Abstract
Autoimmune thyroid diseases (AITD) are complex diseases that develop as a result of interactions between genetic, epigenetic, and environmental factors. Significant progress has been made in our understanding of the genetic and environmental triggers contributing to AITD. The major environmental triggers of AITD include iodine, smoking, medications, pregnancy, and possibly stress. In this review we will focus on two well-documented environmental triggers of AITD, hepatitis C virus (HCV) infection and interferon alpha (IFNa) therapy. Chronic HCV infection has been shown to be associated with increased incidence of clinical and subclinical autoimmune thyroiditis (i.e. the presence of thyroid antibodies in euthyroid subjects). Moreover, IFNa therapy of chronic HCV infection is associated with subclinical or clinical thyroiditis in up to 40% of cases which can be autoimmune, or non-autoimmune thyroiditis. In some cases interferon induced thyroiditis (IIT) in chronic HCV patients may result in severe symptomatology necessitating discontinuation of therapy. While the epidemiology and clinical presentation of HCV and interferon induced thyroiditis have been well characterized, the mechanisms causing these conditions are still poorly understood.
Keywords: Interferon, thyroiditis, autoimmunity
INTRODUCTION
While abundant data point toa strong genetic susceptibility to the development of autoimmune thyroid disease (AITD), including Graves’ disease (GD) and Hashimoto’s thyroiditis (HT) (reviewed in (1)), environmental factors also play an important role. Since monozygotic twins do not show 100% concordance for AITD non-genetic factors must also play a role. Indeed, a recent twin study estimated that about 20% of the liability to the development of GD is attributable to non-genetic factors (2). The environmental factors postulated to precipitated AITD include iodine (3;4), medications, such as amiodarone and interferon alpha (5), infections (6), smoking, and possibly stress (reviewed in (7)). Recently, HCV infection (8) and interferon alpha (IFNa) therapy (9) emerged as the most substantiated environmental triggers of AITD.
HEPATITIS C VIRUS INFECTION
Infection and AITD
One of the most intriguing environmental triggers of autoimmune thyroid diseases is infection (reviewed in (10)). Evidence supporting infectious cause of AITD include seasonality in the incidence of AITD (11), geographic variation (12), and serological evidence for a recent bacterial or viral infection (13). Several infectious agents have been implicated in the pathogenesis of AITD including Yersinia enterocolitica (14–17), Coxsackie B virus (18), retroviruses (19–23), Helicobacter pylori (24;25) and Borrelia (26). However, by far the most consistent association with hepatitis C (8).
Epidemiological Studies
Earlier studies of patients with chronic hepatitis C showed mixed results, with some supporting an association of HCV infection with clinical or subclinical AITD disorders (27–31), and others not (32–34). It is now clear that some of the earlier studies were negative because of the use of less sensitive thyroid antibody assays and the lack of control for factors which may affect the development of thyroid autoimmunity, mainly iodine intake. Indeed, one of the largest and well-controlled studies of HCV and thyroiditis demonstrated that both hypothyroidism and thyroid autoimmunity were significantly more common in patients with hepatitis C compared to controls (8;31). Further evidence for this association came from a recent study that found that the prevalence of non autoimmune hypothyroidism, as well as the presence of Tg-Ab, was higher in untreated children with HCV compared to healthy non-HCV infected controls. This increased prevalence was not associated with other parameters (family history of autoimmune diseases, duration of HCV infection, viral genotype, viral load or liver function) except active HCV infection (35). In two earlier studies from France of patients with hepatitis C infection who had not received IFN alpha therapy, the incidence of thyroid antibodies and/or dysfunction was significantly higher in the patients than in the controls (27;28). Overall, in most studies examining the frequency of thyroid disorders in hepatitis C patients approximately 10% of the patients had positive thyroid antibodies (TAb) prior to initiation of interferon therapy (36–40). Moreover, pooling of data from all studies on HCV infection and thyroid autoimmunity demonstrated a significant increase in the risk of thyroiditis in HCV patients (41). Therefore, HCV infection is the only infectious agent that is clearly associated with an increased risk for autoimmune thyroiditis.
Potential mechanisms
While many potential mechanisms exist for the association of infection and autoimmunity, two main hypotheses have the strongest evidence, molecular mimicry and bystander activation (42). The molecular mimicry hypothesis suggests that sequence similarities between viral proteins and self proteins can cause a cross-over immune response to self antigens which are mimicked by infectious agents’ proteins (43). Some studies suggested molecular mimicry between Yersinia proteins (44) or Borrelia proteins (26) and thyroid antigens exists, these data have not been confirmed.
The bystander activation hypothesis is based on the fact that viral infection of a certain tissue can induce local inflammation (e.g. by cytokine release). This low level inflammation, according to the bystander activation hypothesis, can cause activation of autoreactive resident T-cells that were suppressed by peripheral tolerance mechanisms such as Treg cells (45). Recent data favor the bystander activation as the predominant mechanism by which viral agents trigger autoimmunity in autoimmune thyroiditis (46). Hence, it is possible that HCV can trigger autoimmune thyroiditis by infecting thyroid cells, and causing the release of pro-inflammatory mediators. The release of cytokines can then trigger AITD by bystander activation mechanisms.
