Abstract
“Humanized” mouse models created by engraftment of immunodeficient mice with human hematolymphoid cells or tissues are an emerging technology with broad appeal across multiple biomedical disciplines. However, investigators wishing to utilize humanized mice with engrafted functional human immune systems are faced with a myriad of variables to consider. In this study, we analyze HSC engraftment methodologies using three immunodeficient mouse strains harboring the IL2rγnull mutation; NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1null IL2rγnull mice. Strategies compared engraftment of human HSC derived from umbilical cord blood following intravenous injection into adult mice and intracardiac and intrahepatic injection into newborn mice. We observed that newborn recipients exhibited enhanced engraftment as compared to adult recipients. Irrespective of the protocol or age of recipient, both immunodeficient NOD strains support enhanced hematopoietic cell engraftment as compared to the BALB/c strain. Our data define key parameters for establishing humanized mouse models to study human immunity.
Keywords: Humanized mice, SCID, hematopoietic stem cells, IL-2R “common” gamma chain
INTRODUCTION
The discovery of the severe combined immunodeficiency mutation (Prkdcscid, abbreviated as “scid”) in a stock of C.B-17 mice in 1983 [1] led to the assessment of their ability to support engraftment with human peripheral blood mononuclear cells [PBMC, termed Hu-PBL-SCID, 2] or fetal [termed SCID-Hu, 3] or adult [termed Hu-SRC-SCID, 4] hematopoietic stem cells (HSC). These human hematolymphoid engrafted mouse models have been used in multiple biomedical disciplines for the study of human immunobiology and hematopoiesis (reviewed in [5–8]). However, the utility of the early strains of immunodeficient mice transplanted with human hematolymphoid cells was hindered by the inability to achieve multi-lineage hematopoietic engraftment leading to the generation of a functional human immune system [5,6]. In the Hu-PBL-SCID model, although all lineages of peripheral blood mononuclear cells (PBMC) are injected, the majority of human cells engrafted are T cells, with few B cells, myeloid cells or natural killer (NK) cells surviving. In the Hu-SRC-SCID model, the major obstacle was the inability of HSC-engrafted mice to develop all components of a functional human immune system, particularly T cells [5].
A major technological breakthrough occurred when investigators created genetic stocks of scid, Rag1null or Rag2null mice that also harbor mutations in the IL2 receptor common gamma chain (IL2rγ) gene [9–12]. IL2rγ is required for high affinity signaling through multiple cytokine receptors, including IL2, IL4, IL7, IL9, IL15 and IL21 [13]. Immunodeficient mice bearing a mutated IL2rγ gene support much higher levels of human hematolymphoid engraftment than all previous immunodeficient stocks. Multiple immune lineages develop following engraftment of human HSC that can lead to the generation of a functional human immune system [5–8].
Confounding widespread establishment of humanized mouse models in multiple laboratories is the diversity of approaches that have been reported for engrafting human HSC. For example IL2rγnull mice are available on a number of strain backgrounds. The ability of immunodeficient mice to support engraftment of human hematolymphoid cells has been shown to be strongly affected by the genetic background of the host [5,14–17]. In addition to strain background, reports differ significantly with regard to engraftment methodologies, which include intravenous (IV) engraftment into adult mice [11], or intrahepatic (IH) [10], intraperitoneal (IP) [12], and IV [9,18] injection into newborn mice.
In the present study, we compared a number of variables of human HSC engraftment, including strain background, age of recipient, and engraftment route. The three strains of immunodeficient mice tested were NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1null IL2rγnull. To provide a consistent comparison, we used human HSC derived from umbilical cord blood (UCB) in all of our experiments. We matched UCB donors within an individual experiment, allowing direct comparison of the engraftment characteristics in each strain, independent of donor-to-donor variability. We observed that in the variables tested, newborn immunodeficient NOD strain mice provided the optimal hosts with respect to their ability to support human hematopoietic cell engraftment.
MATERIALS AND METHODS
Mice
NOD.Cg-Prkdcscid Il2rgtm1Wjl/Sz (NOD-scid IL2rγnull) and NOD.Cg-Rag1tm1Mom IL2rgtm1Wjl (NOD-Rag1null IL2rγnull) mice have been described previously [11,19]. C.Cg-Rag1tm1Mom Il2rgtm1Wjl (BALB/c-Rag1null IL2rγnull) mice were generated by LDS by crossing C.129S7(B6)-Rag1tm1Mom mice (BALB/c-Rag1null) with C.129S4-Il2rg tm1Wjl (BALB/c-IL2rγnull) mice and then intercrossing the F1 progeny to fix the Rag1null and the IL2rγnull alleles to homozygosity. Mice were housed in a specific pathogen free facility in microisolator cages, given autoclaved food and maintained on acidified autoclaved water and sulfamethoxazole-trimethoprim medicated water (Goldline Laboratories, Ft. Lauderdale, FL), provided on alternate weeks. All animal use was in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts Medical School and The Jackson Laboratory and conformed to the recommendations in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences, 1996).
Engraftment of Mice with Human Hematopoietic Stem Cells
Umbilical cord blood (UCB) was obtained in accordance with the Committee for the Protection of Human Subjects in Research guidelines of the University of Massachusetts Medical School and was provided by the medical staff of the UMass Memorial Umbilical Cord Blood Donation Program. The program educates and consents mothers regarding UCB collection for research and public banking and performs collections at the time of delivery. Red blood cells were removed from UCB by hetastarch (Baxter HealthCare Corp, Deerfield, IL) to reduce RBC content, followed by double-density Percoll gradient centrifugation (1.05/1.077). The recovered cells were then depleted of T cells using commercially available magnetic bead kits according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA or Stem Cell Technologies, Vancouver, BC, Canada). Efficiency of T cell depletion and percentage of CD34+ cells were evaluated by flow cytometry prior to injection into recipient mice and in all experiments revealed less than 0.5% contaminating CD3+ cells in the UCB preparation. T cell-depleted cord blood was suspended in PBS in a volume to deliver 3×104 CD34+ HSC per recipient (50μL for newborn recipients; 500μL for adult recipients).
Recipient mice were engrafted with one of the following engraftment protocols, as indicated in the Results section. Within an individual experiment, mice of each strain received CD34+ stem cells from the same cord blood donor. At least 3 experiments, each with a unique UCB donor were performed. In all cases, recipient mice were evaluated for human hematolymphoid engraftment at 12 to 16 weeks post-injection.
Protocol A: HSC engraftment of adult mice by IV injection
Adult (6–12 week old) NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1null IL2rγnull mice were irradiated with either 240 cGy (scid mice) or 550 cGy (Rag1null mice) using a 137Cs source (GammaCell 40, Atomic Energy of Canada, Ottawa, Canada). Four hours after irradiation, 3×104 human CD34+ HSC were injected IV into the lateral tail vein.
Protocol B: HSC engraftment of newborn mice by intracardiac (IC) injection
NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1null IL2rγnull mice 24 to 48 hours old were irradiated with either 100 cGy (scid mice) or 400 cGy (Rag1null mice). Mice were injected shortly after gamma-irradiation with 3×104 CD34+ HSC via IC injection using a 27 G winged infusion kit attached to a 1cc syringe. Injected pups were returned to their nursing mothers until weaned [20].
