Abstract
Neonatal bladder inflammation has been demonstrated to produce hypersensitivity to bladder re-inflammation as an adult. The purpose of this study was to investigate the effects of neonatal urinary bladder inflammation on adult bladder function and structure. Female Sprague-Dawley rats were treated on postnatal days 14-16 with intravesical zymosan or anesthesia alone. At 12-16 weeks of age, micturition frequency and cystometrograms were measured. Similarly treated rats had their bladders removed for measurement of plasma extravasation following intravesical mustard oil, for neuropeptide analysis (CGRP or SubP), or for detailed histological examination. Rats treated with zymosan as neonates exhibited increased micturition frequency, reduced micturition volume thresholds, greater extravasation of Evan's Blue following intravesical mustard oil administration, and greater total bladder content of CGRP and SubP. In contrast, there were no quantitative histological changes in the thickness, fibrosis or mast cells of bladder tissue due to neonatal zymosan treatments. Functional changes in urologic systems observed in adulthood, coupled with the increased neuropeptide content and neurogenic plasma extravasation in adult bladders, suggest that the neonatal bladder inflammation treatment enhanced the number, function and/or neurochemical content of primary afferent neurons. These data support the hypothesis that insults to the urologic system in infancy may contribute to the development of adult bladder hypersensitivity.
Perspective
Inflammation of the bladder early in life in the rat has multiple sequelae including laboratory measures that suggest an alteration of the neurophysiological substrates related to the bladder. Some painful bladder syndromes in humans have similar characteristics and so may be due to similar mechanisms.
Keywords: developmental, visceral nociception, hyperalgesia, interstitial cystitis
Introduction
Experience with pain or injury can result in short-term and long-term alterations in an organism's responses to noxious stimuli. When such experiences occur early in life, particularly during the development of nociceptive and antinociceptive systems, these alterations may become permanent. Epidemiological studies in humans have identified that hospitalizations or medical visits, particularly during infancy and when coupled with painful events, may be associated with chronic pains in adulthood5,15,28,47. Formal testing of pain sensitivity has demonstrated that children who were repeatedly subjected to painful procedures as infants have altered pain sensitivity when tested later in life13,45,48,51, manifesting either hypo- or hyperalgesia, depending on the age at time of injury and magnitude of the injury.
Preclinical studies of cutaneous systems in rats and mice have demonstrated long-lasting behavioral, physiological, gene-expression and neuroanatomical changes following exposure to inflammation during the neonatal period9. These are particularly apparent following surgery6 or with re-inflammation as an adult3,14,24,32,36,37,39,44. Similar alterations have been demonstrated in gastrointestinal systems; neonatal rat pups exposed to repeated gastric or colonic irritation demonstrate augmented behavioral and physiological responses to non-noxious and noxious distension in adulthood1,25,26,42,49. Notably, hypersensitivity in some studies occurred in the absence of re-inflammation during adulthood and without identifiable ongoing histological changes. In the studies by Al-Chaer et al. and Ruda et al. older rats presented with inflammatory stimuli did not develop chronic hypersensitivity1,39. This suggests that the neonatal period is a particularly vulnerable time during which stimulation may produce long-term changes in visceral sensory systems since it is a period when nociceptive and antinociceptive circuits are developing and noxious stimuli are normally of low intensity or absent altogether.
Our laboratory30 and others2 have conducted similar studies of urological systems using a model of bladder nociception in which urinary bladder distension is used to evoke reliable and reproducible cardiovascular and visceromotor responses (VMRs) that are inhibited by analgesics. Zymosan, a mycotic inflammogen, produces robust cystitis when administered intravesically as demonstrated by the presence of significant plasma extravasation for up to 24 hrs following intravesical bladder administration34. A mild augmentation of VMRs in response to urinary bladder distension occurs in response to zymosan-induced bladder inflammation in adults34. However, zymosan-induced bladder inflammation during the neonatal period leads to robustly enhanced VMRs to pressure-controlled urinary bladder distension (UBD) following acute re-inflammation of the bladder in adulthood35 as well as increased VMRs to intravesical cold stimuli in the absence of inflammation33. Similar to the findings of Al-Chaer and colleagues, zymosan-induced bladder inflammation in adolescent rats has no long-term consequences35. Increased sensitivity of the urinary bladder during adulthood to distension as a sequela of early-in-life inflammatory events could involve multiple mechanisms, including chronic histological changes in bladder structure due to inflammation and alterations of the neural substrates involved with bladder sensation. Evidence of chronic inflammatory changes would include fibrosis and potentially increased mast cell number. This latter possibility has been noted on bladder biopsies in a subset of adult women diagnosed with interstitial cystitis (IC)31 and has been proposed as a source of bladder hypersensitivity41. Alterations in urologic sensory processing as a result of neonatal injury may be an important consideration for understanding the development of painful bladder syndromes such as IC, since they are typically characterized by urinary frequency and urgency and accompanied by pelvic/suprapubic pain. Epidemiologic studies have reported that a subset of adult women diagnosed with IC recall experiencing early-in-life bladder symptoms18.
The primary goal of the current study was to investigate whether changes occur in adult bladder structure and function as a result of neonatal bladder inflammation. This was done by examining the effects of this inflammation on functional responses in the “baseline” state: that without adult re-exposure to inflammation. A secondary goal was to begin examining potential mechanisms that contribute to long-term changes induced by neonatal bladder inflammation through neurochemical and histological examination of adult bladder tissue and by quantification of neurogenic plasma extravasation in adulthood following neonatal treatment.
