Abstract
Several neurodegenerative diseases, including Alzheimer's, Parkinson's, Huntington's and prion diseases, are characterized pathognomonically by the presence of intra- and/or extracellular lesions containing proteinaceous aggregates, and by extensive neuronal loss in selective brain regions. Related non-neuropathic systemic diseases, e.g., light-chain and senile systemic amyloidoses, and other organ-specific diseases, such as dialysis-related amyloidosis and type-2 diabetes mellitus, also are characterized by deposition of aberrantly folded, insoluble proteins. It is debated whether the hallmark pathologic lesions are causative. Substantial evidence suggests that these aggregates are the end state of aberrant protein folding whereas the actual culprits likely are transient, pre-fibrillar assemblies preceding the aggregates. In the context of neurodegenerative amyloidoses, the proteinaceous aggregates may eventuate as potentially neuroprotective sinks for the neurotoxic, oligomeric protein assemblies. The pre-fibrillar, oligomeric assemblies are believed to initiate the pathogenic mechanisms that lead to synaptic dysfunction, neuronal loss, and disease-specific regional brain atrophy.
The amyloid β-protein (Aβ), which is believed to cause Alzheimer's disease (AD), is considered an archetypal amyloidogenic protein. Intense studies have led to nominal, functional, and structural descriptions of oligomeric Aβ assemblies. However, the dynamic and metastable nature of Aβ oligomers renders their study difficult. Different results generated using different methodologies under different experimental settings further complicate this complex area of research and identification of the exact pathogenic assemblies in vivo seems daunting.
Here we review structural, functional, and biological experiments used to produce and study pre-fibrillar Aβ assemblies, and highlight similar studies of proteins involved in related diseases. We discuss challenges that contemporary researchers are facing and future research prospects in this demanding yet highly important field.
Keywords: Amyloid, neurodegeneration, Alzheimer's disease, amyloid β-protein, protein misfolding, pre-fibrillar assemblies, oligomers, toxicity
INTRODUCTION
The amyloid-cascade hypothesis [1], suggesting that amyloid β-protein (Aβ) fibril formation and plaque deposition lead to neuronal dysfunction, dementia, and death in Alzheimer's disease (AD), had guided scientific research into discovery of etiologic and pathogenic mechanisms of AD. However, this hypothesis has been contentiously debated because: 1) fibrillar amyloid burden does not correlate well with neurological dysfunction [2], 2) cognitive impairment in transgenic murine models of AD is observed before and/or independently of amyloid plaque formation [3], 3) plaque-independent pathology can be explained by the neurotoxicity of soluble Aβ assembly intermediates, 4) oligomer-induced memory dysfunction occurs before neuronal death, and 5) brain, plasma, and cerebrospinal fluid (CSF) concentrations of soluble Aβ oligomers correlate with neurodegeneration better than those of fibrils [4]. These observations have led to a burgeoning yet encompassing alternative paradigm hypothesizing that soluble pre-fibrillar protein assemblies, rather than mature fibrillar deposits, act as proximate neurotoxins that cause synaptic dysfunction, neuronal loss, dementia, and death [4–11]. This new hypothesis has been supported by discovery of toxic pre-fibrillar protein assemblies involved in other protein-misfolding diseases, such as, Parkinson's disease, Huntington's disease, transmissible spongiform encephalopathies, amyotrophic lateral sclerosis, poly-glutamine diseases, type-2 diabetes mellitus (T2D), and systemic amyloidoses [7, 12–15].
Diversity and sometimes inaccuracy in nominal definitions, and in structural/functional descriptions of soluble pre-fibrillar Aβ assemblies, along with different methodologies to generate and study these assemblies, are confounding factors in this already-vast and complex research area. Various forms of soluble pre-fibrillar Aβ assemblies (reviewed in [10, 16–18]) including monomeric Aβ conformers [19], secreted cell- and brain-derived low-order oligomers [20–25], Aβ-derived diffusible ligands (ADDLs) [26, 27], protofibrils (PF) [28–31], Aβ*56 [32], paranuclei [33–35], amylospheroids [36], annular assemblies [37], amyloid pores [18, 37, 38], and βamy balls [39] have been described. However, despite a global concerted scientific effort, the relationships amongst these Aβ-derived assemblies and their relevance to AD pathogenesis are unclear and the fundamental quest for a unanimous pathogenic "equivalent" active in AD-afflicted brain is ongoing [9].
We begin our discussion in the first step of studying pre-fibrillar Aβ assemblies—protein preparation.
SOURCES AND METHODS OF Aβ PREPARATION
The low physiological concentration and the difficulty of procuring highly pure and homogeneous tissue-derived Aβ have precluded its routine use in experimental studies in vitro. Therefore, synthetic Aβ preparations have emerged as alternatives. Synthetic Aβ is produced either by standard solid-phase peptide synthesis (SPPS) [40, 41] or by recombinant DNA technology [42–45].
