Abstract
Ovarian hormones, including progesterone, are known to have immunomodulatory and neuroprotective effects which may alter the disease course of experimental autoimmune encephalomyelitis (EAE). In the current study, we examined the treatment potential of progesterone beginning at the onset of EAE symptoms. Progesterone treated animals showed reduced peak disease scores and cumulative disease indices, and decreased inflammatory cytokine secretion (IL-2 and IL-17). In addition, increased production of IL-10 was accompanied by increased numbers of CD19+ cells and an increase in CD8+ cells. Decreased chemokine and chemokine receptor expression in the spinal cord also contributed to decreased lesions in the spinal cord.
Keywords: progesterone, estrogen, EAE, multiple sclerosis, B cells
1. INTRODUCTION
Multiple sclerosis (MS) is a devastating neurodegenerative disease, characterized by chronic inflammation and demyelination, which is more common in women than men (Sadovnick, 2009). The increased risk of MS in women is often accompanied by a remission of symptoms when high levels of ovarian hormones are present, such as during pregnancy (Airas et al., 2008; Confavreux et al., 1998). Experimental autoimmune encephalomyelitis (EAE), the animal model of MS, has been used extensively to investigate the hormonal regulation of immunomodulatory pathways involved. Pretreatment with 17β-estradiol or treatment with ethinyl estradiol decreases inflammatory cytokines and demyelination in the spinal cord of EAE mice (Bebo et al., 2001; Ito et al., 2001; Subramanian et al., 2003), while 17β-estradiol also increases regulatory T cells (Polanczyk et al., 2004). Although estrogens may be important contributors, other hormones may be crucial to the immune modulation that occurs. Pretreatment with progesterone decreases disease severity and reduces axonal damage and demyelination (Garay et al., 2007; Garay et al., 2009) although results have been mixed (Kim et al., 1999). There is significant evidence that progesterone can have immunomodulatory and neuroprotective effects in other models (De Leon-Nava et al., 2009; Drew and Chavis, 2000; Gonzalez et al., 2005; Hughes et al., 2008; Otsuki et al., 2001). However, effects of progesterone treatment beginning after EAE onset have not been reported. In the present study, the effects of progesterone treatment after EAE disease onset are explored.
2. MATERIALS AND METHODS
2.1 Animals and hormone treatment
Female C57BL/6 mice were obtained from Harlan Laboratories (Houston, TX) and housed at the Portland VA Medical Center in accordance with institutional guidelines. At 7–8 weeks of age, mice were immunized with 200μg MOG 35–55 peptide (PolyPeptide laboratories; San Diego, CA) and 200μg complete Freund’s adjuvant. Mice received pertussis toxin at the time of immunization (d0; 75ng) and on d2 (200ng). Animals were scored daily for disease severity: 0 = normal, 1 = limp tail, 2 = mild hindlimb weakness, 3 = moderate hindlimb weakness, 4 = severe weakness or partial paralysis, 5 = complete hindlimb paralysis, 6 = moribund.
At the onset of EAE (the first day with a score of 1 or greater), animals were randomly assigned to receive either a subcutaneous progesterone implant (100mg 60 day release, Innovative Research of America; Sarasota, FL) or placebo treatment.
2.2 Spleen and lymph node analyses
Spleen and lymph nodes were collected at D22-25 post-immunization. Details of proliferation and cytokine assays have been published previously (Sinha et al., 2009). For flow cytometry, cells were stained using: CD3-APC, CD4-FITC, CD8-PE, CD11b-APC, CD19-FITC, FOXP3-APC, and PD-1PE. Intracellular staining for FOXP3 and PD-1 was completed after fixation/permeabilization (EBiosciences; San Diego, CA). Cells were then run on a FACSCalibur (Becton Dickson). Calculations of cell number were based on cell yields and percentages of live gated cells.
2.3 Spinal cords
Spinal cords were collected for RT-PCR and histopathology. Total RNA was isolated from spinal cord tissue using the RNeasy mini-kit protocol (Qiagen; Valencia, CA) and converted to cDNA using oligo(dT), random hexamers, and Superscript RT II (Invitrogen; Grand Island, NY). RT-PCR for MIP2, CCR2, CCR5, CCR7, IL-17, TNF-α, IL-23 was run on a Prism 7000 Sequence Detection System (Applied Biosystems; Foster City, CA) using Taqman PCR master mix (Qiagen) and primers from Applied Biosystems. Levels of expression were normalized to β-actin.
