Abstract
Ultraviolet (UV) light is intricately linked to the functional status of the cutaneous immune system. In susceptible individuals, UV radiation can ignite pathogenic inflammatory pathways leading to allergy or autoimmunity. In others, this same UV radiation can be used as a phototherapy to suppress pathogenic cutaneous immune responses. These vastly different properties are a direct result of UV light’s ability to ionize molecules in the skin and thereby chemically alter them. Sometimes these UV-induced chemical reactions are essential, the formation of pre-vitamin D3 from 7-dehydrocholesterol, for example. In other instances they can be potentially detrimental. UV radiation can ionize a cell’s DNA causing adjacent pyrimidine bases to chemically bond to each other. To prevent malignant transformation, a cell may respond to this UV-induced DNA damage by undergoing apoptosis. Although this pathway prevents skin cancer it also has the potential of inducing or exacerbating autoreactive immune responses by exposing the cell’s nuclear antigens. Ultaviolet-induced chemical reactions can activate the immune system by a variety of other mechanisms as well. In response to UV irradiation keratinocytes secrete cytokines and chemokines, which activate and recruit leukocytes to the skin. In some individuals UV-induced chemical reactions can synthesize novel antigens resulting in a photoallergy. Alternatively, photosensitizing molecules can damage cells by initiating sunburn-like phototoxic reactions. Herein we review all types of UV-induced skin reactions, especially those involving the immune system.
Keywords: Ultraviolet radiation, skin, phototoxic reaction, photoallergic reaction, phototherapy, atuoimmunity
The basic principles of light
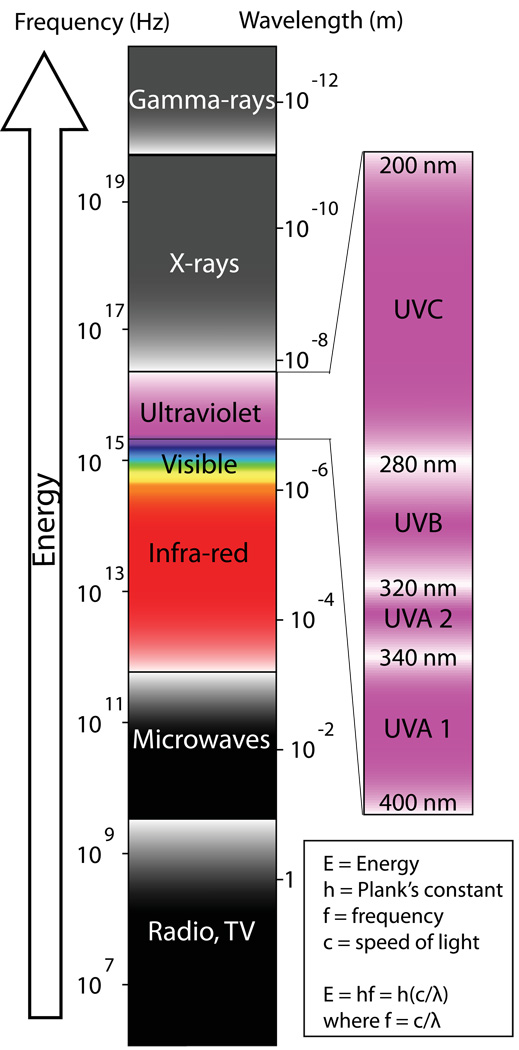
Although light is commonly thought of as something visible, in physics light actually refers to all electromagnetic radiation. The frequency, wavelength, and energy of an electromagnetic wave are related to one another with wavelength being inversely proportional to both frequency and energy (Figure 1). The huge spectrum of electromagnetic radiation can therefore be organized conceptually by decreasing wavelength into radio waves, microwaves, terahertz radiation, infrared radiation, visible light, UV radiation, X-rays, and gamma rays (Figure 1). The sun emits mainly visible light and infrared radiation, but it also emits some UV radiation, which will be the focus of much of the discussion herein.
Figure 1. The electromagnetic spectrum.
Ultraviolet radiation can be further devided into UVC, UVB, and UVA. Wavelength decreases as the frequency of the electromagnetic waves increases. As the frequency increases the energy of the emitted photons also increases. With a shorter wavelength, UVB radiation possesses much more energy than UVA.
UV Radiation
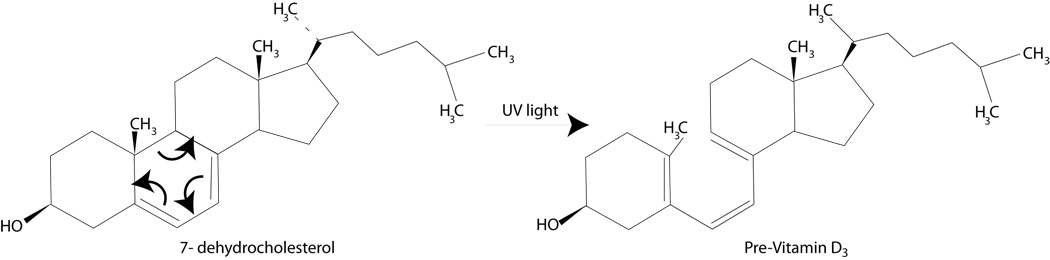
The term ultraviolet means "beyond violet” and refers to electromagnetic radiation with a wavelength shorter than visible violet light but longer than x-rays (Figure 1). Radiation within the UV spectrum can be further divided by wavelength into UVC (200–280 nm), UVB (280–320 nm) and UVA (320–400 nm) (Figure 1). An important property of UV radiation that separates it from the visible spectrum is that UV light can ionize molecules and thereby induce chemical reactions. Ultraviolet light is a strong environmental mutagen and the reactions it induces in the skin can precipitate or exacerbate immune-mediated diseases or even worse, cause a fatal cancer. However, not all UV-induced reactions are pathogenic. For example, in the epidermis, UVB induces a 6-electron conrotatory electrocyclic reaction to synthesize pre-vitamin D3 from 7-dehydrocholesterol, a derivative of cholesterol (Figure 2).
Figure 2. UVB induces a 6-electron conrotatory electrocyclic reaction to synthesize pre vitamin D3 from 7-dehydrocholesterol, a derivative of cholesterol.
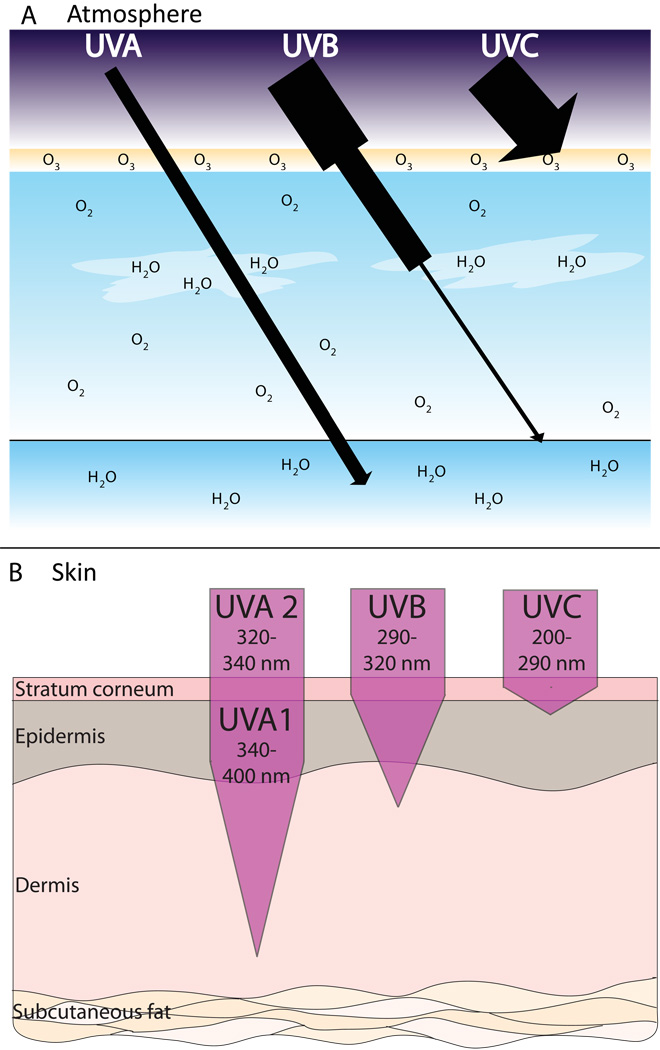
In the earth’s atmosphere, ozone (O3), dioxygen (O2) and water vapor (H2O) selectively filter out both UVC and UVB radiation (Figure 3A). Due to this, UVA makes up about 95% of the UV radiation that reaches the earth. Currently, almost no UVC radiation penetrates the atmosphere and approximately 90% of the UVB is absorbed, depending on geographic location and time of day. As the ozone layer is depleted by chlorofluorocarbon (CFC) pollution, more UVB and even some UVC radiation will reach the earth.
