Abstract
Although it has been accepted that depression and pain are common comorbidities, their interaction is not fully understood. The present study was aimed to investigate the effects of depression on both evoked pain behavior (thermal induced nociception and hyperalgesia) and spontaneous pain behavior (formalin pain) in rats. An unpredictable chronic mild stress (UCMS) paradigm was employed to develop a classical depression. The emotional behaviors were assessed by sucrose preference test, open field test, and elevated plus-maze test. The results showed that the depressed rats always exhibited stronger tolerance to noxious thermal stimulation under both normal and complete Freund’s adjuvant (CFA)-induced chronic pain conditions, when compared to non-depressed animals. Interestingly, the spontaneous nociceptive behaviors induced by formalin injection were significantly enhanced in rats exposed to UCMS in comparison to those without UCMS. Systemic administration of antidepressant fluoxetine significantly restored the nociceptive behaviors to normal level in depressed animals. An additional finding was that the inflammatory rats tended to display depressive-like behaviors without being exposed to UCMS. These results demonstrated that depression can have different effects on stimulus-evoked pain and spontaneous pain, with alleviation in the former while aggravation in the latter.
Perspective: The present study provides evidence that depression can have divergent effects on stimulus-evoked and spontaneous pain by confirming that rats exposed to chronic mild stress tend to exhibit decreased pain sensitivity to experimental stimuli but increased intensity of ongoing pain. This may contribute to further understanding of the perplexing relationship between clinical depression and chronic pain.
Keywords: depression, fluoxetine, pain, thermal hyperalgesia, unpredictable chronic mild stress
Introduction
For the past 20 years, the intricate relationship between pain and depression has attracted increasing attention in all areas of research due to its multiple applications. In clinical practice, depression is often associated with an increased incidence of clinical pain complaints and, thus, the comorbidity of pain and depression has been suggested to be a common phenomenon 21, 24. Previous studies indicate that pain and depression share common neuroanatomical pathways and neurobiological substrates, which might explain the increased vulnerability to pain in depressive patients and vice versa. A population-based study found that 43.4% of patients with depression had at least one painful physical symptom, which was four times the rate of patients who did not suffer from depression. The results also showed that the prevalence of depression in patients with pain symptoms was higher than those without pain symptoms 30. To describe the relationship between pain and depression, some experiments on patients with depressive disorders have been performed. Most studies regarding depressed patients found increased pain thresholds 5, 14, 22, 23, while a few reports described a decrease in the experimentally evoked pain 27, 29. Thus, the correlation between depression and pain is still a matter of debate and has not been fully understood.
Animal studies showed similar results in that the nociceptive behaviors were either reduced 33 or enhanced 3, 47 in subjects exposed to chronic environmental stress, a condition that has been demonstrated to cause depression. The inconsistency concerning the effect of depression on nociceptive behaviors may be due to the use of various animal models and varied experimental procedures. Lautenbacher and Krieg proposed an interesting hypothesis of a global impairment of the sensory system in depression which involves: (a) hypoalgesia to phasic experimental pain due to diminished spinal and brainstem transmission, and (b) hyperalgesia to endogenous painful sensations (e.g. clinical complaints of headache, stomach pain, etc.) because of the insufficient activation of inhibitory systems 22.
The unpredictable chronic mild stress (UCMS) animal model is one of the classical depression models introduced by Willner et al. 44. The paradigm consists of several mild stressors which are analogous to those associated with humans. In this paradigm, animals are subjected to a variety of unpredictable mild stressors everyday. After several weeks, the animals show a decrease in consumption of a palatable sweet solution, known as anhedonia, which is the core character of depression 45. In our study, the UCMS procedure was utilized as an animal model of depression, based on well-established methods found in the literature with modifications 44. We aimed to explore the effect of depression on both evoked pain behavior (thermal induced nociception and hyperalgesia) and spontaneous pain behavior (formalin pain, which is closer to clinical persistent pain). We hypothesized that: (a) the thermal nociception and hyperalgesia will be attenuated whereas the formalin pain exacerbated after weeks of UCMS exposure, and (b) the nociceptive behaviors of UCMS-treated rats will be restored to normal levels in response to an antidepressant treatment.
Materials and methods
Animals
Male Wistar rats (purchased from the Academy of Military Medical Science, Beijing, China) weighing 220–250 g at the beginning of experiment were used. All rats were housed individually with food and water freely available and maintained under a standard 12/12 h light/dark cycle (lights on at 07:00 am), with ambient temperature set at 22 ± 2°C. Animals were softly handled 3–5 minutes per day by the experimenter before the onset of the experiment. All experimental procedures were approved by the International Review Board of the Institute of Psychology, Chinese Academy of Sciences.
Experimental design
Rats were randomly assigned to two groups, control group and UCMS group. The control and UCMS-exposed animals were kept in separated rooms in order to independently manipulate the environments. Testing was performed during the light cycle. Rats were allowed to acclimate for at least one week. Then rats in the UCMS group were subjected to six weeks of mild, unpredictable stressors. Body weights were measured weekly during the UCMS procedure.
Three studies were performed. The first study examined the effect of chronic stress exposure on the pain-related behaviors (including thermal thresholds of acute and chronic pain, and formalin-induced licking behavior). The experimental protocol was shown in Table 1. The depression-related behaviors were assessed before (baseline) and after the UCMS procedure by the sucrose consumption, the open-field, and the elevated plus-maze (EPM) tests. Both control and UCMS groups were further divided into 3 subgroups, which respectively received thermal acute pain, complete Freund’s adjuvant (CFA)-induced hyperalgesia, and formalin tests. The acute thermal pain test and formalin test were performed both before (baseline) and after UCMS procedure (the 6th week). CFA was injected during the second weekend. The thermal hyperalgesia was evaluated once every week until the end of the experiment. The control group received the same treatment except that it was not exposed to UCMS. The hypothalamic-pituitary-adrenal (HPA) activity (the adrenal weight and the blood level of plasma corticosterone) was examined after the completion of all behavioral tests.
Table 1.
