Abstract
Secondary HIV prevention, or “positive prevention,” is concerned with reducing HIV transmission risk behavior and optimizing the health and quality of life of people living with HIV/AIDS (PLWHA). The association between mental health and HIV transmission risk (i.e., sexual risk and poor medication adherence) is well established, although most of this evidence is observational. Further, a number of efficacious mental health treatments are available for PLWHA yet few positive prevention interventions integrate mental health treatment. We propose that mental health treatment, including behavioral and pharmacologic interventions, can lead to reductions in HIV transmission risk behavior and should be a core component of secondary HIV prevention. We present a conceptual model and recommendations to guide future research on the effect of mental health treatment on HIV transmission risk behavior among PLWHA.
Keywords: HIV prevention, Mental health, Secondary HIV prevention, Positive prevention, Methodology
Introduction
In the United States, there are over 1 million people living with HIV/AIDS (PLWHA) [1], and approximately 56,000 new HIV infections annually [2]. While the overall incidence rate has stabilized in the past few years, new infections remain concentrated among African–Americans and men who have sex with men [2]. Globally, approximately 33 million people are living with HIV and 2.7 million are infected each year, with the majority of new infections in Sub-Saharan Africa [1]. Improved HIV treatments and access to care have dramatically increased survival and improved quality of life, and many PLWHA can now lead productive lives [3, 4]. This shift in prognosis makes it imperative to address psychosocial issues and long term risk reduction throughout the lifespan, which underscores the importance of secondary HIV prevention.
Secondary HIV prevention, or “positive prevention,” is concerned with reducing transmission risk behavior among PLWHA and optimizing their health and quality of life. While the majority of PLWHA respond to their HIV diagnosis by adopting lower risk sexual behaviors [5, 6], a substantial proportion continue to engage in high risk sexual behaviors [5, 7–9] that put others in the community at risk for HIV infection [10, 11]. In addition to sexual risk behaviors, it is increasingly recognized that poor adherence to antiretroviral therapy (ART), and to HIV clinical care and treatment more generally, is associated with higher risk of HIV transmission due to failure to suppress HIV viral levels to undetectable levels [12–15]. Poor adherence is also associated with poorer clinical outcomes for PLWHA [16–18] and has high societal costs (e.g., tertiary treatment, lost productivity) [19, 20]. Adherence to care and treatment remains a challenge for many HIV infected patients [9, 21,22] and deserves attention in secondary HIV prevention efforts.
As the HIV/AIDS epidemic continues to evolve, identifying effective secondary HIV prevention interventions is a critical priority for stemming the spread of the virus and improving the long-term quality of life for PLWHA. Meta-analytic reviews have found that secondary HIV prevention interventions are successful in reducing sexual transmission risk behavior, with the most effective interventions including both motivational and prevention skill-building components [23, 24]. However, the field of research on secondary HIV prevention interventions is relatively new, and more sophisticated models and rigorous evaluations are needed to help identify the most effective means to reduce transmission risk behaviors. Fisher and Smith identified future directions for research in this area, including the need for integrated intervention models that include moderating variables such as mental health and substance abuse [25].
The importance of mental health considerations in secondary HIV prevention was advanced by Grossman and Gordon, who acknowledged the absence of empirical data on intervention models that integrate HIV risk reduction and mental health treatment [26]. In this paper, we build on that argument and propose a conceptual model to guide research on the effect of mental health treatment on transmission risk behavior among PLWHA, including both sexual risk behavior and adherence to HIV care and treatment. While not a systematic review, we present accumulating evidence that suggests mental health treatment, primarily cognitive behavioral and pharmacological interventions, improves mental health in PLWHA, and that better mental health is associated with less transmission risk behavior. We conclude by offering scientific questions and methodological recommendations for future mental health research aimed at reducing HIV transmission risk behavior. We hope that this will provide direction for research aimed at understanding how mental health treatment provides added, and potentially essential, value to secondary HIV prevention.
Conceptual Model
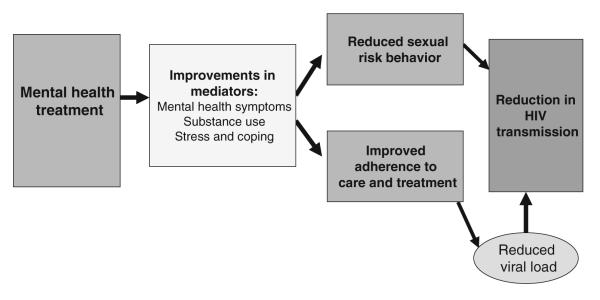
We propose that mental health treatment can lead to substantial reductions in HIV transmission risk behavior among PLWHA and should be a core component of secondary HIV prevention. The conceptual model linking mental health treatment and HIV transmission is shown in Fig. 1. For the purpose of this paper, we broadly define mental health as psychiatric disorders, psychiatric symptoms, psychological distress, and maladaptive coping, and mental health treatment as any intervention (including but not limited to behavioral, psychosocial or pharmacological) that intends to improve mental health. As we will illustrate, interventions to improve mental health may reduce HIV transmission risk behavior, with improvements in mental health symptoms, reductions in substance use, reductions in stress, and increased use of effective coping skills serving as potential mediators. However, specific mental health variables may be differentially associated with outcomes related to sexual risk behavior and adherence to HIV care and treatment.
Fig. 1.
Positive prevention model showing hypothesized effects of mental health treatment on HIV transmission risk behavior
Prevalence of Psychiatric Disorders is High in PLWHA
Persons living with HIV/AIDS have disproportionately high rates of psychiatric disorders, with mood and anxiety disorders being the most common. In a nationally representative US sample of individuals receiving care for HIV infection, 48% screened positive for one or more psychiatric disorders in the past year (36% major depression, 27% dysthymia, 16% generalized anxiety, and 11% panic)—rates far higher than those in the general US population [7]. In a meta-analysis, HIV-infected adults were twice as likely to have major depressive disorder compared to HIV-negative adults [27]. Other clinic based studies have found similarly high rates of both lifetime and current mood and anxiety disorders and symptoms among PLWHA [28–32]. Although the rates of posttraumatic stress and bipolar disorders among PLWHA have not been systematically assessed in large cohort studies, evidence suggests that rates of these disorders are also elevated relative to the general population [32–38].
Mental Health is Associated with Transmission Risk Behavior
The HIV research field has traditionally considered sexual risk behaviors and adherence to antiretroviral medication and HIV care separately. However, because both behaviors contribute to the spread of HIV infection and share common correlates, integration of the two has recently been advocated [39]. In particular, people with psychiatric disorders, including depression and substance use, are more likely to engage in unprotected sexual intercourse, despite knowing their HIV status [40–43], and are more likely to have poor adherence to antiretroviral therapy [44, 45] and difficulties attending regular clinic appointments [18, 46,47], which can result in an inability to suppress viral load and therefore a greater likelihood of transmitting the virus to others [12–15]. A failure to address these behaviors together may result in a missed opportunity for effective secondary HIV prevention.