Materials and Methods
General
Timed-pregnant Sprague-Dawley rats were obtained (Harlan; Prattville, AL) and housed individually on a 6AM-6PM light-dark cycle. After birth, female pups were maintained and randomly assigned to Neonatal Zymosan or Neonatal Anesthesia groups, and subsequently given treatments for three consecutive days beginning on postnatal day 14 (P14-P16). This time period is generally accepted as the end of the neonatal period and is equivalent to infancy or early childhood in humans. Pups remained housed with their mothers during treatments until weaning at P21 and were subsequently housed two to three per cage. All studies were approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and conform to the guidelines of the International Association for the Study of Pain.
Early-in-life procedures
Female rat pups were halothane-anesthetized (5% induction, 1-2% maintenance) via mask on days P14-P16, had their urethras swabbed with iodine-povidone solution and were kept warm with a heating pad. On each day of treatment the Neonatal Zymosan groups had a 24-gauge angiocatheter placed transurethrally into the urinary bladder and secured with surgical tape. Intravesical zymosan (0.1 ml; 1% solution in saline; Sigma-Aldrich, St. Louis) was administered, allowed to dwell for 30 min then drained prior to catheter removal. The Neonatal Anesthesia groups remained anesthetized for 30 min but had no intravesical catheter placement or treatment. All rats were given ampicillin (50 mg/kg, s.c.) on each treatment day. An intravesical saline group was not examined as previous studies demonstrated no physiologically important differences between saline-treated groups and those treated with anesthesia alone35.
Adult procedures
All rats were between 12-16 wks of age and 215-320 g at the time of adult testing. All testing procedures were performed during daylight hours except for micturition frequency which was assessed during the 12 hr dark period. Five Neonatal Zymosan subgroups of rats and five Neonatal Anesthesia subgroups of rats were used: one set for tests of somatic sensitivity, micturition frequency and transurethral cannulation CMG measures; a second set for transabdominal cannulation CMG measures; a third set for studies of mustard oil-induced neurogenic plasma extravasation; a fourth set for bladder neuropeptide analysis; and the final set for histological examination of the bladder. To minimize variability in responses17, CMG testing was performed in most rats during proestrus as assessed by daily vaginal smears for at least two cycles per the method of Long and Evans27. Effects of the estrous cycle were not formally studied and so estrous cycle was not controlled for in the neuropeptide analysis studies, in the histological studies or in those of somatic sensitivity or micturition frequency.
Somatic sensitivity
Thermal sensitivity was assessed using the method described by Hargreaves et al12. Rats were confined within a plexiglass cage (24cm × 45cm × 15cm) on an elevated piece of glass 3 mm thick. A radiant heat source (50W projector lamp) was positioned beneath the glass floor and focused to strike the glabrous skin of the hindpaw. The latency to withdraw the hindpaw during stimulation, defined as the Hindpaw Thermal Threshold, was recorded to the nearest 0.1 s. One trial was conducted on each hindpaw and the mean was used for statistical analysis. Mechanical sensitivity was assessed using a set of calibrated von Frey nylon monofilaments, which required 0.27-250 g of pressure to bend. Filaments were applied in a graded manner to the lateral edge of each hindpaw until it was withdrawn. The Hindpaw Mechanical Threshold was defined as the stimulus that elicited a hindpaw withdrawal reflex on 2 of 3 applications. The mean of the response thresholds from the left and right hindpaws was used for statistical analysis.
Rate of Micturition
Micturition frequency was determined by placing each rat in a metabolic cage with ad libitum access to food and water for a 12 hr dark period. The frequency of micturition episodes was measured by counting the number of urine spots collected on moving blotter paper beneath the cages similar to previous studies35.
Cystometrograms (CMGs)
Neonatal Zymosan or Neonatal Anesthesia rats underwent CMG testing using either a transurethral or transabdominal cannulation procedure similar to methods described previously.e.g.38,43 Prior to cannulation, all rats were anesthetized with a dose of urethane (1.25 g/kg, i.p.) and allowed to breathe spontaneously throughout surgical preparation and testing. Body temperature was maintained at ~37°C throughout testing using a heating pad. For the transurethral preparation, a 22-gauge angiocatheter was placed into the bladder via the urethra and secured with a purse-string suture around the distal urethral orifice. Free flow of blood-free urine was ensured following catheter placement. For transabdominal cannulation, a 2 cm midline abdominal incision was made followed by a small incision in the bladder dome. Saline-filled PE50 tubing was inserted and held in place with a purse-string suture. In both preparations, following surgery the bladder was emptied and room-temperature saline was slowly infused (0.05 ml/min). Intravesical pressure was measured using an in-line, low-volume pressure transducer and T-assembly. Baseline intravesical pressure was recorded for one min prior to the start of each infusion. Micturition Threshold (MT) was defined as the intravesical volume immediately preceding the onset of the first contractile response of magnitude ≥10 mmHg. Rats not exhibiting a response ≥10 mmHg during the infusion period were assigned the maximal MT value of 1.0 ml.
Visceromotor Reflexes
Abdominal electromyograms (EMG) were recorded in rats that underwent transurethral CMG testing. In these rats, platinum EMG wires were sutured through the right abdominal oblique musculature superior to the inguinal ligament and a femoral arterial catheter was inserted. Rectified EMG responses were quantified for each rat as change from baseline (mean rectified EMG during infusion period – mean baseline rectified EMG) and are presented as group mean Δ score ± SEM.