Experimental protocol
| Time after UCMS | Types of pain |
||
|---|---|---|---|
| (weeks) | Acute pain | Chronic inflammatory pain | Formalin pain |
| −1 | EPM; OF; PT | EPM; OF; PT | EPM; OF |
| 1 | |||
| 2 | CFA injection | ||
| 3 | PT | ||
| 4 | PT | ||
| 5 | PT | ||
| 6 | EPM; OF; PT | EPM; OF; PT | EPM; OF; Ft |
EPM: elevated plus-maze test; OF: open field test; PT: pain threshold test. Ft: formalin test.
The second study investigated whether the stress-induced changes in pain behavior could be reversed by antidepressant fluoxetine. Rats were injected i.p. daily with fluoxetine from the third week until the end of the UCMS procedure. The control group was treated with saline. The reason that the antidepressant treatment began in the middle but not at the beginning of the protocol was that we needed to make sure that the rats exhibited depressive behaviors and the model was successful prior to antidepressant treatment 37. The protocols for acute thermal pain and formalin pain were the same as in the first study. In the CFA pain test, rats were injected with CFA at the end of the six-week UCMS procedure. The thermal hyperalgesia was measured 24 h following CFA injection.
The third study was done to observe whether rats with chronic pain tend to develop depressive-like behaviors. Rats were divided into a control group and an experimental group, receiving intraplantar injection of saline and CFA, respectively. The behavioral reactivity to various paradigms (sucrose preference, locomotor activity in the open field, EPM) was measured, and HPA activity examined during a 6-week period of observation.
UCMS procedure and behavioral tests
Table 2 describes the schedule of stressor administration. Stressors were unpredictable in their nature, duration, and frequency. Stress procedures included, in a pseudorandom order: 22- and 40-h periods of water deprivation, 20- and 22-h periods of food deprivation, one 1-h period of empty water bottle (exposed to empty water bottle immediately after one 40-h period of water deprivation), one 3-h period of restricted access to food (two small pieces of pellet in each cage) following one 20-h period of food deprivation, 8- and 16-h periods of cage tilt (45°), 7- and 8-h periods of strobe light, two 16-h periods of soiled bedding, one 16-h of group housing (8 rats in a cage), two 16-h periods overnight illumination, 2- and 5-h periods of intermittent white noise (75 dB), two 16-h periods of novel odor, two 30 min periods of exposure to a hot room (40°C) and two 30-min periods of exposure to a cold room (10°C) (Table 2).
Table 2.
Procedure of unpredictable chronic mild stress
| Time | Types of stress |
|---|---|
| Monday | |
| 09:00 | Clean cages, dry cages, commence strobe light |
| 16:00 | End strobe light and commence hot room (40°C) for 30 min |
| 17:00 | Commence water deprivation and paired housing |
| Tuesday | |
| 09:00 | End paired housing and commence cage tilting |
| 17:00 | End cage tilting and expose to novel odor. Switch lights to on overnight |
| Wednesday | |
| 09:00 | Remove novel odor and exposed to empty water bottle for 1 h |
| 10:00 | Restore water |
| 12:00 | Commence cold room (10°C) for 30 min |
| 17:00 | Soil cage |
| Tuesday | |
| 07:00 | Reverse the light/dark cycle for 24 h |
| 09:00 | Clean cage, commence food deprivation and exposed to hot room (40°C) for 30 min |
| 17:00 | Commence crowding (8 rats per cage) |
| Friday | |
| 09:00 | End crowding and commence strobe light |
| 17:00 | End strobe light, restricted access to food (two small pieces of pellet in each cage) for 3 h |
| 20:00 | Restore food and switch lights to on overnight |
| Saturday | |
| 09:00 | Commence cold room (10°C) for 30 min |
| 16:00 | Remove water and food |
| 17:00 | Commence cage tilting and exposure to novel odour |
| Sunday | |
| 09:00 | End cage tilting and remove novel odour |
| 14:00 | Sucrose preference test for 1 h |
| 15:00 | Restore water and food |
| 17:00 | Soil cage |
Sucrose preference tests were performed before (baseline) and weekly after UCMS. At the start of the experiment, the animals were first trained to drink a 1% sucrose solution, by exposing them to sucrose instead of water for 48 h. Then, the rats received a series of sucrose preference tests, preceded by 22 h food and water deprivation. Each animal was presented simultaneously with 2 bottles, one containing sucrose solution (1%) and the other containing water. The percent preference for sucrose consumption was calculated according to the following formula: % sucrose preference = (sucrose solution consumption/ (sucrose solution consumption + water consumption)) × 100.
The open field test was applied to analyze the free locomotion and exploration of rats. The open field apparatus consisted of a circular area (180 cm in diameter and 50 cm wall height) in a quiet room with dim illumination (40 W). An object - a blue cylinder (7 cm high and 15 cm in diameter) - was put into the center of the open field. The animals were placed individually into the center facing the same position of the wall in all of the tests. Each animal was recorded for 5 min to monitor the distance it traveled and the times it explored the object. A video recording camera was mounted to the ceiling above the observation chamber to record and input the activity in a computer. Rat behavior was recorded and analyzed using a computer-based system Etho Vision (Noldus Information Technology b. v., Netherland). In the interval between each two animal tests, the apparatus was cleaned with ethanol and water, to remove olfactory cues.
The EPM test was carried out to measure the emotional reactivity of rats. The instrument consisted of two open arms (50.8 cm×10.2 cm×1.3 cm) and two closed arms (50.8 cm×10.2 cm×4.6 cm), arranged perpendicularly, and was elevated to a height of 72.4 cm above the floor. The arms were connected by a central square (10.2 cm×10.2 cm). The apparatus was in a quiet room exposed to dim illumination. Rats were placed individually in the center of the maze facing an open arm, and observed for 5 min. The time spent in open arms was recorded and analyzed by a computer-based system (MED-VPM-RS, Med Associate Inc., USA).
Radiant heat was employed to induce acute pain and to access the hyperalgesia related to chronic pain. Animals were placed into a Plexiglas chamber on a glass floor and allowed to acclimate for at least 30 min. Then, a radiant heat stimulus was applied to the plantar surface of the hind paw. The paw withdraw latency (PWL) induced by the radiant heat was used as a measure of pain thresholds and thermal hyperalgesia. Five trials were conducted on each hind paw at an interval of 5 min. Because of considerable variability in the first latency measurement, the average of the last four PWL measurements was used to determine the PWL.