Figure 3. Penetrating abilities of UVA, UVB and UVC.
A) Approximately 90% of UVB and nearly all of UVC is absorbed by O3, O2, and H2O in the Earth’s atmosphere. UVA makes up 95% of the UV radiation that reaches the Earth. B) With its longer wavelength, UVA can penetrate deeper into the skin than UVB.
Although relatively little reaches the earth’s surface, UVB radiation possesses much more energy than UVA (remember that energy is inversely proportional to wavelength). By some estimates UVB is 1,000 times more erythematogenic than UVA. In other words, although more UVA reaches the earth, it is mainly UVB that burns the skin. In contrast, the ability to penetrate the skin is proportional to the wavelength of the radiation. Thus, UVA, with its longer wavelength, can penetrate deep into the dermis of the skin while UVB is absorbed superficially in the epidermis (Figure 3B). This fact will be important later during our discussion of UV-induced inflammatory reactions.
The cutaneous immune system: a brief overview
An intact skin barrier is our first defense against invading microbes and environmental allergens. The importance of the skin’s barrier function has been highlighted recently by evidence linking atopic dermatitis and allergy to mutations in filaggrin, a major barrier-forming protein responsible for organizing keratin filaments in the skin [1]. However, the skin is not merely a physical barrier to microbial pathogens and allergens. When breached, components of the innate immune system such as defensins and cathelicidins are poised to prevent pathogen invasion; these are antimicrobial cationic proteins and peptides, respectively.
In addition to a very complex innate immune system, the skin also hosts a highly efficient adaptive immune system equipped with a specialized antigen presenting cell, the Langerhans cell, as well as unique T cells bearing skin-homing receptors. Receptors that target T cells for migration to the skin include the cutaneous lymphocyte antigen (CLA) and the chemokine receptors CCR4 and CCR6.
When activated keratinocytes can interact so closely with skin-resident immune cells that they can be considered components of the cutaneous immune system. Specifically, keratinocytes can secrete cytokines, chemokines [2], and express costimulatory adhesion molecules such as the intercellular adhesion molecule 1 (ICAM-1). They can also express major histocompatibility complex (MHC) class II molecules, allowing them to present antigen to T cells.
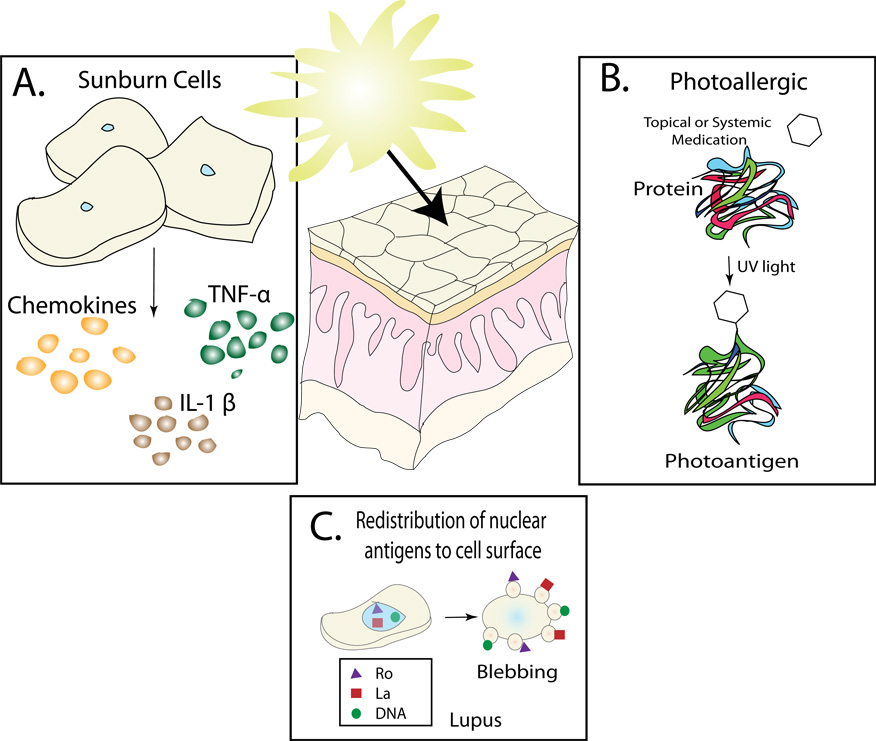
Ultraviolet radiation can activate components of the cutaneous immune system and precipitate an inflammatory response by a variety of mechanisms. For example, UV radiation can: (1) directly activate keratinocytes and other cells to release inflammatory mediators such as cytokines and chemokines, (2) cause redistribution and release of sequestered autoantigens from UV-damaged cells, (3) alter self-proteins to make them more immunogenic (Figure 4), (4) enhance the immunogenicity of externally applied molecules, and (5) chemically alter systemically administered medications whose distribution includes the skin. We will discuss each of these in detail below.
Figure 4. Mechanisms by which UV radiation can activate the immune system.
A) In response to UV irradiation keratinocytes secrete a variety of different cytokines including TNF-α and IL-1β. B) Ionizing radiation from ultraviolet light can induce chemical reactions in the skin. Depicted here is a drug forming a covalent bond to a self-protein resulting is a novel antigen. In this example the drug can be considered a hapten. C) In response to UV-induced DNA damage, keratinocytes undergo apoptosis as a definitive mechanism to prevent malignant transformation. Following UV irradiation there is a redistribution of nuclear antigens to the surface of the keratinocyte.
UV radiation causes DNA damage, apoptosis and release of autoantigens from keratinocytes
When UV radiation strikes the skin, its ionizing energy can be absorbed. There are a variety of molecules within the skin that can absorb this energy and be chemically altered by it. One of the major UV-absorbing molecules is DNA itself. Over time, the absorption of ionizing radiation by a cell’s DNA will result in the accumulation of genetic mutations and eventually the malignant transformation of the cell. The immune system is responsible for destroying these UV-induced skin malignancies. Evidence in support of this is the fact that immunosuppressed individuals are predisposed to developing aggressive skin cancers. However, cancer surveillance by the immune system is more of a final safeguard rather than a primary method of preventing skin cancer; many earlier defenses must fail before malignant transformation occurs.