Tissue preparation for histological evaluation
Bladders from rats in the Neonatal Zymosan or Neonatal Anesthesia groups were histologically examined for evidence of altered bladder morphology. At 12 wks of age, rats were euthanized by exsanguination while anesthetized with isoflurane (5%) and the urinary bladder was excised. Bladder tissue was fixed in 10% formalin for 24 hrs. Then, each bladder was blotted dry and weighed prior to being sectioned transversely. Paraffin-embedded transverse sections (8μm) were cut and mounted on slides. Three slides with four slices of tissue each were made from the bladder base and midsection from each animal. Each slide was then stained with either [1] hematoxylin and eosin for gross histological examination, [2] Gomori's trichrome for evaluation of chronic fibrosis in the form of elevated collagen content (dyed green), or [3] toluidine blue in order to assess for mast cells. The histological preparations were performed by the Histopathology Core Service of the UAB Comparative Pathology Laboratory. For each slice of tissue, digital micrographs were taken using a Leica microscope and camera (Hamamatsu, Japan) with a 2.5X and 10X objective lens. Computer images were examined using Image J software (NIH, Bethesda, MD) by an observer blinded to the treatment group. Tissue thickness measures represent means of measurements taken from eight different slices (four from base of bladder, four from midsection) unless one of these two subregions is stated. Endothelial, lamina propria and submucosal thicknesses were measured at sites between villi. Muscularis thickness is a composite measure of all muscle layers taken at two different sites per slice in the midsections and at the thickest muscular layer (detrusor) in the basal sections.
Percent connective tissue was measured as the area of green pixels in Gomori's Trichrome stained slides as a percentage of total area of tissue; the measure for each rat was the mean of measures in four transverse sections at either the base of the bladder or in the mid-section. Mast cell counts were performed using high power evaluation in a similar number of transverse sections stained with Toluidine blue and quantified per whole slice and within the lamina propria of subregions.
Neurogenic plasma extravasation
Acute neurogenic plasma extravasation evoked by the intravesical administration of the TRPA1 receptor agonist mustard oil, was assessed in adult rats in order to determine whether neonatal inflammation produced changes in the magnitude of this neuropeptide-induced phenomena in adulthood. Rats were anesthetized with urethane (1.25 mg/kg, i.p.). Using the method of Koltzenburg and McMahon19, a bolus of Evan's Blue (EB; 50 mg/kg) was administered via jugular vein and after 10 min of circulation, intravesical mustard oil (0.2 ml, 2.5% in olive oil) was administered via a 22-gauge transurethral catheter and allowed to dwell for 30 min. Rats were then administered pentobarbital (100 mg/kg, i.v.), perfused transcardially with normal saline (500 ml), and bladders were blotted dry and placed in 10 ml of dimethyl sulfoxide for EB extraction. The amount of EB was quantified spectrophotometrically (620 nm) and normalized to the dry weight of the bladder tissue.
Neuropeptide Analysis
Rats were anesthetized with inhaled isoflurane (5%) and euthanized by exsanguination. Urinary bladders were immediately removed and frozen in liquid nitrogen. Subsequently bladders were weighed and homogenized in 5 μl/mg of extraction buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.5% deoxycholic acid, protease inhibitor cocktail) and centrifuged at 14,000 X g for 10 min at 4°C. The supernatant was removed and total protein was measured using the Pierce BCA Protein Assay Reagent Kit (Rockford, IL, USA). Aliquots of 200 μg protein were used for ELISA analysis using standard ELISA kits for Calcitonin Gene Related Peptide (CGRP) and Substance P (SubP) (Phoenix Pharmaceuticals, Inc., Burlingame, CA). Total bladder content of each neuropeptide was calculated.
Statistical analysis
Statistics are presented as group mean ± standard error of the mean (SEM) unless otherwise stated. Independent sample t-tests were used to analyze thermal somatic response thresholds, micturition frequency, Δ rectified EMG, EB extravasation, neurochemical content and histological measures. MTs and mechanical somatic response thresholds were compared using Mann-Whitney U tests, and the percentage of rats from each treatment group exhibiting contractile responses of magnitude ≥10 mmHg during CMG was compared using a chi-square test of independence. Statistical significance for all analyses was set at p<0.05.
Results
Bladder Weights
A comparison of bladder weights in the multiple subexperiments did not demonstrate any significant differences between Neonatal Zymosan and Neonatal Anesthesia rats except for an isolated difference noted between the two treatment groups undergoing neuropeptide analysis; larger bladders were present in that particular Neonatal Zymosan subgroup.
Somatic nociceptive measures
Previous studies have demonstrated that bladder inflammatory events can alter somatic nociceptive measures in adult rats,16 and so somatic sensory testing was performed in the current study to examine these phenomena. Somatic sensitivity did not differ between groups (Table 1).
Table 1.