Chronic inflammatory pain was induced by a subcutaneous injection into the unilateral hind paw of 100 µl of CFA (Sigma, MO, USA). The thermal thresholds were measured before UCMS procedure as the baseline and at 7, 14, 21, 28 days after CFA injection.
In the formalin test, 50 µl of 5% formalin was injected into the rat hind paw. The rats were immediately returned to the testing chamber and the behaviors were recorded by a computer-based video recording system for 60 min. The time spent licking the injected paw was calculated in 5 min epochs.
Blood corticosterone level and adrenal weight
At the end of the experiments, the rats were decapitated. Blood samples from trunk vessel were immediately collected for corticosterone determination. Meanwhile, the adrenals were quickly removed and weighed. The adrenal weight is expressed relative to body weight (in mg / g body weight). The blood samples were centrifuged at 3000 rpm for 15 min to obtain cell-free plasma and then frozen at −80° to store. The levels of plasma corticosterone were measured by enzyme linked immunosorbent assay (ELISA) using a commercial kit (Rapidbio Lab, Calabasas, California, USA).
Antidepressant treatment
The selective serotonin reuptake inhibitor fluoxetine was purchased from Sigma-Aldrich (St. Louis, Missouri) and dissolved in saline (0.9% NaCl) immediately before application. Separate subgroups of stressed and non-stressed animals were treated chronically with daily i.p. injection of either fluoxetine (10 mg/kg /day) or normal saline (NS) (at 5:00 PM) from day 22 through day 42.
Statistical analysis
GraphPad prism 4.0 and Statistica 5.1 were used for statistical analyses and graph generation. Data affected by two or three factors were analyzed with multi-factor analysis of variance (ANOVA). Duncan’s test was used for post hoc test. Student’s t-test was employed when two groups were compared. Relationships between nociceptive behaviors and sucrose preference were examined with Pearson correlation coefficients. The data was presented as means ± SEM. The statistical significance was set at P < 0.05.
Results
UCMS model
Body weights were measured weekly during the UCMS procedure. Initially the mean body weight of rats did not differ between UCMS and control groups (226.8 ± 1.7 g vs. 229.0 ± 2.3 g, t(93) = 0.8345, P = 0.4061). Over the six-week experiment, a consistent reduction in the weight gain was observed in the UCMS-exposed rats, and two-way ANOVA revealed significant difference between the two groups (F(5, 558) = 101.6, P < 0.001) (Fig. 1A).
Fig 1.
The depressive-like behaviors after six weeks of UCMS procedure. (A) Body weight. Over the six-week UCMS exposure, the body weights in the UCMS group were significantly decreased (n = 46 – 47). (B) Sucrose preference. Two weeks after UCMS, there was a significant reduction in sucrose consumption in the UCMS-exposed rats (n = 47 – 48). (C) Locomotor behaviors. The UCMS-treated rats displayed significantly higher level of activity and lower level of exploratory behavior compared to the control group (n = 24). (D) EPM test. The time spent in the open arms significantly prolonged in the UCMS group in comparison to that in the control group (n = 25 – 26). (E) Activity of HPA. The adrenal weights in the UCMS group were significantly increased, but the concentration of plasma corticosterone did not change (n = 17 – 18). Data are presented as mean ± SEM. * P < 0.05; ** P < 0.01; *** P < 0.001, compared to their respective control group.
The results of sucrose preference test are shown in Fig. 1B. Significant reduction was found in sucrose consumption after two weeks of UCMS, which was maintained throughout the observation period (six weeks) (F(5, 546) = 5.699, P < 0.001). This indicates that the depression animal model has been successfully established.
The locomotor (travel distance) and exploratory behavior in the open field were also significantly altered following UCMS exposure. As shown in Fig. 1C, UCMS-exposed rats showed significantly higher level of locomotor activity (3367 ± 278 vs 2757 ± 192 cm, P < 0.01) and lower level of exploratory activity (5.68 ± 0.74 vs 8.00 ± 0.74, P < 0.05) compared with the control group. This suggests that rats exposed to UCMS exhibited depressive behaviors.
Anxiety behaviors of rats were measured by the time spent in the open arms in EPM. As shown in Fig. 1D, the baseline level did not show significant difference between control and UCMS groups. By contrast, UCMS-exposed rats preferred to spend more time in the open arms compared to the control rats (83.57 ± 8.85 vs 31.08 ± 3.51 s, P < 0.05). The increased EPM open-arm time during UCMS exposure indicates that the chronic mild stress has an anxiolytic effect but not an anxiogenic effect.
In addition, UCMS-treated rats displayed remarkable adrenal hypertrophy (0.189 ± 0.010 vs 0.136 ± 0.005 g/kg, t(50) = 1.388, P < 0.001) compared with control rats (Fig. 1E), suggesting an increased activity of HPA axis. Unlike the results obtained in adrenal weight examination, no significant difference was found in the plasma levels of corticosterone between UCMS and control groups (t(33) = 1.983, P = 0.056). This may be due to an adaptive response of corticosterone release during the 6-week period of UCMS procedure.
The effect of depression on pain behaviors
The effect of depression on evoked pain behaviors
Acute thermal pain tests were performed before (baseline) and six weeks after UCMS. As can be seen in Fig 2A, UCMS exposure produced significantly changed PWLs compared to the control group (7.71 ± 0.19 vs 6.37 ± 0.11 s, P < 0.01), suggesting that depressed subjects had higher pain thresholds in response to experimental painful stimuli.
Fig. 2.
The effect of depression on the evoked versus spontaneous pain behaviors. (A) Acute thermal evoked pain. The paw withdrawal latency (PWL) to noxious heat stimuli was significantly increased in the stressed rats (n = 16). (B) Thermal-evoked hyperalgesia in chronic pain state. During the UCMS procedure, CFA was injected into the rat hind paw to induce chronic inflammatory pain. The PWL was significantly higher in the UCMS-exposed rats than the nonstressed ones (n = 16). (C1-2) Spontaneous pain. Subcutaneous injection of formalin into the rat hind paw was used to produce spontaneous pain. Compared with the control rats, the licking behaviors in the UCMS rats were significantly enhanced in the early and late phases as well as in the interphase (n = 16 – 17). Data are presented as mean ± SEM. * P < 0.05; ** P < 0.01; *** P < 0.001, compared to their respective control group.