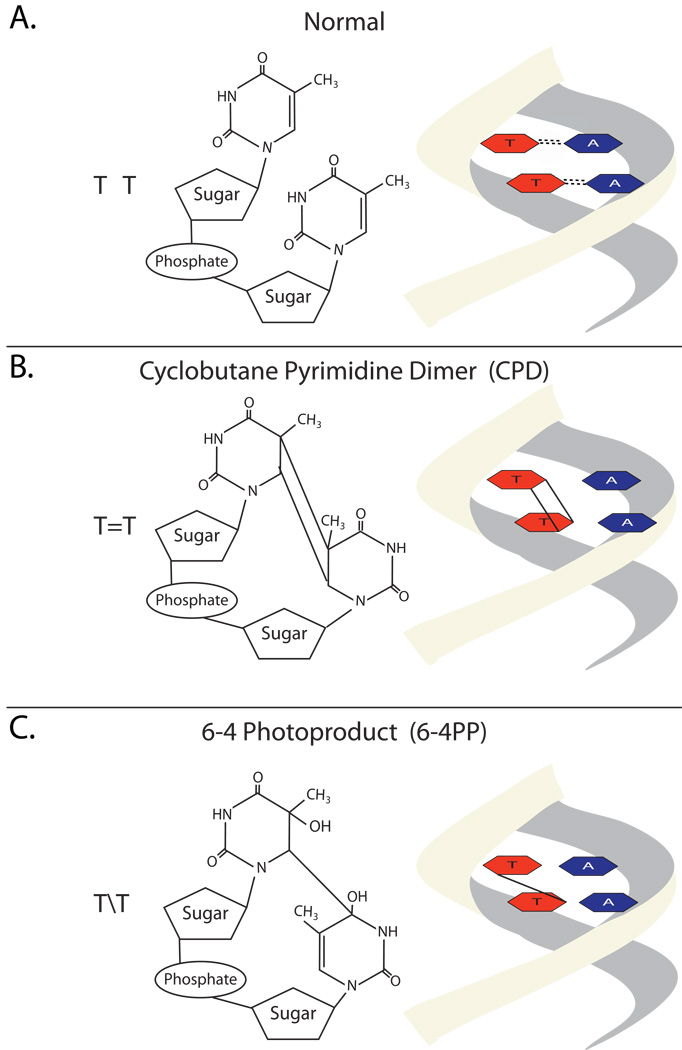
The first UV defense is melanin, a UV-absorbing pigment that is able to dissipate UV radiation as harmless heat. This pigment is made in melanocytes and then transferred to keratinocytes via long dendritic processes. UV radiation that escapes melanin absorption may induce DNA damage by either creating reactive oxygen species or by directly inducing chemical reactions within DNA. For example, ionizing UV radiation can induce the creation of cyclobutane pyrimidine dimers (CPD) within DNA. Figure 5 depicts two adjacent thymine residues being altered in this fashion. In addition to CPD dimers, UV radiation can also induce the formation of (6-4) photoproducts (6-4PP) (Figure 5). UVB is the most common cause of 6-4PPs. To prevent malignant transformation, cells have specific defense mechanisms to seek out pyrimidine dimers for repair. Patients with xeroderma pigmentosa develop multiple skin cancers because they have a defect in a nucleotide excision repair system that normally recognizes and repairs UV-induced mutations [3]. Even in people with normal DNA repair systems some dimers fail to be corrected, eventually resulting in a permanent DNA mutation. As the cell accumulates more DNA mutations, malignant transformation will eventually occur. For example, squamous cell carcinomas and their precancerous precursors have mutational hotspots within the p53 tumor suppressor gene that correspond to sites where sunlight-induced CPD dimers occur [4, 5]. Thus, there is a direct link between CPD dimer formation and skin cancer.
Figure 5. Formation of cyclobutane pyrimidine dimers and (6-4) photoproducts photoproducts.
A) Two normal thymidine residues. B&C) DNA can absorb the ionizing radiation of ultraviolet light and undergo chemical modifications including the formation of cyclobutane pyrimidine dimers (CPD) or 6-4 photoproducts (6-4PPs).
In addition to their role in causing cancer, UV-induced CPDs and 6-4PPs have multiple other effects on cells as well. In melanocytes, they up-regulate melanin production and invoke the tanning response [6]. Also, CPDs can cause cell cycle arrest and 6-4PPs can induce apoptosis [7]. Thus, UV-induced DNA damage will signal the cell to stop dividing and if needed to undergo apoptosis. In keratinocytes cell surface death receptors such as Fas respond to UV-induced damage and trigger apoptosis [8]. In fact, Fas can initiate UV-induced apoptosis even in the absence of its ligand [9]. As part of the keratinocyte’s program for cell death, cytochrome c and other pro-apoptotic factors are released from the mitochondria. This signals the formation of the apoptosome and subsequent activation of caspase-9, which in turn cleaves caspases-3 and/or -7.
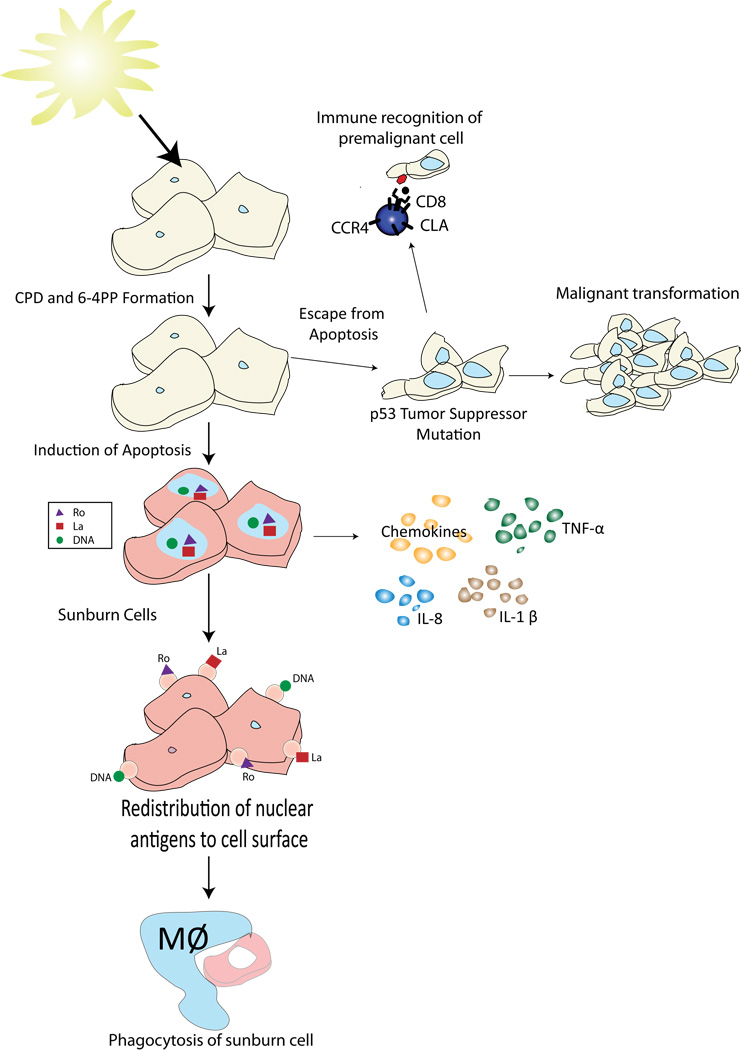
The term sunburn cell refers to keratinocytes undergoing apoptosis (Figure 6) [10, 11]. It is important to understand that programmed cell death of UV-damaged skin cells is a definitive cancer-preventing pathway. Transgenic animals designed to be resistant to the development of UV-induced sunburn cells can tolerate more UV radiation without burning, but they are predisposed to developing skin cancers [5, 12].
Figure 6. Following UV irradiation there are several pathways to eliminate DNA-damaged keratinocytes.
Damaged cells can undergo apoptosis and be engulfed by macrophages. To facilitate this process UV-irradiated cells secrete pro-inflammatory cytokines. These cytokines help recruit the leukocytes to the skin that participate in the disposal of the dying keratinocytes. T cells can also recognize and destroy the damaged keratinocytes. Receptors that target T cells for migration to the skin include the cutaneous lymphocyte antigen (CLA) and the chemokine receptors CCR4 and CCR6. Finally, if a DNA damaged keratinocye escapes these mechanisms a malignancy may develop. These malignancies almost always have mutations in the p53 tumor suppressor gene.
In the setting of sunburn, large numbers of keratinocytes will undergo apoptosis to prevent malignant transformation (Figure 5). The skin is chalked full of Langerhans cells that can easily pick up autoantigens released by dying keratinocytes. Therefore, disposing of UV-damaged cells without igniting autoimmunity is a delicate balance. Fortunately, there are multiple safeguards in place to prevent the induction of autoimmunity. For example, as a keratinocyte undergoes apoptosis, it attempts to keep everything private and ordered. During the initial 24 hours following the induction of apoptosis, the cells release only low levels of nuclear material [13]. This provides a window for the keratinocytes to self-process their cellular contents and then be cleared by phagocytosis. Autoimmunity is favored if there is a defect in cellular processing of the autoantigens [14], an increase in nucleosomal antigen release [13] or a decrease in clearance of the apoptotic cells [15] (Figure 6). Thus, although exposure to potential autoantigens in the setting of UV-induced apoptosis is common, efficient processing and disposal mechanisms minimize the antigenicity of this material.