Effect of Neonatal Bladder Treatments on Measures of Nociception and Cystometric Measures
| Measure | Neonatal Zymosan | Neonatal Anesthesia |
|---|---|---|
| Somatic Nociceptive Measures | ||
| N | 7 | 8 |
| Hindpaw Thermal Threshold (sec) | 8.4 ± 0.5 | 8.2 ± 0.2 |
| Hindpaw Mechanical Threshold (gm) | 128.5 ± 7.6 | 129.7 ± 18.6 |
| Visceral Nociceptive Measures | ||
| N | 7 | 10 |
| Abdominal Electromyographic Activity During Bladder Filling (Δ rectified mV from baseline) | 270 ± 100* | −70 ± 110 |
| Transurethral Cystometric Measures | ||
| N | 13 | 12 |
| Micturition Volume Threshold (ml) | 0.80 ± 0.09* | 0.99 ± 0.01 |
| Resting Pressure (mm Hg) | 2.626± 0.362 | 2.568 ± 0.298 |
| Transabdominal Cystometric Measures | ||
| N | 5 | 5 |
| Micturition Volume Threshold (ml) | 0.150 ± 0.052* | 0.488 ± 0.144 |
| Resting Pressure (mm Hg) | 8.935 ± 2.331 | 7.202 ± 1.895 |
| Peak Pressure (mm Hg) | 32 ± 1 | 31 ± 3 |
Values are Mean ± SEM
indicates statistically significant difference from Neonatal Anesthesia group with with p<0.05; Student's t-test and Mann-Whitney U tests
Micturition frequency
Micturition frequency of adult rats in the Neonatal Zymosan and Neonatal Anesthesia groups is presented in Figure 1A. Rats in the Neonatal Zymosan group had a significantly greater number of micturition episodes compared to the Neonatal Anesthesia group during the 12 hr night-time measure.
Figure 1.
Effects of neonatal intravesical zymosan. Female rats were anesthetized on days P14-16 of life and treated with either intravesical zymosan (Zymosan) or only anesthetized (Anesthesia) which resulted in multiple changes as adults including increased spontaneous micturition (A), increased neurogenic plasma extravasation produced by intravesical mustard oil and measured as an increase in the extravasation of intravascular Evan's Blue dye (B) and increased total bladder content of the neuropeptides CGRP (C) and Substance P (D). Number of animals studied are as indicated. * indicates a statistically significant difference (p<0.05)
Cystometric testing
Cystometric measures for adult rats in the Neonatal Zymosan and Neonatal Anesthesia groups are presented in Table 1. Neonatal Zymosan treated rats exhibited significantly lower group mean MTs compared to those treated with anesthesia using both the transurethral and transabdominal cannulation techniques. A significantly greater percentage of rats from the zymosan treatment group exhibited contractile responses ≥10 mmHg during transurethral CMG testing compared to the anesthesia treatment group (55% versus 8%, respectively; χ2(1)=6.33, p<.05). During transabdominal CMG testing all rats from both neonatal treatment groups exhibited contractile responses ≥10 mmHg which allowed for a measure of mean peak pressures during contractions. The MTs were significantly lower in both neonatal treatment groups when measured using a transabdominal catheter as opposed to the transurethral catheter (P<0.01; Student's t-test), consistent with previous published literature43. The peak pressure measures did not differ statistically between the two neonatal treatment groups. Resting pressures did not differ statistically between the two neonatal treatment groups although the resting pressures obtained using the transabdominal cannulation technique were statistically lower than those measured using the transurethral cannulation technique. EMG responses were reliably measured in rats that underwent transurethral CMG testing. Group mean Δ rectified EMG data during urinary bladder distension for adult rats treated with zymosan or anesthesia as neonates are presented in Table 1. Rats in the Neonatal Zymosan group demonstrated enhanced EMG responses during bladder filling relative to the one min baseline period, and this difference was significantly greater in comparison with the responses during filling from the Neonatal Anesthesia group. Group mean EMG responses from Neonatal Zymosan and Neonatal Anesthesia groups during the one min baseline period (pre-CMG) did not differ.
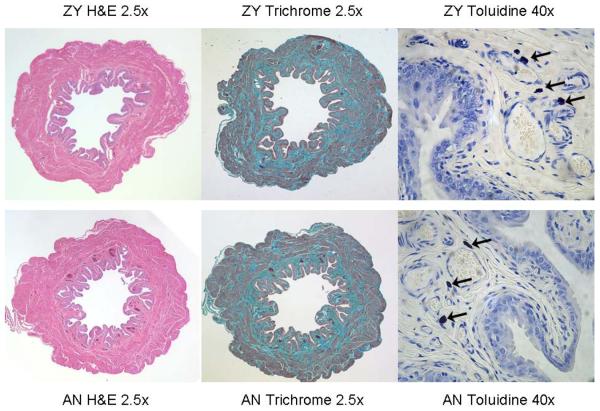
Histological measures
Table 2 presents histological data from adult rats in the Neonatal Zymosan and Neonatal Anesthesia groups. Typical examples of stained tissues are given in Figure 2. Data from hematoxylin and eosin-stained tissue indicated that neonatal zymosan treatment did not result in any statistically significant alterations in either the number of urothelial cell layers or the thickness of any structural layers within the bladders obtained from adult rats when compared with those of rats in the Neonatal Anesthesia group. There also were no significant differences between groups in the ratio of connective tissue to total bladder area as indicated by data from Gomori's Trichrome-stained tissue or in the total number of mast cells in each tissue specimen as indicated by Toluidine Blue, although there was a trend for mast cell numbers in the basal transverse sections to be increased in the Neonatal Zymosan group.
Table 2.