Protocol C: HSC engraftment of newborn mice by IH injection
This protocol was performed identically to Protocol B, except that mice were injected via IH injection [20].
Antibodies
For analysis of human cell populations in engrafted mice, anti-human CD3, CD4, CD8, CD34, and CD45, anti-mouse CD45 fluorochrome-conjugated monoclonal antibodies (mAbs), and appropriate isotype control Abs were obtained from BD PharMingen (San Diego, CA). Antibodies were conjugated with FITC, PE, PerCP, APC, Alexa 405, Pacific Blue, or Alexa700.
Flow Cytometry
Single-cell suspensions of bone marrow, spleen, and thymus were prepared from nonengrafted and human HSC engrafted mice. Whole blood was collected in EDTA. Red blood cells in bone marrow and spleen were removed by lysis with a hypotonic solution. Cells counts were determined using a Coulter Counter (Beckman, Miami, FL). Single cell suspensions of 1×106 cells in a 50μL volume or 75μL of whole blood were pre-incubated with rat anti-mouse FcR11b (clone 2.4G2) to block Fc binding. Cells were incubated for 30 min at 4°C in appropriate dilutions of antibodies as determined in preliminary experiments. For analysis of engrafted mice, PerCP-conjugated anti-mouse CD45 mAb and APC-conjugated anti-human CD45 mAb were included. All cells that were negative for murine CD45 and positive for human CD45 were analyzed for the presence of multi-lineage differentiation markers. Labeled single cell suspensions were washed in RPMI and fixed in 1% paraformaldehyde in PBS. The staining protocol of whole blood was performed according to manufacturer's instructions (BD Biosciences, San Jose, CA). At least 50,000 events were acquired on BD Biosciences LSRII or FACSCalibur instruments (BD Biosciences). Data analysis was performed with FlowJo (Tree Star, Inc., Ashland, OR) software.
Histopathological analysis
Tissues recovered from non-engrafted and HSC engrafted mice were fixed in 10% buffered formalin. H&E and human CD45 staining of tissue sections were preformed as previously described [11].
Statistical Methods
Parametric data were compared by one-way ANOVA with Bonferroni post-tests to compare individual pair-wise groupings and nonparametric data were compared by a Kruskal-Wallis test with Dunns post-test to compare individual pair-wise groupings. Significant differences were assumed for p values <0.05. All statistical analyses were performed using GraphPad Prism software (version 4.0c, GraphPad, San Diego, CA).
RESULTS
Development of BALB/c-Rag1nullIL2rγnull Mice
The optimal mouse strains (described in Table 1) that support engraftment of human HSC harbor mutations in the IL2 receptor common gamma chain (IL2rγnull) and are available on a number of genetic backgrounds, including NOD-scid, NOD-Rag1null and BALB/c-Rag2null [10,11,19]. As the BALB/c-Rag2null IL2rγnull stocks are not commercially available, we generated a BALB/c-Rag1null IL2rγnull stock by crossing the BALB/c-Rag1null strain with the BALB/c-IL2rγnull strain. These parental stocks were obtained from the Repository at The Jackson Laboratory (http://www.jax.org/). Null mutation of either the Rag-1 or the Rag-2 genes results in the inability to generate mouse T or B cells with rearranged antigen receptors [21,22]. As expected, BALB/c-Rag1null IL2rγnull mice are completely devoid of T, B and NK cells (data not shown) and we have shown previously that they can be engrafted with human PBMC, although not as efficiently as NOD-scid IL2rγnull mice [23].
Table 1.
Immunodeficient mouse models for engraftment of human hematopoietic stem cells*
| Common Stain Name | Mutant Allele(s) | Immunological Phenotype | Human HSC Engraftment | Caveats of Model | Ref. |
|---|---|---|---|---|---|
| NOD strains | |||||
| NOD-scid | Prkdcscid | -No mature T and B cells | -Moderate engraftment | -Residual innate immunity Shortened life span due to the development of thymic lymphomas | [11] |
| -Low innate immunity including reduced NK cell function | -Radiation sensitive | ||||
| NOD-scid B2mnull | Prkdcscid B2mtm1Unc-J | -No mature T and B cells | -Heightened engraftment | -Residual innate immunity | [28,38] |
| -Lack of MHC class I due to β2m deletion, severely reduced NK cell function | -Severely decreased life span due to accelerated development of thymic lymphomas; short-lived | ||||
| -Radiation sensitive | |||||
| NOD/LtSz-scid IL2rγnull | Prkdcscid IL2rgtm1Wjl | -No mature T cells, B cells or NK cells, further reduction of innate immunity | -Very high engraftment | -Long life span | [11,18] |
| -Multiple lineages of lymphoid and myeloid cells | -Complete absence of IL2R γ-chain | ||||
| -Resistant to lymphoma development | |||||
| -Radiation sensitive | |||||
| NOD/Shi-scid IL2rγnull | Prkdcscid IL2rgtm1sug | -Similar to NOD/LtSz-scid IL2rγnull | -Very high engraftment | -Similar to NOD/LtSz-scid IL2rγnull | [9] |
| -Multiple lineages of lymphoid and myeloid cells | -IL2R γ-chain targeted mutation results in truncation of IL2r gamma chain; not complete deletion | ||||
| NOD.Cg-scid IL2rγnull | Prkdcscid IL2rgtm1Wjl | -Similar to NOD/LtSz-scid IL2rγnull | -Very high engraftment | -Similar to NOD/LtSz-scid IL2rγnull | [32] |
| Tg(HLA-A2.1)Enge/Dvs | -Transgenic expression of genomic HLA-A2.1 | -Multiple lineages of lymphoid and myeloid cells Supports HLA-restricted T cell selection | |||
| NOD/LtSz -scid IL2rγnull | Prkdcscid IL2rgtm1Wjl | -Similar to NOD/LtSz-scid IL2rγnull | -Very high engraftment | -Similar to NOD/LtSz-scid IL2rγnull | [33] |
| Tg(HLA-A2/H2D/β2M) | -Transgenic expression of chimeric (HHD) HLA-A2.1 covalently linked to human β2m | -Multiple lineages | -Human CD8 T cell selection on HLA-A2 | ||
| Dvs/Sz | |||||
| NOD-Rag1null | Rag1tm1Mom | -No mature T and B cells | -Low and variable engraftment | -Not radiation sensitive | [39] |
| -Reduced NK cell activity | |||||
| NOD-Rag1null Prfnull | Rag1tm1Mom prftm1Sdz | -No mature T and B cells | -Low and variable engraftment | -Not radiation sensitive | [29] |
| -Lack of perforin, diminished NK cell-mediated cytotoxicity | |||||
| NOD-Rag1null IL2rγnull | Rag1tm1Mom IL2rgtm1Wjl | -No mature T and B cells | -Very high engraftment | -Not radiation sensitive | [19] |
| -No NK cells | -Multiple lineages | ||||
| -IL-2rγ-chain deficient, further reduction of innate immunity | |||||
| H2d-based strains | |||||
| CB17-scid | Prkdcscid | Mature mouse T and B cells develop with age (referred to as leakiness) High NK cell activity | -Very low level of engraftment | -Radiation sensitive | [1] |
| -No functional immune system | |||||
| CB17-scid beige | Prkdcscid Lystbg | -No mature T and B cells | -Low level of engraftment | -Radiation sensitive | [30] |
| -Reduced NK cell function | |||||
| BALB/c-scid bg | Prkdcscid Lystbg | -Similar CB17-scid bg | -Low level of engraftment | -Radiation sensitive | [14] |
| -Reduced NK cell cytotoxicity | |||||
| BALB/c-Rag1null IL2rγnull | Rag1tm1Mom IL2rgtm1Wjl | -Similar to MOD-Rag1null IL2rγnull | High engraftment | -Lower levels of peripheral T cells | [23] |
| -Multiple lineages | -Not radiation sensitive | ||||
| BALB/c-Rag2null IL2rγnull | Rag2tm1Fwa IL2rgtm1Sug | -Similar to NOD-Rag1null IL2rγnull | -High engraftment | -Lower levels of peripheral T cells | [10] |
| -Multiple lineages | -Not radiation sensitive | ||||
| H2d-Rag2null IL2rγnull | Rag2tm1Fwa IL2rgtm1Krf | -Similar to NOD-Rag1null IL2rγnull | -High engraftment | -Not radiation sensitive | [40,41] |
| -Multiple lineages | |||||
B2m, b2-microglobulin, bg, beige; Foxn1, forkhead box N1; HSC, hematopoietic stem cells; IL, interleukin; Il2rg, interleukin-2 receptor γ-chain; lyst, lysosomal trafficking regulator; NK, natural killer; NOD, non-obese diabetic; nu, nude; prf1, perforin 1; Prkdc protein kinase, DNA activated, catalytic polypeptide; Rag recombination-activating gene; scid, severe combined immunodeficiency; Tg, transgenic
Engraftment of Adult Immunodeficient Mice with Human HSC
IV injection of human HSC into adult, irradiated mice has been used to establish humanized mice. We have previously shown that this approach is suitable for establishing high levels of human hematolymphoid engraftment into adult NOD-scid IL2rγnull mice and NOD-Rag1null IL2rγnull mice [11,19]. In contrast, it has been reported that reproducible engraftment of adult BALB/c-Rag2null IL2rγnull mice with HSC [24] or PBMC [25] requires macrophage ablation by treatment with chlodronate-containing liposomes.