One revelation in our understanding of autoimmunity was the discovery that autoantigens targeted in systemic lupus erythematosus (SLE) are clustered on the surface of apoptotic cells (Figure 6) [16]. Likewise, UV radiation induces the redistribution of nuclear autoantigens to the surface of keratinocytes [17]. For example, SS-A/Ro and SS-B/La are found on the surface of keratinocytes following UV exposure. The presence of Ro on the surface of UV-exposed keratinocytes is of particular interest because anti-Ro antibodies are indicative of enhanced photosensitivity [18]. They are seen in many patients with SLE and in the overwhelming majority of patients with subacute cutaneous lupus erythematosus (SCLE). Binding of anti-Ro antibodies to UV-exposed keratinocytes may increase cell death via complement-mediated cytotoxicity, resulting in the premature release of nuclear antigens before they have been adequately processed. Naively one might predict that knocking out Ro in an experimental system would result in less autoimmunity. Surprisingly, this is not the case. Animals deficient in Ro are more sensitive to UV radiation and develop a spontaneous lupus-like autoimmune disease [19]. Thus, Ro evidently plays a role in cell survival following UV exposure. Some have suggested that it may sequester ribonuclear proteins following DNA damage and thereby help to prevent autoimmunity [19].
Immunosuppressive effects of UV light
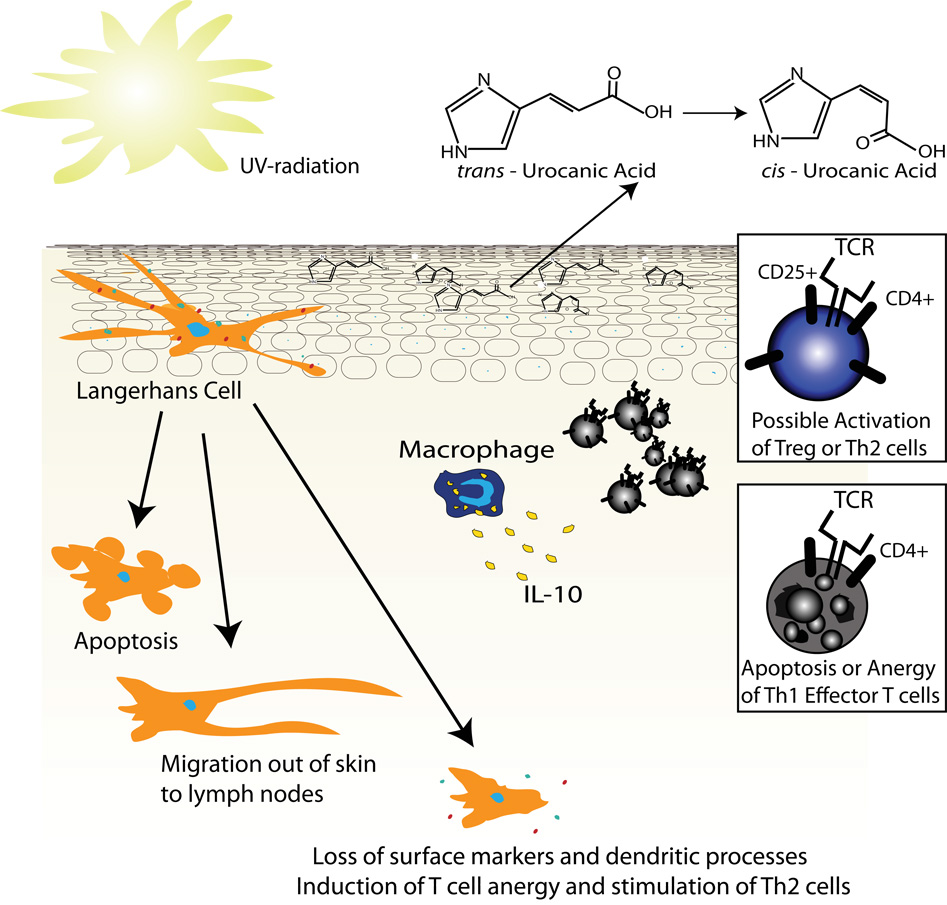
Although UV light can induce strong inflammatory responses in susceptible individuals, under chronic or minimally erythematogenic doses its immunosuppressive effects are dominant. The mechanism of this immunosuppression involves both cellular components and soluble mediators. Langerhans cells are very sensitive to UV radiation. These cells are antigen presenting dendritic cells that have a cell surface phenotype poised to simulate T cells, including autoreactive and allergen-specific T cells [20]. Highlighting their role as antigen presenting cells, Langerhans cells express molecules such as: MHC class II, lymphocyte function-associated antigen-3 (LFA-3), ICAM-1, ICAM-3, B7, CD1a and CD40. In the absence of Langerhans cells a state of antigen unresponsiveness predominates. For example, Langerhans cell density correlates with the ability of the hapten DNFB to induce contact hypersensitivity [21]. These cells are more sensitive to UV radiation than keratinocytes. This helps prevent immune recognition of nuclear antigens released by dying keratinocytes. Prior to the appearance of UV induced sunburn cells, Langerhans cells in the skin are lost or at least severely altered. Langerhans cell loss following UV exposure is partially due to UV-induced apoptosis [22]. However, UV radiation also induces these cells to migrate out of the skin to draining lymph nodes [23, 24] and there is a dramatic reduction of the immunologic markers expressed by Langerhans cells following UV exposure [25, 26]. Reduced markers include: MHC class II, ICAM, and B7. Lastly, UV radiation alters the shape of Langerhans cells, destroying their dendritic processes (Figure 7) [23]. Thus, the residual Langerhans cells after UV exposure have drastically altered antigen-presenting capabilities. Some believe that UV-treated Langerhans cells preferentially stimulate T helper type 2 (Th2) cells and suppress T helper type 1 (Th1) cells, which might explain some of UV light’s immunosuppressive effects [27]. Studies have also demonstrated that UVB can induce T cell apoptosis directly [28].
Figure 7. Mechanisms of UV-induced immunosuppression.
UV irradiation disrupts Langerhans cell function by: inducing apoptosis, altering their surface phenotype, or by increasing their migration to the lymph nodes. The altered Langerhans cells can induce anergy or deletion of Th1 cells. Urocanic acid is present in large amounts in the epidermis. UV radiation can induce the isomerization of trans-urocanic acid to the cis-isomer that has immunosuppressive properties. Lastly, skin-infiltrating macrophages secrete IL-10, an anti-inflammatory cytokine.
Following UV exposure a systemic immunosuppressive state is induced that cannot be explained solely by local loss of Langerhans cells and T cells. For example, UV radiation on the dorsum of a mouse will prevent a delayed-type hypersensitivity (DTH) response on the mouse’s ventral surface [29]. This state of global immunosuppresion is due in part to soluble inflammatory mediators. One of these molecules is IL-10; IL-10 is an anti-inflammatory cytokine that can inhibit the production of proinflammatory cytokines such as IFN-γ. It also can inhibit the surface expression of MHC class II and co-stimulatory molecules that are important for T cell activation and antigen recognition. Following UV exposure, some IL-10 may be secreted by keratinocytes [30], but the majority likely comes from skin-infiltrating bone marrow derived macrophages that can express large amounts of IL-10 (Figure 7) [31]. These cells begin infiltrating the skin as early as six hours following UV exposure, reaching their peak numbers at 48 to 72 hours [32].
Apart from IL-10 there are likely many other immunosuppressive mechanisms induced by UV radiation. For example, urocanic acid is found within the epidermis at relatively high levels because the enzyme, urocanase, that usually processes this molecule is missing in the skin [33]. The absorption spectrum that causes the transformation of urocanic acid from its trans to cis isomer closely matches the absorption spectrum that induces immunosuppression. This identified the cis isomer of urocanic acid as a possible immunosuppressive agent. Correlating with this observation, the administration of UV-irradiated urocanic acid suppresses DTH to herpes simplex virus in the mouse [34]. Still other mechanisms of UV-induced immunosuppression include the induction of CD4+ CD25+ T regulatory cells [35].
Ultraviolet light induces inflammation
In contrast to the immunosuppressive mechanisms just described, UV radiation can also activate the immune system. The activation is mediated in part through UV-induced cytokine production, though the mechanism by which this occurs is debatable. It is possible that DNA damage is the inducing signal. This theory has been investigated by a variety of groups.