Histological measures of bladder tissue in adult rats that received neonatal treatments
| Measure | Neonatal Zymosan (n=6) |
Neonatal Anesthesia (n=6) |
|---|---|---|
| # urothelial layers1 | 3.1 ± 0.3 | 3.0 ± 0.2 |
| Urothelial thickness (mm)1 | 0.033 ± 0.004 | 0.032 ± 0.003 |
| Laminae Propria thickness (mm)1 | 0.023 ± 0.003 | 0.023 ± 0.002 |
| Submucosa thickness (mm)1 | 0.071 ± 0.006 | 0.074 ± 0.006 |
| Muscularis thickness-midsection (mm)1 | 0.60 ± 0.05 | 0.57 ± 0.03 |
| Muscularis thickness-base (mm)1 | 0.76 ± 0.04 | 0.73 ± 0.04 |
| Percent connective tissue - midsection2 | 42.4 ± 2.5 | 42.9 ± 2.2 |
| Percent connective tissue - base2 | 47.3 ± 2.1 | 48.9 ± 2.0 |
| Mast cells-midsection-total (#/slice)3 | 26 ± 4 | 24 ± 2 |
| Mast cells-base-total (#/slice)3 | 38 ± 7 | 29 ± 5 |
| Mast cells-midsection-lamina propria (#/slice)3 | 9 ± 1 | 8 ± 2 |
| Mast cells-base-lamina propria (#/slice)3 | 9 ± 1 | 8 ± 1 |
Values are Mean ± SEM; there were no statistical differences between groups
All measurements performed by observer blinded to treatment group on photo images using NIH Image J software. Additional methods described in text.
measured in Hematolylin & Eosin stained slides
measured in Gomori's Trichrome stained slides
measured in Toluidine Blue stained slides
Figure 2.
Examples of Hematoxylin & Eosin (left panels), Gomori's Trichrome (middle panels) and Toluidine Blue (right panels) stains in Neonatal Zymosan (upper panels) and Neonatal Anesthesia (lower panels) treatment groups. Arrows in panels at right indicate individual mast cells. Comparisons of the two treatment groups demonstrated no quantitative differences.
Neurogenic plasma extravasation
Rats in the Neonatal Zymosan group had significantly greater plasma extravasation of EB following administration of mustard oil as adults than those in the Neonatal Anesthesia group (Figure 1B) indicating a greater neuropeptide-induced vascular permeability.
Neuropeptide Content
Both total bladder CGRP and SubP content were significantly greater in rats in the Neonatal Zymosan groups than in the Neonatal Anesthesia groups (Figures 1C&1D).
Discussion
The most important finding of the present study was that neonatal urinary bladder inflammation resulted in adult bladder hypersensitivity in the absence of gross histological changes. This finding is of clinical significance since urinary symptoms (urgency, frequency) that accompany chronic conditions such as painful IC may similarly occur in the absence of obvious urinary bladder histopathology. Patients with IC have altered micturition with low volume bladder capacity and increased frequency similar to rats in the present study that experienced neonatal urinary bladder inflammation.
Previous work from our laboratory has demonstrated that neonatal exposure to urinary bladder inflammation significantly enhances bladder nociception in adulthood following re-inflammation of the bladder35. This phenomenon relates, in part, to failed inhibitory control systems associated with bladder sensation8. The present studies confirm our previous findings and provide evidence of functional bladder hypersensitivity even in the absence of re-exposure to bladder inflammation as an adult. This finding is similar to that noted by Al-Chaer et al. in studies of colonic nociception1,25 and others in studies of gastric nociception26,42 where increased responsiveness to distension was noted without any additional treatment. We did not observe functional changes in somatic nociceptive systems using measures of hindpaw sensation. This is also consistent with our previous findings.35
In the present studies, bladder hypersensitivity was manifested as increased micturition frequency and reduced MTs with increased abdominal EMG responses during volume-controlled CMG testing. The most parsimonious explanation for the increased micturition frequency is that it is due to the decreased bladder capacity which was measured as the reduced MT. It is unlikely that the increase in micturition frequency observed in the Neonatal Zymosan group was due to increased water intake since we have observed similar increases in micturition frequency in animals tested as part of an ongoing study (data not shown here) but have failed to observe differences in the volume of water intake between neonatal treatment groups. Likewise, chronic inflammatory histological changes in adult bladder tissue following neonatal zymosan treatment did not account for this hypersensitivity as there was no evidence of chronic fibrosis and the number of mast cells was not significantly increased. This leaves neurogenic mechanisms as the most likely source of hypersensitivity.
This view is supported by evidence that alterations in the neural substrates associated with bladder sensation were produced by the neonatal zymosan treatments. Neonatal zymosan treatment resulted in enhanced EB extravasation from adult bladder tissue following administration of intravesical mustard oil, indicating enhanced neurogenically-mediated plasma extravasation. This extravasation has been mechanistically related to the release of primary afferent neurotransmitters10,19 and so indicates that there was a difference in either the number, chemosensitivity or content of vasoactive neurotransmitters within the bladder. An increase in both CGRP and SubP content, the two main neuropeptides of bladder afferents, was confirmed in the present study using an ELISA analysis of bladder tissue. A difference in neuropeptide content could be due to either the presence of more primary afferents within the bladder tissue containing similar amounts of these neuropeptides or the same number of primary afferents each containing more these neuropeptides. These results are congruent with observations in humans with interstitial cystitis of primary afferent nerve proliferation at suburothelial sites7. Quantitative immunohistochemical studies of bladder CGRP and SubP containing afferents which are ongoing in our laboratory have results to date suggesting an increase in subendothelial neuropeptide content (unpublished results). Neuropeptide content has been demonstrated to change with other bladder manipulations, such as outlet obstruction in juvenile guinea pigs4. However, neuropeptide changes with bladder outlet obstruction were associated with decreased, rather than increased CGRP and SubP containing primary afferents, a result opposite to that noted in the present study.