For histology, spinal cords sections were stained using luxol fast blue- periodic acid shiff for semi-quantitative analysis of infiltration and demyelination.
2.4 Serum progesterone levels
Blood was collected via cardiac puncture at the time of sacrifice and assayed for progesterone by RIA (Beckman-Coulter/Diagnostic Systems Laboratory; Webster, TX).
3. RESULTS
3.1 Disease severity
Cumulative disease index and peak disease scores were significantly reduced in animals receiving progesterone treatment beginning at onset compared to control animals (Figure 1A and 1B).
Figure 1.
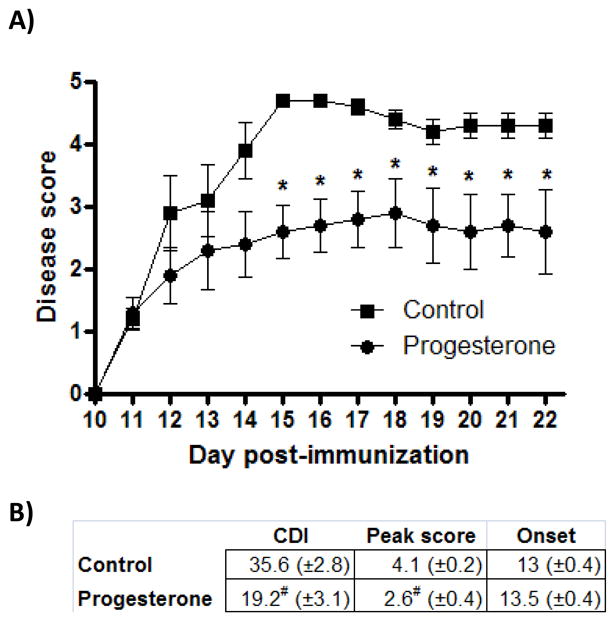
A) Animals receiving progesterone at disease onset had reduced disease severity compared to controls. Significant differences were present from D15-22 (p<.04). Data are representative of experiments completed 3 times, containing 6–8 animals per group. * indicates p<.04. B) Cumulative disease index (CDI) through D22 post-immunization and peak disease scores were significantly reduced in animals receiving progesterone treatment beginning at the onset of disease symptoms. N=21 per group. # indicates significant difference compared to control group (p<.001).
3.2 Proliferation and cytokine secretion
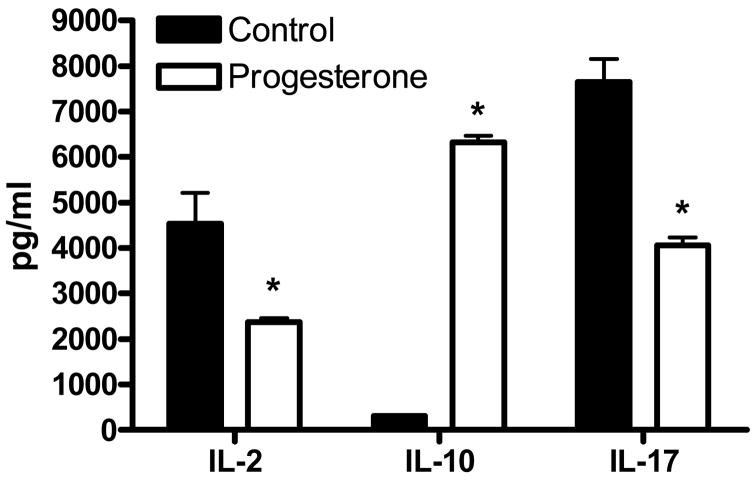
Cells isolated from the spleens of progesterone treated animals secreted reduced amounts of the IL-2 and IL-17 (p<.03), while levels of IL-10 were increased (p<.01; Figure 2). In response to antigen, cells from the spleen and lymph node of progesterone treated animals showed reduced levels of proliferation (data not shown).
Figure 2.
Splenocytes isolated from animals treated with progesterone secreted significantly less IL-2 and IL-17 and increased levels of IL-10 in the presence of MOG35-55 peptide compared to controls. * indicates p<.03.
3.3 Flow cytometry
Progesterone treated animals had increased numbers of CD3+, CD11b+, and CD19+ cells in the spleen (p<.05) and a trend for an increase in CD8+ cells (p<.08). CD19+ cells were increased in the lymph node (p<.01, Figure 3). There were no significant differences in the number of CD4+ (Control: 8.2 × 106 [+/−1.5], Progesterone: 10.0 × 106 [+/−1.0]), CD4+FOXP3+ (Control: 1.5 × 106 [+/− 0.3], Progesterone: 2.0 × 106 [+/−0.2]), or CD4+PD1+ (Control: 0.8 × 106 [+/−0.2], Progesterone: 1.4 × 106 [+/−0.4] cells.