Exposure to UV radiation induces the activation of nuclear factor kappa B (NF-κB). This in turn induces the secretion of a variety of inflammatory mediators including: IL-1, IL-6, TNF-α and VEGF. Furthermore, blocking NF-κB inhibits the secretion of these molecules [36]. The hypothesis that UV-induced DNA damage is responsible for NF-κB activation was directly tested. Investigators found that enucleated cells were fully responsive to UV-induced NF-κB activation. Thus, a signal cascade based upon UV-induced DNA damage seems unlikely for NF-κB [37]. In contrast to NF-κB, the wavelength dependence (action spectrum) for UV-induced TNF-α production correlates with the in vivo action spectra for UV-induced CPD formation [38]. In other words, the production of TNF-α correlates with the amount of DNA damage, making a link between the two likely.
Together with IL-1, TNF was one of the first cytokines found to be upregulated in the skin following UV irradiation [39, 40]. It was subsequently discovered that keratinocytes and mast cells were the sources of TNF-α [41, 42]. Once expressed, TNF-α has effects on a variety of cell types. It increases MHC class I expression on endothelial cells and dermal fibroblasts [43]; induces the production of IL-1α [44]; increases the expression of adhesion molecules, including ICAM-1, VCAM-1 and E-selectin [45]; and it promotes formation of sunburn cells [46]. By upregulating adhesion meolcules on endothelial cells, TNF-α helps support the migration of neutrophils and macrophages to UV-exposed skin. Although these skin-infiltrating macrophages can secrete IL-10, they are also effective at antigen presentation [32]. Thus, if their local inflammatory milieu changes, they can become more pathogenic, capable of participating in an autoreactive immune response.
Given that TNF-α supports the formation of sunburn cells, it is not surprising that polymorphisms within its promoter have been linked to lupus. The TNF-α -308A promoter polymorphism increases susceptibility to SLE and SCLE. When a reporter gene was placed under the regulation of this polymorphism an abnormal photoregulation of gene expression was observed; the combination of UVB plus IL-1α resulted in a dramatic increase in transcription over wild type levels. This finding helps to link together the TNF-α -308A promoter polymorphism with UV photosensitivity, formation of sunburn cells, and the photosensitive diseases SLE and SCLE [47].
It has been proposed that UV damage can be considered a “danger” signal. Recent data linking IL-1β production to “inflammasomes” supports this notion. IL-1β is normally expressed in human keratinocytes as an inactive precursor. Upon exposure to UV radiation, the precursor is then cleaved by caspase-1 to yield active IL-1β. Caspase-1 activation is dependent upon its recruitment to inflammasomes, which links UV-induced cytokine production to the innate immune system. Inflammasomes are composed of NOD-like receptor (NLR)-family proteins [48]. These intracellular proteins contain a nucleotide-binding oligomerization domain called NACHT and several leucine rich repeats domains that may bind to microbial ligands similar to Toll-like receptor family proteins. The relationship between inflammasomes and UV-induced IL-1β production demonstrates that this pathway can also sense tissue injury. This is especially interesting in light of the recent findings linking NLR proteins to autoimmune disease [49]. IL-1β is a proinflammatory cytokine that has also been linked to a variety of inflammatory diseases, including contact hypersensitivity [50].
Keratinocytes have also been shown to produce a variety of other cytokines in response to UV radiation. Interleukin-8 is a chemotactic cytokine that is upregulated in response to UV exposure [51]. This cytokine is partially responsible for the dramatic influx of inflammatory cells into the skin following UV irradiation. Some other UV-induced cytokines have very specific action spectra. For example, IL-12 is upregulated in response to UVA, but not UVB, exposure [52].
Phytophotodermatitis, UV-induced altered self, and other UV eruptions
Photosensitizers can absorb photons of a particular wavelength, enter a transient activation state, and then elicit a chemical reaction by transferring the photon’s energy to another molecule. By capturing photons and releasing their energy, photosensitizers increase the sensitivity of the skin to light. The physical manifestation of this increased photosensitivity will depend on the specific photosensitizer; some will reduce the threshold to sunburn while others may induce a photoallergy in susceptible individuals.
Photosensitizers may be a variety of substances including: light-sensitizing botanicals, such as the psoralens responsible for phytophotodermatitis; systemically distributed medications, such as tetracyclines; endogenous light absorbing molecules, such as porphyrins; and externally applied chemicals, such as aminolevulenic acid, the chemical used in photodynamic therapy (Figure 8). The action spectrum for most photosensitizers affecting the skin lies in the UVA range but may also include UVB or even visible light. Importantly, UVA can penetrate deeper into the dermis to excite photosensitizers whose distribution does not include the more superficial epidermis (Figure 3B).
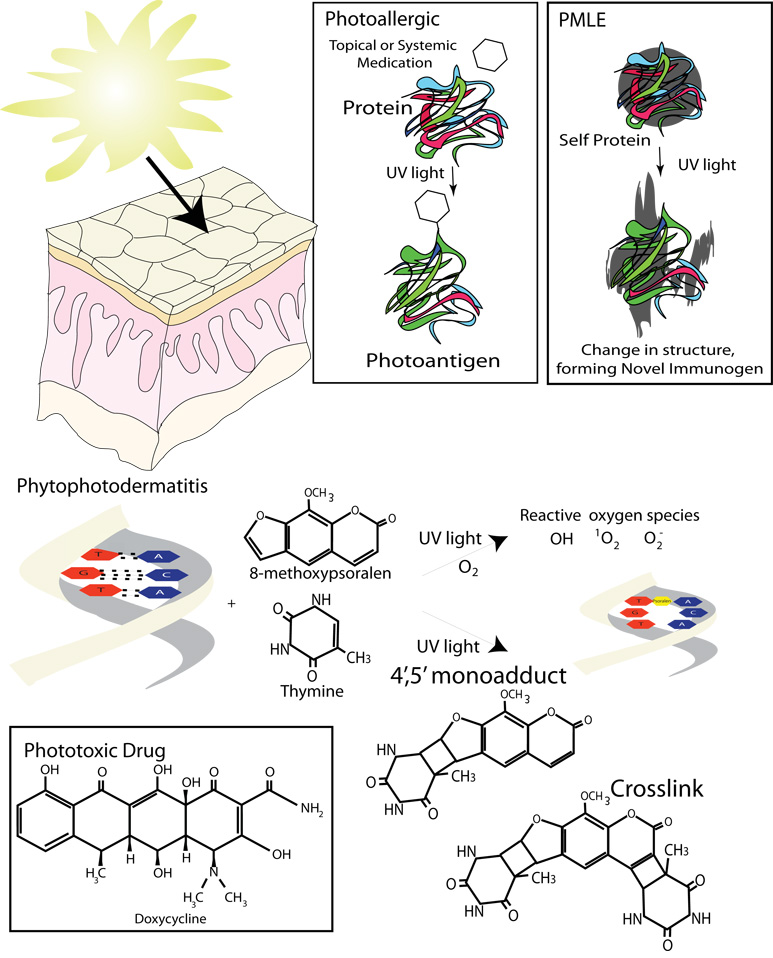
Figure 8. Phototoxic and photoallergic reactions.
In photoallergic reactions small photosensitizer can act as a haptens using UV light to attach themselves to larger molecules, forming photoallergens. In PMLE it is believed that there is an endogenous protein or other molecule that can absorb ultraviolet light and be chemically modified by it. The resulting antigen then initiates an immune response. Phytophotodermatitis and phototoxic drug eruptions are examples of phototoxic reactions. In these types of reactions reactive oxygen species can be synthesized by the energy released from light absorbing photosensitizers, such as psoralens. Psoralens can also bind to DNA forming monoadducts or crosslinks.