For CFA-injected rats, PWL was accessed weekly after inoculation. The injected paw looked red and tumid immediately after the injection. The rats showed limping and paw elevation, and kept licking and shaking the inflamed hindpaw. As shown in Fig 2B, UCMS-treated rats exhibited significantly longer PWL to noxious thermal stimuli than control animals in ANOVA (Group effect: F(1, 120) = 26.75; P < 0.001), consistent with the result of acute thermal pain.
The effect of depression on spontaneous pain behavior
Subcutaneous injection of formalin into the hind paw produced a typical biphasic pattern of licking behavior, as shown in Fig. 2C1. Compared with the control rats, the licking behaviors in the UCMS rats were significantly enhanced throughout the entire hour of observation (Group effect: F(1, 372) = 77.68). Cumulative licking time clearly showed the increase in phase I (0–5 min, 120.2 ± 6.7 s vs 94.1 ± 5.3 s, t(31) = 3.1000, P < 0.01), interphase (5–15 min, 104.2 ± 12.7 s vs 46.7 ± 5.7 s (control), t(31) = 4.212, P < 0.001), and phase II (15–60 min, 992.9 ± 41.3 s vs 531.8 ± 27.6 s (control), t(31) = 9.3800, P < 0.001, see Fig. 2C2). These results indicate that spontaneous pain behavior was exacerbated after UCMS exposure.
Correlation between behaviors of depression and pain
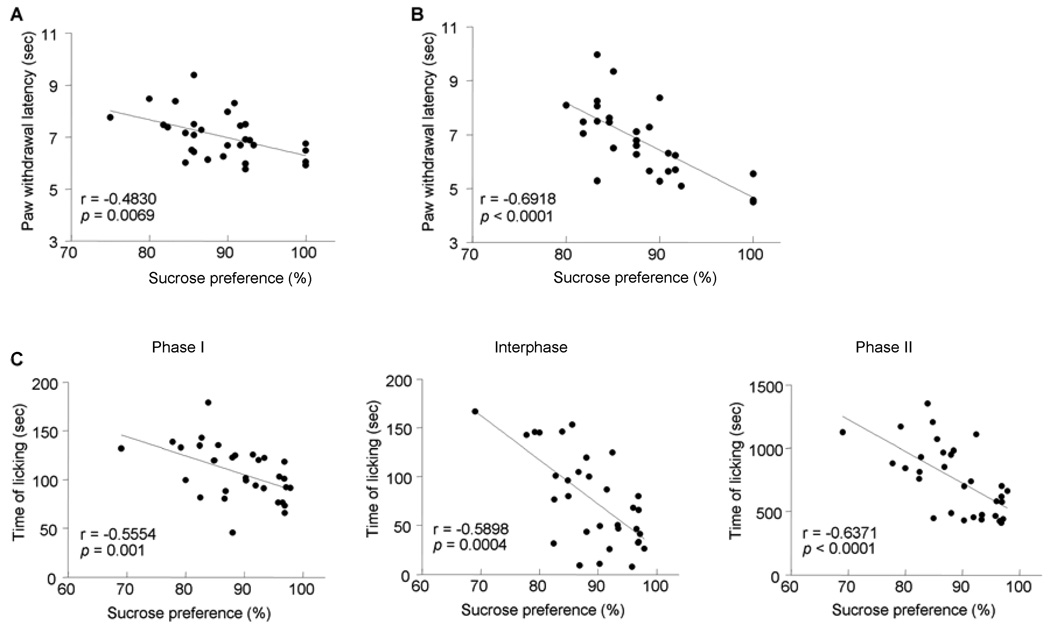
To investigate the seemingly inconsistent results for evoked and spontaneous pain, Pearson correlations were computed between the pain and depressive behaviors. Significant negative correlations were found between sucrose preference and pain thresholds in both acute pain (r = −0.4830, P = 0.0069, see Fig. 3A) and thermal hyperalgesia (r = −0.6918, P < 0.0001, see Fig. 3B). This indicates that the sensation and reaction to environmental noxious events was weakened in the depressed subjects. A significant correlation was found between sucrose preference and licking time in formalin pain with correlation coefficients of r = −0.5554, −0.5898, and −0.6371 for phase I, interphase, and phase II, respectively (Fig. 3C). The fact that the spontaneous pain was intensified in depressive condition implies that depression may bring a heightened awareness of persistent, unavoidable pain, therefore serving to maintain or exaggerate ongoing pain. These results confirmed that depression is associated with decreased sensitivity to experimental pain and increased clinical pain symptoms.
Fig. 3.
The correlation between nociceptive behaviors and sucrose preference. There is a negative correlation between sucrose preference and thermal pain thresholds in both the acute (A) and chronic pain (B) states. Also, the intensity of spontaneous pain induced by formalin is negtively correlated with sucrose consumption in the early and late phases as well as in the interphase (C).
Fluoxetine reversed depression-induced changes in pain behavior
Sucrose preference test
As shown in Fig. 4, sucrose preference was significantly decreased three weeks after UCMS exposure (Group effect: F(1, 225) = 44.96, P < 0.001), consistent with prior findings. While not affecting sucrose intake in the control rats, fluoxetine treatment for three consecutive weeks produced apparent beneficial effects on the UCMS rats, demonstrated by improved sucrose consumption, in comparison to those treated with saline.
Fig. 4.
The therapeutical effect of fluoxetine on depression. Rats recieved fluoxetine (10 mg/kg/day, IP) administration for consecutive 21 days. Fluoxetine treatment significantly reversed sucrose intake reduction in the UCMS-exposed rats in comparison with those administered with normal saline Data are presented as mean ± SEM. * P < 0.05, *** P < 0.001, compared to their respective non-UCMS group; # P < 0.05, ###, P < 0.001, compared to their respective NS-treated group. n = 12.