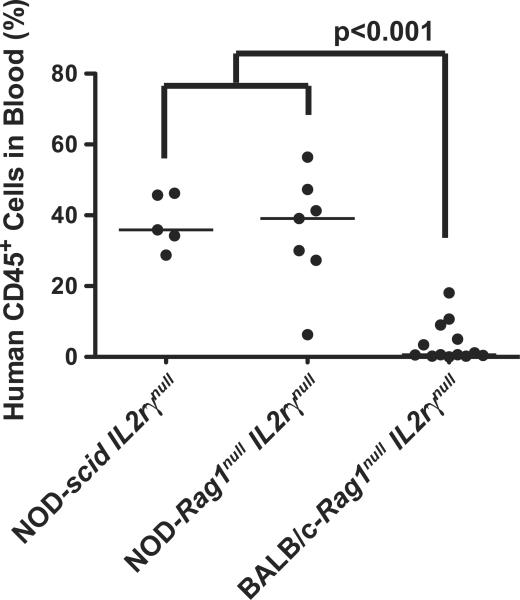
We first compared directly the ability of the three strains of adult IL2rγnull mice to be engrafted following the IV administration of HSC into irradiated adult recipients. Mice 6–12 weeks of age were sublethally irradiated with 240 cGy (scid mice) or 550 cGy (Rag1null mice) and injected IV with 3×104 CD34+ cells from the same UCB donor. NOD-scid IL2rγnull mice received a lower dose of radiation because the scid mutation results in increased radiosensitivity [19]. Twelve weeks later, engraftment levels were determined by quantifying the percentages of human-CD45+ cells in the peripheral blood. We found that immunodeficient NOD strain background mice engrafted at significantly higher levels as compared to BALB/c-Rag1null IL2rγnull mice (Figure 1). Engraftment levels in the blood of the adult recipients were reflective of the relative engraftment in the spleen, both within an individual recipient and among various strains (data not shown).
Figure 1. HSC engraftment in adult IL2rγnull strains.
Adult mice were irradiated and injected IV via the tail vein with T cell depleted UCB containing 3×104 CD34+ cells as described in the Materials and Methods (Protocol A). At 12–16 weeks after HSC injection, the percentages of human CD45+ cells in the peripheral blood were determined by flow cytometry. The solid lines represent the median value for each group. Each dot represents an individual animal.
HSC Engraftment in Newborn NOD-scid IL2rγnull Mice Injected via the Intracardiac Route and adult NOD-scid IL2rγnull Mice Injected via the tail vein
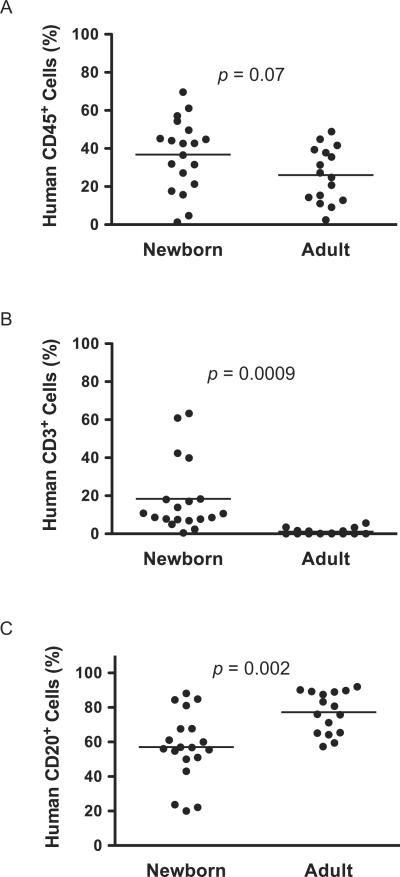
As an alternative to adult engraftment with HSC, many investigators have chosen to deliver HSC to newborn mice [10,18]. Using NOD-scid IL2rγnull mice, we compared directly the engraftment levels obtained in peripheral blood of mice engrafted as newborns (IC injection) versus adults (IV injection). While overall human CD45+ engraftment did not differ statistically between these two cohorts of mice, the data trended towards higher engraftment in newborns (Figure 2A). However, the ability to support human T cell development was dramatically improved in newborn NOD-scid IL2rγnull mice as compared to their adult counterparts (Figure 2B). In addition, both newborn and adult mice engrafted at high levels with human B cells (Figure 2C).
Figure 2. HSC-engraftment in newborn and adult NOD-scid IL2rγnull mice.
Newborn and adult NOD-scid IL2rγnull mice were irradiated and engrafted with CD34+ cells, as described in the Materials and Methods. Twelve to 16 weeks later HSC-engraftment was evaluated by determining the percentages of human CD45+ (A), CD3+ T cells (B) and CD20+ B cells (C) cells in the peripheral blood. The proportion of T cells and B cells is shown as a percentage of the human CD45+ cells. The solid lines represent the median value for each group. Each dot represents an individual animal.