Many studies have found that depression symptoms are associated with greater sexual risk behaviors among PLWHA [40–43] and those at risk for HIV infection [48–50]. Anxiety disorders, including social anxiety and posttraumatic stress, are also associated with increased sexual risk behaviors [51–54]. While two meta-analytic reviews of the literature found no relationship between negative affect (measured as depression or anxiety symptoms) and sexual risk behavior [55, 56], the range of effect sizes across studies was −.41 to .55. A relatively even distribution was displayed with a third of studies showing little effect, a third showing significant negative effects, and a third showing significant positive effects, suggesting important mediators and moderators may have been overlooked. Further, many of these studies used cross-sectional designs and global measures of affect, which may underestimate the influence of mental health because affect itself fluctuates. Additionally, global measures do not accurately identify clinically significant mental health problems and thus may not capture the relationship between psychiatric disorders and sexual risk [57]. Evidence exists to support a potential U-shaped relationship between sexual risk behavior and depression, with increased sexual risk behavior associated with moderate but not severe depression [58–60].
Poor adherence to ART has also been consistently associated with depression [44, 45], as well as posttraumatic stress disorder (PTSD) and other anxiety disorders [33, 61, 62]. This is consistent with findings that mental health influences medication adherence in other chronic medical conditions [63, 64]. Recent systematic reviews of both quantitative and qualitative research among PLWHA highlight the importance of depression, psychological distress, and other mental health concerns in influencing adherence [65, 66]. Mental health disorders also appear to reduce the likelihood of receiving antiretroviral therapy (ART) [67–70], and are associated with more intermittent care and missed appointments [18, 46, 47].
Efficacy of Mental Health Treatment Among PLWHA
A substantial literature exists to demonstrate that mental health symptoms and disorders in PLWHA respond to both cognitive behavioral [71–73] and pharmacological intervention [74]. Based on the available meta-analytic reviews of randomized controlled trial data, the effects of behavioral health interventions and antidepressant medications on a variety of psychological symptoms among PLWHA are well supported.
Behavioral Health Interventions
Behavioral health interventions, often referred to as mental health interventions, are defined here as the mental health interventions most commonly conducted with PLWHA, including cognitive behavioral therapy, behavioral therapy, supportive psychotherapy, and coping and stress management interventions. Three meta-analytic reviews provide evidence that behavioral health interventions improve mental health outcomes among PLWHA. The first examined randomized controlled trials that assessed the efficacy of group psychotherapy for treating depression among PLWHA [72]. The intervention approaches consisted of cognitive behavioral therapy (five trials), coping effectiveness training (one trial), and supportive psychotherapy (two trials). Utilizing data from a total of 665 participants from eight studies, a significant decrease in depressive symptoms (d = 0.38, CI: 0.23–0.53) was found for group psychotherapy overall and for cognitive behaviorally based interventions specifically (d = 0.37, CI: 0.18–0.56).
The second review focused on the impact of cognitive behavioral interventions on symptoms of depression, anxiety, anger, and stress [73]. All of the trials included in the analysis assessed the efficacy of interventions that highlighted cognitive appraisal or restructuring techniques, and most included coping and stress management skills training, using a small group format for intervention delivery. Drawing on data from a combined 1,246 participants in 15 trials, aggregate effect sizes provided evidence of significant improvements on all four mental health outcomes: depression (d = 0.33), anxiety (d = 0.30), anger (d = 1.00) and stress (d = 0.43).
Lastly, a meta-analysis of the effects of stress management interventions on psychosocial outcomes among PLWHA utilized a broader approach and examined intervention trials that included one or more of the following components: coping and interpersonal skills training, relaxation exercises, HIV/AIDS education, social support, and physical exercise planning and practice [71]. Aggregating across data for 3,077 participants from 35 studies, the broad array of stress-management interventions included in the analysis were found to significantly improve anxiety (d = 0.31, CI: 0.14–0.48) and depressive symptoms (d = 0.29, CI: 0.18–0.41), distress (d = 0.19, CI: 0.06–0.33), and quality of life (d = 0.16, CI: 0.05–0.27).
The weight of the evidence from these three reviews suggests that a variety of behavioral health interventions, delivered in both small group and individual formats for varying numbers of sessions (range of 3–15), significantly improve mental health symptoms—especially those associated with depression and anxiety—among PLWHA. The reviews highlight several common factors that are likely to affect psychiatric distress, most notably cognitive restructuring and reappraisal, and the development of coping and stress management skills. However, the reviews also identify several limitations of the collective literature on mental health treatment for PLWHA including a focus on mainly white male participants; insufficient follow-up time; an absence of secondary outcomes (e.g., sexual behavior, substance use and medication adherence); and failure to include an attention-matched treatment control condition.
Pharmacological Interventions
Standard pharmacological interventions are also efficacious in treating depression in PLWHA. A meta-analysis of double-blinded randomized placebo-controlled trials of antidepressant-based depression treatment in PLWHA estimated a pooled effect size of 0.57 (95% CI: 0.28-0.85) [74]. The seven contributing studies, with a combined 494 participants, tested both tricyclic and selective serotonin reuptake inhibitors and followed participants for 6–16 weeks. As with the behavioral health intervention trials, nearly all focused on white men; further research with women and minorities and longer follow-up times were noted in the review as significant needs.
In sum, behavioral health and pharmacologic interventions are effective in treating mental health symptoms among PLWHA, with small to moderate effect sizes [75]. Locating them in the context of a broader research base, the effect sizes are somewhat lower than those found for behavioral health (d ≥ 0.60) [76, 77], and higher than those found for standard pharmacological treatments (d = 0.20–0.39) for depression in general clinical samples [78, 79]. Moreover, the empirical support for the efficacy of a range of different cognitive, behavioral, and interpersonal supportive interventions in PLWHA echoes that for the treatment of depression and anxiety in general clinical samples [80, 81]. However, it is important to note that comparisons of effect sizes for studies among PLWHA and general clinical samples are imperfect at best, because trials with PLWHA generally have targeted reductions in mental health symptoms, often experienced in the context of multiple other stressors or diagnoses, whereas general clinical population trials have typically targeted successful remission of mental health disorders in individuals with more normative life experiences and without comorbid psychiatric diagnoses.
Support for Mental Health Treatment as Positive Prevention
Given the association between poor mental health and higher HIV transmission risk behavior, and the efficacy of standard mental health treatments for PLWHA, it is plausible that effective mental health interventions, even if not intended as positive prevention, may have the secondary effect of reducing HIV transmission risk behaviors. Indeed, a body of evidence is beginning to emerge in support of our hypothesis for both sexual risk behaviors [82–87] and adherence to antiretroviral therapy [88, 89]. These interventions have many similar characteristics in that they are multi-session psychotherapeutic interventions built on cognitive behavioral therapy (CBT) techniques focused on the development of skills such as cognitive restructuring and reappraisal, coping and stress management. Although the purported causal mechanisms of change have not been explored, we hypothesize that such behavioral health interventions are effective at reducing HIV transmission risk behavior because of improvements in mental health. Specifically, mental health treatments that decrease mental health symptoms and substance use and/or improve stress management and coping skills may in turn lead to a reduction in sexual risk behavior and improvements in HIV treatment adherence (Fig. 1). In the following sections, we describe mental health intervention trials that examined HIV transmission risk behavior as an outcome to support our proposed model of positive prevention. To date, few pharmacological interventions have reported on risk behavior outcomes.