Could HCV infect thyroid cells? A recent study demonstrated HCV virions inside thyroid follicular cells (47) suggesting that this could be a potential mechanism. However, even if HCV cannot infect thyroid cells, viral proteins that are shed from virions may also have important physiological consequences. For example, it was shown that HCV E2 proteins can induce apoptosis (48;49), and upregulate the pro-inflammatory cytokine interleukin 8 (IL-8) (50). These data suggested that HCV envelope proteins themselves could significantly impact the thyroid environment and contribute to thyroid dysfunction. Moreover, we have recently shown that the HCV virus can activate cytokine secretion by thyroid cells (51). We examined whether the HCV receptor, CD81, was expressed and functional on human thyroid cells. We found significant levels of CD81 mRNA and protein on human thyroid cells in primary cultures. Moreover, incubation of human thyroid cells with HCV envelope glycoprotein E2 resulted in E2 binding to thyroid cells and activation of IL-8 secretion (51). These findings suggest that the mere binding of HCV envelope proteins to thyroid cells is sufficient to trigger cytokine secretion and activation of resident T-cells. This activation, in genetically susceptible individuals, can trigger autoimmune thyroiditis through a bystander mechanism.
INTERFERON INDUCED THYROIDITIS (IIT)
Interferon alpha (IFNa) is a type I interferon that has been widely used as a therapeutic agent mostly, for infectious and malignant diseases (52). IFNa binds to interferon receptors, and activates various signaling pathways, including the JAK-STAT pathway, and the MAP kinase pathway leading to transcription of target proteins which mediate its immune and anti-tumor effects (53–55). One of the most remarkable successes of IFNa as a therapeutic agent has been in the treatment of chronic hepatitis C, where the combination of IFNa+Ribavirin induces remission in up to 50% of patients (56). However, IFNa therapy can cause numerous and wide-ranging side effects, including severe complications that can result in morbidity and discontinuation of therapy (57). Thyroiditis is among the commonest side-effects of IFNa therapy; in fact, subclinical thyroiditis occurs in 20–40% of and clinical thyroiditis in 5–10% of patients (9). Up until recently, little was known about the mechanisms causing interferon induced thyroiditis (IIT); however, recently significant progress has been made in our understanding of the etiology of IIT.
Epidemiological Studies
Since the first description of IIT in 1985 (58) numerous studies have confirmed the strong association between IFNa treatment and the development of thyroiditis (reviewed in (9)). In view of the varying manifestations of IIT we have recently proposed a new classification of IIT into autoimmune IIT and non-autoimmune IIT (59). Autoimmune IIT can manifest as clinical disease, i.e. Graves’ disease (GD) or Hashimoto’s thyroiditis (HT), or as subclinical disease, i.e. the production of thyroid autoantibodies (TAb) without abnormal thyroid functions. Non-autoimmune IIT can manifest as destructive thyroiditis, or non-autoimmune hypothyroidism (59).
The commonest clinical manifestation of autoimmune IIT is Hashimoto’s thyroiditis (HT) (60–62). HT commonly develops in individuals that had positive TAb before receiving IFNa. It was estimated that the positive predictive value of elevated TPO antibodies before IFNa therapy for the development of HT was 67%, a relatively high number (38). HT can also develop de novo in HCV patients receiving IFNa even if they did not have positive TAb prior to therapy. These data may suggest a triggering effect for IFNa in individuals with genetic susceptibility to the development of AITD (59).
Graves’ disease (GD) is a less common clinical manifestation of autoimmune IIT (38;63). In one series only 6/321 patients with hepatitis B or C treated with developed GD (63). In most cases of GD the thyrotoxicosis did not resolve after discontinuation of IFNa therapy (36;63;63;64), again suggesting that IFNa may have triggered GD in individuals predisposed to develop the disease.
Subclinical AITD may also develop with IFNa therapy. Subclinical AITD manifests by the production of TAb without clinical disease. The incidence of de novo development of TAb is about 10–40% (30;36–38;65), and the majority of individuals who develop “de novo” TAb on IFNa therapy remain positive when the treatment course is completed (62).
Non-autoimmune IIT is as common as autoimmune IIT comprising approximately 50% of IIT cases. Non-autoimmune IIT usually manifests as destructive thyroiditis (DT), likely due to direct effects of IFNa on the thyroid gland. DT starts with an early thyrotoxic phase, caused by the release of preformed thyroid hormones, and then progresses to a late hypothyroid phase, with complete resolution in most cases (59). Some patients develop permanent hypothyroidism usually if they had prior TAb (66). Patients with destructive thyroiditis have negative TSH-receptor antibodies (TRAb) and low thyroid radioactive iodine uptake (59). On re-treatment with IFNa patients frequently develop recurrent thyroiditis, and therefore, careful monitoring of thyroid functions is recommended if another course of IFNa is given (67). Non-autoimmune IIT can also manifest by clinical hypothyroidism with no detectable TAb (36;38;61;62;64). This again suggest a direct effect of IFNa on the thyroid gland.