Figure 3.
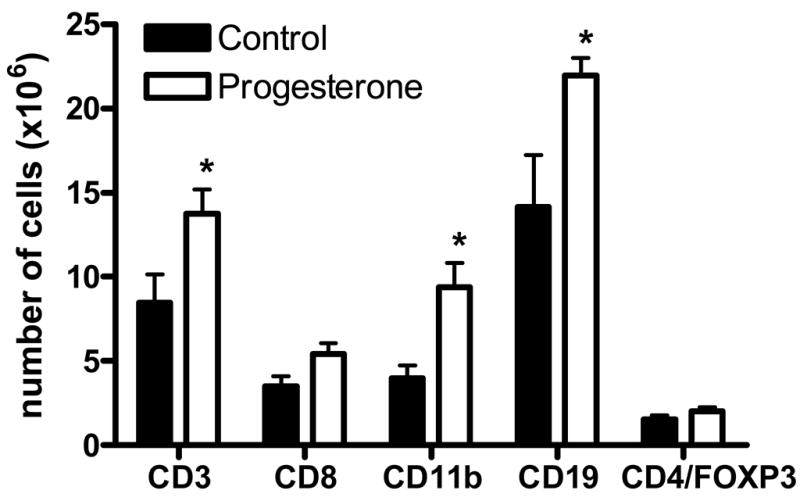
There were significant increase in the number of CD3+, CD11b+, and CD19+ cells in the spleen of progesterone treated animals. There was also a trend for an increase in CD8+ cells (p<.08). Increases in CD19+ cells in the spleen were accompanied by a significant increase within the lymph node (data not shown). * indicates p<.05.
3.4 Real-time PCR
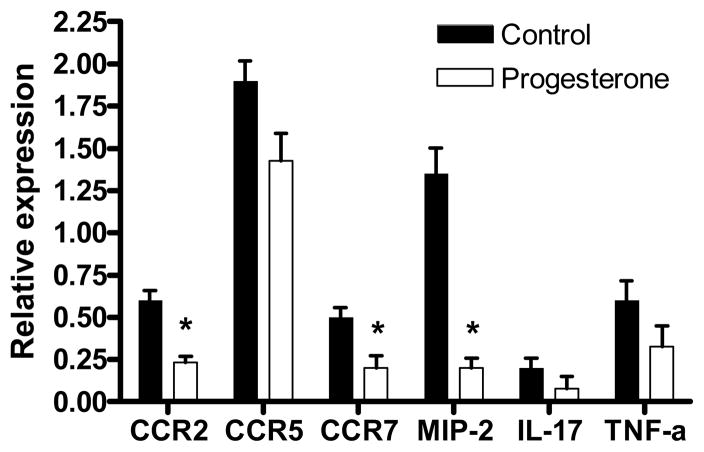
Levels of mRNA for cytokines and chemokine receptors were analyzed in the spinal cord. While levels of IL-17 and TNF-α did not differ between groups, there were significant decreases in CCR2, CCR7, and MIP-2/CXCL2 (Figure 4).
Figure 4.
Levels of mRNA expression for CCR2, CCR7, and MIP-2 were significantly decreased in spinal cords from animals receiving progesterone treatment after disease onset compared to controls. * indicates p<.03.
3.5 Histology
Progesterone reduced cellular infiltration into the spinal cord compared to control animals (data not shown).
3.6 Serum progesterone levels
Progesterone levels were significantly different between groups (p<.001): 76ng/ml (SEM=10.0) in the progesterone group (which is similar to peak levels seen during pregnancy in the mouse (Holinka et al., 1979)), compared to 3.5ng/ml in the control group (SEM= 1.1). Estrogen levels were frequently below the range of detection (<5pg/ml) and did not differ between groups.
4. DISCUSSION
After the onset of disease symptoms, progesterone treatment decreased the secretion of pro-inflammatory cytokines (IL-2 and IL-17) and increased secretion of the anti-inflammatory cytokine IL-10. In addition, decreased cytokine and chemokine receptor expression (MIP-2, CCR2, CCR7) in the spinal cord may work to reduce cellular infiltration and damage in the CNS. While previous studies have demonstrated a protective effect of progesterone against EAE (Garay et al., 2007; Garay et al., 2009), the current study is the first to demonstrate the effectiveness of progesterone treatment started at the onset of EAE.