The Aβ sequence is recognized as a "difficult" target for SPPS owing to its high hydrophobicity and innate propensity to aggregate [46, 47]. To overcome these issues, various deprotecting agents [46], novel solvent systems for coupling [47], and solid-support modifications [48] have been employed to augment synthetic yield and improve purity of crude Aβ peptides. Recently, application of an O-N acyl migration reaction, also called "O-acyl isopeptide" chemistry was proposed as an efficient alternative SPPS route for obtaining Aβ with increased solubility and purity [49, 50].
Similarly, tailored recombinant expression systems have been used to produce Aβ in high yields. A strategy to increase solubility and facilitate purification is production of Aβ peptides as fusions with sequences affording high solubility, followed by cleavage of the fusion protein and purification by high-performance liquid chromatography (HPLC) [42–44,51].
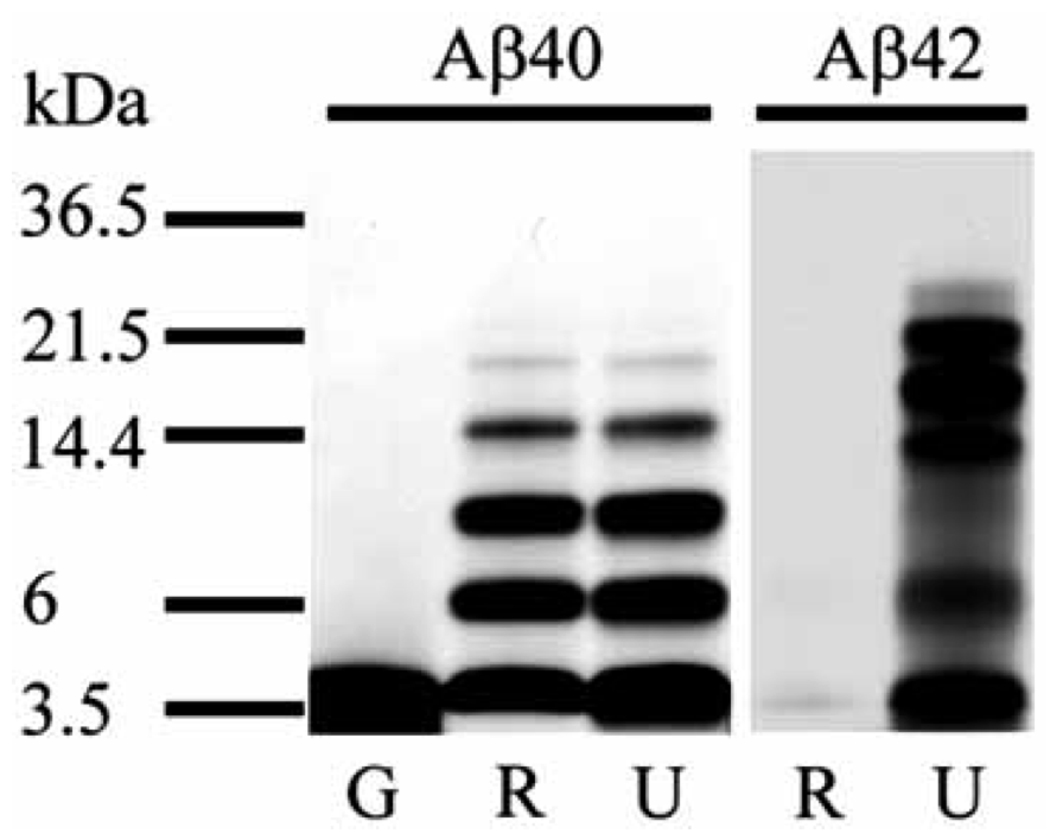
Because self-association of Aβ is believed to be central in the pathogenesis of AD, extensive research has been dedicated to developing methodologies to characterize Aβ assemblies structurally and biologically [52]. Multiple studies have shown that many of the neurotoxic effects of Aβ assemblies can be recapitulated by synthetic Aβ in vitro and in vivo [53]. However, differences in peptide quality, presence of trace contaminants in Aβ preparations from different sources, and compositional variation of Aβ preparations, even from the same source, have been a serious problem leading to irreproducible or discrepant study outcomes [54–56]. For example, under identical conditions, an Aβ oligomer-specific monoclonal antibody was shown to react only with oligomers derived from recombinant Aβ but not those derived from chemically synthesized Aβ [57]. In our hands, photo-induced cross-linking of unmodified proteins (PICUP) using Aβ obtained from different sources, but prepared identically, yielded distinct results (Fig. (1)).
Fig. 1. Comparison of photo-cross-linking using Aβ peptides from different sources.