Potential mechanisms
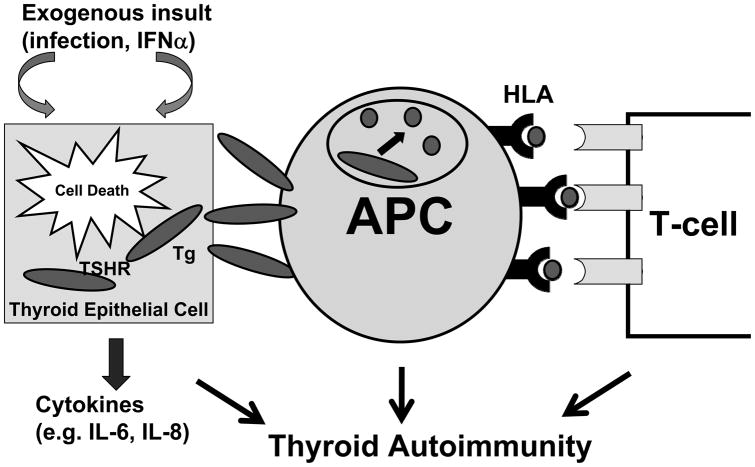
IFNa is a critical cytokine in the immune response to infectious agents. Therefore, it seems likely that its immune effects will trigger autoimmunity when given in pharmacological doses. However, this assumption cannot explain the predilection of interferon induced inflammation to the thyroid and the development of non-autoimmune thyroiditis. Therefore, we and others have proposed a direct effect of IFNa on the thyroid as a complementary mechanism to the immune effects, triggering thyroiditis (Figure 1) (9;68).
Figure 1.
IIT develops in genetically predisposed individuals by direct effects of IFNa on the thyroid cell, as well as by immune effects. The HCV infection and IFNa therapy cause secretion of cytokines from thyroid cells and thyroid cell death with release of thyroid antigens. These antigens can be picked by antigen presenting cells (APC’s) and presented to resident T-cells in the thyroid triggering autoimmune thyroiditis.
Immune effects of IFNa
IFNa activates the JAK-STAT pathway upon binding to its receptor (69), leading to activation of a large number of interferon-stimulated genes (ISGs) including cytokine and adhesion molecule genes (70;71). These combined effects can trigger an autoimmune response in a genetically susceptible individual. Among the key immune effects of IFNa the most important ones include: increasing the expression of MHC class I antigens on cells including thyroid epithelial cells (38), switching the immune response to a Th1 pattern (72) resulting in the secretion of interferon gamma and IL-2, two potent proinflammatory cytokines (73), enhancing the activity of lymphocytes, macrophages, and NK cells (52;70;74), activating neutrophils and monocytes (70), inducing the release of cytokines, such as IL-6 (70), and decreasing T regulatory cell function (75;76).
Thyroid toxic effects of IFNa
In recent years the dogma that IFNa causes thyroiditis only by immune mechanisms has been challenged (9;59). Indeed, IFNa has been shown to have several thyroid-specific effects. We have recently tested the expression levels of the TSHR, Tg, TPO, and NIS genes in a rat thyroid cell line. Our results showed an early (24 hours) increase in the levels of TSHR, Tg, TPO, and NIS with a later decrease (at 48 hrs) in the levels of TPO and NIS, but not TSHR (77). Another group also showed a late decrease in TSH-induced gene expression of thyroglobulin (Tg), TPO, and sodium iodide symporter (NIS) in cultured human thyrocytes (68). Moreover, we have shown that IFNa induced thyroid cell death by necrosis and not by apoptosis (77). Increased thyroid cell death was also recently reported by another group (78). Taken together these data challenge the dogma that thyroiditis is solely an result of immune stimulation by IFNa. It is more likely that direct thyroid toxic effects may trigger thyroidal inflammation that combined with genetic predisposition and immune stimulatory effects of IFNa trigger thyroiditis.
Genetic predisposition to IIT
Solid data support a strong genetic component in the etiology of AITD (reviewed in (1;79)). Therefore, it is possible that IFNa may trigger IIT in genetically predisposed individuals (59). Support for this hypothesis comes from epidemiological observations showing variations in the prevalence of IIT in different ethnic populations (80) and between males and females, with females showing a higher prevalence of IIT (81). The female predisposition to IIT could be due to an X-chromosome susceptibility locus, or due to the effects of estrogens (82).
Since TAb may be a biomarker for genetic predisposition to AITD and they are genetically inherited (83), the fact that positive TAb prior to IFNa therapy is a risk factor for IIT supports the notion of a strong genetic susceptibility to the development of IIT. We have studied the genetic predisposition to IIT in the thyroiditis-prone NOD-H2h4 mouse (84). NOD-H2h4 mice treated with IFNa for eight showed an increased frequency thyroiditis and/or thyroid antibodies, compared to the saline–injected group, (46.2% vs. 30.8%); however, this difference was not statistically significant possibly due to the small groups of mice tested (84;84).