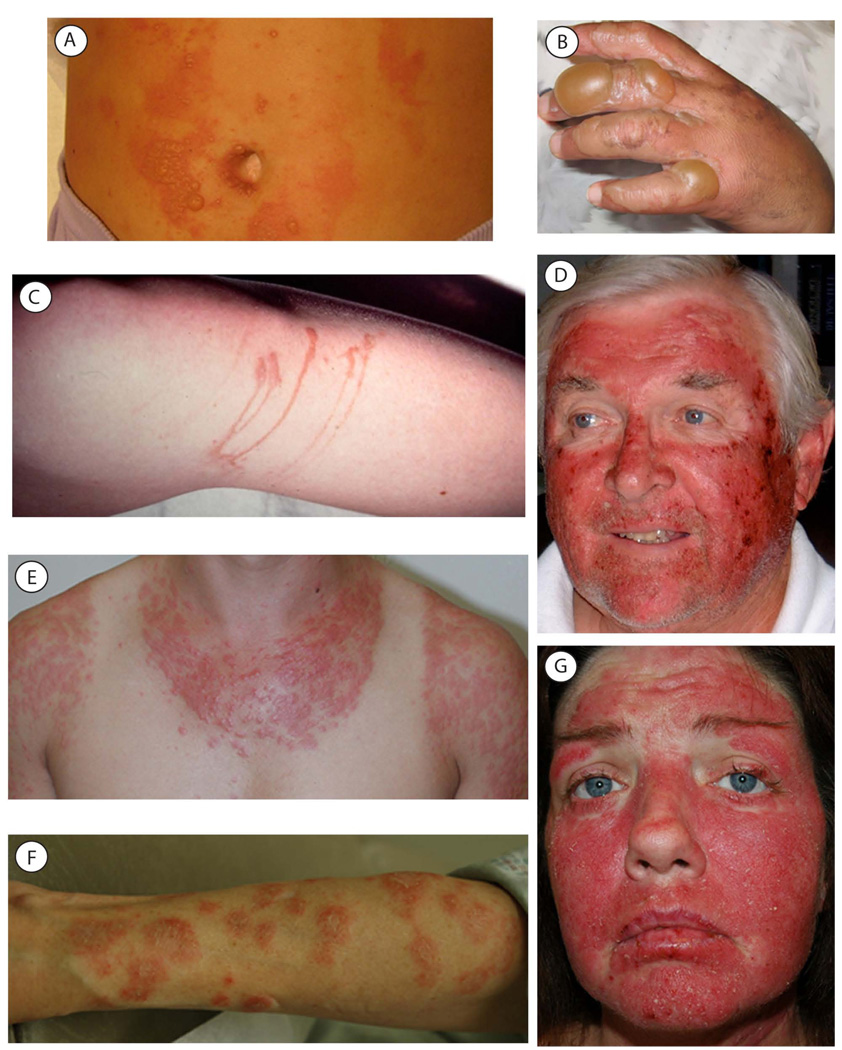
In broad terms, photosensitizers induce two types of reactions: phototoxic and photoallergic (Figure 8). Phototoxic reactions are sunburn type reactions that occur in all individuals exposed to the photosensitizer. They occur quickly, within a few hours of sun exposure, and unlike photoallergic reactions, do not require an allergic sensitization period. Clinically, the eruption is similar to an exaggerated sunburn with erythema and possibly some blistering (Figure 9). The erythema usually resolves within a few days, but a residual postinflammatory hyperpigmentation may last for several weeks. The two main types of phototoxic reactions are phytophotodermatitis and phototoxic drug.
Figure 9.
A) Phytophotodermatitis in response to lemonade and UV radiation; The linear areas of this rash reveal a likely external cause. B) Picture of hand of the same patient with phytophotodermatitis. The eruption is similar to an exaggerated sunburn, presenting with erythema and bullae. C) A milder case of phytophotodermatitis; The sometimes linear nature of the eruption is more apparent in this photo. D) Phototoxic drug eruption; Note the erythematous photodistributed plaque with hemorrhagic crust. E) Polymorphous light eruption; Notice the photodistribution of this rash. F) Subacute cutaneous lupus; Annular plaques with a ridge of scale and some central clearing or hyperpigmentation. The patient has a positive anti-Ro titer. G) Systemic lupus erythematosus; Acute photodistributed erythematous plaque with very light scale. The patient has a positive anti-Ro titer. Photos A, B and D are courtesy of Daniel B. Eisen, M.D.
In phytophotodermatitis the photosensitizer is a botanical substance, most commonly a psoralen (furocoumarins), which is ingested or externally applied to the skin. Plants in the families Umbelliferae (e.g. parsley) and Rutaceae (e.g. limes) are common sources of photoactive psoralens. In the skin, the psoralen causes cellular damage upon excitation by UVA radiation. This damage is caused by either direct photosensitizer-target interactions or indirectly through reactive oxygen species. The most common type of direct psoralen-target interaction is the formation of DNA-psoralen photoadducts that are induced by UVA radiation. In this example, the psoralen absorbs UVA energy and then interacts directly with DNA to form a cross-link or a single strand adduct. Alternatively, the psoralen can transfer the photon’s energy to oxygen creating reactive oxygen species (e.g. 1O2, O2−, .OH). The reactive oxygen species can then injure cells by interacting directly with: cellular DNA; lipid-rich membranes, including the plasma and mitochondrial membranes; and enzymes vital for cell survival, e.g. cytochrome P450 enzymes (Figure 8) [53]. Psoralens can be found abundantly in our environment making phytophotodermatitis extremely common, especially amongst chefs and gardeners.
Phototoxic drug reactions can be due to topical or internal exposure to photosensitizing medications. Oral medications causing phototoxic reactions include but are not limited to: tetracyclines, especially doxycycline and demeclocycline; fluoroquinones; sulfa medications, e.g. lasix; amiodarone; and NSAIDS, all types. Phototoxic drug reactions are usually dose-dependent, both in terms of the medication’s dose and the amount of UV exposure. To avoid phototoxic drug reactions, patients should be educated on the risk of sun sensitivity when they start the medication.
In contrast to phototoxic reactions, photoallergic reactions are true immunologic reactions. Similar to the above description, the photosensitizer absorbs light energy and then transfers this energy to another molecule that then induces a chemical reaction. This time, however, the result is the creation of a novel allergen, a photoallergen. For the most part the proteins involved in the formation of these photoallergens are unknown. It is possible that the photosensitizer acts like a hapten. When irradiated, the energy absorbed is used to chemically bond the photosensitizer/hapten to a self-protein, resulting in a novel immunogenic compound. After repeat exposure, a DTH reaction to the new photoallergen occurs. As is the case in other DTH responses, there is a delay in onset, sometimes in the range of weeks. This makes it difficult for patients to associate UV exposure with the eruption, which makes the photodistribution of the rash key to its diagnosis.
In photoallergic reactions the photoallergen can be external, in which case the reaction would be called photoallergic contact dermatitis, or it may be internal as seen in photoallergic drug reactions. Clinically, the lesions of photoallergic reactions are eczematous pruritic plaques distributed over sun-exposed areas. Histopathologically they show features of contact dermatitis. Although cross reactivity to similar antigens may occur, photoallergic reactions are not as common as phototoxic reactions. Implicated medications include: thiazide diuretics, sulfonamide antibiotics, sulfonylureas, phenothiazides, quinine, quinidine, tricyclic antidepressants, antimalarials, and NSAIDs.
There are also a variety of immune mediated DTH-like reactions that occur hours or days after sun exposure in which the photoallergen is unknown but believed to be an endogenous self molecule. These photodermatoses are called idiopathic or primary photosensitivity disorders. Among this group, polymorphous light eruption (PMLE) is the most common. PMLE is characterized by an erythematous, mildly pruritic, papulovesicular eruption that appears on sun-exposed skin, especially the chest and upper extremities. Following sun exposure there is a delay in the onset of these eruptions, from hours to days. Thus, some patients do not attribute their rash to sun exposure. Once present, the eruption will last for days. PMLE is most common in the spring and summer and may recur annually. A variant of PMLE, called juvenile spring eruption, presents with vesicles on helices of ears of young boys. Most authorities consider UVA to be the main cause of PMLE, but UVB, or occasionally even visible light, may also be responsible. Mild cases of PMLE are prevented and treated with photoprotection. More severe cases, ironically, may require phototherapy.
Actinic prurigo (AP) is a persistent HLA-restricted variant of PMLE. Its incidence is highest in the Native American population, especially young Native American girls. Clinically, it differs from PMLE by: (1) the presence of papules and nodules that are sometimes lichenified or excoriated due to pruritis, (2) a distribution that may include non-sun-exposed areas, and (3) the presence of cheilitis or conjunctivitis that may accompany the skin lesions or be the only presentation of the disease.
Chronic actinic dermatitis (CAD) is another type of idiopathic or primary photosensitivity disorder. This persistent eczematous dermatitis of chronically sun-exposed skin commonly affects middle-age to elderly men. CAD can be caused by UVA, UVB, or even visible ight, which makes strict sun avoidance essential.