The lack of histological changes in the current study is consistent with multiple clinical pain conditions which are defined as “functional” in nature. There was a suggestion of an increase in mast cell counts and potentially of some bladder muscle mass, but none of the changes approached statistical significance at the timepoint in development at which they were measured. It is possible that with a sustained alteration in peripheral nervous system structures, there could be an eventual alteration in other bladder structures.
Collectively, these data extend our earlier results demonstrating that early-in-life exposure to bladder inflammation can lead to a hypersensitive bladder in adulthood and suggest a role for a peripheral mechanism. Peripherally, primary nociceptive afferents can become hypersensitive following inflammation52, and mechanically insensitive afferents can acquire polymodal transducer ability following acute inflammation29. Hypersensitivity also may occur because neonatal bladder inflammation produces long-term changes in central nociceptive substrates and/or “primes” either peripheral or central substrates to be affected by a subsequent bladder stimuli. Neuroplasticity of central mechanisms has been demonstrated in somatic and gastrointestinal systems following early-in-life inflammation as reflected by both peripheral and central changes. Specifically, increased branching of primary afferents at both peripheral and central locations, increased spontaneous and evoked activity of dorsal horn neurons, and alterations in the balance between descending inhibitory and excitatory systems have all been implicated as factors contributing to cutaneous hypersensitivity39 and bladder hypersensitivity8. Collectively, these data give evidence that in the urological systems, there are both peripheral and central components to adult hypersensitivity induced by early-in-life events.
These findings are of clinical significance because bladder infections are common during infancy, particularly in the female population which is especially vulnerable to long-term consequences of neonatal inflammation21. Preventative strategies that might block the secondary effects of bladder inflammation would seem prudent. These strategies might target any number of possible secondary changes resulting from bladder inflammation that can have long term consequences including increased nerve growth factor expression20, a strengthening of excitatory synapses23, altered microglial activation40 and alterations in vanilloid50, GABAergic11 and purinergic53 mechanisms. Interventions that affect these mechanisms during critical periods of development might block the secondary hypersensitivity. Using the present neonatal inflammation system, it may be possible to examine preventative therapeutics, such as analgesic treatments used by others during cutaneous inflammatory events22 and to trial novel “reactive” treatments such as electro-acupuncture, which has been examined in colonic hypersensitivity models46.
In summary, our findings show that a brief, local inflammatory insult of the urinary bladder during the neonatal period is capable of creating a hypersensitive adult bladder with altered primary afferents. These data support the hypothesis that early-in-life inflammatory insults to the urologic system, as seen in other systems, may contribute to the development of certain clinical adult hypersensitivity states. Identification of potential risk factors and the mechanisms contributing to adult hypersensitivity following early-in-life inflammation may aid in the diagnosis of painful bladder conditions and provide useful insights into potential preventative strategies or treatments for such conditions.
Acknowledgements
Supported by DK51413, DK073218 and DK078655.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterol. 2000;119:1276–1285. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 2.Blatt LK, Lashinger ESR, Laping NJ, Su X. Evaluation of pressor and visceromotor reflex responses to bladder distension in urethane anesthetized rats. Neurourol Urodynam. 2008 Nov 24; doi: 10.1002/nau.20650. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Blom JMC, Benatti C, Alboni S, Capone G, Ferraguti C, Brunello N, Tascedda F. Early postnatal chronic inflammation produces long-term changes in pain behaviour and N-methyl-D-Aspartate receptor subtype gene expression in the central nervous system of adult mice. J Neurosci Res. 2006;84:1789–1798. doi: 10.1002/jnr.21077. [DOI] [PubMed] [Google Scholar]
- 4.Chertin B, Rolle U, Cascio S, Puri P. Alterations in cholinergic and neuropeptide innervation of urinary bladder following partial bladder outlet obstruction. Pediatr Surg Int. 2003;19:427–431. doi: 10.1007/s00383-002-0937-6. [DOI] [PubMed] [Google Scholar]
- 5.Chitkara DK, vanTilburg MAL, Blois-Martin N, Whitehead WE. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chu Y-C, Chan K-H, Tsou M-Y, Lin S-M, Hsieh Y-C, Tao Y-X. Mechanical pain hypersensitivity after incisional surgery is enhanced in rats subjected to neonatal peripheral inflammation. Anesthesiol. 2007;106:1204–1212. doi: 10.1097/01.anes.0000267604.40258.d1. [DOI] [PubMed] [Google Scholar]