Synthetic Aβ from Global Peptide (G) and the UCLA Biopolymers Laboratory (U), and recombinant Aβ from rPeptide (R) were prepared in 10 mM sodium phosphate, pH 7.4 at 2 mg/ml nominal concentration and filtered through a 10-kDa molecular-weight cut-off filter [77]. Each filtered peptide was cross-linked using PICUP [73]. The resulting cross-linked oligomers were subjected to SDS-PAGE and silver-staining. The data suggest that Aβ40 from Global peptide contained contaminants that prevented cross-linking and that Aβ42 from rPeptide aggregated during the filtration step and was hardly detectable.
Neither SPPS nor recombinant methods can produce 100% pure Aβ. Failure sequences, oxidation of Met35, and racemization may occur during various SPPS steps [52]. In recombinant preparations where a fusion protein is enzymatically cleaved to release Aβ, it is important to verify that the cleavage product is not contaminated with the uncleaved fusion protein, the cleaving enzyme, or adventitious proteolytic fragments [52]. Practically, it is important that the researcher verifies chemical purity of the preparation and ensures removal of residual components which could complicate solvation and stock preparation, potentially alter the biophysical and biological features of the peptide, and render concentration measurements error-prone [52].
METHODS USED TO STUDY PRE-FIBRILLAR Aβ ASSEMBLIES
Important biological functions of oligomeric Aβ assemblies have spurred extensive efforts to characterize them structurally. The non-crystalline nature of the oligomers and their slow tumbling time in aqueous solutions preclude high-resolution structural determination by X-ray crystallography and solution-state NMR, respectively. Moreover, the metastable nature of Aβ oligomers and their existence in rapidly changing mixtures have made their structural characterization particularly difficult. To address these issues, multiple low-resolution methodologies have been used to assess various structural features of pre-fibrillar Aβ assemblies. Here, we outline some of the key methods used to provide structural and biophysical information on pre-fibrillar Aβ assemblies.
ATOMIC-RESOLUTION TECHNIQUES
NMR Spectroscopy
Solution-state NMR is a powerful high-resolution technique for determining peptide and protein structure in solution. Typically, the structure is calculated based on distances and angles obtained through measurements of nuclear Over-hauser effect and spin-spin scalar coupling interactions as constraints for computer-generated models. As mentioned above, peak broadening due to slow tumbling times currently precludes solving the structure of Aβ oligomers using solution-state NMR. However, multiple NMR studies have assessed structural properties of Aβ monomers. For instance, studies by Lee et al. introduced the concept of "plaque competence," which defines the propensity of near-physiological concentrations of soluble Aβ to deposit onto authentic amyloid plaques in vitro [58]. The plaque competence assay identified a central 26-residue fragment (Tyr10–Met35) which was deemed necessary to mimic plaque-deposition characteristics of the full-length Aβ [58]. Preliminary NMR conformational analysis showed that this 26-residue fragment had a different conformation from a plaque-incompetent fragment (Asp1–Lys28) [58]. Further NMR studies also confirmed that the central 26 residues of Aβ were sufficient to mimic amyloidogenic properties of Aβ [59]. It was reported that the central hydrophobic cluster of full-length Aβ, and Aβ(10–35), both adopted well-defined, albeit irregular, conformations in solution, whereas the C- and N-terminal flanking regions of the full-length Aβ were partially disordered [59].
NMR studies also have highlighted differences between Aβ40 and Aβ42. Solution-state NMR studies of non-oxidized [60] or Met-oxidized [61, 62] Aβ40 and Aβ42 show that the C-terminus of Aβ42 is more rigid compared to that of Aβ40, likely due to the extended hydrophobic C-terminus of Aβ42. Similarly, a study combining molecular dynamics and NMR experiments, showed that the C-terminus of Aβ42 is more structured than that of Aβ40 [63]. NMR studies also revealed that common C-terminal peptide segments within Aβ40 and Aβ42 have distinct structures, which may be relevant to the strong disease-association of elevated Aβ42 production [64].
X-Ray Crystallography
X-ray crystallography examines atomic structures of crystals by X-ray diffraction techniques (reviewed in [65]). The signal is intensified by the coherent alignment and lattice repeat of the crystal. The wavelength of the light used in X-ray crystallography is usually around 1.5 Å, about the length of a C-C bond. Use of X-rays with this wavelength theoretically allows resolution of individual atoms. Recently, Sawaya et al. reported that 33 peptide segments derived from 14 different amyloidogenic proteins formed amyloid-like fibrils, microcrystals, or both [66] and used X-ray crystallography to examine the atomic organization of molecules within microcrystals of these peptides. Microcrystals of 2 Aβ segments were resolved, Gly37–Ala42 and Met35–Val40. The authors suggested that the structural organization of these peptides within the crystals is similar to those of Aβ fibrils and concluded that the fundamental unit of amyloid-like fibrils is a steric zipper arrangement formed by two tightly interdigitated β-sheets [66].