Phototherapy: Inhibiting the Immune System with Light
Phototherapy is defined as the use of UV radiation in the treatment of skin disease. There are many types of phototherapy including: broadband UVB (280–320 nm), narrowband UVB (311-313nm), UVA1 (340–400 nm), and combination therapy of psoralen plus UVA (PUVA). Weighing the type of disease being treated, the depth of disease pathology, and the risk of skin carcinogenesis helps to determine the best phototherapy treatment modality for a particular patient. Once the appropriate modality has been chosen, the treatment is then individualized based upon the patient’s minimal erythema dose (MED). MED is defined as the minimum amount of radiation that produces erythema 24 hours after exposure.
Use of phototherapy with psoralens revolutionized the treatment of patients with severe psoriasis. Although the psoralens used in PUVA photochemotherapy are highly purified medications, their mechanism of action is the same as the environmental psoralens responsible for phytophotodermatitis. By titrating the exact dose of psoralen and the amount and type of UV radiation, the physician is able to maximize the immunosuppressive effects of the treatment and minimize the risk of photoburn. PUVA is the most powerful phototherapy and just about any immune-mediated skin disease, including cutaneous T cell lymphoma, will respond to PUVA therapy. The main concern associated with PUVA is the increased risk of skin cancer, including melanoma [54].
Take-home messages:
The ultraviolet spectrum is further divided by wavelength into UVC (200–280 nm), UVB (280–320 nm) and UVA (320–400 nm). More UVA reaches the Earth’s surface but it is mainly UVB that causes sunburn. Almost no UVC reaches the Earth’s surface.
UV radiation causes local and systemic immunosuppression by: disrupting Langerhans cell function, inducing the formation of the cis isomer of urocanic acid, inducing anergy or deletion of Th1 cells, and promoting the influx of IL-10-secreting macrophages.
Ultraviolet radiation induces keratinocytes and other cells to produce a variety of cytokines, including IL-1β and TNF-α.
Transgenic animals designed to be resistant to the development of UV-induced sunburn cells can tolerate more UV radiation without burning, but they are predisposed to developing skin cancers. Sunburn cells are essential to prevent skin cancer but may induce or exacerbate an autoimmune disease state because their nuclear antigens are redistributed to their cell surface.
Photosensitizers, absorb photons and release their energy. They induce two types of skin reactions: phototoxic and photoallergic. Phototoxic reactions are sunburn type reactions whereas photoallergic reactions are true immune mediated reactions that occur in sensitized individuals. UVA plays an important role in both phototoxic and photoallergic reactions.
Phototherapy is the use of UV radiation in the treatment of skin disease. Types of phototherapy include broadband UVB (280–320 nm), narrowband UVB (311–313nm), UVA1 (340–400 nm), and combination therapy of psoralen plus UVA (PUVA).
Acknowledgments
This work was supported by the National Institutes of Health (NIH), Burroughs Wellcome Fund (BMF) and Howard Hughes Medical Institute (HHMI). EM is an early career awardee of the BWF and the HHMI. Dalila Maverakis helped prepare the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, Goudie DR, Sandilands A, Campbell LE, Smith FJ, O'Regan GM, Watson RM, Cecil JE, Bale SJ, Compton JG, DiGiovanna JJ, Fleckman P, Lewis-Jones S, Arseculeratne G, Sergeant A, Munro CS, El Houate B, McElreavey K, Halkjaer LB, Bisgaard H, Mukhopadhyay S.McLean WH. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat Genet. 2006;38:441–446. doi: 10.1038/ng1767. [DOI] [PubMed] [Google Scholar]
- 2.Svobodova A, Walterova D.Vostalova J. Ultraviolet light induced alteration to the skin. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2006;150:25–38. doi: 10.5507/bp.2006.003. [DOI] [PubMed] [Google Scholar]
- 3.Cleaver JE. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968;218:652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- 4.Tommasi S, Denissenko M.F.Pfeifer GP. Sunlight induces pyrimidine dimers preferentially at 5-methylcytosine bases. Cancer Res. 1997;57:4727–4730. [PubMed] [Google Scholar]
- 5.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T.Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 6.Eller MS, Yaar M.Gilchrest BA. DNA damage and melanogenesis. Nature. 1994;372:413–414. doi: 10.1038/372413a0. [DOI] [PubMed] [Google Scholar]
- 7.Lo HL, Nakajima S, Ma L, Walter B, Yasui A, Ethell D.W.Owen LB. Differential biologic effects of CPD and 6-4PP UV-induced DNA damage on the induction of apoptosis and cell-cycle arrest. BMC Cancer. 2005;5:135. doi: 10.1186/1471-2407-5-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leverkus M, Yaar M.Gilchrest BA. Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp Cell Res. 1997;232:255–262. doi: 10.1006/excr.1997.3514. [DOI] [PubMed] [Google Scholar]
- 9.Aragane Y, Kulms D, Metze D, Wilkes G, Poppelmann B, Luger T.A.Schwarz T. Ultraviolet light induces apoptosis via direct activation of CD95 (Fas/APO-1) independently of its ligand CD95L. J Cell Biol. 1998;140:171–182. doi: 10.1083/jcb.140.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olson R.L.Everett MA. Epidermal apoptosis: cell deletion by phagocytosis. J Cutan Pathol. 1975;2:53–57. doi: 10.1111/j.1600-0560.1975.tb00208.x. [DOI] [PubMed] [Google Scholar]
- 11.Kerr JF, Wyllie A.H.Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aziz MH, Sundling KE, Dreckschmidt N.E.Verma AK. Protein kinase Cepsilon inhibits UVR-induced expression of FADD, an adaptor protein, linked to both Fas- and TNFR1-mediated apoptosis. J Invest Dermatol. 2009;129:2011–2021. doi: 10.1038/jid.2008.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Nieuwenhuijze AE, van Lopik T, Smeenk R.J.Aarden LA. Time between onset of apoptosis and release of nucleosomes from apoptotic cells: putative implications for systemic lupus erythematosus. Ann Rheum Dis. 2003;62:10–14. doi: 10.1136/ard.62.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz H.G.Moroy T. Features of systemic lupus erythematosus in Dnase1-deficient mice. Nat Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 15.Botto M. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp Clin Immunogenet. 1998;15:231–234. doi: 10.1159/000019076. [DOI] [PubMed] [Google Scholar]
- 16.Casciola-Rosen LA, Anhalt G.Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa F, Kashihara-Sawami M, Lyons M.B.Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol. 1990;94:77–85. doi: 10.1111/1523-1747.ep12873930. [DOI] [PubMed] [Google Scholar]
- 18.Scheinfeld N.Deleo VA. Photosensitivity in lupus erythematosus. Photodermatol Photoimmunol Photomed. 2004;20:272–279. doi: 10.1111/j.1600-0781.2004.00094.x. [DOI] [PubMed] [Google Scholar]
- 19.Xue D, Shi H, Smith JD, Chen X, Noe DA, Cedervall T, Yang DD, Eynon E, Brash DE, Kashgarian M, Flavell R.A.Wolin SL. A lupus-like syndrome develops in mice lacking the Ro 60-kDa protein, a major lupus autoantigen. Proc Natl Acad Sci U S A. 2003;100:7503–7508. doi: 10.1073/pnas.0832411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stingl G, Katz SI, Shevach EM, Rosenthal A.S.Green I. Analogous functions of macrophages and Langerhans cells in the initiation in the immune response. J Invest Dermatol. 1978;71:59–64. doi: 10.1111/1523-1747.ep12544055. [DOI] [PubMed] [Google Scholar]
- 21.Toews GB, Bergstresser P.R.Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 22.Meunier L, Bata-Csorgo Z.Cooper KD. In human dermis, ultraviolet radiation induces expansion of a CD36+ CD11b+ CD1− macrophage subset by infiltration and proliferation; CD1+ Langerhans-like dendritic antigen-presenting cells are concomitantly depleted. J Invest Dermatol. 1995;105:782–788. doi: 10.1111/1523-1747.ep12326032. [DOI] [PubMed] [Google Scholar]