Mental Health Interventions that Impact Sexual Risk Behavior
The first mental health intervention studies that examined sexual HIV transmission risk behavior were actually conducted early in the HIV epidemic. Prior to the existence of ART, stress management interventions were proposed to improve immune functioning and increase longevity among PLWHA [90–92]. One such study examined the impact of eight weekly sessions of stress management training (consisting of relaxation training, stress management skill-building, and behavioral contracting for health habit change) on immune function and sexual risk behavior among 64 HIV-infected gay men randomly assigned to either a treatment condition or a waitlist control [83]. At the post intervention assessment, no difference in immune function was detected, but participants in the treatment condition reported significantly fewer sexual partners than control participants. Similarly, eight sessions of either a CBT group intervention (consisting of stressor appraisal, cognitive restructuring, behavioral practice and contracting) or a social support group intervention were compared to a waitlist control [85]. Study participants were 68 HIV-infected men experiencing significant depressive symptoms. No clear pattern of differential change was evident for sexual behavior, as all groups showed reductions in frequency of unprotected intercourse at 3-month follow-up. However, support group participants exhibited a trend toward reductions in unprotected receptive anal intercourse, and participants in the CBT group showed significantly greater reductions in substance use. Despite limitations, including small sample sizes and limited follow up periods, these studies provided early support that mental health interventions might reduce sexual HIV transmission risk behavior. Yet, despite the suggestion that “the ability of this kind of treatment to reduce multiple partner sex and unsafe sex should be explored further” (p. 886) [83], very little research was subsequently conducted in this area in the past 20 years.
Much later in the epidemic, and following the availability of antiretroviral therapy, mental health interventions focusing on PLWHA who had experienced childhood sexual abuse (CSA) were developed [87, 93]. CSA has emerged as a key risk factor for HIV infection [94, 95] and PLWHA with histories of CSA engage in more HIV risk behaviors than those without such histories [96, 97], leading to greater likelihood of HIV transmission to others [98]. Thus, intervention models targeting PLWHA with CSA histories have specifically focused on sexual risk reduction as a treatment goal.
To date, three such intervention trials have been described in the literature. First, the Enhanced Sexual Health Intervention (ESHI) combined CBT approaches (e.g., problem-solving strategies, communication skills, relaxation training, cognitive restructuring) with gender and culturally specific concepts (e.g., collectivism, interconnectedness) for reducing both HIV risk and traumatic stress [87]. There were 147 women randomly assigned to either the 11 session group intervention (plus weekly phone calls) or a waitlist control condition (one information session plus the weekly telephone calls). Results indicated that, after adjusting for covariates in a post-only comparison, women receiving the intervention were more likely to report sexual risk reduction than women in the control condition. Shortcomings of the trial included the lack of an attention matched control condition, use of a dichotomous outcome measure for sexual risk reduction, and outcome assessment at post-intervention only. This intervention was later adapted for African American and Latino men who have sex with men [84]. A total of 137 men were randomized into the six session Sexual Health Intervention for Men (S-HIM) or an attention-matched health promotion intervention condition that focused on health behaviors other than sexual health. All participants reported reductions in sexual risk behavior and number of partners, with S-HIM participants reporting significant reductions in sexual risk behavior immediately post intervention. However, both groups reported reductions in sexual risk behavior and depression at the six-month follow-up period, with no differences shown between conditions.
A third study strengthens the evidence that targeted mental health interventions reduce sexual risk behavior [82]. This intervention (Living in the Face of Trauma; LIFT) included HIV positive men and women with histories of CSA and consisted of 15 group sessions that were grounded in CBT related to traumatic stress, and specifically, stress and coping theory [99, 100]. The primary focus was to develop adaptive skills for coping with a history of sexual trauma and the stress of HIV infection, with risk and health protective behaviors addressed in the context of sexual abuse and related sequelae, rather than skills training specific to HIV risk reduction [82, 93, 101]. Participants were 117 men and 130 women randomly assigned to the LIFT intervention or to an attention matched treatment control (interpersonal support group therapy). Participants who received the LIFT intervention achieved significantly greater reductions in traumatic stress immediately following the intervention [101]. Over the one-year follow-up period, participants in the LIFT intervention condition had a significantly greater reduction in frequency of unprotected sexual intercourse with all partners, and specifically with HIV-negative and serostatus unknown partners [82], as well as significantly greater decreases in frequency of alcohol use, any hazardous alcohol use, and any cocaine use [102].
Finally, the Healthy Living Project was a positive prevention intervention trial targeting high risk PLWHA but not specifically targeting people with a mental health disorder or trauma history, thus providing potential generalizability to a broader high risk PLWHA community [86]. The individual-level 15-session intervention delivered over 15 months combined three separate modules, including a behavioral health module focused on coping skills, a module on sexual risk behavior prevention, and a module on adherence to ART. A total of 936 HIV-infected men (79%) and women recruited in four US cities were randomly assigned to the intervention condition or a waitlist no treatment control condition and followed for 25 months (10 month follow-up post intervention completion). All participants had engaged in unprotected anal or vaginal intercourse with an HIV negative or serostatus unknown partner or an HIV positive non-primary partner within 3 months prior to enrollment. Although the intervention had no observed effects on mental health outcomes [103], there was a significant difference in linear trajectories representing the mean number of transmission risk acts between the intervention and control groups across the 25 month follow up period [86]. However, there was not a significant difference at the 25 month assessment point (10 month follow-up). Thus, the effect of the multi-component intervention was evident 5 months post intervention completion, but not maintained at 10 months. The methodological design does not permit conclusions regarding the essential intervention components needed to effect sexual risk behavior change, including whether the coping skills module was necessary but not sufficient to reduce transmission risk behavior. Further, participants in the intervention demonstrated significant reductions in alcohol and other substances across the 25 month follow-up compared with participants in the control condition, and these findings were stronger for women than for men [104]. However, it is not yet known for Healthy Living or LIFT, whether a reduction in substance use was an important mechanism of action for reduction in sexual risk behavior.
Mental Health Interventions that Impact Adherence
Few mental health interventions for PLWHA have measured the impact on adherence to antiretroviral therapy, and none to our knowledge has reported adherence to care more generally (e.g., appointment adherence) as an outcome. We identified three cognitive behavioral intervention trials, two described above. Women who attended eight or more of the 11 Enhanced Sexual Health Intervention group sessions self-reported greater adherence to medication post intervention than those whose attendance was less frequent [87]. The Healthy Living Project showed improvements in self-reported adherence during the intervention period, but these impacts were not maintained across the follow up period [89]. More recently, Safren and colleagues evaluated an intervention that consisted of 10–12 individual sessions of cognitive behavioral therapy for depression specific to chronic illness with an additional focus on adherence to ART, compared to a one-session adherence intervention [88]. The adherence outcome was measured using electronic pill bottle caps. At post assessment, participants in the intervention group had significantly better adherence than those in the control group. Improvements in adherence were maintained at 6- and 12-month follow-ups, and control participants who crossed over to the intervention group also experienced improvements in adherence.