Hydrogen–Deuterium Exchange
Hydrogen–deuterium exchange is a powerful probe of protein structure and dynamics. The method involves the study of exchange rates of labile protons in proteins with deuterons from the solvent, typically D2O. Labile protons are those bonded to nitrogen, sulfur, or oxygen. These protons can exchange with solvent hydrogen or deuterium cations. Labile protons that are solvent-exposed and are not involved in hydrogen bonding exchange rapidly, whereas buried or hydrogen-bonded protons exchange at substantially slower rates. This makes hydrogen–deuterium exchange sensitive to structural rearrangements occurring during protein aggregation. Thus, amide protons buried in the core of oligomeric and higher-order assemblies or hydrogen-bonded in helices and sheets do not exchange readily with solvent deuterons. The exchange rate is detected using NMR and/or mass spectrometry. For study of rapidly changing assemblies, mass spectrometric detection of exchange may be advantageous because NMR requires longer times (hours) to record the spectra, making the study of short-lived oligomers difficult. In addition, NMR requires prior assignment of the protons and is generally limited to proteins smaller than 25 kDa. An additional advantage of mass spectrometric detection is requirement of substantially smaller amounts of protein. However, assignment of specific exchanging protons using mass spectrometry requires tandem mass spectrometry and can be a daunting task, whereas for a previously assigned NMR spectrum, identification of exchanging protons is straight-forward. Hydrogen–deuterium exchange coupled with mass spectrometry was used to map structural differences in Aβ PF and fibrils [67].
DETERMINATION OF OLIGOMER-SIZE DISTRIBUTION
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE is a routine and inexpensive method enabling separation of proteins based on their electrophoretic mobility which is affected by a combination of the primary, secondary, tertiary, and quaternary structures of proteins. In this method, protein mixtures are electrophoresed after treatment with SDS. SDS binds proteins via its hydrophobic dodecyl tail, leaving its sulfate group solvent-exposed, and thus creating a negatively-charged envelope that "coats" protein molecules [68]. In most cases, SDS binding denatures secondary and non-disulfide-linked tertiary structures, negatively charging proteins approximately uniformly and proportionally to their mass. Under these conditions, electrophoretic migration of proteins through the gel matrix is governed directly by the molecular mass of the protein. Without SDS, different proteins of the same mass may electrophorese distinctly due to differences in overall charge (different isoelectric points) and folding.
Importantly, the effect of SDS on all proteins is not equivalent. In many cases, SDS can induce or stabilize secondary and quaternary structures. SDS may cause dissociation of some oligomers or conversely induce oligomerization and aggregation. Therefore, resolution of apparently monomeric or low-molecular-weight (LMW) oligomeric components in a protein mixture does not necessarily indicate existence of such components under native conditions, i.e., without SDS. Aβ is an amphipathic protein known to form SDS-stable oligomers [30]. Indeed, SDS-induced assembly of Aβ into insoluble aggregates has been capitalized on to purify Aβ from brain homogenates [69]. When treated with SDS, Aβ assembles rapidly into high-molecular-mass aggregates [57]. During electrophoresis of Aβ40, these aggregates dissociate completely and only a monomer is observed following staining, whereas electrophoresis of Aβ42 yields apparent trimeric and tetrameric components [33]. Essentially identical monomer-trimer-tetramer distributions are observed when different preparations of Aβ42, including "monomeric", oligomeric, and fibrillar Aβ42 are analyzed by SDS-PAGE [70]. Thus, despite its wide use, SDS-PAGE is not a reliable method for characterization and size determination of non-covalently associated Aβ oligomers.
Photo-Induced Cross-Linking of Unmodified Proteins (PICUP)
PICUP is a method originally developed to study stable protein homo- and hetero-oligomers [71]. PICUP was used by us and others to study oligomer size distribution of Aβ [72] and a variety of other proteins, including amyloidogenic proteins [73]. PICUP generates covalent bonds between closely interacting polypeptide chains within ≤1 s exposure to visible light without pre facto chemical modifications of the native sequence and without using spacers. The cross-linking is induced by rapid photolysis of a tris-bipyridyl Ru(II) complex in the presence of an electron acceptor. Illumination causes generation of a Ru(III) ion, which subsequently abstracts an electron from, and produces a carbon radical within, the polypeptide. The radical reacts rapidly with adjacent susceptible groups and forms covalent bonds. Therefore, PICUP stabilizes oligomer populations by covalent cross-linking, and "freezes" molecular interactions that exist before cross-linking. The mechanism, protocol, and limitations of PICUP were discussed in detail elsewhere [71, 73].