- 7.Christmas TJ, Rode J, Chapple CR, Milroy EJG, Turner-Warwick RT. Nerve proliferation in interstitial cystitis. Virchows Archiv A. 1990;416:447–451. doi: 10.1007/BF01605152. [DOI] [PubMed] [Google Scholar]
- 8.DeBerry J, Ness TJ, Robbins MT, Randich A. Inflammation-induced enhancement of the visceromotor reflex to urinary bladder distension: modulation by endogenous opioids and the effects of early-in-life experience with bladder inflammation. J Pain. 2007;8:914–923. doi: 10.1016/j.jpain.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald M. The development of nociceptive circuits. Nature. 2005;6:507–520. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 10.Gepetti P, Nassini R, Materazzi S, Benemei S. The concept of neurogenic inflammation. BJU Int. 2008;101(Suppl 3):2–6. doi: 10.1111/j.1464-410X.2008.07493.x. [DOI] [PubMed] [Google Scholar]
- II.Hathaway G, Harrop E, Baccei M, Walker S, Moss A, Fitzgerald M. A postnatal switch in GABAergic control of spinal cutaneous reflexes. Eur J Neurosci. 2006;23:112–118. doi: 10.1111/j.1460-9568.2005.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargreaves K, Dubner R, Brown R, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 13.Hermann C, Hohmeister J, Demirakca S, Zohsel K, Flor H. Long-term alteration of pain sensitivity in school-aged children and early pain experiences. Pain. 2006;125:278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 14.Hohmann AG, Neely MH, Pina J, Nackley AG. Neonatal chronic hind paw inflammation alters sensitization to intradermal capsaicin in adult rats: a behavioural and immunocytochemical study. J Pain. 2005;6:798–808. doi: 10.1016/j.jpain.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Howell S, Poulton R, Talley NJ. The natural history of childhood abdominal pain and its association with adult irritable bowel syndrome: birth-cohort study. Am J Gastroenterol. 2005;100:2071–2078. doi: 10.1111/j.1572-0241.2005.41753.x. [DOI] [PubMed] [Google Scholar]
- 16.Jaggar SI, Scott HCF, Rice ASC. Inflammation of the rat urinary bladder is associated with a referred thermal hyperalgesia which is nerve growth factor dependent. B J Anesth. 1999;83:442–448. doi: 10.1093/bja/83.3.442. [DOI] [PubMed] [Google Scholar]
- 17.Johnson OL, Berkley KJ. Estrous influences on micturition thresholds of the female rat before and after bladder inflammation. Am J Physiol Regul Integr Comp Physiol. 2002;282:R289–R294. doi: 10.1152/ajpregu.2002.282.1.R289. [DOI] [PubMed] [Google Scholar]
- 18.Jones CA, Nyberg L. Epidemiology of interstitial cystitis. Urology. 1997;49:2–9. doi: 10.1016/s0090-4295(99)80327-6. [DOI] [PubMed] [Google Scholar]
- 19.Koltzenburg M, McMahon SB. Plasma extravasation in the rat urinary bladder following mechanical, electrical and chemical stimuli: evidence for a new population of chemosensitive primary sensory afferents. Neurosci Lett. 1986;72:352–356. doi: 10.1016/0304-3940(86)90540-9. [DOI] [PubMed] [Google Scholar]
- 20.Lamb K, Gebhart GF, Bielfeldt K. Increased nerve growth factor expression triggers bladder overactivity. J Pain. 2004;5:150–156. doi: 10.1016/j.jpain.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 21.LaPrairie JL, Murphy AZ. Female rats are more vulnerable to the long-term consequences of neonatal inflammatory injury. Pain. 2007;132(Suppl 1):S124–S133. doi: 10.1016/j.pain.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.LaPrairie JL, Johns ME, Murphy AZ. Pre-emptive morphine analgesia attenuates the long-term consequences of neonatal inflammation in male and female rats. Ped Res. 2008;64:625–630. doi: 10.1203/PDR.0b013e31818702d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Baccei ML. Excitatory synapses in the rat superficial dorsal horn are strengthened following peripheral inflammation during early postnatal development. Pain. 2009;143:56–64. doi: 10.1016/j.pain.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Lidow MS, Song ZM, Ren K. Long-term effects of short-lasting early local inflammatory insult. Neuroreport. 2001;12:399–403. doi: 10.1097/00001756-200102120-00042. [DOI] [PubMed] [Google Scholar]
- 25.Lin C, Al-Chaer ED. Long-term sensitization of primary afferents in adult rats exposed to neonatal colon pain. Brain Res. 2003;971:73–82. doi: 10.1016/s0006-8993(03)02358-8. [DOI] [PubMed] [Google Scholar]
- 26.Liu LS, Winston JH, Shenoy MM, Song GQ, Chen JD, Pasricha PJ. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterol. 2008;134:2070–2079. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 27.Long JA, Evans HM. The estrous cycle in the rat and its associated phenomena. Mem Univ Calif. 1922;6:1–148. [Google Scholar]
- 28.Mallen DC, Peat G, Thomas E, Croft PR. Is chronic pain in adulthood related to childhood factors? A population-based case-control study of young adults. J Rheumatol. 2006;33:2286–2290. [PubMed] [Google Scholar]
- 29.Michaelis M, Habler H-J, Janig W. Silent afferents: a separate class of primary afferents? Clin Exp Pharm Physiol. 1996;23:99–105. doi: 10.1111/j.1440-1681.1996.tb02579.x. [DOI] [PubMed] [Google Scholar]
- 30.Ness TJ, Lewis-Sides A, Castroman P. Characterization of pressor and visceromotor reflex responses to bladder distension in rats: sources of variability and effect of analgesics. J Urol. 2001;165:968–974. [PubMed] [Google Scholar]