Finally, there is some observational evidence that being prescribed—and adhering to—antidepressant medication is accompanied by improvements in adherence to antiretroviral medications [105–109]. Whether this impact is a result of reductions in depression or represents a selection effect (e.g., more adherent individuals being prescribed antidepressants) is unclear.
Directions for Future Research
In sum, evidence supports the efficacy of mental health treatment, both behavioral and pharmacological, in reducing psychiatric symptoms and psychological distress among PLWHA. In addition, secondary HIV prevention or positive prevention interventions, focused primarily on skills training to change behavior, have demonstrated efficacy for reducing sexual risk behavior. Surprisingly, few positive prevention intervention trials have been conducted that either combine behavior change skills training and mental health interventions or examine the potential effect of mental health treatment on transmission risk behavior, including sexual risk behavior or adherence to HIV care and treatment. However, as reviewed above, accumulating evidence suggests that mental health treatment may also reduce transmission risk behavior. At a minimum, addressing mental health may provide added value to positive prevention approaches. As we suggest, mental health treatment likely provides another effective approach to reducing HIV transmission. More than 25 years into the HIV/AIDS epidemic, we recommend that mental health treatment that addresses HIV transmission risk behavior become a research priority for HIV prevention. As shown in Fig. 1, our model proposes that mental health treatment for PLWHA leads to reductions in HIV transmission risk behavior by addressing the underlying mental health issues and psychosocial context in which sexual risk behavior and poor treatment adherence occurs.
Scientific Research Questions
To best evaluate the effect of mental health treatment on HIV transmission risk behavior, we offer a number of recommendations related to research questions and study methodology. Given the limited number of intervention trials that have examined mental health and its impact on HIV transmission risk behavior, more studies need to be conducted to explore the potential effect of mental health treatment on transmission risk outcomes. This includes expanding beyond cognitive behavioral interventions tailored to HIV/AIDS to different types of mental health intervention, including psychiatric medication. An important conceptual issue is whether mental health interventions must be tailored specifically to HIV-related issues (e.g., HIV-related stigma and shame, long term effects of traumatic experiences) or whether more standard mental health treatment (e.g., antidepressant medication, interpersonal therapy for depression) can produce transmission risk behavior change. Related to this, research should consider whether a multi-component intervention that incorporates HIV prevention skills training with mental health treatment provides greater benefit than a mental health intervention that contextualizes HIV but does not directly incorporate HIV risk reduction skills. Further, research should evaluate if sequential components of varying content have more or less of an impact than a treatment with integrated components. These conceptual questions regarding intervention content would foster innovative experimental interventions to be tested in future trials that include multiple treatment designs.
Study Design Recommendations to Evaluate the Effects of Mental Health Interventions as Positive Prevention
Comparison Groups
An important issue to consider regarding mental health treatment research is an appropriate comparison or control condition for such intervention trials. First, given that the current state of the science in both mental health and positive prevention interventions for PLWHA provides us with a number of efficacious interventions, we recommend that experimental interventions be compared to available treatments. At minimum, comparison conditions should be attention matched, with no treatment or waitlist control conditions acceptable only as a third condition when comparing two other intervention conditions. Second, in order to ascertain the potential added value of mental health treatment in HIV prevention, the optimal comparison condition for a mental health intervention trial may likely be a proven positive prevention intervention. Thus, an optimal (albeit costly) study design might be a fourcondition study comparing: (1) an integrated treatment that addresses mental health, coping, adherence and risk reduction; (2) a mental health and/or coping intervention; (3) a behavioral skills risk reduction intervention; and (4) a waitlist control.
Inclusion Criteria and Primary Outcomes
Other methodological issues for consideration include the appropriate selection of inclusion criteria and primary outcomes for such trials. Inclusion criteria for mental health treatment trials addressing HIV transmission risk may require a specified level of psychological distress or psychiatric disorder, sexual risk behavior or nonadherence, or a combination of these criteria. It is reasonable to prioritize psychological distress as a primary inclusion criterion since mental health would be the primary focus of the intervention. However, if the study goal is to identify the mediating effect of mental health on transmission risk behavior, elevated risk behavior is also an important inclusion criterion. Future research should also examine whether mental health approaches to HIV prevention are appropriate only for those with psychiatric diagnoses, severe psychological distress, or a mental health history associated with risk behavior, or whether mental health interventions benefit the broader population of PLWHA. In contrast, exclusion criteria regarding substance abuse or dependence and personality disorders, often utilized in psychotherapy research, are likely inappropriate for mental health intervention trials among PLWHA given the high prevalence of multiple psychiatric diagnoses in this population [110] (i.e., such exclusion criteria would severely limit the generalizability of such research).
Maintenance of Effects and Generalizability
Based on recommendations from previous meta-analyses and guidance for “best evidence,” research designs must include a follow-up period post intervention completion of sufficient length (at minimum 6 months, preferably 12 months or more) so as to ascertain sustainability of intervention effects. If, in fact, the effects of mental health treatment are not maintained, future research can examine the relative merits of intervention length and the utility of booster sessions, and potentially generate innovative approaches to preventing relapse. Lastly, studies must be conducted that include participants representative of the current demography of PLWHA, including women, racial and ethnic minorities, MSM of color, heterosexual men, and those living in low resource settings.
Mediational and Component Analysis
To increase understanding of how mental health treatments “work,” there is also a need to design and implement research trials that will allow for an analysis of intervention components. Mechanisms of action, such as cognitive reappraisal or affect regulation, should be examined to understand how mental health interventions impact the pathways of behavior change with regard to HIV transmission risk behavior. Such causal mechanisms may differ for sexual risk reduction and adherence to care and treatment. For example, reductions in substance use and improvement in coping skills may lead to reductions in sexual risk behavior (e.g., unprotected intercourse, particularly with HIV-negative partners), while adherence to ART and HIV care and treatment may be primarily influenced by improvements in depressive symptomatology. Designs with multiple treatment conditions, like the fourcondition factorial design described above, are needed to compare and potentially dismantle intervention approaches and related study outcomes. For example, component analysis provides an opportunity to determine if mental health treatment is as effective as or possibly more effective than HIV prevention and/or adherence skills training for PLWHA, or if it is only efficacious when tailored to the context of HIV risk transmission.
Equally important are: (a) the use of sufficient sample size to adequately examine mechanisms of change, (b) the selection of theoretically based assessments to measure potential mediating and moderating variables, (c) application of statistical methods and multilevel modeling that accounts for nested effects of group level influence, and (d) sufficient follow up to examine change over time. Further, to assess the impact of mental health treatment on transmission risk behavior, study design and measure selection must also account for the impact of psychiatric medication on sexual functioning and the potential association between adherence to psychiatric medication and ART adherence.
Translation of Research to Practice
As with any prevention intervention with public health significance, mental health treatments to reduce transmission risk behavior should be evaluated in trial methodologies that enhance the translation of research into practice. While a number of design issues are to be considered, especially pressing areas that exist with regard to the dissemination and implementation of mental health treatment are the level of training needed for intervention delivery, the availability of mental health professionals, and the most appropriate setting for implementation. Thus, a key question is: Can HIV prevention specialists, when given appropriate training and supervision, deliver such mental health interventions, or are trained and licensed mental health professionals required? This is especially important in resource poor settings, where mental health providers are extremely limited or nonexistent [111]. Finally, related to these concerns, addressing social and cultural context is essential in all HIV prevention interventions, but may be especially complex when such interventions address mental health.