Size-Exclusion Chromatography (SEC)
SEC (gel permeation chromatography) fractionates solutes based on their Stokes (hydrodynamic) radii. When solutes of different sizes pass through an SEC column packed with porous material, larger molecules spend less time interacting with the solid phase and elute faster, whereas smaller molecules diffuse into the pores and therefore spend more time interacting with the solid phase and elute later. SEC affords an SDS-independent separation mechanism and covers a molecular mass range of ~103–106 Da. However, SEC provides lower resolution than SDS-PAGE and molecular-mass estimations of polypeptides can be inaccurate if the elution profiles are sensitive to the protein conformations. SEC analysis of Aβ assemblies does not resolve LMW oligomers but can distinguish between PF and small oligomers [30]. At this resolution, SEC may be useful for studying the kinetics involved in conversion of LMW Aβ to PF (or dissociation of PF into LMW Aβ). In addition to its use as an analytical method [39], SEC has been used extensively to purify fractions of particular Aβ assemblies [30, 33, 38, 74–76]. Description of the basic instrumentation and utilization of SEC for preparation of aggregate-free Aβ was published previously [77].
Analytical Ultracentrifugation (AU)
AU is a versatile technique used to characterize the hydrodynamic and thermodynamic properties of proteins or macromolecules. AU combines an ultracentrifuge and an optical detection system capable of measuring the sample concentration inside the centrifuge cell during sedimentation. Coupled with data-analysis software, AU can determine sample purity and molecular mass in the native state, measure sedimentation and diffusion coefficients, characterize assembly–disassembly mechanisms of complex analytes, determine subunit stoichiometry, detect and characterize macromolecular conformational changes, and measure equilibrium constants and thermodynamic parameters for self- and hetero-associating assemblies. Two types of experiments are commonly performed using ultracentrifugation–sedimentation-velocity [78, 79] and sedimentation-equilibrium [80], Sedimentation-equilibrium experiments can analyze a mixture of moieties of various molecular masses. After each analyte reaches its equilibrium, high-molecular-mass species locate towards the bottom of the cell, whereas low-molecular-mass species dominate at the top. The equilibrium data can be fitted to calculated models for the distribution of the solutes. Using this type of analysis, Huang et al. have reported that Aβ40 existed as an equilibrium mixture of monomers, dimers and tetramers at neutral pH [81]. However, other equilibria, including monomer–dimer, monomer–trimer, or monomer–tetramer, produced equivalent residuals [81] hindering precise determination of the oligomerization state of the peptide.
Dynamic Light Scattering (DLS) Spectroscopy
DLS, also known as quasielastic light scattering or photon-correlation spectroscopy, is a non-invasive analytical method for determination of diffusion coefficients of particles undergoing Brownian motion in solution. DLS measures the temporal dependence of light scattering emanated from an analyte in solution over 10−7−1 s. Fluctuations in the intensity of the scattered light relate to the rate of the Brownian motion which is correlated to the diffusion coefficients of the particles. In a mixture of analytes, a distribution of diffusion coefficients is obtained. The data are processed to determine the particle hydrodynamic radii which relate to the diffusion coefficients using the Stokes-Einstein equation. DLS has an intrinsic bias for large aggregates because the intensity of the scattered light is proportional to the square of the particle size [82], Therefore, DLS is well suited to measure minute amounts of aggregated proteins (<0.01% by weight) on the background of monomers and small oligomers. Because DLS allows monitoring protein assembly without manipulation or consumption of the analyte, it has been used widely to study Aβ aggregation and assembly processes [33, 75, 83–87].
For proteins larger than ~500 kDa or for extended proteins (rod-like/unfolded), scattering varies significantly with angle. Determining scattering at additional angles (multi-angle laser light scattering or MALLS) allows direct measures of mass (≤MDa) and radius (a measure of geometric size). Because the light-scattering signal is directly proportional to protein concentration and molecular mass, a combination of the DLS signal and concentration measurements using refractive index or absorbance, allows calculation of the molecular mass of each component when proteins are fractionated chromatographically. DLS can resolve the monomeric or dimeric state of a protein, but it cannot distinguish among small oligomers when their hydrodynamic radii differ by less than a factor of 2 [88]. Consequently, DLS is less useful for analyzing individual small oligomers than SEC-MALLS, PICUP coupled with SDS-PAGE, or sedimentation velocity. Detailed accounts of the theory and practice of DLS and its application to study of Aβ were given by Lomakin et al. [82, 89, 90]. Solution state and size distribution of ADDLs has been assessed recently by Hepler et al. using SEC coupled with MALLS [70].
Ion-Mobility Spectrometry-Mass Spectrometry (IMS-MS)
IMS-MS is a mass-spectrometric method that can resolve molecules of identical mass-to-charge (m/z) values which differ by assembly state or conformation. In IMS-MS, ions are carried by a weak, uniform electric field through a drift cell in which they collide at low velocity with a low-pressure inert gas (typically helium). The analyte ions quickly reach equilibrium resulting in a constant drift velocity. At equilibrium, the mobility of the ions is inversely proportional to their collisional cross-section. Thus, ions with compact structures drift fast through the cell, whereas ions with large cross-sections move more slowly. The ions exit the drift cell, pass through a mass filter, and are detected as a function of time, producing an "arrival-time" distribution. Protein oligomers often have identical m/z ratio (i.e., a singly-charged monomer would have the same m/z as a doubly-charged dimer, triply-charged trimer, etc.). IMS-MS analysis can resolve these species yielding an oligomer size distribution. IMS-MS studies of AB have shown that freshly prepared LMW Aβ40 contained monomers, dimers, trimers, and tetramers, whereas similarly prepared solutions of Aβ42 comprised oligomers up to a dodecamer [91]. These results accord with earlier observations of distinct oligomer size distributions of Aβ40 and Aβ42 by PICUP [33] and may explain differences in neurotoxic effects of the two Aβ alloforms.