- 23.Dandie GW, Clydesdale GJ, Jacobs I.Muller HK. Effects of UV on the migration and function of epidermal antigen presenting cells. Mutat Res. 1998;422:147–154. doi: 10.1016/s0027-5107(98)00186-9. [DOI] [PubMed] [Google Scholar]
- 24.Vink AA, Strickland FM, Bucana C, Cox PA, Roza L, Yarosh D.B.Kripke ML. Localization of DNA damage and its role in altered antigen-presenting cell function in ultraviolet-irradiated mice. J Exp Med. 1996;183:1491–1500. doi: 10.1084/jem.183.4.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aberer W, Schuler G, Stingl G, Honigsmann H.Wolff K. Ultraviolet light depletes surface markers of Langerhans cells. J Invest Dermatol. 1981;76:202–210. doi: 10.1111/1523-1747.ep12525745. [DOI] [PubMed] [Google Scholar]
- 26.Weiss JM, Renkl AC, Denfeld RW, de Roche R, Spitzlei M, Schopf E.Simon JC. Low-dose UVB radiation perturbs the functional expression of B7.1 and B7.2 co-stimulatory molecules on human Langerhans cells. Eur J Immunol. 1995;25:2858–2862. doi: 10.1002/eji.1830251022. [DOI] [PubMed] [Google Scholar]
- 27.Simon JC, Mosmann T, Edelbaum D, Schopf E, Bergstresser P.R.Cruz PD., Jr In vivo evidence that ultraviolet B-induced suppression of allergic contact sensitivity is associated with functional inactivation of Th1 cells. Photodermatol Photoimmunol Photomed. 1994;10:206–211. [PubMed] [Google Scholar]
- 28.Krueger JG, Wolfe JT, Nabeya RT, Vallat VP, Gilleaudeau P, Heftler NS, Austin L.M.Gottlieb AB. Successful ultraviolet B treatment of psoriasis is accompanied by a reversal of keratinocyte pathology and by selective depletion of intraepidermal T cells. J Exp Med. 1995;182:2057–2068. doi: 10.1084/jem.182.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noonan FP, Bucana C, Sauder D.N.De Fabo EC. Mechanism of systemic immune suppression by UV irradiation in vivo. II. The UV effects on number and morphology of epidermal Langerhans cells and the UV-induced suppression of contact hypersensitivity have different wavelength dependencies. J Immunol. 1984;132:2408–2416. [PubMed] [Google Scholar]
- 30.Enk CD, Sredni D, Blauvelt A.Katz SI. Induction of IL-10 gene expression in human keratinocytes by UVB exposure in vivo and in vitro. J Immunol. 1995;154:4851–4856. [PubMed] [Google Scholar]
- 31.Kang K, Hammerberg C, Meunier L.Cooper KD. CD11b+ macrophages that infiltrate human epidermis after in vivo ultraviolet exposure potently produce IL-10 and represent the major secretory source of epidermal IL-10 protein. J Immunol. 1994;153:5256–5264. [PubMed] [Google Scholar]
- 32.Baadsgaard O, Wulf HC, Wantzin G.L.Cooper KD. UVB and UVC, but not UVA, potently induce the appearance of T6− DR+ antigen-presenting cells in human epidermis. J Invest Dermatol. 1987;89:113–118. doi: 10.1111/1523-1747.ep12580461. [DOI] [PubMed] [Google Scholar]
- 33.De Fabo E.C.Noonan FP. Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology. J Exp Med. 1983;158:84–98. doi: 10.1084/jem.158.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross JA, Howie SE, Norval M, Maingay J.Simpson TJ. Ultraviolet-irradiated urocanic acid suppresses delayed-type hypersensitivity to herpes simplex virus in mice. J Invest Dermatol. 1986;87:630–633. doi: 10.1111/1523-1747.ep12456257. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz A, Maeda A, Wild MK, Kernebeck K, Gross N, Aragane Y, Beissert S, Vestweber D.Schwarz T. Ultraviolet radiation-induced regulatory T cells not only inhibit the induction but can suppress the effector phase of contact hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 36.Abeyama K, Eng W, Jester JV, Vink AA, Edelbaum D, Cockerell CJ, Bergstresser P.R.Takashima A. A role for NF-kappaB-dependent gene transactivation in sunburn. J Clin Invest. 2000;105:1751–1759. doi: 10.1172/JCI9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Devary Y, Rosette C, DiDonato J.A.Karin M. NF-kappa B activation by ultraviolet light not dependent on a nuclear signal. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 38.Walker S.L.Young AR. An action spectrum (290–320 nm) for TNFalpha protein in human skin in vivo suggests that basal-layer epidermal DNA is the chromophore. Proc Natl Acad Sci U S A. 2007;104:19051–19054. doi: 10.1073/pnas.0703385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oxholm A, Oxholm P, Staberg B.Bendtzen K. Immunohistological detection of interleukin I-like molecules and tumour necrosis factor in human epidermis before and after UVB-irradiation in vivo. Br J Dermatol. 1988;118:369–376. doi: 10.1111/j.1365-2133.1988.tb02430.x. [DOI] [PubMed] [Google Scholar]
- 40.Kupper TS, Chua AO, Flood P, McGuire J.Gubler U. Interleukin 1 gene expression in cultured human keratinocytes is augmented by ultraviolet irradiation. J Clin Invest. 1987;80:430–436. doi: 10.1172/JCI113090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kock A, Schwarz T, Kirnbauer R, Urbanski A, Perry P, Ansel J.C.Luger TA. Human keratinocytes are a source for tumor necrosis factor alpha: evidence for synthesis and release upon stimulation with endotoxin or ultraviolet light. J Exp Med. 1990;172:1609–1614. doi: 10.1084/jem.172.6.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh LJ, Davis MF, Xu L.J.Savage NW. Relationship between mast cell degranulation and inflammation in the oral cavity. J Oral Pathol Med. 1995;24:266–272. doi: 10.1111/j.1600-0714.1995.tb01180.x. [DOI] [PubMed] [Google Scholar]
- 43.Collins T, Lapierre LA, Fiers W, Strominger J.L.Pober JS. Recombinant human tumor necrosis factor increases mRNA levels and surface expression of HLA-A,B antigens in vascular endothelial cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1986;83:446–450. doi: 10.1073/pnas.83.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinarello CA, Cannon JG, Wolff SM, Bernheim HA, Beutler B, Cerami A, Figari IS, Palladino MA, O'Connor JV., Jr Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groves RW, Allen MH, Ross EL, Barker J.N.MacDonald DM. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 46.Schwarz A, Bhardwaj R, Aragane Y, Mahnke K, Riemann H, Metze D, Luger T.A.Schwarz T. Ultraviolet-B-induced apoptosis of keratinocytes: evidence for partial involvement of tumor necrosis factor-alpha in the formation of sunburn cells. J Invest Dermatol. 1995;104:922–927. doi: 10.1111/1523-1747.ep12606202. [DOI] [PubMed] [Google Scholar]
- 47.Werth VP, Zhang W, Dortzbach K.Sullivan K. Association of a promoter polymorphism of tumor necrosis factor-alpha with subacute cutaneous lupus erythematosus and distinct photoregulation of transcription. J Invest Dermatol. 2000;115:726–730. doi: 10.1046/j.1523-1747.2000.00118.x. [DOI] [PubMed] [Google Scholar]
- 48.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S.Beer HD. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 49.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, Fain P.R.Spritz RA. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med. 2007;356:1216–1225. doi: 10.1056/NEJMoa061592. [DOI] [PubMed] [Google Scholar]
- 50.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, Kummer JA, Tschopp J.French LE. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 51.Kondo S, Kono T, Sauder D.N.McKenzie RC. IL-8 gene expression and production in human keratinocytes and their modulation by UVB. J Invest Dermatol. 1993;101:690–694. doi: 10.1111/1523-1747.ep12371677. [DOI] [PubMed] [Google Scholar]
- 52.Kondo S.Jimbow K. Dose-dependent induction of IL-12 but not IL-10 from human keratinocytes after exposure to ultraviolet light A. J Cell Physiol. 1998;177:493–498. doi: 10.1002/(SICI)1097-4652(199812)177:3<493::AID-JCP12>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 53.Carraro C.Pathak MA. Studies on the nature of in vitro and in vivo photosensitization reactions by psoralens and porphyrins. J Invest Dermatol. 1988;90:267–275. doi: 10.1111/1523-1747.ep12455986. [DOI] [PubMed] [Google Scholar]
- 54.Stern RS, Nichols K.T.Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA Follow-Up Study. N Engl J Med. 1997;336:1041–1045. doi: 10.1056/NEJM199704103361501. [DOI] [PubMed] [Google Scholar]