse. J Bone Miner Res. 1999;14:700–9. doi: 10.1359/jbmr.1999.14.5.700. [DOI] [PubMed] [Google Scholar]
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, Cossu G, Mavilio F. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–30. doi: 10.1126/science.279.5356.1528. [DOI] [PubMed] [Google Scholar]
- Frank O, Heim M, Jakob M, Barbero A, Schafer D, Bendik I, Dick W, Heberer M, Martin I. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85:737–46. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- Galmiche MC, Koteliansky VE, Briere J, Herve P, Charbord P. Stromal cells from human long-term marrow cultures are mesenchymal cells that differentiate following a vascular smooth muscle differentiation pathway. Blood. 1993;82:66–76. [PubMed] [Google Scholar]
- Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–56. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- Grab DJ, Lanners H, Martin LN, Chesney J, Cai C, Adkisson HD, Bucala R. Interaction of Borrelia burgdorferi with peripheral blood fibrocytes, antigen-presenting cells with the potential for connective tissue targeting. Mol Med. 1999;5:46–54. [PMC free article] [PubMed] [Google Scholar]
- Hao J, Varshney RR, Wang DA. TGF-beta3: A promising growth factor in engineered organogenesis. Expert Opin Biol Ther. 2008;8:1485–93. doi: 10.1517/14712598.8.10.1485. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y. Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem. 2006;281:18015–24. doi: 10.1074/jbc.M600603200. [DOI] [PubMed] [Google Scholar]
- Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–20. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- Hong KM, Burdick MD, Phillips RJ, Heber D, Strieter RM. Characterization of human fibrocytes as circulating adipocyte progenitors and the formation of human adipose tissue in SCID mice. FASEB J. 2005;19:2029–31. doi: 10.1096/fj.05-4295fje. [DOI] [PubMed] [Google Scholar]
- Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185–92. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Igarashi M, Kamiya N, Hasegawa M, Kasuya T, Takahashi T, Takagi M. Inductive effects of dexamethasone on the gene expression of Cbfa1, Osterix and bone matrix proteins during differentiation of cultured primary rat osteoblasts. J Mol Histol. 2004;35:3–10. doi: 10.1023/b:hijo.0000020883.33256.fe. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Kawaguchi H, Kamekura S, Ogata N, Mori Y, Nakamura K, Ikegawa S, Chung UI. Distinct roles of Sox5, Sox6, and Sox9 in different stages of chondrogenic differentiation. J Bone Miner Metab. 2005;23:337–40. doi: 10.1007/s00774-005-0610-y. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Lee SY, Jung JC, Bang OS, Kang SS. TGF-beta3 inhibits chondrogenesis of cultured chick leg bud mesenchymal cells via downregulation of connexin 43 and integrin beta4. J Cell Physiol. 2008;214:345–53. doi: 10.1002/jcp.21202. [DOI] [PubMed] [Google Scholar]
- Jin EJ, Park JH, Lee SY, Chun JS, Bang OS, Kang SS. Wnt-5a is involved in TGF-beta3-stimulated chondrogenic differentiation of chick wing bud mesenchymal cells. Int J Biochem Cell Biol. 2006;38:183–95. doi: 10.1016/j.biocel.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Kato N, Nakayama Y, Nakajima Y, Samoto H, Saito R, Yamanouchi F, Masunaga H, Shimizu E, Ogata Y. Regulation of bone sialoprotein (BSP) gene transcription by lipopolysaccharide. J Cell Biochem. 2006;97:368–79. doi: 10.1002/jcb.20628. [DOI] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of fibrotic disorders. Thromb Haemost. 2009a;101:613–8. [PMC free article] [PubMed] [Google Scholar]
- Keeley EC, Mehrad B, Strieter RM. Fibrocytes: Bringing new insights into mechanisms of inflammation and fibrosis. Int J Biochem Cell Biol. 2009b doi: 10.1016/j.biocel.2009.10.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthritis Cartilage. 2001;9 A:S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–40. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Beck SC, Murphy JM, Barry FP, Chichester CO, Pittenger MF. Chondrogenic differentiation of cultured human mesenchymal stem cells from marrow. Tissue Eng. 1998;4:415–28. doi: 10.1089/ten.1998.4.415. [DOI] [PubMed] [Google Scholar]
- Magne D, Vinatier C, Julien M, Weiss P, Guicheux J. Mesenchymal stem cell therapy to rebuild cartilage. Trends Mol Med. 2005;11:519–26. doi: 10.1016/j.molmed.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Marion NW, Mao JJ. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006;420:339–61. doi: 10.1016/S0076-6879(06)20016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad B, Burdick MD, Strieter RM. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int J Biochem Cell Biol. 2009;41:1708–18. doi: 10.1016/j.biocel.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–8. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- Mierisch CM, Wilson HA, Turner MA, Milbrandt TA, Berthoux L, Hammarskjold ML, Rekosh D, Balian G, Diduch DR. Chondrocyte transplantation into articular cartilage defects with use of calcium alginate: the fate of the cells. J Bone Joint Surg Am. 2003;85-A:1757–67. doi: 10.2106/00004623-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, O'Byrne PM, Strieter RM, Kolb M. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–94. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- Moioli EK, Mao JJ. Chondrogenesis of mesenchymal stem cells by controlled delivery of transforming growth factor-beta3. Conf Proc IEEE Eng Med Biol Soc. 2006;1:2647–50. doi: 10.1109/IEMBS.2006.260861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrugala D, Bony C, Neves N, Caillot L, Fabre S, Moukoko D, Jorgensen C, Noel D. Phenotypic and functional characterisation of ovine mesenchymal stem cells: application to a cartilage defect model. Ann Rheum Dis. 2008;67:288–95. doi: 10.1136/ard.2007.076620. [DOI] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol. 1997;183:108–21. doi: 10.1006/dbio.1996.8487. [DOI] [PubMed] [Google Scholar]