ANALYSIS OF SECONDARY STRUCTURE
Circular Dichroism (CD) Spectroscopy
CD is the change in the absorption of circularly polarized light as a function of wavelength exhibited by optically active molecules. Because secondary structural elements such as α-helices, β-strands, β-turns and disordered regions display characteristic wavelength-dependent dichroism, CD is a useful method to determine protein secondary structure. Secondary structure analysis by CD spectroscopy uses "far-UV" spectra (190–250 nm), in which the chromophores are peptide bonds. The CD signal reflects an average of the entire molecular population. Therefore, CD can only determine the overall proportion of secondary structural elements, but not the amino acid residues involved or the fraction of molecules that have a particular conformation. CD has been used extensively to investigate secondary structure of Aβ peptides and to monitor structural transitions of Aβ during its oligomerization and aggregation [31, 92–95].
Fourier-Transform Infrared (FTIR) Spectroscopy
Complementary to CD, FTIR enables determination of the secondary structure of protein samples as thin films, as solids, or in solution. Characteristic bands in IR spectra of proteins and polypeptides include predominantly amide I and amide II. The amide I band corresponds to the absorption in the vibrational spectrum of the C=O component of the amide bond, whereas the amide II band corresponds to the absorption of the N–H bond. Because C=O and N–H bonds are involved in hydrogen bonding, the absorption wavelength of both the amide I and amide II bands are sensitive to the secondary structure content of proteins. In many cases, instead of a series of well-resolved peaks for each type of secondary structure, one broad peak is observed. However, for proteins that cannot be studied by high-resolution methods, FTIR provides useful structural information. It is thought that FTIR is more sensitive to β-sheet content whereas CD measurements generally tend to underestimate β-sheet relative to α-helix content [96]. FTIR has been used extensively to study the conformational changes of Aβ during assembly [97–101].
MORPHOLOGICAL ANALYSIS
Transmission Electron Microscopy (TEM)
TEM uses a cathode ray which emits a high-voltage electron beam focused by electrostatic and electromagnetic lenses. When the electron beam passes through a thin, electron-transparent specimen, it carries information about the inner structure of the sample as it reaches the TEM imaging system. There, the spatial variation in this information, which creates the image, is magnified by a series of electromagnetic lenses and detected by a fluorescent screen, photographic plate, or a light-sensitive sensor, e.g., a camera. TEM has been used extensively to examine the morphology of pre-fibrillar Aβ assemblies, including LMW Aβ [33, 34], small oligomers [102–105], paranuclei [33, 34], PF [31, 75, 76, 87] and spheroids [36, 39].
Scanning Transmission Electron Microscopy (STEM)
In STEM, an electron beam scans a specimen and scattered electrons are collected by detectors behind the specimen. In a thin proteinaceous specimen, the image intensity is directly proportional to the mass of the irradiated region. Therefore, following background subtraction and calibration, the protein mass and mass-per-length unit (MPL) can be determined quantitatively [106]. STEM has been used to characterize the MPL ratios of Aβ fibrils and PF [107–109].
Atomic-Force Microscopy (AFM)
AFM images high-resolution (≤1 nm) topography of a sample, adsorbed on an atomically flat smooth surface, typically mica. A cantilever tip samples the surface and when the tip contacts a spot with adsorbed sample, an ionic repulsive force bends the cantilever upwards. The extent of bending, measured by a laser reflected onto a split photo detector, is translated to force units. By keeping the force constant while scanning across the surface, the vertical movement of the tip generates the surface contour which is recorded as the topography of the sample. AFM has been modified for specific applications and can be used in different modes. In "tapping mode" (commonly referred to as "intermittent-contact" or "dynamic-force mode"), a stiff cantilever oscillates close to the sample. Part of the oscillation extends into the repulsive regime so that the tip intermittently touches, or "taps," the surface. This mode provides good resolution on soft samples and therefore is useful for investigation of pre-fibrillar Aβ species.
An advantage of AFM over TEM is that it allows continuous monitoring of the growth of oligomers in solution [110]. Multiple studies on the structure of soluble oligomers have used conventional tapping-mode AFM [26, 30, 31, 111–114]. The smallest size of individual oligomers that could be observed by AFM corresponded to a height of ~1–4 nm [101].