Conclusion
Mental health treatment, whether behavioral or pharmacological in nature, effectively improves the mental health of PLWHA. Poor mental health is associated with HIV transmission risk behaviors, and a developing literature suggests that mental health treatment may reduce sexual risk behavior and improve adherence to HIV care and treatment, leading to a reduction in HIV transmission. A priority for future research in positive prevention is to examine the impact of mental health treatment on HIV transmission risk behavior by conducting studies with methodologically rigorous designs that test whether improvements in mental health serve as a mediator of intervention effects and permit an understanding of the causal mechanisms within multi-component, integrated approaches to secondary HIV prevention.
Acknowledgments
This research was partially supported by the Duke University Center for AIDS Research (P30-AI064518) and grant R01-MH078731.
Contributor Information
Kathleen J. Sikkema, Department of Psychology and Neuroscience, Duke University, Box 90086, Durham, NC 27708-0086, USA
Melissa H. Watt, Global Health Institute, Duk University, Durham, NC, USA
Anya S. Drabkin, Department of Psychology and Neuroscience, Duke University, Box 90086, Durham, NC 27708-0086, USA
Christina S. Meade, Global Health Institute, Duk University, Durham, NC, USA; Department of Psychiatry and Behavioral Sciences, Duke University, Durham, NC, USA
Nathan B. Hansen, Yale University School of Medicine, New Haven, CT, USA
Brian W. Pence, Global Health Institute, Duk University, Durham, NC, USA; Department of Community and Family Medicine, Duke University, Durham, NC, USA
References
- 1.UNAIDS UNAIDS; Geneva: Report on the global AIDS epidemic. 2008
- 2.Hall HI, Song R, Rhodes P, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima VD, Harrigan R, Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50(5):529–36. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker JV, Peng G, Rapkin J, et al. CD4 + count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22(7):841–8. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crepaz N, Marks G, Liau A, et al. Prevalence of unprotected anal intercourse among HIV-diagnosed MSM in the United States: a meta-analysis. AIDS. 2009;23(13):1617–29. doi: 10.1097/QAD.0b013e32832effae. [DOI] [PubMed] [Google Scholar]
- 6.Weinhardt L, Kelly JA, Brondino M, et al. HIV transmission risk behavior among men and women living with HIV in 4 cities in the United States. J Acquir Immune Defic Syndr. 2004;36(5):1057–66. doi: 10.1097/00126334-200408150-00009. [DOI] [PubMed] [Google Scholar]
- 7.Bing EG, Burnam MA, Longshore D, et al. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the United States. Arch Gen Psychiatry. 2001;58(8):721–8. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- 8.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta-analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39(4):446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 9.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296(6):679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 10.Boily MC, Baggaley RF, Wang L, et al. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis. 2009;9(2):118–29. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiridou M, Geskus R, de Wit J, Coutinho R, Kretzschmar M. Primary HIV infection as source of HIV transmission within steady and casual partnerships among homosexual men. AIDS. 2004;18(9):1311–20. doi: 10.1097/00002030-200406180-00010. [DOI] [PubMed] [Google Scholar]
- 12.Flaks RC, Burman WJ, Gourley PJ, Rietmeijer CA, Cohn DL. HIV transmission risk behavior and its relation to antiretroviral treatment adherence. Sex Transm Dis. 2003;30(5):399–404. doi: 10.1097/00007435-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty H, Sen PK, Helms RW, et al. Viral burden in genital secretions determines male-to-female sexual transmission of HIV-1: a probabilistic empiric model. AIDS. 2001;15(5):621–7. doi: 10.1097/00002030-200103300-00012. [DOI] [PubMed] [Google Scholar]
- 14.Porco TC, Martin JN, Page-Shafer KA, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004;18(1):81–8. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: do missed visits matter? J Acquir Immune Defic Syndr. 2009;50(1):100–8. doi: 10.1097/QAI.0b013e31818d5c37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima VD, Harrigan R, Murray M, et al. Differential impact of adherence on long-term treatment response among naive HIV-infected individuals. AIDS. 2008;22(17):2371–80. doi: 10.1097/QAD.0b013e328315cdd3. [DOI] [PubMed] [Google Scholar]
- 17.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crackcocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50(1):93–9. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 18.Mugavero MJ, Lin HY, Willig JH, et al. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48(2):248–56. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mullins CD, Whitelaw G, Cooke JL, Beck EJ. Indirect cost of HIV infection in England. Clin Ther. 2000;22(11):1333–45. doi: 10.1016/s0149-2918(00)83030-1. [DOI] [PubMed] [Google Scholar]
- 20.Gardner EM, Maravi ME, Rietmeijer C, Davidson AJ, Burman WJ. The association of adherence to antiretroviral therapy with healthcare utilization and costs for medical care. Appl Health Econ Health Policy. 2008;6(2–3):145–55. doi: 10.1007/bf03256129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. AIDS Care. 2000;12(3):255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 22.Kleeberger CA, Phair JP, Strathdee SA, Detels R, Kingsley L, Jacobson LP. Determinants of heterogenous adherence to HIV-antiretroviral therapies in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 2001;26(1):82–92. doi: 10.1097/00126334-200101010-00012. [DOI] [PubMed] [Google Scholar]
- 23.Crepaz N, Lyles CM, Wolitski RJ, et al. Do prevention interventions reduce HIV risk behaviours among people living with HIV? A meta-analytic review of controlled trials. AIDS. 2006;20(2):143–57. doi: 10.1097/01.aids.0000196166.48518.a0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson BT, Carey MP, Chaudoir SR, Reid AE. Sexual risk reduction for persons living with HIV: research synthesis of randomized controlled trials, 1993 to 2004. J Acquir Immune Defic Syndr. 2006;41(5):642–50. doi: 10.1097/01.qai.0000194495.15309.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher JD, Smith L. Secondary prevention of HIV infection: the current state of prevention for positives. Curr Opin HIV AIDS. 2009;4(4):279–87. doi: 10.1097/COH.0b013e32832c7ce5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman CI, Gordon CM. Mental health considerations in secondary HIV prevention. AIDS Behav. doi: 10.1007/s10461-008-9496-8. [Published online 5 Dec 2008] [DOI] [PubMed] [Google Scholar]