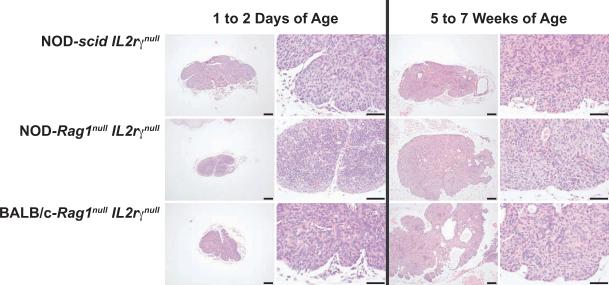
These data document that human T cell levels in HSC-engrafted adult mice are reduced as compared to HSC-engrafted newborn mice. We next questioned whether this engraftment deficiency is related to defects within the thymic architecture of adult mice rendering the thymi as unsuitable environments for the development of human prothymocytes. Histological sections of thymic lobes from unmanipulated newborn and 5–7-week old immunodeficient mice were used to evaluate their overall structure. Thymic lobes from all three immunodeficient strains showed an absence of lymphocytes at 1–2 days (Figure 3). At 5–7 weeks of age, all thymi remained alymphoid and contained hypoplastic cysts, consistent with the report that introduction of the IL2rγnull mutation results in the development of hypoplastic cysts that increase with age in mice with impaired immune systems [26]. Overall, it appeared that the hypoplastic cysts were more prevalent in the thymi of adult BALB/c-Rag1null IL2rγnull mice than in the thymi of the adult immunodeficient NOD strains (Figure 3).
Figure 3. Thymic architecture in non-engrafted newborn and adult IL2rγnull strains.
Histological sections of thymi from non-engrafted immunodeficient mice at 1 to 2 days of age (left two columns, 40X AND 100X) and at 5 to 7 weeks of age (right two columns,40X AND 100X) stained with H&E. Thymi were obtained from indicated strains on the Y axis. Bars = 100uM.
Hematopoietic Cell Engraftment in Newborn Immunodeficient Mice Injected via the Intracardiac Route
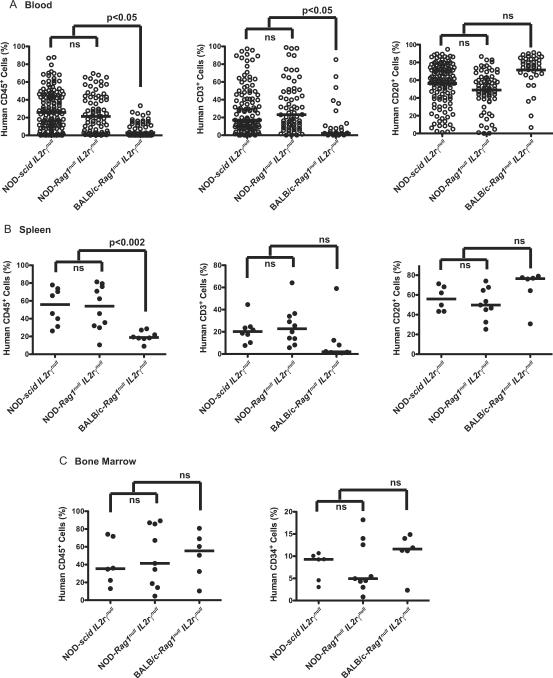
We next sought to determine if the strain-dependent differences in engraftment observed in adult recipients would be recapitulated in newborn mice. One to 2-day-old NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull and BALB/c-Rag1null IL2rγnull mice were irradiated with 100 cGy (scid mice) or 400 cGy (Rag1null mice) and engrafted via IC injection with T cell-depleted UCB containing 3×1044 CD34+ stem cells. Twelve to 16 weeks after injection, mice were analyzed for the presence of human hematolymphoid cells (Figure 4). In agreement with data from adult engrafted mice, we observed that overall human CD45+ and human CD3+ cell engraftment was higher in the blood of immunodeficient NOD strains as compared to the immunodeficient BALB/c strain mice (Figure 4A). The percent of human CD45+ cells that were CD20+ B cells was higher in the blood of mice on the BALB/c background reflecting the decreased percentages of CD3+ T cells that develop in these HSC recipients.
Figure 4. HSC-engraftment in newborn IL2rγnull strains.
Newborn NOD-scid IL2rγnull, NOD-Rag1nullIL2rγnull, and BALB/c-Rag1nullIL2rγnull mice were irradiated and engrafted with CD34+ cells via IC injection as described in the Materials and Methods (Protocol B). Twelve to 16 weeks after HSC injection, the percentages of human CD45+ cells in the peripheral blood (A), spleen (B) and bone marrow (C) were determined by flow cytometry. Percentages of human CD45+ cells in peripheral blood and spleens expressing HuCD3 or HuCD20 were determined by flow cytometry. Percentages of HuCD45+ in bone marrow expressing CD34+ cells were determined by flow cytometry. The data for the peripheral blood are from 97 NOD-scid IL2rγnull mice, 73 NOD-Rag1nullIL2rγnull mice and 49 BALB/c-Rag1nullIL2rγnull mice. The data from spleen and bone marrow are representative of 3 separate experiments. The solid lines represent the median value for each group. Each dot represents an individual animal. The proportion of T cells and B cells is a percentage of the human CD45+ cells.
Human hematopoietic cell engraftment in the spleen and bone marrow was examined in a subset of HSC-engrafted mice (Figures 4B and 4C). The percentages of human CD45+ cells were significantly higher in the spleens of immunodeficient NOD strains as compared to that observed in the immunodeficient BALB/c strain. Percent CD3+ T cell levels in the spleen trended higher in NOD strain mice but this difference was not significant and there were no differences in the percentages of CD20+ B cells between three strains. However, the total numbers of human CD45+ (9 to 11-fold) CD3+ (12–17-fold) and CD20+ (6 to 9-fold) cells recovered from the spleen were significantly higher in NOD strains as compared to the BALB/c strain (Table 2). In contrast to the spleen, the percentages and total numbers of human CD45+ cells engrafted in the bone marrow were similar between the 3 strains (Figure 4C and Table 2). The percentages and numbers of human CD34+ cells in the bone marrow were also not significantly different between the strains (Figure 4C).
Table 2.
Number of human cells in bone marrow and spleen*
| Human CD45+ bone marrow cells (×106) | Human CD45+ spleen cells (×106) | Human CD3+ spleen cells (×106) | Human CD20+ spleen cells (×106) | |
|---|---|---|---|---|
| NOD-scid IL2rγnull | 10±5.9 | 19±14** | 3.6±3.1** | 10.9±8.2** |
| NOD-Rag1null IL2rγnull | 8.5±7.1 | 16±13** | 5.0±7.8** | 6.9±4.1** |
| BALB/c-Rag1null IL2rγnull | 12±6.9 | 1.8±0.7 | 0.30±0.7 | 1.2±0.5 |
Newborn immunodeficient mice received CD34+ HSC by IC injection as described in Protocol B and engraftment was evaluated 12 to 16 weeks later.