- Park JS, Woo DG, Yang HN, Lim HJ, Chung HM, Park KH. Heparin-bound transforming growth factor-beta3 enhances neocartilage formation by rabbit mesenchymal stem cells. Transplantation. 2008;85:589–96. doi: 10.1097/TP.0b013e3181639b3a. [DOI] [PubMed] [Google Scholar]
- Pereira RF, O'Hara MD, Laptev AV, Halford KW, Pollard MD, Class R, Simon D, Livezey K, Prockop DJ. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–7. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picinich SC, Mishra PJ, Glod J, Banerjee D. The therapeutic potential of mesenchymal stem cells. Cell- & tissue-based therapy. Expert Opin Biol Ther. 2007;7:965–73. doi: 10.1517/14712598.7.7.965. [DOI] [PubMed] [Google Scholar]
- Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Tsuchiya T. Enhancement of chondrogenic differentiation of human articular chondrocytes by biodegradable polymers. Tissue Eng. 2001;7:781–90. doi: 10.1089/107632701753337726. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971;53:69–82. [PubMed] [Google Scholar]
- Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–9. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V. The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell. 2001;1:277–90. doi: 10.1016/s1534-5807(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Strieter RM, Keeley EC, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells, fibrocytes, in promoting pulmonary fibrosis. Trans Am Clin Climatol Assoc. 2009a;120:49–59. [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Keeley EC, Hughes MA, Burdick MD, Mehrad B. The role of circulating mesenchymal progenitor cells (fibrocytes) in the pathogenesis of pulmonary fibrosis. J Leukoc Biol. 2009b;86:1111–8. doi: 10.1189/jlb.0309132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai G, Polak JM, Bishop AE, Christodoulou I, Buttery LD. Differentiation of osteoblasts from murine embryonic stem cells by overexpression of the transcriptional factor osterix. Tissue Eng. 2004;10:1456–66. doi: 10.1089/ten.2004.10.1456. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kim UJ, Blasioli DJ, Kim HJ, Kaplan DL. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials. 2005;26:7082–94. doi: 10.1016/j.biomaterials.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Yang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ, Chang TJ, Hung SC. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS One. 2008;3:e1540. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JU, Barthel TS, Nishimura K, Solchaga L, Caplan AI, Goldberg VM, Johnstone B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J Bone Joint Surg Am. 1998;80:1745–57. doi: 10.2106/00004623-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Zou L, Zou X, Li H, Mygind T, Zeng Y, Lu N, Bunger C. Molecular mechanism of osteochondroprogenitor fate determination during bone formation. Adv Exp Med Biol. 2006;585:431–41. doi: 10.1007/978-0-387-34133-0_28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD45-positive fibrocytes undergoing osteogenesis and chondrogenesis. (A) Removal of CD45-negative progenitor cells from purified CD45-postive fibrocytes. Fibrocytes were first isolated from PBMC and cultured as described in Materials and Methods section. After culture, fibrocytes were harvested, then incubated with CD45 magnetic microbeads (Miltenyi Biotec) followed by selection. Total cell proteins from harvested fibrocytes (S), CD45-positive cells (+) or CD45-negative cells (-) were subject to SDS-PAGE followed by immunodetection using anti-human CD45 antibody (BD Pharmingen). (B) Time course of the gene expression of osteonectin and collagen I (fibrocytic marker) from CD45-postive fibrocytes treated with osteogenic media (+) or with (-) control media. Results are representative of 2 experiments. (C) Time course of the gene expression of aggrecan and collagen I from CD45-postive fibrocytes treated with chondrogenic media supplemented with (+) or without (-)TGF-β3 (10 ng/ml). Results are representative of 2 experiments. Nucleotide sequences of collagen I RT-PCR primers are 5′-GAT CTG CGT CTG CGA CAA CG-3′ (forward) and 5′-AAA TTC CTC CGG TTG ATT TC-3′ (reverse).
Inhibition of PKA (i.e., by H89) attenuates the ability of fibrocytes to undergo differentiation to osteoblast-like cells and is associated with a change in the RANKL/OPG ratio to levels similar to control media at day 21. Intensity of the bands from RT-PCR was quantified by densitometer (Quantity-1 software, Bio-Rad). N=6 for each data for quantification of band intensity using densitometer software (*p<0.01).