Scanning Tunneling Microscopy (STM)
STM is a non-optical microscopic technique which employs principles of quantum mechanics. The electron cloud of atoms on the surface of a sample extends a minute distance above the surface. When a probe, with a tip as sharp as a single atom, is brought sufficiently close to such a surface and a small voltage is applied, a strong interaction occurs between the electron cloud on the surface and the tip leading to an electric tunneling current. The magnitude of the current depends exponentially on the distance between the probe and the surface. The tunneling current rapidly increases as the distance between the tip and the surface decreases. This rapid alteration in the current due to changes in distance results in construction of an atomically resolved image when the tip scans the structure. The feedback signal, applied to a piezoelectric element provides a measure of molecular surface contour. STM was used to examine the structure of Aβ40 monomers, dimers and oligomers on a surface of atomically flat gold [115]. At low concentrations (0.5 µM) small globular structures were observed. High-resolution STM measurements of Aβ samples, both immediately following preparation and after 24 h aging, found structures of ~3–4 nm in diameter corresponding to oligomeric Aβ. These results suggested that oligomer formation could potentially proceed through a mechanism involving linear association of monomers [115].
STRUCTURAL AND BIOLOGICAL STUDIES OF PRE-FIBRILLAR Aβ ASSEMBLIES
The use of proper terminology to describe soluble pre-fibrillar Aβ assemblies is crucial to forming consensus in the literature. However, achieving this goal is difficult because various oligomeric forms of Aβ have been described structurally, functionally, or both and the relationship amongst these is unclear. Although all these structures are oligomeric, the use of the term "oligomer" to describe all the assemblies may be misleading for at least three reasons, as discussed before: 1) the structure of each assembly is unique; 2) the pathways leading to the formation of the assemblies or the ultimate path they may take towards fibrillization may differ; and 3) the biological activities of each assembly may differ or similar activities may be mediated through different mechanisms [57]. In the following section, we describe pre-fibrillar Aβ species ranging from monomers to PF (Table (1)). In most cases, we begin with structural characterization of each species followed by discussion of its biological activity.
Table 1.
Summary of Structural and Biological Characteristics of Pre-Fibrillar Aβ Assemblies
| Assembly | Structural characteristics/production | Biological activity |
|---|---|---|
| "Activated monomeric conformer" of Aβ | ||
| Cell-derived Aβ oligomers | ||
| Aβ-derived diffusible ligands (ADDLs) |
|
|
| Protofibrils (PF) |
|
|
| Aβ*56 |
|
|
| Amyloid pores |
"ACTIVATED MONOMERIC CONFORMER" OF Aβ
Structural Characterization
Monomer activation denotes a conformational change preceding Aβ self-assembly that may render monomers toxic, or cause them to nucleate further aggregation, or both. Based on concepts taken from actin polymerization [116] and a kinetic model of Aβ aggregation induced by constant rotary shaking, Taylor et al. introduced the idea of "activated monomeric conformers" of Aβ40 [19], also called "intermediate aggregated species" [117]. It was postulated that this moiety was an oxidative or hydrolytic derivative, or a slowly-folding conformer of intact Aβ40 [19, 117]. It was proposed that the "inactive" monomer slowly converted into the "activated" monomeric conformer, several of which might cooperate to form a growing nidus for oligomerization and progression of aggregation [19]. In these studies, the presence of the active monomeric Aβ conformer was tested by HPLC using acetonitrile and trifluoroacetic acid, which might have caused structural alterations in Aβ sequence by increasing its β-sheet content [118]. Lee et al. have provided evidence that Aβ aggregation intermediates and final structures formed under slowly agitated or quiescent conditions at 37°C differed in their toxicity, stability to denaturant, and apparent morphologies [119], emphasizing that parametrical consideration of methodologies used to prepare Aβ for structural or biological studies and proper methods to assess the assembly state of the resulting preparation are paramount [52]. Similarly, NMR studies emphasize the importance of performing structural studies under physiological conditions [61] rather than "structure-inducing" milieus as reported by Taylor et al. [19].
Other studies also have shown presence of Aβ intermediates, however, the assembly state of these was not determined unambiguously. A study by Chimon et al. described Aβ40 intermediates that contained a β-sheet-rich character and were thought to originate from a monomeric state, preceding PF and fibril formation [120]. Filtration experiments showed that these intermediates were not monomeric but were likely larger than decamers, indicating that unambiguous determination of the assembly intermediates is difficult. By electron microscopy, these intermediates had a spherical morphology similar to ADDLs [121], amylospheroids [36] and βamy balls [39]. NMR studies showed that the intermediate species was well ordered in the hydrophobic core and the C-terminal region [120]. Aβ40 was used in this study at higher concentrations than those found in biological specimens and the possibility that the intermediate could have undergone fibril formation during preparation for NMR studies was not excluded. Similarly, Lim et al. provide evidence for presence of monomeric intermediates using CD and NMR studies of Aβ40 and Aβ42 under both non-amyloidogenic (<5°C) and amyloid-promoting conditions (>5°C) at physiological pH [94]. CD studies of the Aβ peptides suggested that the initially unfolded Aβ peptides at low temperature gradually transformed to β-sheet-containing monomeric intermediates at stronger amyloidogenic conditions (higher temperatures) [94]. However, exclusive presence of monomers after dialysis of 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP)-treated Aβ species against phosphate buffer was not confirmed [94]. Providing formal proof for the presence of Aβ monomers exclusively is difficult and has not been achieved unambiguously in the studies mentioned above. Therefore, whether a critical conformational change in the monomer occurs before self-assembly, or the conformational change and the assembly occur concurrently and co-dependently remains an open question.