- 27.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158(5):725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 28.Lyketsos CG, Hutton H, Fishman M, Schwartz J, Treisman GJ. Psychiatric morbidity on entry to an HIV primary care clinic. AIDS. 1996;10(9):1033–9. doi: 10.1097/00002030-199610090-00015. [DOI] [PubMed] [Google Scholar]
- 29.Olley BO, Seedat S, Stein DJ. Persistence of psychiatric disorders in a cohort of HIV/AIDS patients in South Africa: a 6-month follow-up study. J Psychosom Res. 2006;61(4):479–84. doi: 10.1016/j.jpsychores.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Pence BW, Miller WC, Gaynes BN, Eron JJ., Jr. Psychiatric illness and virologic response in patients initiating highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44(2):159–66. doi: 10.1097/QAI.0b013e31802c2f51. [DOI] [PubMed] [Google Scholar]
- 31.Atkinson JH, Jr, Grant I, Kennedy CJ, Richman DD, Spector SA, McCutchan JA. Prevalence of psychiatric disorders among men infected with human immunodeficiency virus. A controlled study. Arch Gen Psychiatry. 1988;45(9):859–64. doi: 10.1001/archpsyc.1988.01800330091011. [DOI] [PubMed] [Google Scholar]
- 32.Israelski DM, Prentiss DE, Lubega S, et al. Psychiatric comorbidity in vulnerable populations receiving primary care for HIV/AIDS. AIDS Care. 2007;19(2):220–5. doi: 10.1080/09540120600774230. [DOI] [PubMed] [Google Scholar]
- 33.Safren SA, Gershuny BS, Hendriksen E. Symptoms of posttraumatic stress and death anxiety in persons with HIV and medication adherence difficulties. AIDS Patient Care STDS. 2003;17(12):657–64. doi: 10.1089/108729103771928717. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson JH, Higgins JA, Vigil O, et al. Psychiatric context of acute/early HIV infection. The NIMH Multisite Acute HIV Infection Study: IV. AIDS Behav. 2009;13:1061–67. doi: 10.1007/s10461-009-9585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV-positive cigarette smokers: a sample of smokers facing multiple challenges. AIDS Educ Prev. 2009;21(3 Suppl):54–64. doi: 10.1521/aeap.2009.21.3_supp.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez A, Israelski D, Walker C, Koopman C. Posttraumatic stress disorder in women attending human immunodeficiency virus outpatient clinics. AIDS Patient Care STDS. 2002;16(6):283–91. doi: 10.1089/10872910260066714. [DOI] [PubMed] [Google Scholar]
- 37.Cohen MA, Batista SM, Gorman JM. Anxiety disorders. In: Cohen MA, Gorman JM, editors. Comprehensive textbook of AIDS psychiatry. Oxford University Press; New York: 2008. pp. 121–30. [Google Scholar]
- 38.Kelly B, Raphael B, Judd F, et al. Posttraumatic stress disorder in response to HIV infection. Gen Hosp Psychiatry. 1998;20(6):345–52. doi: 10.1016/s0163-8343(98)00042-5. [DOI] [PubMed] [Google Scholar]
- 39.Kalichman SC. Co-occurrence of treatment nonadherence and continued HIV transmission risk behaviors: implications for positive prevention interventions. Psychosom Med. 2008;70(5):593–7. doi: 10.1097/PSY.0b013e3181773bce. [DOI] [PubMed] [Google Scholar]
- 40.Bradley MV, Remien RH, Dolezal C. Depression symptoms and sexual HIV risk behavior among serodiscordant couples. Psychosom Med. 2008;70(2):186–91. doi: 10.1097/PSY.0b013e3181642a1c. [DOI] [PubMed] [Google Scholar]
- 41.Ryan K, Forehand R, Solomon S, Miller C. Depressive symptoms as a link between barriers to care and sexual risk behavior of HIV-infected individuals living in non-urban areas. AIDS Care. 2008;20(3):331–6. doi: 10.1080/09540120701660338. [DOI] [PubMed] [Google Scholar]
- 42.Bousman CA, Cherner M, Ake C, et al. Negative mood and sexual behavior among non-monogamous men who have sex with men in the context of methamphetamine and HIV. J Affect Disord. 2009;119(1–3):84–91. doi: 10.1016/j.jad.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Valverde EE, Cassetti I, Metsch LR, et al. Sex risk practices among HIV-positive individuals in Buenos Aires, Argentina. AIDS Patient Care STDS. 2009;23(7):551–6. doi: 10.1089/apc.2008.0094. [DOI] [PubMed] [Google Scholar]
- 44.Mugavero M, Ostermann J, Whetten K, et al. Barriers to anti-retroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–28. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 45.Gordillo V, del Amo J, Soriano V, Gonzalez-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. AIDS. 1999;13(13):1763–9. doi: 10.1097/00002030-199909100-00021. [DOI] [PubMed] [Google Scholar]
- 46.Mellins CA, Kang E, Leu CS, Havens JF, Chesney MA. Longitudinal study of mental health and psychosocial predictors of medical treatment adherence in mothers living with HIV disease. AIDS Patient Care STDS. 2003;17(8):407–16. doi: 10.1089/108729103322277420. [DOI] [PubMed] [Google Scholar]
- 47.Rajabiun S, Mallinson RK, McCoy K, et al. “Getting me back on track”: the role of outreach interventions in engaging and retaining people living with HIV/AIDS in medical care. AIDS Patient Care STDS. 2007;21(Suppl 1):S20–9. doi: 10.1089/apc.2007.9990. [DOI] [PubMed] [Google Scholar]
- 48.Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry. 2004;161(5):912–4. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- 49.Reisner SL, Mimiaga MJ, Skeer M, Mayer KH. Beyond anal sex: sexual practices associated with HIV risk reduction among men who have sex with men in Boston, Massachusetts. AIDS Patient Care STDS. 2009;23(7):545–50. doi: 10.1089/apc.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klein H, Elifson KW, Sterk CE. Depression and HIV risk behavior practices among at risk women. Womens Health. 2008;48(2):167–88. doi: 10.1080/03630240802313605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hart TA, James CA, Purcell DW, Farber E. Social anxiety and HIV transmission risk among HIV-seropositive male patients. AIDS Patient Care STDS. 2008;22(11):879–86. doi: 10.1089/apc.2008.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reyes JC, Robles RR, Colon HM, et al. Severe anxiety symptomatology and HIV risk behavior among Hispanic injection drug users in Puerto Rico. AIDS Behav. 2007;11(1):145–50. doi: 10.1007/s10461-006-9090-x. [DOI] [PubMed] [Google Scholar]
- 53.Hutton HE, Treisman GJ, Hunt WR, et al. HIV risk behaviors and their relationship to posttraumatic stress disorder among women prisoners. Psychiatr Serv. 2001;52(4):508–13. doi: 10.1176/appi.ps.52.4.508. [DOI] [PubMed] [Google Scholar]
- 54.Reisner SL, Mimiaga MJ, Safren S, Mayer K. Stressful or traumatic life events, post-traumatic stress disorder (PTSD) symptoms, and HIV sexual risk taking among men who have sex with men. AIDS Care. 2009;21(12):1481–9. doi: 10.1080/09540120902893258. [DOI] [PubMed] [Google Scholar]
- 55.Crepaz N, Marks G. Are negative affective states associated with HIV sexual risk behaviors? A meta-analytic review. Health Psychol. 2001;20(4):291–9. doi: 10.1037//0278-6133.20.4.291. [DOI] [PubMed] [Google Scholar]