In recent years several susceptibility genes for thyroid autoimmunity have been identified, including HLA-DR, CTLA-4, PTPN22, FOXP3, thyroglobulin, and TSHR (85–87). It is likely that some of these genes also contribute to the genetic susceptibility to IIT. Indeed, two small studies showed HLA associations of IIT (88;89). We recently tested several candidate genes for association with IIT. Our preliminary data, in a small cohort, showed evidence for association of IIT with polymorphisms in the CTLA-4 and CD40 genes (90). Taken together, this preliminary evidence supports a genetic role in the etiology of IIT.
CONCLUSIONS
One of the commonest complications of IFNa therapy for chronic hepatitis C infection is interferon induced thyroiditis (IIT) (9). It is likely that IFNa triggers thyroiditis in genetically predisposed individuals by bothdirect thyroid-toxic mechanisms and immune-modulatory mechanisms (7). It is likely that the HCV infection itself contributes to the initiation of thyroid autoimmunity (51). Since IIT is very common in HCV patients receiving IFNa therapy all patients should undergo routine thyroid screening. Hopefully, in the future pharmacogenomic approaches will be used to identify patients predisposed to IIT prior to the initiation of IFNa therapy (91).
Acknowledgments
This work was supported in part by: DK61659 from NIDDK and a VA Merit Award (to YT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huber A, Menconi F, Corathers S, Jacobson EM, Tomer Y. Joint genetic susceptibility to type 1 diabetes and autoimmune thyroiditis: from epidemiology to mechanisms. Endocr Rev. 2008;29(6):697–725. doi: 10.1210/er.2008-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brix TH, Kyvik KO, Christensen K, Hegedus L. Evidence for a major role of heredity in Graves’ disease: a population- based study of two Danish twin cohorts. J Clin Endocrinol Metab. 2001;86(2):930–934. doi: 10.1210/jcem.86.2.7242. [DOI] [PubMed] [Google Scholar]
- 3.Rose NR, Bonita R, Burek CL. Iodine: an environmental trigger of thyroiditis. Autoimmun Rev. 2002;1(1–2):97–103. doi: 10.1016/s1568-9972(01)00016-7. [DOI] [PubMed] [Google Scholar]
- 4.Li HS, Jiang HY, Carayanniotis G. Modifying effects of iodine on the immunogenicity of thyroglobulin peptides. J Autoimmun. 2007;28(4):171–176. doi: 10.1016/j.jaut.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Oppenheim Y, Ban Y, Tomer Y. Interferon induced Autoimmune Thyroid Disease (AITD): a model for human autoimmunity. Autoimmun Rev. 2004;3(5):388–393. doi: 10.1016/j.autrev.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Tomer Y, Davies TF. Infections and autoimmune endocrine disease. Baillière’s Clin Endocrinol Metab. 1995;9:47–70. doi: 10.1016/s0950-351x(95)80819-1. [DOI] [PubMed] [Google Scholar]
- 7.Tomer Y, Huber A. The etiology of autoimmune thyroid disease: a story of genes and environment. J Autoimmun. 2009;32(3–4):231–239. doi: 10.1016/j.jaut.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomer Y, Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? Am J Med. 2004;117(1):60–61. doi: 10.1016/j.amjmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36(4):1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomer Y, Davies TF. Infection, Thyroid Disease and Autoimmunity. Endocr Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 11.Cox SP, Phillips DI, Osmond C. Does infection initiate Graves disease? A population based 10 year study. Autoimmunity. 1989;4(1–2):43–49. doi: 10.3109/08916938909034358. [DOI] [PubMed] [Google Scholar]
- 12.Phillips DI, Barker DJ, Rees Smith B, Didcote S, Morgan D. The geographical distribution of thyrotoxicosis in England according to the presence or absence of TSH-receptor antibodies. Clin Endocrinol (Oxf) 1985;23:283–287. doi: 10.1111/j.1365-2265.1985.tb00225.x. [DOI] [PubMed] [Google Scholar]
- 13.Valtonen VV, Ruutu P, Varis K, Ranki M, Malkamaki M, Makela PH. Serological evidence for the role of bacterial infections in the pathogenesis of thyroid diseases. Acta Med Scand. 1986;219:105–111. doi: 10.1111/j.0954-6820.1986.tb03283.x. [DOI] [PubMed] [Google Scholar]
- 14.Wenzel BE, Heesemann J, Wenzel KW, Scriba PC. Antibodies to plasmid-encoded proteins of enteropathogenic Yersinia in patients with autoimmune thyroid disease [letter] Lancet. 1988;1(8575–6):56. doi: 10.1016/s0140-6736(88)91034-3. [DOI] [PubMed] [Google Scholar]