p<0.01 as compared to BALB/c-Rag1null IL2rγnull mice
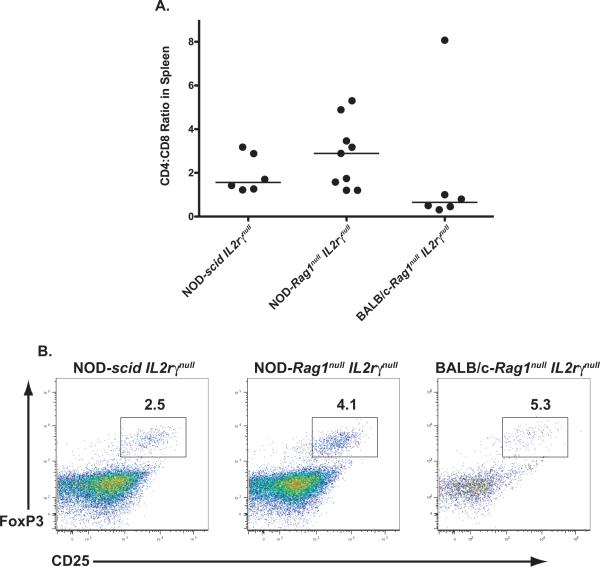
The CD4+ and CD8+ T cell ratios of the CD3+ population of human CD45 cells in the spleen of the 3 strains of mice ranged from 0.3 to 8.1 with the median values being 1.5 for NOD-scid IL2rγnull mice, 2.8 for NOD-Rag1null IL2rγnull mice and 0.65 for BALB/c-Rag1null IL2rγnull mice (Figure 5A). Human CD4 T cells with a regulatory T cell phenotype (CD25+ and Foxp3+) were also detected in the 3 strains of mice as indicated by the representative flow cytometry histograms (Figure 5B).
Figure 5. Comparison of peripheral T cell subsets in HSC-engrafted newborn IL2rγnull strains.
Newborn mice of the indicated strains were irradiated and engrafted with CD34+ cells via IC injection, as described in Materials and Methods (Protocol B). (A) The CD4 to CD8 ratio of human CD3+ T cells was determined in the spleens of engrafted mice. Solid lines represent the median value for each group. Each point represents an individual animal. (B) Putative T-regulatory cell populations were identified in the spleen of each group by staining for FoxP3 and CD25. For analysis, samples were gated on CD4+ cells and representative dot plots are shown in panel B.
Thymic Engraftment in HSC-injected Newborn Mice
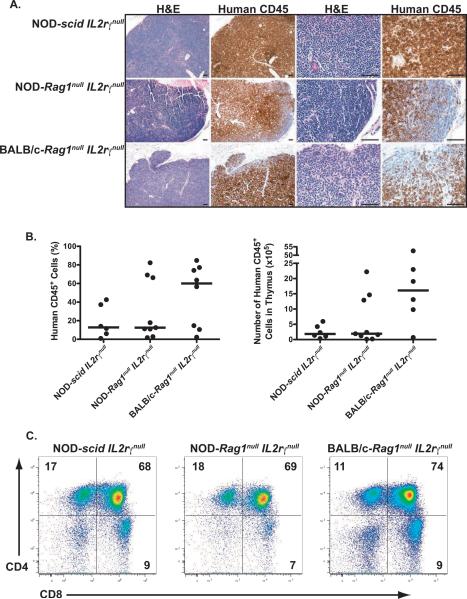
Because of the large discrepancy of T cell numbers in the spleens of immunodeficient NOD and BALB/c strains of mice, we next investigated the levels of thymic engraftment to determine if the reduced numbers of peripheral T cells in the BALB/c strain was due to reduced levels of intrathymic development of human T cells. Histological analysis revealed similar cell numbers and disorganized structure in the thymi of HSC-engrafted immunodeficient NOD and BALB/c strain mice (Figure 6A). Moreover, the percent human CD45+ engraftment was not significantly different between the 3 strains although there was a trend towards higher numbers of human CD45+ cells in BALB/c-Rag1null IL2rγnull mice (Figure 6B). Representative flow cytometry histograms of thymocyte subsets in the three strains of HSC-engrafted immunodeficient mice are shown in Figure 6C. Similar percentages of each of the major populations of human thymic T cells were detected among the strains with a majority of the cells, as expected, being double-positive for CD4 and CD8 with small populations of single positive cells.
Figure 6. Thymic engraftment in HSC-inected newborn IL2rγnull strains.
Newborn mice were irradiated and injected via the IC route with CD34+ cells as described in Materials and Methods. Twelve to 16 weeks later, the thymi were recovered and processed for histology. (A) Histological sections of thymi from engrafted mice were evaluated following H&E and human CD45 staining; Bars = 100μM. (B) Levels of human CD45+ cells (both percentage and number) in the thymi were determined by FACS, with the solid lines represent the median value for each group. Each dot represents an individual animal. (C) Representative histograms following staining with anti-HuCD4 and anti-HuCD8 are shown for each strain.
Hematopoietic Cell Engraftment in Newborn Immunodeficient Mice Injected via the Intrahepatic Route
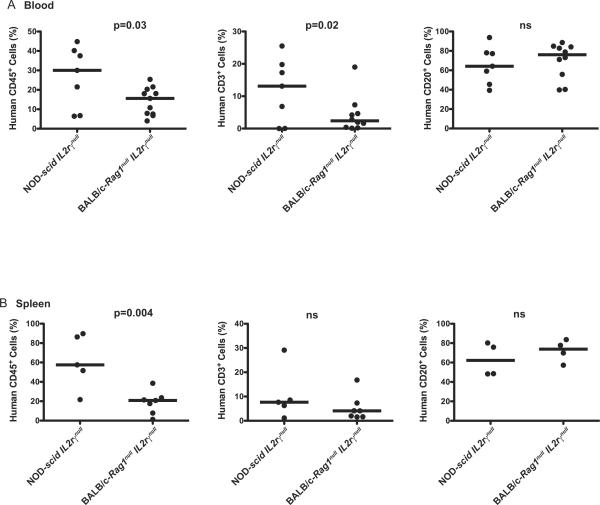
As the original description of engrafting BALB/c-Rag2null IL2rγnull mice with human HSC utilized an alternate newborn engraftment methodology, IH injection [10], we sought to compare the hematopoietic cell engraftment achieved in mice using this route of injection. When NOD-scid IL2rγnull and BALB/c-Rag1null IL2rγnull mice were irradiated and injected with 3×104 CD34+ stem cells via the IH route, a higher percentage of human CD45+ cells were detected in the peripheral blood and spleens of NOD-scid IL2rγnull mice as compared to BALB/c-Rag1null IL2rγnull mice (Figure 7A and 7B). Again, the percent of human CD3+ T cells was significantly higher in the blood of NOD-scid IL2rγnull mice as compared to BALB/c-Rag1null IL2rγnull mice but was not significantly different in the spleens. Human B cell engraftment was similar in both blood and spleen among the strains.
Figure 7. Intrahepatic injection of HSC into newborn IL2rγnull strains.
Newborn mice were irradiated and injected via the IH route with CD34+ cells as described in Materials and Methods. Twelve to 16 weeks after HSC engraftment, the percentages of human CD45+, CD3+ and CD20+ cells in peripheral blood (A) and spleen (B) were determined by flow cytometry. The solid lines represent the median value for each group. The proportion of T cells and B cells is a percentage of the human CD45+ cell numbers. Each dot represents an individual animal.
DISCUSSION
In this study we have compared NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1nullIL2rγnull strains of mice for their ability to support engraftment of human HSC from the same cord blood donor within individual experiments. We observed that newborn mice engrafted at higher levels than adult mice, and that in all parameters tested, NOD strain mice provided the optimal environment for engraftment of human hematopoietic cells. Although all strains of newborn mice engrafted with human HSC generated equivalent numbers and phenotypes of human thymocytes and bone marrow cells, NOD strain mice exhibited significantly higher numbers of human T cells and B cells in their spleens. No differences were observed in newborn NOD-scid IL2rγnull mice when engrafted via the IH route as compared to the IC route. These data suggest that newborn immunodeficient NOD IL2rγnull mice represent the optimal recipients for engraftment of human HSC.