Evoked and spontaneous pain behaviors
Systemic administration of fluoxetine significantly affects the PWL of rats in both acute pain (F(1, 44) = 37.99, P < 0.001, see Fig 5A) and thermal hyperalgesia (F(1, 44) = 96.06, P < 0.001, see Fig 5B). Post hoc analysis revealed that fluoxetine per se had an antinociceptive effect (i.e., elevated pain thresholds) in normal (control) rats (12.40 ± 0.48 vs 10.10 ± 0.27 s, P < 0.001, Fig 5A; 6.53 ± 0.28 vs 4.19 ± 0.07 s, P < 0.001, Fig 5B). Furthermore, UCMS-exposed rats exhibited stronger tolerance to noxious thermal stimulation (13.84 ± 0.53 vs 10.10 ± 0.27 s, P < 0.001, Fig 5A; 6.48 ± 0.25 vs 4.19 ± 0.07 s, P < 0.001, Fig 5B) compared with control rats, consistent with the previous finding that depression is associated with decreased sensitivity on experimental pain. Most importantly, the thermal nociceptive thresholds in UCMS-exposed rats were decreased (13.84 ± 0.53 vs 10.82 ± 0.41 s, P < 0.001, Fig 5A; 6.48 ± 0.25 vs 4.57 ± 0.21s, P < 0.001, Fig 5B) and approached normal level following fluoxetine treatment (10.82 ± 0.41 vs 10.10 ± 0.27 s, P = 0.25, Fig 5A; 4.57 ± 0.21 vs 4.19 ± 0.07 s, P =0.10, Fig 5B), reflecting the therapeutic effect of fluoxetine as an antidepressant.
Fig. 5.
The effect of fluoxetine on the depression-induced changes in pain behavior. In both acute (A) and chronic pain (B) states, the elevated nociceptive thermal thresholds by UCMS exposure were reversed and approached normal level following systemic administration of fluoxetine, indicating the therapeutic effect of fluoxetine as an antidepressant. In addition, the PWLs of rats in the control group were increased by fluoxetine treatment, suggesting an antinociceptive effect of fluoxetine per se. (C1-2) In spontaneous pain state, the enhanced licking behaviors by UCMS exposure were reversed by fluoxetine. Additionally, in control rats, the licking behaviors were suppressed by fluoxetine injection. Data are presented as mean ± SEM. * P < 0.05;** P < 0.01; *** P < 0.001, compared to their respective control group; # P < 0.05; ## P < 0.01; ### P < 0.001, compared to their respective NS-treated group. n = 9–10.
The formalin-induced spontaneous pain behaviors in both UCMS and control rats were suppressed by fluoxetine administration (for Fig. 5C1, F(1, 28) = 42.80, P <0.001; for Fig. 5C2, F(1, 33) = 11.28, P = 0.002 for Phase I, F(1, 32) = 68.13, P < 0.001 for interphase, and F(1, 33) = 41.57, P < 0.001 for Phase II), confirming the antinociceptive effect of fluoxetine on normal rats and anti-depression effect on depressive ones.
The chronic pain-induced depression
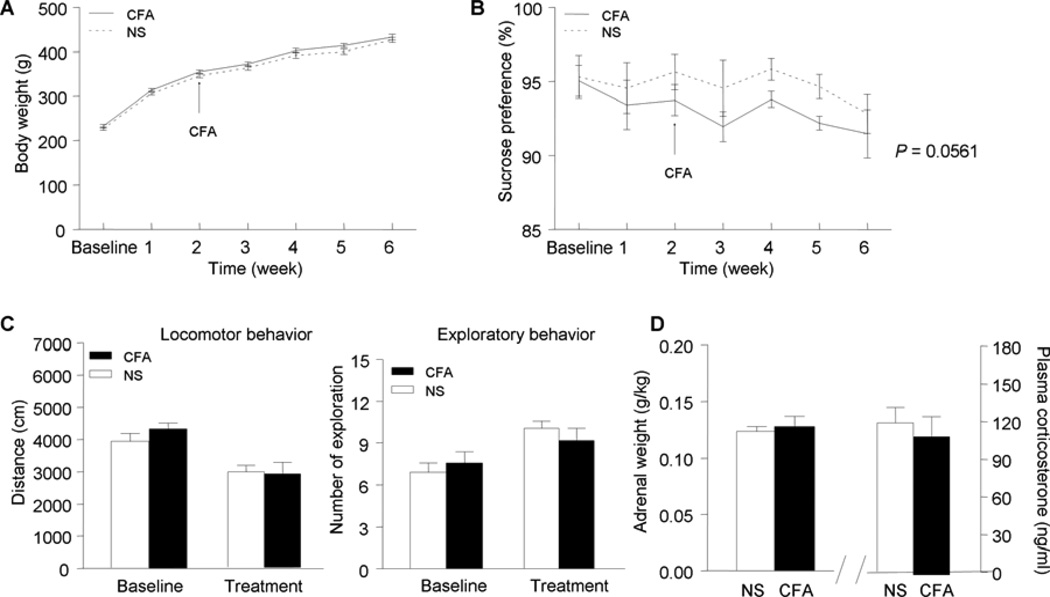
The possibility that chronic pain may cause depression was also examined in this study (Fig. 6). It was found that the rats with chronic inflammatory pain exhibited some depressive-like behaviors, for example, decreased sucrose consumption (P = 0.0561). These results support the idea that individuals suffering from chronic pain may be more likely to develop depressive disorders.
Fig. 6.
The depressive-like behaviors resulted from chronic inflammatory pain. The rats with chronic inflammatory pain showed lower sucrose preference (near significant level, B). Other behavioral indices, including body weight (A), OF test (C), and HPA activity (D), did not show significant changes during the development of chronic pain. Data are presented as mean ± SEM. ** P < 0.01, compared to their respective NS-treated group. n = 16.