- 15.Corapcioglu D, Tonyukuk V, Kiyan M, Yilmaz AE, Emral R, Kamel N, et al. Relationship between thyroid autoimmunity and Yersinia enterocolitica antibodies. Thyroid. 2002;12(7):613–617. doi: 10.1089/105072502320288483. [DOI] [PubMed] [Google Scholar]
- 16.Brix TH, Hansen PS, Hegedus L, Wenzel BE. Too early to dismiss Yersinia enterocolitica infection in the aetiology of Graves’ disease: evidence from a twin case-control study. Clin Endocrinol (Oxf) 2008;69(3):491–496. doi: 10.1111/j.1365-2265.2008.03227.x. [DOI] [PubMed] [Google Scholar]
- 17.Chatzipanagiotou S, Legakis JN, Boufidou F, Petroyianni V, Nicolaou C. Prevalence of Yersinia plasmid-encoded outer protein (Yop) class-specific antibodies in patients with Hashimoto’s thyroiditis. Clin Microbiol Infect. 2001;7(3):138–143. doi: 10.1046/j.1469-0691.2001.00221.x. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer MH, Donadi EA, Tambascia MA, Magna LA, Prigenzi LS. Relationship between HLA antigens and infectious agents in contributing towards the development of Graves’ disease. Immunol Invest. 1998;27(1–2):17–29. doi: 10.3109/08820139809070887. [DOI] [PubMed] [Google Scholar]
- 19.Nagasaka A, Nakai A, Oda N, Kotake M, Iwase K, Yoshida S. Reverse transcriptase is elevated in the thyroid tissue from Graves’ disease patients. Clin Endocrinol (Oxf) 2000;53(2):155–159. doi: 10.1046/j.1365-2265.2000.01051.x. [DOI] [PubMed] [Google Scholar]
- 20.Jaspan JB, Luo H, Ahmed B, Tenenbaum S, Voss T, Sander DM, et al. Evidence for a retroviral trigger in Graves’ disease. Autoimmunity. 1995;20(2):135–142. doi: 10.3109/08916939509001938. [DOI] [PubMed] [Google Scholar]
- 21.Jaspan JB, Sullivan K, Garry RF, Lopez M, Wolfe M, Clejan S, et al. The interaction of a type A retroviral particle and class II human leukocyte antigen susceptibility genes in the pathogenesis of Graves’ disease. J Clin Endocrinol Metab. 1996;81(6):2271–2279. doi: 10.1210/jcem.81.6.8964863. [DOI] [PubMed] [Google Scholar]
- 22.Yokoi K, Kawai H, Akaike M, Mine H, Saito S. Presence of human T-lymphotropic virus type II-related genes in DNA of peripheral leukocytes from patients with autoimmune thyroid diseases. J Med Virol. 1995;45(4):392–398. doi: 10.1002/jmv.1890450407. [DOI] [PubMed] [Google Scholar]
- 23.Tomoyose T, Komiya I, Takara M, Yabiku K, Kinjo Y, Shimajiri Y, et al. Cytotoxic T-lymphocyte antigen-4 gene polymorphisms and human T-cell lymphotrophic virus-1 infection: their associations with Hashimoto’s thyroiditis in Japanese patients. Thyroid. 2002;12(8):673–677. doi: 10.1089/105072502760258640. [DOI] [PubMed] [Google Scholar]
- 24.de Luis DA, Varela C, de La CH, Canton R, de Argila CM, San Roman AL, et al. Helicobacter pylori infection is markedly increased in patients with autoimmune atrophic thyroiditis. J Clin Gastroenterol. 1998;26(4):259–263. doi: 10.1097/00004836-199806000-00008. [DOI] [PubMed] [Google Scholar]
- 25.Figura N, Di Cairano G, Lore F, Guarino E, Gragnoli A, Cataldo D, et al. The infection by Helicobacter pylori strains expressing CagA is highly prevalent in women with autoimmune thyroid disorders. J Physiol Pharmacol. 1999;50(5):817–826. [PubMed] [Google Scholar]
- 26.Benvenga S, Guarneri F, Vaccaro M, Santarpia L, Trimarchi F. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid. 2004;14(11):964–966. doi: 10.1089/thy.2004.14.964. [DOI] [PubMed] [Google Scholar]
- 27.Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, et al. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18(2):253–257. [PubMed] [Google Scholar]
- 28.Ganne-Carrie N, Medini A, Coderc E, Seror O, Christidis C, Grimbert S, et al. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14(2):189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, et al. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158(13):1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 30.Preziati D, La Rosa L, Covini G, Marcelli R, Rescalli S, Persani L, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132(5):587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 31.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 32.Loviselli A, Oppo A, Velluzzi F, Atzeni F, Mastinu GL, Farci P, et al. Independent expression of serological markers of thyroid autoimmunity and hepatitis virus C infection in the general population: results of a community-based study in north-western Sardinia. J Endocrinol Invest. 1999;22(9):660–665. doi: 10.1007/BF03343626. [DOI] [PubMed] [Google Scholar]
- 33.Metcalfe RA, Ball G, Kudesia G, Weetman AP. Failure to find an association between hepatitis C virus and thyroid autoimmunity. Thyroid. 1997;7(3):421–424. doi: 10.1089/thy.1997.7.421. [DOI] [PubMed] [Google Scholar]