The engraftment of human HSC into mice and subsequent development of a human immune system requires the elimination of murine adaptive immunity and significant reduction of innate immunity (Table 1). Initial efforts to “humanize mice” focused on models in which the adaptive immune response was abrogated, including mice bearing the scid, Rag1null or Rag2null mutation [5]. The CB17-scid mouse, which does not develop T or B cells but has moderate NK cell activity [1], was the first model used extensively to engraft human HSC, but the overall engraftment was extremely low and these mice did not develop a functional human immune system [4]. Further experimentation revealed that strain background can dramatically impact the engraftment of HSC in immunodeficient recipient mice [27]. For example NOD-scid mice show significantly higher levels of engraftment with human HSC as compared to CB17-scid mice [11]. However overall engraftment levels were still low in these models, and this was attributed to the innate immune system, including NK cell function. Thus the next objective was to develop genetic stocks of mice with a weakened innate immune system in addition to absence of adaptive immunity. The focus was to introduce specific mutations to reduce NK cell activity in NOD mice bearing the scid, Rag1 null or Rag2 null mutations. The mutant genes included β2mnull [28], to reduce NK development, perforin [29], to reduce NK cell cytotoxicity and Lsytbg [30], which interferes with NK cell degranulation. While the introduction of these mutations successfully increased the engraftment levels as compared to NOD-scid mice, overall engraftment was variable and still not optimal [5].
The next significant advancement in humanized mouse models was creating stocks of scid, Rag1null or Rag2null mice that expressed targeted mutations within the IL2rγ gene, either by a complete deletion or truncation of the receptor [9,10,12,18]. Immunodeficient mice harboring mutations within the IL2rγ gene show high levels of engraftment with human HSC and develop multiple human immune cell lineages [5]. In addition efforts are underway to improve the IL2rγ-deficient models for the development of functional human immune systems including the transgenic expression of human HLA, both class I and II, and human-specific cytokines and growth factors and by the additional deletion of murine genes to further dampen the innate immune system [31]. Two recent studies have documented the generation of HLA-A2-restricted CD8 T cell responses within A2-transgeneic NOD-scid IL2rγnull mice engrafted with HLA-A2 HSC and then infected with either EBV or Dengue virus [32,33]. However, the creation of humanized mice is still complicated due to the diverse protocols used to engraft HSC, including the growing number of mouse models available and the choices of recipient age, injection route and source of human HSC.
For the data presented in this study, we have used T cell-depleted human UCB, delivered at a dose of 3×104 CD34+ cells per recipient. T cell depletion is required to prevent the development of graft-versus-host disease in recipient mice mediated by the T cells present in unfractionated cord blood. The dose we chose is a stringent test of engraftment, as many studies have published CD34+ doses of 1×105 or greater [5–8]. However, in a practical sense, the fact that our lowered CD34+ cell dose leads to robust human hematolymphoid engraftment means that many mice can potentially be engrafted from a single UCB sample, where 1×106 or more CD34+ cells can often be obtained. We have not specifically tested the engraftment levels supported by the three strains when the cord blood sample is enriched for CD34+ cells by either lineage depletion or CD34+ positive selection. In our laboratory, we have found that more CD34+ cells are required when injecting lineage-depleted cord blood or CD34+ purified cells in order to achieve engraftment levels similar to that achieved following the injection of T cell-depleted cord blood cells (unpublished observations).
HSC can be obtained from many different sources in addition to umbilical cord blood, including G-CSF mobilized peripheral blood [11], bone marrow [4], and fetal liver [3]. A recent report suggested that UCB was equivalent to fetal liver CD34+ cells in their scid repopulating activity in immunodeficient mice [14]. We chose umbilical cord blood to do these comparisons as it is readily available and has suitable numbers of CD34+ HSC to engraft large cohorts of mice. While the human immune systems created from cord blood-derived HSC are extremely useful in studying basic aspects of human immunology, there are advantages for using HSC derived from alternative sources. For example, CD34+ cells can be isolated from the peripheral blood or bone marrow of patients with pre-existing immunologic abnormalities, such as autoimmune diseases, to study the development of clinical disease. However there are no guarantees that HSC derived from a patient would manifest the same disease in the humanized mouse models.
Three strains have emerged as the preferred hosts for human HSC engraftment: NOD.Cg-PrkdcscidIl2rgtm1Wjll (commonly referred to as NSG mice) [11,18], NOD.Cg-PrkdcscidIl2rgtm1Sug (commonly referred to as NOG mice) [9,34] and C.129(Cg)-Rag2tm1FwaIl2rgtm1Sug (abbreviated as BALB/c-Rag2null IL2rγnull) [10] mice. As the BALB/c-Rag2null IL2rγnull strain is not commercially available, we generated our own stock that is similar to the published version, except that the Rag1null mutation is employed instead of Rag2null. However, the phenotype of Rag1null and Rag2null mice are identical [21,22] and our stock of BALB/c-Rag1null IL2rγnull mice has a phenotype that is predicted from the existing BALB/c-Rag2null IL2rγnull strain and from our own NOD-scid IL2rγnull and NOD-Rag1null IL2rγnull mice.
Using this new stock of mice, we compared directly the role of the NOD and BALB/c strain mice bearing the Rag1null mutation for their ability to support human HSC engraftment. In the majority of cases, NOD-Rag1null IL2rγnull and NOD-scid IL2rγnull mice support higher levels of human HSC engraftment in the blood and spleen, and importantly for studies of human immunity, higher levels of T cell development. As previously reported [5–8], we confirmed multilineage development of all hematopoietic cells in all engrafted mice irrespective of the strain or protocol of engraftment (unpublished observations). The major differences in lineage-specific engraftment that were observed were in the T cell population between NOD and BALB/c strain mice. In contrast, human CD45+ engraftment in the bone marrow and thymus between the strains was similar. The peripheral T cell lymphopenia in HSC engrafted BALB/c-Rag1null IL2rγnull mice could result from a survival defect of human cells or a lower proliferation rate of human cells compared with the immunodeficient NOD mice. We are currently investigating these possibilities. The direct comparison of newborn and adult BALB/c-Rag1null IL2rγnull mice as was done for NOD-scid IL2rγnull mice was not performed as neither strain exhibited human HSC engraftment at levels observed in the NOD strains. However, we must caution that there are multiple substrains of BALB/c mice, and the BALB/c-Rag1null IL2rγnull stock we have generated is not identical to that developed in the laboratory of Manz [10], and may display different engraftment characteristics.
The reasons for differences in strain background are not well understood, but it is clear that modifying genes are contributing to the ability of the host to support engraftment. Although NK cells are important factors in determining human HSC engraftment and vary considerably between background strains, no functional NK cells develop in mice with a mutated IL2rγ gene [5–8]. An important advance in this regard is the recent report documenting the role of the signal regulatory protein α (Sirpα) gene in human HSC engraftment [35]. Polymorphisms in this gene product alter its ability to bind human CD47 and promote human HSC engraftment in immunodeficient mice. Continued screening for host genetic pathways will likely lead to further insights on the role of mouse genetic polymorphisms on their ability to support human hematopoietic engraftment.