Discussion
In the present study, we investigated the effect of unpredictable mild stress on evoked pain (thermal stimulus induced pain and hyperalgesia) and spontaneous pain (formalin pain) in rats. The results confirmed previous findings that depressed subjects tend to exhibit decreased pain sensitivity to experimental stimuli but increased intensity of ongoing pain. Furthermore, chronic treatment with selective serotonin reuptake inhibitor fluoxetine reversed the depression-related behavioral changes in UCMS-exposed rats. Finally, rats with chronic inflammatory pain exhibited some depressive-like behaviors, supporting the idea that chronic pain may bring about clinical depression.
In contrast to the high frequency of clinical pain complaints, most authors reported that depressive patients were less sensitive to experimental pain 5, 6,15, 22, 23. For example, Bär et al investigated 30 patients suffering major depressive disorders and found hypoalgesic responses to cutaneous thermal or electrical stimuli 5. Lautenbacher et al observed that the pressure-pain thresholds of the forearm were significantly higher in depressed patients than in healthy subjects 23. Animal studies also demonstrated that rats submitted to a chronic unpredictable stress paradigm had increased nociceptive thresholds in the tail-flick test 33. In our study, we found that the rat model of depression exhibited less sensitivity to noxious radiant heat applied on the hindpaw in both acute and chronic pain states, consistent with previous findings. This indicates that depression may inhibit evoked pain. Inconsistent data were also reported concerning the response of depressed subjects to experimental pain. The tolerance to ischemic pain produced by a tourniquet was found to be reduced in depressed patients 32. Both mechanical allodynia and depression-like behavior were exacerbated after peripheral nerve injury in Wistar-Kyoto (WKY) rats 47. The possible explanation for these controversial findings might be the use of non-unified protocols for pain threshold measurements as well as the application of different kinds of stimuli.
Ward et al. supposed that the stress-evoked release of endogenous opioids might account for the hypoalgesia in major depressive disorder 43. Data obtained by Frew et al. supported this notion and offered evidence that systemical administration with μ-opioid receptor antagonist naltrexone intensified cold- and shock-induced pain in depressive patients 12. Bär et al revealed that depressed patients displayed elevated thermal pain thresholds and demonstrated activation of similar cortical structures between depressed patients and healthy subjects during pain processing, including contralateral primary and secondary somatosensory cortices, insula, anterior cingulate cortex, and supplementary motor area. More important, a relative hyperactivation of the prefrontal cortices was found during the application of painful stimuli in depressive disorder patients compared to the healthy controls 7, indicating that the prefrontal area may play an important role in linking pain and depression.
In our experiment, the relationship between spontaneous pain and depression was found to be totally different from evoked pain condition. In contrast to the reduced pain sensitivity, the ongoing pain produced by subcutaneous formalin injection was significantly intensified in UCMS-exposed rats. This suggests that depression can facilitate the spontaneous pain. Clinical studies have shown that depression is closely associated with an increased frequency of clinical pain complaints 8, 46. It is well established that low levels of central serotonin (5-HT) and norepinephrine (NE) are related to the state of depression 10, 25. Also, it has long been known that serotonergic and noradrenergic projections are involved in the descending modulation of pain in addition to regulating mood, recognition, and attention. Thereby, it is possible that the painful physical symptoms in depressed subjects are caused by the dysfunction of descending 5-HT and NE pathways 41. An additional explanation for the exaggeration of ongoing pain in depressed subjects may be the emotional effect. As a negative affect, depression may enhance the emotional response to intense pain (e.g., formalin-induced pain) while thresholds remain unchanged.
The selective serotonin reuptake inhibitor (SSRI) fluoxetine is widely used in clinical practice for the treatment of depression 42. It inhibits the reuptake of 5-HT by presynaptic neurons, increases the amount of 5-HT present in the synapse, and helps normalize the transmission of neuronal signals. In the current study, fluoxetine produced apparent therapeutic effects on the depressed rats, demonstrated by improved sucrose consumption in comparison to the saline treated group. After chronic treatment with fluoxetine, the decreased or increased nociceptive behaviors in evoked or spontaneous pain conditions in the UCMS-exposed rats were all restored to normal level (i.e., control level), confirming that the altered pain perception was related to the depressive state. Besides, fluoxetine was also found effective in relieving pain in rats without depression, as has been found in previous studies 1, 38, 40. The antinociceptive effect of fluoxetine was thought to be mediated by the descending serotoninergic inhibitory pathways. Possibly, the role of 5-HT in analgesia is distinctly different from the development of depression.
In clinical study, depression seems to be more common in patients with chronic pain than in those suffering any other chronic illness 2. A review of European literature reported that the percentage of patients with depressive disorder ranged from 23.1% (with pain) and 8.8% (without pain) to 41.0% and 14.5%, respectively 13. Although there is much clinical evidence that people with chronic pain are much more likely to have a depressive disorder, data from animal studies are very scarce. In the present study, we found that rats injected with CFA displayed a decrease (nearly significant) in sucrose consumption. This indicates that chronic pain may lead to depressive-like behaviors.
UCMS model is one of the classical depression models. In the current study, chronic stress resulted in a significant decrease of sucrose preference in rats, demonstrating that the animal model has been successfully established. The open filed test showed that the stressed rats displayed higher locomotor activities, reflecting the psychomotor agitation observed in some depressed humans 17, 28. The exploratory behaviors of the stressed rats exhibited lower activity, suggesting a change in the emotionality 39. Anxiety behaviors were evaluated by the time spent in the open arms in EPM. It has been reported that anxiety and depression are often comorbid with each other 11, 19, 35. However, the increased EPM open-arm time during UCMS exposure in this study indicates an anxiolytic effect of chronic mile stress but not an anxiogenic effect. It is difficult to understand the conflicting observations of decreased and increased anxiety-like behavior in the EPM test after UCMS 9, 36, 16, 26. Very recently, Kompagne et al. (2008) reported a consistent result and interpreted the increased EPM open-arm time as reflecting the dampened emotional processing of the sensory input but not a less anxious state 20. We also examined the HPA activity and found that the adrenal weight was increased, indicating the hyperactivity of HPA in the depressive state 3. Meanwhile, the plasma level of corticosterone remained unchanged after UCMS exposure. It has been proposed that there is an adaptive response of HPA axis in the presence of high-prolonged glucocorticoid concentrations 4, 31. Previous studies measuring the corticosterone level weekly after UCMS also demonstrated that the corticosterone level increased at the beginning, decreased at the second week, and returned to baseline level at the end of the sixth week of UCMS 39.