- 34.Boadas J, Rodriguez-Espinosa J, Enriquez J, Miralles F, Martinez-Cerezo FJ, Gonzalez P, et al. Prevalence of thyroid autoantibodies is not increased in blood donors with hepatitis C virus infection. J Hepatol. 1995;22(6):611–615. doi: 10.1016/0168-8278(95)80216-9. [DOI] [PubMed] [Google Scholar]
- 35.Indolfi G, Stagi S, Bartolini E, Salti R, De Martino M, Azzari C, et al. Thyroid function and anti-thyroid autoantibodies in untreated children with vertically acquired chronic hepatitis C virus infection. Clin Endocrinol (Oxf) 2008;68(1):117–121. doi: 10.1111/j.1365-2265.2007.03009.x. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe U, Hashimoto E, Hisamitsu T, Obata H, Hayashi N. The risk factor for development of thyroid disease during interferon-alpha therapy for chronic hepatitis C. Am J Gastroenterol. 1994;89(3):399–403. [PubMed] [Google Scholar]
- 37.Carella C, Amato G, Biondi B, Rotondi M, Morisco F, Tuccillo C, et al. Longitudinal study of antibodies against thyroid in patients undergoing interferon-alpha therapy for HCV chronic hepatitis. Horm Res. 1995;44(3):110–114. doi: 10.1159/000184606. [DOI] [PubMed] [Google Scholar]
- 38.Roti E, Minelli R, Giuberti T, Marchelli S, Schianchi C, Gardini E, et al. Multiple changes in thyroid function in patients with chronic active HCV hepatitis treated with recombinant interferon-alpha. Am J Med. 1996;101(5):482–487. doi: 10.1016/s0002-9343(96)00259-8. [DOI] [PubMed] [Google Scholar]
- 39.Marazuela M, Garcia-Buey L, Gonzalez-Fernandez B, Garcia-Monzon C, Arranz A, Borque MJ, et al. Thyroid autoimmune disorders in patients with chronic hepatitis C before and during interferon-alpha therapy. Clin Endocrinol (Oxf) 1996;44(6):635–642. doi: 10.1046/j.1365-2265.1996.751768.x. [DOI] [PubMed] [Google Scholar]
- 40.Carella C, Mazziotti G, Morisco F, Rotondi M, Cioffi M, Tuccillo C, et al. The addition of ribavirin to interferon-alpha therapy in patients with hepatitis C virus-related chronic hepatitis does not modify the thyroid autoantibody pattern but increases the risk of developing hypothyroidism. Eur J Endocrinol. 2002;146(6):743–749. doi: 10.1530/eje.0.1460743. [DOI] [PubMed] [Google Scholar]
- 41.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Ghinoi A, Rotondi M, et al. Thyroid disorders in chronic hepatitis C virus infection. Thyroid. 2006;16(6):563–572. doi: 10.1089/thy.2006.16.563. [DOI] [PubMed] [Google Scholar]
- 42.Benoist C, Mathis D. Autoimmunity. The pathogen connection. Nature. 1998;394(6690):227–228. doi: 10.1038/28282. [DOI] [PubMed] [Google Scholar]
- 43.Oldstone MBA. Molecular mimicry and autoimmune diseases. Cell. 1987;50:819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- 44.Shenkman L, Bottone EJ. Antibodies to Yersinia enterocolitica in thyroid disease. Ann Intern Med. 1976;85:735–739. doi: 10.7326/0003-4819-85-6-735. [DOI] [PubMed] [Google Scholar]
- 45.Fournie GJ, Mas M, Cautain B, Savignac M, Subra JF, Pelletier L, et al. Induction of autoimmunity through bystander effects. Lessons from immunological disorders induced by heavy metals. J Autoimmun. 2001;16(3):319–326. doi: 10.1006/jaut.2000.0482. [DOI] [PubMed] [Google Scholar]
- 46.Arata N, Ando T, Unger P, Davies TF. By-stander activation in autoimmune thyroiditis: studies on experimental autoimmune thyroiditis in the GFP+ fluorescent mouse. Clin Immunol. 2006;121(1):108–117. doi: 10.1016/j.clim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Bartolome J, Rodriguez-Inigo E, Quadros P, Vidal S, Pascual-Miguelanez I, Rodriguez-Montes JA, et al. Detection of hepatitis C virus in thyroid tissue from patients with chronic HCV infection. J Med Virol. 2008;80(9):1588–1594. doi: 10.1002/jmv.21269. [DOI] [PubMed] [Google Scholar]
- 48.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188(8):1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 49.Balasubramanian A, Ganju RK, Groopman JE. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J Infect Dis. 2006;194(5):670–681. doi: 10.1086/505708. [DOI] [PubMed] [Google Scholar]
- 50.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278(37):35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 51.Akeno N, Blackard JT, Tomer Y. HCV E2 protein binds directly to thyroid cells and induces IL-8 production: a new mechanism for HCV induced thyroid autoimmunity. J Autoimmun. 2008;31(4):339–344. doi: 10.1016/j.jaut.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfeffer LM, Dinarello CA, Herberman RB, Williams BR, Borden EC, Bordens R, et al. Biological properties of recombinant alpha-interferons: 40th anniversary of the discovery of interferons. Cancer Res. 1998;58(12):2489–2499. [PubMed] [Google Scholar]