We have found that engraftment of newborn rather than adult mice with HSC is optimal for several reasons. First, for studies of immune function where a robust T cell population is required, newborn injection is clearly superior to adults as hosts in their ability to support T cell development. While the mechanism underlying the superiority of the newborn host in generating human T cells is unclear, it is likely due to thymic organogenesis in these mice. In the absence of developing T cells, untreated scid mice rapidly undergo thymic hypoplasia, so that by the time HSC are administered to adult mice, thymic architecture is severely altered. It has been reported that introduction of the IL2rγnull gene results in the development of hypoplastic cysts that increase with age in immunocompetent mice [26]. We extended this observation to IL2rγnull scid and IL2rγnull Rag1null mice, and did not observe large differences in the appearance of these hypoplastic cysts with age between strains. In contrast, in newborn mice, HSC can be delivered before thymic dysplasia occurs and the HSC inoculum can potentially contribute thymic organizing cells to thymus organogenesis. This is suggested by the apparent lower appearance of hypoplastic cysts we observed in human HSC-engrafted mice. It is also possible that the higher ratio of HSC number to body mass achievable with the same number of cells in newborn mice as compared to adult mice supports enhanced human T cell development. Previous studies with umbilical cord blood derived HSC have shown that increasing the cell dose (cells/kg) results in superior engraftment and survival for human recipients [36], and this may benefit T cell development in newborn mice.
Several approaches have been used to engraft newborn immunodeficient mice with human HSC, including IH [10], IV (facial vein) [37] and IC (this report). We believe that engraftment success of IV injection through the facial vein and IC injection are essentially identical, as they both introduce the HSC directly into the circulation. However, we favor IC injection as it is a rapid, relatively simple technique and does not require microscopic visualization of the injection site. We compared engraftment in newborn NOD-scid IL2rγnull and BALB/c-Rag1null IL2rγnull mice established by IH versus IC injection routes to determine if either route promoted higher levels of engraftment. Interestingly, the route of injection did not influence the results in either strain, as both methodologies readily led to equivalent human HSC engraftment within an immunodeficient mouse strain. Our laboratory favors the IC injection, as the “flash” of blood that is often observed during injection provides confirmation of delivery of the HSC into the intended anatomical site [20].
In summary, we have compared directly human HSC engraftment in NOD-scid IL2rγnull, NOD-Rag1null IL2rγnull, and BALB/c-Rag1null IL2rγnull mice. We observed that human HSC engraft more efficiently in newborn mice as compared to adult mice, and that both immunodeficient NOD strains tested displayed enhanced hematopoietic cell engraftment as compared to the immunodeficient BALB/c strain. Although all strains permitted the engraftment of multi-lineage cell populations, significantly fewer human T and B cells were present in the spleen of BALB/c mice as compared to both NOD strains. We conclude that NOD-scid IL2rγnull and NOD-Rag1null IL2rγnull strains engraft at higher levels and generate more human T cells following HSC engraftment than do the BALB/c-Rag1null IL2rγnull mice. These data identify key variables for establishing humanized mouse models for the study of human immunity.
ACKNOWLEDGMENTS
We thank Jean Leaf, Linda Paquin, and Allison Ingalls for technical assistance. This work was supported by National Institutes of Health research grants AI46629, DK53006, HL077642, CA34196, an institutional Diabetes Endocrinology Research Center (DERC) grant DK32520 and grants from the Beta Cell Biology Consortium of NIDDK, grants from the Juvenile Diabetes Foundation, International and a grant from the University of Massachusetts Center for AIDS Research, P30 AI042845. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bosma GC, Custer RP, Bosma MJ. A severe combined immunodeficiency mutation in the mouse. Nature. 1983;301:527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- 2.Mosier DE, Gulizia RJ, Baird SM, Wilson DB. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988;335:256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- 3.McCune JM, Namikawa R, Kaneshima H, Shultz LD, Lieberman M, Weissman IL. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988;241:1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- 4.Lapidot T, Pflumio F, Doedens M, Murdoch B, Williams DE, Dick JE. Cytokine stimulation of multilineage hematopoiesis from immature human cells engrafted in SCID mice. Science. 1992;255:1137–1141. doi: 10.1126/science.1372131. [DOI] [PubMed] [Google Scholar]
- 5.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat.Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 6.Legrand N, Ploss A, Balling R, Becker PD, Borsotti C, Brezillon N, Debarry J, de JY, Deng H, Di Santo JP, Eisenbarth S, Eynon E, Flavell RA, Guzman CA, Huntington ND, Kremsdorf D, Manns MP, Manz MG, Mention JJ, Ott M, Rathinam C, Rice CM, Rongvaux A, Stevens S, Spits H, Strick-Marchand H, Takizawa H, van Lent AU, Wang C, Weijer K, Willinger T, Ziegler P. Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host.Microbe. 2009;6:5–9. doi: 10.1016/j.chom.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manz MG. Human-hemato-lymphoid-system mice: opportunities and challenges. Immunity. 2007;26:537–541. doi: 10.1016/j.immuni.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J.Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 9.Ito M, Hiramatsu H, Kobayashi K, Suzue K, Kawahata M, Hioki K, Ueyama Y, Koyanagi Y, Sugamura K, Tsuji K, Heike T, Nakahata T. NOD/SCID/gamma(c)(null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood. 2002;100:3175–3182. doi: 10.1182/blood-2001-12-0207. [DOI] [PubMed] [Google Scholar]
- 10.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 11.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2rγnull mice engrafted with mobilized human hematopoietic stem cell. J.Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 12.Gimeno R, Weijer K, Voordouw A, Uittenbogaart CH, Legrand N, Alves NL, Wijnands E, Blom B, Spits H. Monitoring the effect of gene silencing by RNA interference in human CD34+ cells injected into newborn RAG2−/− gammac−/− mice: functional inactivation of p53 in developing T cells. Blood. 2004;104:3886–3893. doi: 10.1182/blood-2004-02-0656. [DOI] [PubMed] [Google Scholar]