A limitation of the experimental design should be addressed here. The completion of this experiment needs a long period of time (4–5 months). Study 1 and 2 were carried out in spring (March/April) and summer (June/July), respectively. Although the thermal stimuli used in both studies were of the same intensity, the pain thresholds (i.e., PWLs) varied between the control groups in Study 1 and 2 (6.37 ± 0.11 vs. 10.10 ± 0.27 s). The seasonal effect was thought to be a reason affecting pain thresholds. Pöllmann and Harris (1978) have reported significant circannual rhythm of pain sensitivity in human subjects 34. They elicited pain with a cold stimulus and found that the pain thresholds were significantly higher in cold season (winter) than in warm season (spring). Thus, it is reasonable that the pain thresholds to heat stimuli are higher in summer and lower in winter/spring, as the present study has shown. The same reasoning is also applicable for the formalin data, for that formalin test is a model of inflammatory pain, which can be affected by seasonal temperature changes.
In conclusion, our studies demonstrated that: (a) depression inhibited evoked but facilitated spontaneous pain behaviors in rats; and (b) persistent pain may cause depressive behaviors. These findings suggest that the underlying mechanisms of the modulation of the evoked and spontaneous pain perception in the depressive state are different, and that there is a strong interactive relationship between pain and depression. Future studies may be designed to identify the different roles of 5-HT in mediating chronic pain and depression, and the separate areas, specific transmitters, or signaling molecules associated with evoked and spontaneous pain in depressed state, thus to help build better strategies for the treatment of depression and chronic pain.
Acknowledgments
This work was funded by a NNSF grant (30700223), and a grant for young scientists (07CX051005) from the Chinese Academy of Sciences to JYW, NNSF grants (30570577 and 30770688), the 100 Talented Plan of the Chinese Academy of Sciences, a grant from the 863 project (2006AA02Z431), and a grant from NIH Fogarty International Center (1R03TW008038) to FL. The authors have no potential conflicts of interests to be disclosed.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Institution where the work was done: Institute of Psychology, Chinese Academy of Sciences
References
- 1.Abdel-Salam OM, Baiuomy AR, Arbid MS. Studies on the anti-inflammatory effect of fluoxetine in the rat. Pharmacol Res. 2004;49:119–131. doi: 10.1016/j.phrs.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- 3.Andre J, Zeau B, Pohl M, Cesselin F, Benoliel JJ, Becker C. Involvement of cholecystokininergic systems in anxiety-induced hyperalgesia in male rats behavioral and biochemical studies. J Neurosci. 2005;25:7896–7904. doi: 10.1523/JNEUROSCI.0743-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azpiroz A, Fano E, Garmendia L, Arregi A, Cacho R, Beitia G, Brain PF. Effects of chronic mild stress (CMS) and imipramine administration, on spleen mononuclear cell proliferative response, serum corticosterone level and brain norepinephrine content in male mice. Psychoneuroendocrinology. 1999;24:345–361. doi: 10.1016/s0306-4530(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 5.Bär KJ, Brehm S, Boettger MK, Boettger S, Wagner G, Sauer H. Pain perception in major depression depends on pain modality. Pain. 2005;117:97–103. doi: 10.1016/j.pain.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 6.Bär KJ, Brehm S, Boettger MK, Wagner G, Boettger S, Sauer H. Decreased sensitivity to experimental pain in adjustment disorder. Eur J Pain. 2006;10:467–471. doi: 10.1016/j.ejpain.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Bär KJ, Wagner G, Koschke M, Boettger S, Boettger MK, Schlosser R, Sauer H. Increased prefrontal activation during pain perception in major depression. Biol Psychiatry. 2007;62:1281–1287. doi: 10.1016/j.biopsych.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54:399–409. doi: 10.1016/s0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 9.D’Aquila PS, Brain P, Willner P. Effects of chronic mild stress on performance in behavioural tests relevant to anxiety and depression. Physiol Behav. 1994;56:861–867. doi: 10.1016/0031-9384(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 10.Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61 Suppl 1:5–12. [PubMed] [Google Scholar]
- 11.Dunlop BW, Davis PG. Combination Treatment With Benzodiazepines and SSRIs for Comorbid Anxiety and Depression: A Review. Prim Care Companion. J Clin Psychiatry. 2008;10:222–228. doi: 10.4088/pcc.v10n0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frew AK, Drummond PD. Stress-evoked opioid release inhibits pain in major depressive disorder. Pain. 2008;139:284–292. doi: 10.1016/j.pain.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Cebrian A, Gandhi P, Demyttenaere K, Peveler R. The association of depression and painful physical symptoms--a review of the European literature. Eur Psychiatry. 2006;21:379–388. doi: 10.1016/j.eurpsy.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Gormsen L, Jensen TS, Bach FW, Rosenberg R. (Pain and depression) Ugeskr Laeger. 2006;168:1967–1969. [PubMed] [Google Scholar]
- 15.Graff-Guerrero A, Pellicer F, Mendoza-Espinosa Y, Martinez-Medina P, Romero-Romo J, de la Fuente-Sandoval C. Cerebral blood flow changes associated with experimental pain stimulation in patients with major depression. J Affect Disord. 2008;107:161–168. doi: 10.1016/j.jad.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gronli J, Murison R, Fiske E, Bjorvatn B, Sorensen E, Portas CM, Ursin R. Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav. 2005;84:571–577. doi: 10.1016/j.physbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–190. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- 19.Hirschfeld RM. The Comorbidity of Major Depression and Anxiety Disorders: Recognition and Management in Primary Care. Prim Care Companion J Clin Psychiatry. 2001;3:244–254. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kompagne H, Bardos G, Szenasi G, Gacsalyi I, Harsing LG, Levay G. Chronic mild stress generates clear depressive but ambiguous anxiety-like behaviour in rats. Behav Brain Res. 2008;193:311–314. doi: 10.1016/j.bbr.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Kunzel HE, Schuld A, Pollmacher T. (Chronic pain and depression) Versicherungsmedizin. 2006;58:67–72. [PubMed] [Google Scholar]