- 31.Peeker R, Enerback L, Fall M, Aldenborg F. Recruitment, distribution and phenotypes of mast cells in interstitial cystitis. J Urol. 2000;163:1009–1015. [PubMed] [Google Scholar]
- 32.Peng YB, Ling QD, Ruda MA, Kenshalo ER. Electrophysiological changes in adult rat dorsal horn neurons after neonatal peripheral inflammation. J Neurophysiol. 2003;90:73–80. doi: 10.1152/jn.01019.2002. [DOI] [PubMed] [Google Scholar]
- 33.Randich A, Mebane H, Ness TJ. Ice water testing reveals hypersensitivity in adult rats that experienced neonatal bladder inflammation: implications for painful bladder syndrome/interstitial cystitis. J. Urol. 2009 May 16; doi: 10.1016/j.juro.2009.02.107. epub. PMID 19447422. [DOI] [PubMed] [Google Scholar]
- 34.Randich A, Uzzell T, Cannon R, Ness TJ. Inflammation and enhanced nociceptive responses to bladder distension produced by intravesical zymosan in the rat. BMC Urol. 2006;6:2. doi: 10.1186/1471-2490-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Randich A, Uzzell T, DeBerry JJ, Ness TJ. Neonatal urinary bladder inflammation produces adult bladder hypersensitivity. J Pain. 2006;7:469–479. doi: 10.1016/j.jpain.2006.01.450. [DOI] [PubMed] [Google Scholar]
- 36.Ren K, Anseloni V, Zou S-P, Wade EB, Novikova SI, Ennis M, Traub RJ, Gold MS, Dubner R, Lidow MS. Characterization of basal and re-inflammation-associated long-term alteration in pain responsivity following short-lasting neonatal local inflammatory insult. Pain. 2004;110:588–596. doi: 10.1016/j.pain.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Ren K, Novikova SI, He F, Dubner R, Lidow MS. Neonatal local noxious insult affects gene expression in the spinal cord of adult rats. Molecular Pain. 2005;1:27. doi: 10.1186/1744-8069-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rice ASC. Topical spinal administration of a nitric oxide synthase inhibitor prevents the hyper-reflexia associated with a rat model of persistent visceral pain. Neurosci Lett. 1995;187:111–114. doi: 10.1016/0304-3940(95)11356-8. [DOI] [PubMed] [Google Scholar]
- 39.Ruda MA, Ling Q-D, Hohmann AG, Peng YB, Tachibana T. Altered nociceptive neuronal circuits after neonatal peripheral inflammation. Science. 2000;289:628–631. doi: 10.1126/science.289.5479.628. [DOI] [PubMed] [Google Scholar]
- 40.Saab CY, Wang J, Gu C, Garner KN, Al-Chaer ED. Microglia: a newly discovered role in visceral hypersensitivity? Neuron Glia Biol. 2007;2:2781–277. doi: 10.1017/S1740925X07000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sant GR, Kempuraj D, Marchand JE, Theoharides TC. The mast cell in interstitial cystitis: role in pathophysiology and pathogenesis. Urology. 2007;69(Suppl 4A):34–40. doi: 10.1016/j.urology.2006.08.1109. [DOI] [PubMed] [Google Scholar]
- 42.Smith C, Nordstrom E, Sengupta JN, Miranda A. Neonatal gastric suctioning results in chronic visceral and somatic hyperalgesia: role of corticotrophin releasing factor. Neurogastroenterol Motil. 2007;19:692–699. doi: 10.1111/j.1365-2982.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 43.Smith PP, Hurtado E, Smith CP, Boone TB, Somogyi GT. Comparison of cystometric methods in female rats. Neurourol Urodynam. 2008;27:324–329. doi: 10.1002/nau.20512. [DOI] [PubMed] [Google Scholar]
- 44.Tachibana T, Ling Q-D, Ruda MA. Increased Fos induction in adult rats that experienced neonatal peripheral inflammation. Neuroreport. 2001;12:925–927. doi: 10.1097/00001756-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 45.Taddio A, Katz J. The effects of early pain experience in neonates on pain responses in infancy and childhood. Paediatr Drugs. 2005;7:245–257. doi: 10.2165/00148581-200507040-00004. [DOI] [PubMed] [Google Scholar]
- 46.Tian S-L, Wang X-Y, Ding C-H. Repeated electro-acupuncture attenuates chronic visceral hypersensitivity and spinal NMDA receptor phosphorylation in rat irritable bowel syndrome model. Life Sci. 2008;83:356–363. doi: 10.1016/j.lfs.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 47.Walker SM. Pain in children: recent advances and ongoing challenges. Br J Anaesth. 2008;101:101–110. doi: 10.1093/bja/aen097. [DOI] [PubMed] [Google Scholar]
- 48.Walker SM, Franck LS, Fitzgerald M, Myles J, Stocks J, Marlow N. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141:79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 49.Wang J, Gu C, Al-Chaer E. Altered behaviour and digestive outcomes in adult male rats primed with minimal colon pain as neonates. Behav Brain Funct. 2008;4:28. doi: 10.1186/1744-9081-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterol. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 51.Wollgarten-Hadamek I, Hohmeister J, Demirakca S, Zohsel K, Flor H, Hermann C. Do burn injuries during infancy affect pain and sensory sensitivity in later childhood? Pain. 2009;141:165–172. doi: 10.1016/j.pain.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 52.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 53.Xu G-Y, Shenoy M, Winston JH, Mittal S, Pasricha PJ. P2X receptor-mediated visceral hyperalgesia in a rat model of chronic visceral hypersensitivity. Gut. 2008;57:1230–1237. doi: 10.1136/gut.2007.134221. [DOI] [PubMed] [Google Scholar]