- 56.Crepaz N, Marks G. Towards an understanding of sexual risk behavior in people living with HIV: a review of social, psychological, and medical findings. AIDS. 2002;16(2):135–49. doi: 10.1097/00002030-200201250-00002. [DOI] [PubMed] [Google Scholar]
- 57.Kalichman SC, Weinhardt L. Negative affect and sexual risk behavior: comment on Crepaz and Marks (2001) Health Psychol. 2001;20(4):300–1. [PubMed] [Google Scholar]
- 58.Chesney MA, Koblin BA, Barresi PJ, et al. An individually tailored intervention for HIV prevention: baseline data from the EXPLORE study. Am J Public Health. 2003;93(6):933–8. doi: 10.2105/ajph.93.6.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rogers G, Curry M, Oddy J, Pratt N, Beilby J, Wilkinson D. Depressive disorders and unprotected casual anal sex among Australian homosexually active men in primary care. HIV Med. 2003;4(3):271–5. doi: 10.1046/j.1468-1293.2003.00155.x. [DOI] [PubMed] [Google Scholar]
- 60.Reisner SL, Mimiaga MJ, Skeer M, et al. Clinically significant depressive symptoms as a risk factor for HIV infection among black MSM in Massachusetts. AIDS Behav. 2009;13(4):798–810. doi: 10.1007/s10461-009-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meade CS, Hansen NB, Kochman A, Sikkema KJ. Utilization of medical treatments and adherence to antiretroviral therapy among hiv-positive adults with histories of childhood sexual abuse. AIDS Patient Care STDS. 2009;23(4):259–66. doi: 10.1089/apc.2008.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vranceanu AM, Safren SA, Lu M, et al. The relationship of post-traumatic stress disorder and depression to antiretroviral medication adherence in persons with HIV. AIDS Patient Care STDS. 2008;22(4):313–21. doi: 10.1089/apc.2007.0069. [DOI] [PubMed] [Google Scholar]
- 63.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–7. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization World Health Organization; Geneva: Adherence to long-term therapies: evidence for action. 2003
- 65.Ammassari A, Trotta MP, Murri R, et al. Correlates and predictors of adherence to highly active antiretroviral therapy: overview of published literature. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S123–7. doi: 10.1097/00126334-200212153-00007. [DOI] [PubMed] [Google Scholar]
- 66.Vervoort SC, Borleffs JC, Hoepelman AI, Grypdonck MH. Adherence in antiretroviral therapy: a review of qualitative studies. AIDS. 2007;21(3):271–81. doi: 10.1097/QAD.0b013e328011cb20. [DOI] [PubMed] [Google Scholar]
- 67.Mocroft A, Madge S, Johnson AM, et al. A comparison of exposure groups in the EuroSIDA study: starting highly active antiretroviral therapy (HAART), response to HAART, and survival. J Acquir Immune Defic Syndr. 1999;22(4):369–78. doi: 10.1097/00126334-199912010-00008. [DOI] [PubMed] [Google Scholar]
- 68.Fairfield KM, Libman H, Davis RB, Eisenberg DM. Delays in protease inhibitor use in clinical practice. J Gen Intern Med. 1999;14(7):395–401. doi: 10.1046/j.1525-1497.1999.08198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr. 2005;38(1):96–103. doi: 10.1097/00126334-200501010-00017. [DOI] [PubMed] [Google Scholar]
- 70.Turner BJ, Fleishman JA, Wenger N, et al. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. J Gen Intern Med. 2001;16(9):625–33. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Scott-Sheldon LA, Kalichman SC, Carey MP, Fielder RL. Stress management interventions for HIV+ adults: a meta-analysis of randomized controlled trials, 1989 to 2006. Health Psychol. 2008;27(2):129–39. doi: 10.1037/0278-6133.27.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Himelhoch S, Medoff DR, Oyeniyi G. Efficacy of group psychotherapy to reduce depressive symptoms among HIV-infected individuals: a systematic review and meta-analysis. AIDS Patient Care STDS. 2007;21(10):732–9. doi: 10.1089/apc.2007.0012. [DOI] [PubMed] [Google Scholar]
- 73.Crepaz N, Passin WF, Herbst JH, et al. Meta-analysis of cognitive-behavioral interventions on HIV-positive persons’ mental health and immune functioning. Health Psychol. 2008;27(1):4–14. doi: 10.1037/0278-6133.27.1.4. [DOI] [PubMed] [Google Scholar]
- 74.Himelhoch S, Medoff DR. Efficacy of antidepressant medication among HIV-positive individuals with depression: a systematic review and meta-analysis. AIDS Patient Care STDS. 2005;19(12):813–22. doi: 10.1089/apc.2005.19.813. [DOI] [PubMed] [Google Scholar]
- 75.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed Erlbaum; Hillsdale: 1988. [Google Scholar]
- 76.Cuijpers P, Van Straten A, Warmerdam L, Smits N. Characteristics of effective psychological treatments of depression: a metaregression analysis. Psychother Res. 2008;18(2):225–36. doi: 10.1080/10503300701442027. [DOI] [PubMed] [Google Scholar]
- 77.Robinson LA, Berman JS, Neimeyer RA. Psychotherapy for the treatment of depression: a comprehensive review of controlled outcome research. Psychol Bull. 1990;108(1):30–49. doi: 10.1037/0033-2909.108.1.30. [DOI] [PubMed] [Google Scholar]
- 78.Katzman MA, Tricco AC, McIntosh D, et al. Paroxetine versus placebo and other agents for depressive disorders: a systematic review and meta-analysis. J Clin Psychiatry. 2007;68(12):1845–59. doi: 10.4088/jcp.v68n1204. [DOI] [PubMed] [Google Scholar]
- 79.Moncrieff J, Wessely S, Hardy R. Active placebos versus anti-depressants for depression. Cochrane Database Syst Rev. 2004;1 doi: 10.1002/14651858.CD003012.pub2. CD003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol. 2008;76(6):909–22. doi: 10.1037/a0013075. [DOI] [PubMed] [Google Scholar]
- 81.Hunot V, Churchill R, de Lima M Silva, Teixeira V. Psychological therapies for generalised anxiety disorder. Cochrane Database Syst Rev. 2007;1 doi: 10.1002/14651858.CD001848.pub4. CD001848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sikkema KJ, Wilson PA, Hansen NB, et al. Effects of a coping intervention on transmission risk behavior among people living with HIV/AIDS and a history of childhood sexual abuse. J Acquir Immune Defic Syndr. 2008;47(4):506–13. doi: 10.1097/QAI.0b013e318160d727. [DOI] [PubMed] [Google Scholar]
- 83.Coates TJ, McKusick L, Kuno R, Stites DP. Stress reduction training changed number of sexual partners but not immune function in men with HIV. Am J Public Health. 1989;79(7):885–7. doi: 10.2105/ajph.79.7.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams JK, Wyatt GE, Rivkin I, Ramamurthi HC, Li X, Liu H. Risk reduction for HIV-positive African American and Latino men with histories of childhood sexual abuse. Arch Sex Behav. 2008;37(5):763–72. doi: 10.1007/s10508-008-9366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly JA, Murphy DA, Bahr GR, et al. Outcome of cognitive-behavioral and support group brief therapies for depressed, HIV-infected persons. Am J Psychiatry. 1993;150(11):1679–86. doi: 10.1176/ajp.150.11.1679. [DOI] [PubMed] [Google Scholar]