- 22.Lautenbacher S, Krieg JC. Pain perception in psychiatric disorders: a review of the literature. J Psychiatr Res. 1994;28:109–122. doi: 10.1016/0022-3956(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 23.Lautenbacher S, Spernal J, Schreiber W, Krieg JC. Relationship between clinical pain complaints and pain sensitivity in patients with depression and panic disorder. Psychosom Med. 1999;61:822–827. doi: 10.1097/00006842-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 24.Leo RJ. Chronic pain and comorbid depression. Curr Treat Options Neurol. 2005;7:403–412. doi: 10.1007/s11940-005-0032-0. [DOI] [PubMed] [Google Scholar]
- 25.Mann JJ, Malone KM. Cerebrospinal fluid amines and higher-lethality suicide attempts in depressed inpatients. Biol Psychiatry. 1997;41:162–171. doi: 10.1016/s0006-3223(96)00217-x. [DOI] [PubMed] [Google Scholar]
- 26.Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27:549–561. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- 27.Merskey H. The Effect of Chronic Pain Upon the Response to Noxious Stimuli by Psychiatric Patients. J Psychosom Res. 1965;8:405–419. doi: 10.1016/0022-3999(65)90083-8. [DOI] [PubMed] [Google Scholar]
- 28.Mineur YS, Belzung C, Crusio WE. Effects of unpredictable chronic mild stress on anxiety and depression-like behavior in mice. Behav Brain Res. 2006;175:43–50. doi: 10.1016/j.bbr.2006.07.029. [DOI] [PubMed] [Google Scholar]
- 29.Moroz BT, Nuller IuL, Ustimova IN, Andreev BV. (Study of pain sensitivity based on the indicators of electro- odontometry in patients with depersonalization and depressive disorders) Zh Nevropatol Psikhiatr Im S S Korsakova. 1990;90:81–82. [PubMed] [Google Scholar]
- 30.Ohayon MM, Schatzberg AF. Using chronic pain to predict depressive morbidity in the general population. Arch Gen Psychiatry. 2003;60:39–47. doi: 10.1001/archpsyc.60.1.39. [DOI] [PubMed] [Google Scholar]
- 31.Pignatelli D, Maia M, Castro AR, da Conceicao Magalhaes M, Vivier J, Defaye G. Chronic stress effects on the rat adrenal cortex. Endocr Res. 2000;26:537–544. doi: 10.3109/07435800009048567. [DOI] [PubMed] [Google Scholar]
- 32.Pinerua-Shuhaibar L, Prieto-Rincon D, Ferrer A, Bonilla E, Maixner W, Suarez-Roca H. Reduced tolerance and cardiovascular response to ischemic pain in minor depression. J Affect Disord. 1999;56:119–126. doi: 10.1016/s0165-0327(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 33.Pinto-Ribeiro F, Almeida A, Pego JM, Cerqueira J, Sousa N. Chronic unpredictable stress inhibits nociception in male rats. Neurosci Lett. 2004;359:73–76. doi: 10.1016/j.neulet.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 34.Pollmann L, Harris PH. Rhythmic changes in pain sensitivity in teeth. Int J Chronobiol. 1978;5:459–464. [PubMed] [Google Scholar]
- 35.Rihmer Z, Szadoczky E, Furedi J, Kiss K, Papp Z. Anxiety disorders comorbidity in bipolar I, bipolar II and unipolar major depression: results from a population-based study in Hungary. J Affect Disord. 2001;67:175–179. doi: 10.1016/s0165-0327(01)00309-3. [DOI] [PubMed] [Google Scholar]
- 36.Rossler AS, Joubert C, Chapouthier G. Chronic mild stress alleviates anxious behaviour in female mice in two situations. Behav Processes. 2000;49:163–165. doi: 10.1016/s0376-6357(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 37.Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 38.Sawynok J, Reid A. Antinociception by tricyclic antidepressants in the rat formalin test: differential effects on different behaviours following systemic and spinal administration. Pain. 2001;93:51–59. doi: 10.1016/S0304-3959(01)00291-3. [DOI] [PubMed] [Google Scholar]
- 39.Silberman DM, Ayelli-Edgar V, Zorrilla-Zubilete M, Zieher LM, Genaro AM. Impaired T-cell dependent humoral response and its relationship with T lymphocyte sensitivity to stress hormones in a chronic mild stress model of depression. Brain Behav Immun. 2004;18:81–90. doi: 10.1016/s0889-1591(03)00109-0. [DOI] [PubMed] [Google Scholar]
- 40.Singh VP, Jain NK, Kulkarni SK. On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res. 2001;915:218–226. doi: 10.1016/s0006-8993(01)02854-2. [DOI] [PubMed] [Google Scholar]
- 41.Stahl S, Briley M. Understanding pain in depression. Hum Psychopharmacol. 2004;19 Suppl 1:S9–S13. doi: 10.1002/hup.619. [DOI] [PubMed] [Google Scholar]
- 42.Stokes PE, Holtz A. Fluoxetine tenth anniversary update: the progress continues. Clin Ther. 1997;19:1135–1250. doi: 10.1016/s0149-2918(97)80066-5. [DOI] [PubMed] [Google Scholar]
- 43.Ward NG, Bloom VL, Dworkin S, Fawcett J, Narasimhachari N, Friedel RO. Psychobiological markers in coexisting pain and depression: toward a unified theory. J Clin Psychiatry. 1982;43:32–41. [PubMed] [Google Scholar]
- 44.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology (Berl) 1987;93:358–364. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 45.Willner P. Validity: reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 46.Wilson KG, Mikail SF, D’Eon JL, Minns JE. Alternative diagnostic criteria for major depressive disorder in patients with chronic pain. Pain. 2001;91:227–234. doi: 10.1016/S0304-3959(00)00440-1. [DOI] [PubMed] [Google Scholar]
- 47.Zeng Q, Wang S, Lim G, Yang L, Mao J, Sung B, Chang Y, Lim JA, Guo G. Exacerbated mechanical allodynia in rats with depression-like behavior. Brain Res. 2008;1200:27–38. doi: 10.1016/j.brainres.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]