- 13.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu.Rev.Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 14.Lepus CM, Gibson TF, Gerber SA, Kawikova I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold KC, Donis RO, Bothwell AL, Pober JS, Harding MJ. Comparison of human fetal liver, umbilical cord blood, and adult blood hematopoietic stem cell engraftment in NOD-scid/gammac−/−, Balb/c-Rag1−/−gammac−/−, and C.B-17-scid/bg immunodeficient mice. Hum.Immunol. 2009;70:790–802. doi: 10.1016/j.humimm.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christianson SW, Greiner DL, Schweitzer IB, Gott B, Beamer GL, Schweitzer PA, Hesselton RM, Shultz LD. Role of natural killer cells on engraftment of human lymphoid cells and on metastasis of human T-lymphoblastoid leukemia cells in C57BL/6J-scid mice and in C57BL/6J-scid bg mice. Cell.Immunol. 1996;171:186–199. doi: 10.1006/cimm.1996.0193. [DOI] [PubMed] [Google Scholar]
- 16.Greiner DL, Shultz LD, Yates J, Appel MC, Perdrizet G, Hesselton RM, Schweitzer I, Beamer WG, Shultz KL, Pelsue SC, Leif JH, Rajan TV. Improved engraftment of human spleen cells in NOD/LtSz-scid/scid mice as compared with C.B-17-scid/scid mice. Am.J.Path. 1995;146:888–902. [PMC free article] [PubMed] [Google Scholar]
- 17.Greiner DL, Hesselton RA, Shultz LD. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor gamma chain null mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearson T, Shultz LD, Miller D, King M, Laning J, Fodor W, Cuthbert A, Burzenski L, Gott B, Lyons B, Foreman O, Rossini AA, Greiner DL. Non-obese diabetic-recombination activating gene-1 (NOD-Rag1 null) interleukin (IL)-2 receptor common gamma chain (IL2r gamma null) null mice: a radioresistant model for human lymphohaematopoietic engraftment. Clin.Exp.Immunol. 2008;154:270–284. doi: 10.1111/j.1365-2249.2008.03753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson T, Greiner DL, Shultz LD. Creation of “humanized” mice to study human immunity. Curr.Protoc.Immunol. 2008 doi: 10.1002/0471142735.im1521s81. Chapter 15, Unit. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 22.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 23.King M, Pearson T, Rossini AA, Shultz LD, Greiner DL. Humanized mice for the study of type 1 diabetes and beta cell function. Ann.N.Y.Acad.Sci. 2008;1150:46–53. doi: 10.1196/annals.1447.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rozemuller H, Knaan-Shanzer S, Hagenbeek A, van Bloois L, Storm G, Martens AC. Enhanced engraftment of human cells in RAG2/gammac double-knockout mice after treatment with CL2MDP liposomes. Exp.Hematol. 2004;32:1118–1125. doi: 10.1016/j.exphem.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canninga-van Dijk MR, Weijer K, Spits H, Storm G, van Bloois L, Rijkers G, Martens AC, Ebeling SB. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mononuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102:2522–2531. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 26.Ikebe M, Miyakawa K, Takahashi K, Ohbo K, Nakamura M, Sugamura K, Suda T, Yamamura K, Tomita K. Lymphohaematopoietic abnormalities and systemic lymphoproliferative disorder in interleukin-2 receptor gamma chain-deficient mice. Int.J.Exp.Pathol. 1997;78:133–148. doi: 10.1046/j.1365-2613.1997.230356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hesselton RM, Greiner DL, Mordes JP, Rajan TV, Sullivan JL, Shultz LD. High levels of human peripheral blood mononuclear cell engraftment and enhanced susceptibility to human immunodeficiency virus type 1 infection in NOD/LtSz-scid/scid mice. J.Inf.Dis. 1995;172:974–982. doi: 10.1093/infdis/172.4.974. [DOI] [PubMed] [Google Scholar]
- 28.Christianson SW, Greiner DL, Hesselton RM, Leif JH, Wagar EJ, Schweitzer IB, Rajan TV, Gott B, Roopenian DC, Shultz LD. Enhanced human CD4+ T cell engraftment in β2-microglobulin-deficient NOD-scid mice. J.Immunol. 1997;158:3578–3586. [PubMed] [Google Scholar]
- 29.Shultz LD, Banuelos S, Lyons B, Samuels R, Burzenski L, Gott B, Lang P, Leif J, Appel M, Rossini A, Greiner DL. NOD/LtSz-Rag1nullPrf1null mice: a new model system with increased levels of human peripheral leukocyte and hematmopoietic stem cell engraftment. Transplantation. 2003;76:1036–1042. doi: 10.1097/01.TP.0000083041.44829.2C. [DOI] [PubMed] [Google Scholar]
- 30.Mosier DE, Stell KL, Gulizia RJ, Torbett BE, Gilmore GL. Homozygous scid/scid;beige/beige mice have low levels of spontaneous or neonatal T cell-induced B cell generation. J.Exp.Med. 1993;177:191–194. doi: 10.1084/jem.177.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manz MG, Di Santo JP. Renaissance for mouse models of human hematopoiesis and immunobiology. Nat.Immunol. 2009;10:1039–1042. doi: 10.1038/ni1009-1039. [DOI] [PubMed] [Google Scholar]
- 32.Strowig T, Gurer C, Ploss A, Liu YF, Arrey F, Sashihara J, Koo G, Rice CM, Young JW, Chadburn A, Cohen JI, Munz C. Priming of protective T cell responses against virus-induced tumors in mice with human immune system components. J.Exp.Med. 2009;206:1423–1434. doi: 10.1084/jem.20081720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaiswal S, Pearson T, Friberg H, Shultz LD, Greiner DL, Rothman AL, Mathew A. Dengue virus infection and virus-specific HLA-A2 restricted immune responses in humanized NOD-scid IL2rgammanull mice. PLoS.ONE. 2009;4:e7251. doi: 10.1371/journal.pone.0007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yahata T, Ando K, Miyatake H, Uno T, Sato T, Ito M, Kato S, Hotta T. Competitive repopulation assay of two gene-marked cord blood units in NOD/SCID/gammac(null) mice. Mol.Ther. 2004;10:882–891. doi: 10.1016/j.ymthe.2004.07.029. [DOI] [PubMed] [Google Scholar]
- 35.Takenaka K, Prasolava TK, Wang JC, Mortin-Toth SM, Khalouei S, Gan OI, Dick JE, Danska JS. Polymorphism in Sirpa modulates engraftment of human hematopoietic stem cells. Nat.Immunol. 2007;8:1313–1323. doi: 10.1038/ni1527. [DOI] [PubMed] [Google Scholar]
- 36.Gluckman E, Rocha V. Donor selection for unrelated cord blood transplants. Curr.Opin.Immunol. 2006;18:565–570. doi: 10.1016/j.coi.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa F, Livingston AG, Wingard JR, Nishikawa S, Ogawa M. An assay for long-term engrafting human hematopoietic cells based on newborn NOD/SCID/beta2-microglobulin(null) mice. Exp.Hematol. 2002;30:488–494. doi: 10.1016/s0301-472x(02)00784-1. [DOI] [PubMed] [Google Scholar]
- 38.Lapidot T. Mechanism of human stem cell migration and repopulation of NOD/SCID and B2mnull NOD/SCID mice. The role of SDF-1/CXCR4 interactions. Ann.N.Y.Acad.Sci. 2001;938:83–95. doi: 10.1111/j.1749-6632.2001.tb03577.x. [DOI] [PubMed] [Google Scholar]
- 39.Shultz LD, Lang PA, Christianson SW, Gott B, Lyons B, Umeda S, Leiter E, Hesselton R, Wagar EJ, Leif JH, Kollet O, Lapidot T, Greiner DL. NOD/LtSz-Rag1null mice: An immunodeficient and radioresistant model for engraftment of human hematolymphoid cells, HIV infection, and adoptive transfer of NOD mouse diabetogenic T cells. J.Immunol. 2000;164:2496–2507. doi: 10.4049/jimmunol.164.5.2496. [DOI] [PubMed] [Google Scholar]
- 40.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, Yamamura K, Sugamura K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor gamma chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 41.Jacobs H, Krimpenfort P, Haks M, Allen J, Blom B, Demolliere C, Kruisbeek A, Spits H, Berns A. PIM1 reconstitutes thymus cellularity in interleukin 7- and common gamma chain-mutant mice and permits thymocyte maturation in Rag- but not CD3gamma-deficient mice. J.Exp.Med. 1999;190:1059–1068. doi: 10.1084/jem.190.8.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]