- 86.Healthy Living Project Team Effects of a behavioral intervention to reduce risk of transmission among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;44(2):213–21. doi: 10.1097/QAI.0b013e31802c0cae. [DOI] [PubMed] [Google Scholar]
- 87.Wyatt GE, Longshore D, Chin D, et al. The efficacy of an integrated risk reduction intervention for HIV-positive women with child sexual abuse histories. AIDS Behav. 2004;8(4):453–62. doi: 10.1007/s10461-004-7329-y. [DOI] [PubMed] [Google Scholar]
- 88.Safren SA, O’Cleirigh C, Tan JY, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected individuals. Health Psychol. 2009;28(1):1–10. doi: 10.1037/a0012715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson MO, Charlebois E, Morin SF, Remien RH, Chesney MA. Effects of a behavioral intervention on antiretroviral medication adherence among people living with HIV: the healthy living project randomized controlled study. J Acquir Immune Defic Syndr. 2007;46(5):574–80. doi: 10.1097/qai.0b013e318158a474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Antoni MH, Baggett L, Ironson G, et al. Cognitive-behavioral stress management intervention buffers distress responses and immunologic changes following notification of HIV-1 seropositivity. J Consult Clin Psychol. 1991;59(6):906–15. doi: 10.1037//0022-006x.59.6.906. [DOI] [PubMed] [Google Scholar]
- 91.Goodkin K, Fuchs I, Feaster DJ, Leeka J, Rishel DD. Life stressors and coping style are associated with immune measures in HIV-1 infection—a preliminary report. Int J Psychiatry Med. 1992;22:155–72. doi: 10.2190/BD46-F4JD-K8TW-RUFH. [DOI] [PubMed] [Google Scholar]
- 92.McCain NL, Zeller JM, Cella DF, Urbanski PA, Novak RM. The influence of stress management training in HIV disease. Nurs Res. 1996;45:246–53. doi: 10.1097/00006199-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 93.Sikkema KJ, Hansen NB, Tarakeshwar N, Kochman A, Tate DC, Lee RS. The clinical significance of change in trauma-related symptoms following a pilot group intervention for coping with HIV-AIDS and childhood sexual trauma. AIDS Behav. 2004;8(3):277–91. doi: 10.1023/B:AIBE.0000044075.12845.75. [DOI] [PubMed] [Google Scholar]
- 94.Greenberg J. Childhood sexual abuse and sexually transmitted diseases in adults: a review of and implications for STD/HIV programmes. Int J STD AIDS. 2001;12(12):777–83. doi: 10.1258/0956462011924380. [DOI] [PubMed] [Google Scholar]
- 95.Senn TE, Carey MP, Vanable PA. Childhood and adolescent sexual abuse and subsequent sexual risk behavior: evidence from controlled studies, methodological critique, and suggestions for research. Clin Psychol Rev. 2008;28(5):711–35. doi: 10.1016/j.cpr.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kalichman SC, Sikkema KJ, DiFonzo K, Luke W, Austin J. Emotional adjustment in survivors of sexual assault living with HIV/AIDS. J Trauma Stress. 2002;15(4):189–296. doi: 10.1023/A:1016247727498. [DOI] [PubMed] [Google Scholar]
- 97.O’Leary A, Purcell D, Remien RH, Gomez C. Childhood sexual abuse and sexual transmission risk behaviour among HIV-positive men who have sex with men. AIDS Care. 2003;15(1):17–26. doi: 10.1080/0954012021000039725. [DOI] [PubMed] [Google Scholar]
- 98.Mimiaga MJ, Noonan E, Donnell D, et al. Childhood sexual abuse is highly associated with HIV risk-taking behavior and infection among MSM in the EXPLORE study. J Acquir Immune Defic Syndr. 2009;51(3):340–8. doi: 10.1097/QAI.0b013e3181a24b38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lazarus R, Folkman S. Stress, appraisal and coping. Springer; New York: 1984. [Google Scholar]
- 100.Folkman S, Chesney M, McKusick L, Ironson G, Johnson D, Coates T. Translating coping theory into an intervention. In: Eckenrode J, editor. The social context of coping. Plenum Press; New York: 1991. pp. 239–60. [Google Scholar]
- 101.Sikkema KJ, Hansen NB, Kochman A, et al. Outcomes from a group intervention for coping with HIV/AIDS and childhood sexual abuse: reductions in traumatic stress. AIDS Behav. 2007;11:49–60. doi: 10.1007/s10461-006-9149-8. [DOI] [PubMed] [Google Scholar]
- 102.Meade C, Drabkin AS, Wilson PA, Hansen NB, Kochman A, Sikkema K. Reductions in alcohol and cocaine use following a group coping intervention for HIV positive adults with childhood sexual abuse histories. CFAR Social and Behavioral Sciences Research Network Meeting; Boston. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carrico AW, Chesney MA, Johnson MO, et al. Randomized controlled trial of a cognitive-behavioral intervention for HIV-positive persons: an investigation of treatment effects on psychosocial adjustment. AIDS Behav. 2009;13(3):555–63. doi: 10.1007/s10461-008-9429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong FL, Rotheram-Borus MJ, Lightfoot M, et al. Effects of behavioral intervention on substance use among people living with HIV: the healthy living project randomized controlled study. Addiction. 2008;103(7):1206–14. doi: 10.1111/j.1360-0443.2008.02222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar V, Encinosa W. Effects of antidepressant treatment on antiretroviral regimen adherence among depressed HIV-infected patients. Psychiatr Q. 2009;80:131–41. doi: 10.1007/s11126-009-9100-z. [DOI] [PubMed] [Google Scholar]
- 106.Tsai A, Weiser S, Petersen M, Raglans K, Bangsberg D. Effect of antidepressant medication treatment on antiretroviral adherence and HIV-1 RNA viral load in HIV+ homeless and marginally housed individuals. 16th Conference on retroviruses and opportunistic infections; Montreal. 2009. [Google Scholar]
- 107.Yun LW, Maravi M, Kobayashi JS, Barton PL, Davidson AJ. Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr. 2005;38(4):432–8. doi: 10.1097/01.qai.0000147524.19122.fd. [DOI] [PubMed] [Google Scholar]
- 108.Walkup J, Wei W, Sambamoorthi U, Crystal S. Antidepressant treatment and adherence to combination antiretroviral therapy among patients with AIDS and diagnosed depression. Psychiatr Q. 2008;79(1):43–53. doi: 10.1007/s11126-007-9055-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Horberg MA, Silverberg MJ, Hurley LB, et al. Effects of depression and selective serotonin reuptake inhibitor use on adherence to highly active antiretroviral therapy and on clinical outcomes in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47(3):384–90. doi: 10.1097/QAI.0b013e318160d53e. [DOI] [PubMed] [Google Scholar]
- 110.Gaynes BN, Pence BW, Eron JJ, Jr, Miller WC. Prevalence and comorbidity of psychiatric diagnoses based on reference standard in an HIV+ patient population. Psychosom Med. 2008;70(4):505–11. doi: 10.1097/PSY.0b013e31816aa0cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.World Health Organization World Health Organization; Geneva: The World Health Report 2001: mental health–new understanding, new hope. 2001