- 53.Parmar S, Platanias LC. Interferons: mechanisms of action and clinical applications. Curr Opin Oncol. 2003;15(6):431–439. doi: 10.1097/00001622-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 54.Jonasch E, Haluska FG. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6(1):34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 55.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenhaver DH, Niesel DW, Klimpel GR, et al. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266(10):1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]
- 56.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Goncales FL, Jr, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 57.Russo MW, Fried MW. Side effects of therapy for chronic hepatitis C. Gastroenterology. 2003;124(6):1711–1719. doi: 10.1016/s0016-5085(03)00394-9. [DOI] [PubMed] [Google Scholar]
- 58.Fentiman IS, Thomas BS, Balkwill FR, Rubens RD, Hayward JL. Primary hypothyroidism associated with interferon therapy of breast cancer. Lancet. 1985;1(8438):1166. doi: 10.1016/s0140-6736(85)92475-4. [DOI] [PubMed] [Google Scholar]
- 59.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: Toward a new classification. Hepatology. 2006;43(4):661–672. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 60.Martocchia A, Labbadia G, Paoletti V, Gargano S, Grossi A, Trabace S, et al. Hashimoto’s disease during interferon-alpha therapy in a patient with pre-treatment negative anti-thyroid autoantibodies and with the specific genetic susceptibility to the thyroid disease. Neuro Endocrinol Lett. 2001;22(1):49–52. [PubMed] [Google Scholar]
- 61.Baudin E, Marcellin P, Pouteau M, Colas-Linhart N, Le Floch JP, Lemmonier C, et al. Reversibility of thyroid dysfunction induced by recombinant alpha interferon in chronic hepatitis C. Clin Endocrinol (Oxf) 1993;39(6):657–661. doi: 10.1111/j.1365-2265.1993.tb02423.x. [DOI] [PubMed] [Google Scholar]
- 62.Carella C, Mazziotti G, Morisco F, Manganella G, Rotondi M, Tuccillo C, et al. Long-term outcome of interferon-alpha-induced thyroid autoimmunity and prognostic influence of thyroid autoantibody pattern at the end of treatment. J Clin Endocrinol Metab. 2001;86(5):1925–1929. doi: 10.1210/jcem.86.5.7459. [DOI] [PubMed] [Google Scholar]
- 63.Wong V, Fu AX, George J, Cheung NW. Thyrotoxicosis induced by alpha-interferon therapy in chronic viral hepatitis. Clin Endocrinol (Oxf) 2002;56(6):793–798. doi: 10.1046/j.1365-2265.2002.01553.x. [DOI] [PubMed] [Google Scholar]
- 64.Lisker-Melman M, Di Bisceglie AM, Usala SJ, Weintraub B, Murray LM, Hoofnagle JH. Development of thyroid disease during therapy of chronic viral hepatitis with interferon alfa. Gastroenterology. 1992;102(6):2155–2160. doi: 10.1016/0016-5085(92)90348-3. [DOI] [PubMed] [Google Scholar]
- 65.Imagawa A, Itoh N, Hanafusa T, Oda Y, Waguri M, Miyagawa J, et al. Autoimmune endocrine disease induced by recombinant interferon-alpha therapy for chronic active type C hepatitis. J Clin Endocrinol Metab. 1995;80(3):922–926. doi: 10.1210/jcem.80.3.7883851. [DOI] [PubMed] [Google Scholar]
- 66.Weetman AP, Smallridge RC, Nutman TB, Burman KD. Persistent thyroid autoimmunity after subacute thyroiditis. J Clin Lab Immunol. 1987;23:1–6. [PubMed] [Google Scholar]
- 67.Parana R, Cruz M, Lyra L, Cruz T. Subacute thyroiditis during treatment with combination therapy (interferon plus ribavirin) for hepatitis C virus. J Viral Hepat. 2000;7(5):393–395. doi: 10.1046/j.1365-2893.2000.00247.x. [DOI] [PubMed] [Google Scholar]
- 68.Caraccio N, Giannini R, Cuccato S, Faviana P, Berti P, Galleri D, et al. Type I interferons modulate the expression of thyroid peroxidase, sodium/iodide symporter, and thyroglobulin genes in primary human thyrocyte cultures. J Clin Endocrinol Metab. 2005;90(2):1156–1162. doi: 10.1210/jc.2004-1173. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen KB, Watford WT, Salomon R, Hofmann SR, Pien GC, Morinobu A, et al. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science. 2002;297(5589):2063–2066. doi: 10.1126/science.1074900. [DOI] [PubMed] [Google Scholar]
- 70.Corssmit EP, de Metz J, Sauerwein HP, Romijn JA. Biologic responses to IFN-alpha administration in humans. J Interferon Cytokine Res. 2000;20(12):1039–1047. doi: 10.1089/107999000750053690. [DOI] [PubMed] [Google Scholar]