The increased presence of B cells may play a prominent role in the treatment effects of progesterone in the current study, which is in line with protective immunomodulatory effects of B cells demonstrated previously (Fillatreau et al., 2002; Matsushita et al., 2006; Mauri et al., 2003). CD19 deficient mice show worse EAE severity and greater CNS pathology compared to wild-type mice, which may be the result of increased TNF-α and decreased IL-10 (Matsushita et al., 2006). In the present study, B cell numbers were increased in progesterone treated animals, likely contributing to the increased secretion of IL-10 and decreased disease severity. T cells or CD8+ cells alone treated with progesterone can also increase production of IL-10 (Enomoto et al., 2007; Miyaura and Iwata, 2002). Inhibition of antigen presenting cells due to increased levels of IL-10 prevents further IL-12 production and Th1 differentiation, giving it a potentially crucial role in disease inhibition by progesterone.
Conflicting evidence regarding the role of CD8+ cells in EAE makes the increased numbers following progesterone treatment particularly interesting. Infiltrating CD8+ cells have been implicated in lesion development in multiple sclerosis (Babbe et al., 2000) and EAE (Matsushita et al., 2006; Sun et al., 2001). Evidence suggests that a population of CD8+ dendritic cells may have a regulatory role in reducing EAE severity in Lewis rats by increasing IFN-γ and nitric oxide production (Pettersson et al., 2004). While a beneficial roll of IFN-γ is counter to the view of Th1-mediated EAE, it is not without precedence (Chu et al., 2000; Willenborg et al., 1999). CD8+ cells also exert a pregnancy protective effect when exposed to progesterone (Blois et al., 2004), lending further support to the protective immunomodulatory potential of CD8+ cells. Given the differential effects of CD8+ subpopulations, further work will aim to elucidate the CD8+ subtype responsible for the protective effects of progesterone.
Cross-reactivity between progesterone and the glucocorticoid receptor (Kontula et al., 1983) makes it possible that some effects of progesterone seen in the current study are due to glucocorticoid activity. However, the expression of progesterone receptors on bone marrow derived dendritic cells (Butts et al., 2008) and splenocyte populations (including CD4+, CD19+, CD8+, natural killer cells, and macrophages) does allow for the direct action of progesterone on these cell types (De Leon-Nava et al., 2009). The release of progesterone-induced blocking factor from cells (including γδTCR+ and CD8+ cells) after interaction of progesterone with the progesterone receptor (Szekeres-Bartho and Wegmann, 1996), may also contribute to immunomodulatory effects.
While estrogen and progesterone have some overlapping effects, their mechanisms are quite different. Estrogens work to reduce the Th1 cytokine environment and decrease chemokine receptor expression (Bebo et al., 2001; Matejuk et al., 2001; Polanczyk et al., 2003; Polanczyk et al., 2004; Polanczyk et al., 2007; Subramanian et al., 2003), and 17β-estradiol also increases the FOXP3+ and PD-1+ regulatory T cell populations to inhibit disease (Polanczyk et al., 2004; Polanczyk et al., 2006; Polanczyk et al., 2007). Although progesterone treatment at disease onset decreased Th1 cytokines, as well as cytokine and chemokine receptor expression, there were no changes in regulatory T cells. As a result, it is likely that estrogens and progestins have overlapping modes of action while also affecting distinctly different immunomodulatory pathways.
By examining mechanisms of progesterone treatment in established EAE, we can gain a better understanding of mechanisms that may be involved in the remission of MS during pregnancy. The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART’MUS) trial is currently ongoing using a 19-nor-progesterone derivative (Nomegestrol acetate) and estrogen combination (Vukusic et al., 2009). Differences in receptor activity and specificity between progestin derivatives (Sitruk-Ware, 2004), alone and in combination with estrogens, may result in differing immunological and neuroprotective effects however (Ciriza et al., 2006; Gomez et al., 1998; Kaushic et al., 2003; Otsuki et al., 2001). Further investigation into the effects of progesterone and its derivatives in the treatment of established EAE is necessary to more fully understand the mechanisms involved.
Acknowledgments
Dr. Yates is a Postdoctoral Fellow of the National Multiple Sclerosis Society, and this work was supported in part by National Multiple Sclerosis Society Postdoctoral Fellowship FG1832-A-1, National Multiple Sclerosis Society grant RG3405-B-5; NIH grants NS45445, and NS49210; and the Biomedical Laboratory R&D Service, Department of Veterans Affairs.