- 71.You X, Teng W, Shan Z. Expression of ICAM-1, B7.1 and TPO on human thyrocytes induced by IFN-alpha. Chin Med J (Engl) 1999;112(1):61–66. [PubMed] [Google Scholar]
- 72.Farrar JD, Murphy KM. Type I interferons and T helper development. Immunol Today. 2000;21(10):484–489. doi: 10.1016/s0167-5699(00)01710-2. [DOI] [PubMed] [Google Scholar]
- 73.Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112(3):1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- 74.Corssmit EP, Heijligenberg R, Hack CE, Endert E, Sauerwein HP, Romijn JA. Effects of interferon-alpha (IFN-alpha) administration on leucocytes in healthy humans. Clin Exp Immunol. 1997;107(2):359–363. doi: 10.1111/j.1365-2249.1997.269-ce1161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krause I, Valesini G, Scrivo R, Shoenfeld Y. Autoimmune aspects of cytokine and anticytokine therapies. Am J Med. 2003;115(5):390–397. doi: 10.1016/s0002-9343(03)00390-5. [DOI] [PubMed] [Google Scholar]
- 76.Lindahl P, Leary P, Gresser I. Enhancement by interferon of the expression of surface antigens on murine leukemia L 1210 cells. Proc Natl Acad Sci U S A. 1973;70(10):2785–2788. doi: 10.1073/pnas.70.10.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akeno N, Tomer Y. Dissecting the mechanisms of interferon induced thyroiditis (IIT): Direct effects of interferon alpha on thyroid epithelial cells. The 89th Meeting of the Endocrine Society; Toronto, Canda. June 2007; 2007. [Google Scholar]
- 78.Caraccio N, Cuccato S, Pratesi F, Dardano A, Ursino S, Chimenti D, et al. Effect of type I interferon(s) on cell viability and apoptosis in primary human thyrocyte cultures. Thyroid. 2009;19(2):149–155. doi: 10.1089/thy.2008.0290. [DOI] [PubMed] [Google Scholar]
- 79.Tomer Y, Davies TF. Searching for the autoimmune thyroid disease susceptibility genes: From gene mapping to gene function. Endocr Rev. 2003;24:694–717. doi: 10.1210/er.2002-0030. [DOI] [PubMed] [Google Scholar]
- 80.Dalgard O, Bjoro K, Hellum K, Myrvang B, Bjoro T, Haug E, et al. Thyroid dysfunction during treatment of chronic hepatitis C with interferon alpha: no association with either interferon dosage or efficacy of therapy. J Intern Med. 2002;251(5):400–406. doi: 10.1046/j.1365-2796.2002.00974.x. [DOI] [PubMed] [Google Scholar]
- 81.Prummel MF, Laurberg P. Interferon-alpha and autoimmune thyroid disease. Thyroid. 2003;13(6):547–551. doi: 10.1089/105072503322238809. [DOI] [PubMed] [Google Scholar]
- 82.Grossman CJ, Roselle GA, Mendenhall CL. Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol. 1991;40(4–6):649–659. doi: 10.1016/0960-0760(91)90287-f. [DOI] [PubMed] [Google Scholar]
- 83.Ban Y, Greenberg DA, Davies TF, Jacobson E, Concepcion E, Tomer Y. Linkage analysis of thyroid antibody production: evidence for shared susceptibility to clinical autoimmune thyroid disease. J Clin Endocrinol Metab. 2008;93(9):3589–3596. doi: 10.1210/jc.2008-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oppenheim Y, Kim G, Ban Y, Unger P, Concepcion E, Ando T, et al. The effects of alpha interferon on the development of autoimmune thyroiditis in the NOD H2h4 mouse. Clin Dev Immunol. 2003;10(2–4):161–165. doi: 10.1080/10446670310001642177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: From epidemiology to etiology. J Autoimmun. 2008;30(1–2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ban Y, Tozaki T, Tobe T, Ban Y, Jacobson EM, Concepcion ES, et al. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: An association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28:201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Kakizaki S, Takagi H, Murakami M, Takayama H, Mori M. HLA antigens in patients with interferon-alpha-induced autoimmune thyroid disorders in chronic hepatitis C. J Hepatol. 1999;30(5):794–800. doi: 10.1016/s0168-8278(99)80131-7. [DOI] [PubMed] [Google Scholar]
- 89.Kryczka W, Brojer E, Kowalska A, Zarebska-Michaluk D. Thyroid gland dysfunctions during antiviral therapy of chronic hepatitis C. Med Sci Monit. 2001;7 (Suppl 1):221–225. [PubMed] [Google Scholar]
- 90.Jacobson EM, Chaudhry S, Mandac JC, Concepcion E, Tomer Y. Immune-regulatory gene involvement in the etiology of interferon induced thyroiditis (IIT) Thyroid. 2006;16:926. [Google Scholar]
- 91.Ross CJ, Katzov H, Carleton B, Hayden MR. Pharmacogenomics and its implications for autoimmune disease. J Autoimmun. 2007;28(2–3):122–128. doi: 10.1016/j.jaut.2007.02.008. [DOI] [PubMed] [Google Scholar]