The authors would like to thank Eva Niehaus for her assistance in manuscript preparation, as well as Francis Pau and the staff of the Endocrinology Technology and Support Lab at the Oregon National Primate Research Center at OHSU for completing RIA assays.
References
- Airas L, Saraste M, Rinta S, Elovaara I, Huang YH, Wiendl H. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: relevance of natural killer cells. Clin Exp Immunol. 2008;151:235–243. doi: 10.1111/j.1365-2249.2007.03555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babbe H, Roers A, Waisman A, Lassmann H, Goebels N, Hohlfeld R, Friese M, Schroder R, Deckert M, Schmidt S, Ravid R, Rajewsky K. Clonal expansions of CD8(+) T cells dominate the T cell infiltrate in active multiple sclerosis lesions as shown by micromanipulation and single cell polymerase chain reaction. J Exp Med. 2000;192:393–404. doi: 10.1084/jem.192.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebo BF, Jr, Fyfe-Johnson A, Adlard K, Beam AG, Vandenbark AA, Offner H. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166:2080–2089. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- Blois SM, Joachim R, Kandil J, Margni R, Tometten M, Klapp BF, Arck PC. Depletion of CD8+ cells abolishes the pregnancy protective effect of progesterone substitution with dydrogesterone in mice by altering the Th1/Th2 cytokine profile. J Immunol. 2004;172:5893–5899. doi: 10.4049/jimmunol.172.10.5893. [DOI] [PubMed] [Google Scholar]
- Butts CL, Bowers E, Horn JC, Shukair SA, Belyavskaya E, Tonelli L, Sternberg EM. Inhibitory effects of progesterone differ in dendritic cells from female and male rodents. Gend Med. 2008;5:434–447. doi: 10.1016/j.genm.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CQ, Wittmer S, Dalton DK. Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med. 2000;192:123–128. doi: 10.1084/jem.192.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciriza I, Carrero P, Frye CA, Garcia-Segura LM. Reduced metabolites mediate neuroprotective effects of progesterone in the adult rat hippocampus. The synthetic progestin medroxyprogesterone acetate (Provera) is not neuroprotective. J Neurobiol. 2006;66:916–928. doi: 10.1002/neu.20293. [DOI] [PubMed] [Google Scholar]
- Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339:285–291. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- De Leon-Nava MA, Nava K, Soldevila G, Lopez-Griego L, Chavez-Rios JR, Vargas-Villavicencio JA, Morales-Montor J. Immune sexual dimorphism: effect of gonadal steroids on the expression of cytokines, sex steroid receptors, and lymphocyte proliferation. J Steroid Biochem Mol Biol. 2009;113:57–64. doi: 10.1016/j.jsbmb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Drew PD, Chavis JA. Female sex steroids: effects upon microglial cell activation. J Neuroimmunol. 2000;111:77–85. doi: 10.1016/s0165-5728(00)00386-6. [DOI] [PubMed] [Google Scholar]
- Enomoto LM, Kloberdanz KJ, Mack DG, Elizabeth D, Weinberg A. Ex vivo effect of estrogen and progesterone compared with dexamethasone on cell-mediated immunity of HIV-infected and uninfected subjects. J Acquir Immune Defic Syndr. 2007;45:137–143. doi: 10.1097/QAI.0b013e3180471bae. [DOI] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Garay L, Deniselle MC, Lima A, Roig P, De Nicola AF. Effects of progesterone in the spinal cord of a mouse model of multiple sclerosis. J Steroid Biochem Mol Biol. 2007;107:228–237. doi: 10.1016/j.jsbmb.2007.03.040. [DOI] [PubMed] [Google Scholar]
- Garay L, Deniselle MC, Meyer M, Costa JJ, Lima A, Roig P, Denicola AF. Protective effects of progesterone administration on axonal pathology in mice with experimental autoimmune encephalomyelitis. Brain Res. 2009;1283:177–185. doi: 10.1016/j.brainres.2009.04.057. [DOI] [PubMed] [Google Scholar]
- Gomez F, Ruiz P, Briceno F, Lopez R, Michan A. Treatment with progesterone analogues decreases macrophage Fcgamma receptors expression. Clin Immunol Immunopathol. 1998;89:231–239. doi: 10.1006/clin.1998.4602. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Tseng YC, Finch CE. Reproductive aging in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol Reprod. 1979;20:1201–1211. doi: 10.1095/biolreprod20.5.1201. [DOI] [PubMed] [Google Scholar]
- Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. J Immunol. 2008;180:2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167:542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- Kaushic C, Ashkar AA, Reid LA, Rosenthal KL. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52:1230–1238. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- Kontula K, Paavonen T, Luukkainen T, Andersson LC. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem Pharmacol. 1983;32:1511–1518. doi: 10.1016/0006-2952(83)90474-4. [DOI] [PubMed] [Google Scholar]
- Matejuk A, Adlard K, Zamora A, Silverman M, Vandenbark AA, Offner H. 17 beta-estradiol inhibits cytokine, chemokine, and chemokine receptor mRNA expression in the central nervous system of female mice with experimental autoimmune encephalomyelitis. J Neurosci Res. 2001;65:529–542. doi: 10.1002/jnr.1183. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med. 2003;197:489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyaura H, Iwata M. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J Immunol. 2002;168:1087–1094. doi: 10.4049/jimmunol.168.3.1087. [DOI] [PubMed] [Google Scholar]
- Otsuki M, Saito H, Xu X, Sumitani S, Kouhara H, Kishimoto T, Kasayama S. Progesterone, but not medroxyprogesterone, inhibits vascular cell adhesion molecule-1 expression in human vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2001;21:243–248. doi: 10.1161/01.atv.21.2.243. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Wu XC, Ciumas C, Lian H, Chirsky V, Huang YM, Bjelke B, Link H, Xiao BG. CD8alpha dendritic cells and immune protection from experimental allergic encephalomyelitis. Clin Exp Immunol. 2004;137:486–495. doi: 10.1111/j.1365-2249.2004.02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk M, Zamora A, Subramanian S, Matejuk A, Hess DL, Blankenhorn EP, Teuscher C, Vandenbark AA, Offner H. The protective effect of 17beta-estradiol on experimental autoimmune encephalomyelitis is mediated through estrogen receptor-alpha. Am J Pathol. 2003;163:1599–1605. doi: 10.1016/s0002-9440(10)63516-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–2230. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J Neurosci Res. 2006;84:370–378. doi: 10.1002/jnr.20881. [DOI] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Vandenbark AA, Offner H. Treg suppressive activity involves estrogen-dependent expression of programmed death-1 (PD-1) Int Immunol. 2007;19:337–343. doi: 10.1093/intimm/dxl151. [DOI] [PubMed] [Google Scholar]
- Sadovnick AD. European Charcot Foundation Lecture: the natural history of multiple sclerosis and gender. J Neurol Sci. 2009;286:1–5. doi: 10.1016/j.jns.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Sinha S, Subramanian S, Miller L, Proctor TM, Roberts C, Burrows GG, Vandenbark AA, Offner H. Cytokine switch and bystander suppression of autoimmune responses to multiple antigens in experimental autoimmune encephalomyelitis by a single recombinant T-cell receptor ligand. J Neurosci. 2009;29:3816–3823. doi: 10.1523/JNEUROSCI.5812-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitruk-Ware R. Pharmacological profile of progestins. Maturitas. 2004;47:277–283. doi: 10.1016/j.maturitas.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Subramanian S, Matejuk A, Zamora A, Vandenbark AA, Offner H. Oral feeding with ethinyl estradiol suppresses and treats experimental autoimmune encephalomyelitis in SJL mice and inhibits the recruitment of inflammatory cells into the central nervous system. J Immunol. 2003;170:1548–1555. doi: 10.4049/jimmunol.170.3.1548. [DOI] [PubMed] [Google Scholar]
- Sun D, Whitaker JN, Huang Z, Liu D, Coleclough C, Wekerle H, Raine CS. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- Szekeres-Bartho J, Wegmann TG. A progesterone-dependent immunomodulatory protein alters the Th1/Th2 balance. J Reprod Immunol. 1996;31:81–95. doi: 10.1016/0165-0378(96)00964-3. [DOI] [PubMed] [Google Scholar]
- Vukusic S, Ionescu I, El-Etr M, Schumacher M, Baulieu EE, Cornu C, Confavreux C. The Prevention of Post-Partum Relapses with Progestin and Estradiol in Multiple Sclerosis (POPART’MUS) trial: rationale, objectives and state of advancement. J Neurol Sci. 2009;286:114–118. doi: 10.1016/j.jns.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham SA, Staykova MA, Ramshaw IA, Cowden WB. IFN-gamma is critical to the control of murine autoimmune encephalomyelitis and regulates both in the periphery and in the target tissue: a possible role for nitric oxide. J Immunol. 1999;163:5278–5286. [PubMed] [Google Scholar]