Biological Activity
In cell biological studies, toxicity and apoptotic activity were enhanced when the concentration of the "activated monomeric conformer" described by Taylor et al. was maximal during the aggregation continuum [19]. Similarly, in electrophysiological experiments, Aβ species described as monomeric conformers obtained between ~60–120 min during rotary-shaken aggregation inhibited neuronal action potentials [122]. This neurotoxicity was attributed only to the "active" monomeric conformers detected at 60 min after the initiation of aggregation but not to inactive monomers or fully formed aggregates [19, 117, 122]. In these studies, the kinetics of aggregation was monitored by turbidity and the loss of the low-molecular-mass starting material was monitored by HPLC [19]. The sensitivity of turbidity measurements for detection of small particles is substantially lower than that of DLS and it is therefore unlikely that the method can distinguish monomers from small oligomers. Also of note is that biological activities similar to those obtained by Taylor et al. [19] have been described for Aβ oligomers [53], thus it is unclear that these toxicity data can be ascribed to monomeric Aβ.
BRAIN- AND CELL-DERIVED LOW-ORDER Aβ OLIGOMERS
Structural Characterization
Dimeric and trimeric assemblies of Aβ have been isolated from amyloid plaque cores [20], cerebrospinal fluid (CSF) [22], and human cortical homogenates [21], and produced by cells transfected with amyloid precursor protein (APP) [23]. Dimeric and trimeric Aβ (apparent SDS-PAGE mobility corresponding to ~9 and ~13.5 kDa) purified from neuritic plaques and leptomeningeal mural amyloid, were characterized by SEC, AFM, and electron microscopy [104]. Matrix-assisted laser-desorption ionization (MALDI) mass spectrometry was used to determine the mass of the "dimeric" components [104]. However, to extract Aβ from tissue sources, SDS was included in extraction buffers and the possibility that these Aβ species could form during the extraction process was not excluded. Immunoaffinity purification, HPLC, and MALDI analyses of CSF from patients suffering from meningitides and other neurological conditions including dementia, revealed Aβ species of various length, including two truncated trimer species, (Asp1–Met35)3 and (His6–Ala42)3 and an Aβ40 dimer, (Asp1–Val40)2 [22]. Aβ in CSF was shown to be associated with high-density lipoprotein particles in an apparent monomeric form detected by SDS-PAGE [123, 124]. Similarly, 24 and 27% of brain Aβ40 and Aβ42, respectively, were shown to be concentrated in lipid-raft extracts as dimers determined by SDS-PAGE in the Tg2576 murine model of AD [125]. However, as discussed above, SDS-PAGE is not a reliable method for Aβ size determination [57].
Walsh et al. have reported "natural" Aβ oligomers that are "SDS-stable" low-order oligomers (dimers, trimers, and tetramers) detected in the conditioned media and/or lysates of cells [23, 24, 126–128]. These oligomers are produced by Chinese hamster ovarian cells that express human mutant (V717F, V717I, or E693Q, cells referred to as 7PA2), or wild-type APP [23, 129]. Low-abundance species of masses ~10, 14 and sometimes 16 kDa, which were reactive with antibodies against Aβ also were detected in the culture media of these cells [23], The nature of these low-order oligomers is not fully understood but the observations that they are not disassembled by several types of denaturants suggests that they may be covalently linked [24].
Biological Activity
Initially, biological effects of oligomeric Aβ fractions purified from neuritic plaques and leptomeningeal mural deposits were investigated in an astroglial-neuronal co-culture system believed to approximate in vivo conditions [104]. Neuronal viability was compromised by AD-derived Aβ only when microglial cells were co-cultured, indicating that toxicity was mediated through a microglia-dependent mechanism [104].
Ease of maintenance and fast growth-rate of 7PA2 cells facilitated investigations of the biological activities of "natural", cell-derived Aβ oligomers. Intracerebroventricular microinjection of small volumes (~1.5 µL containing ~3 ng/mL Aβ) of SEC-fractionated cell-culture media to anesthetized wild-type rats was shown to inhibit hippocampal long-term potentiation (LTP) [25, 130]. This inhibition was predominantly mediated by Aβ trimers in wild-type murine hippocampal brain slices, whereas dimers and tetramers had intermediate potencies, and monomers were apparently ineffective [128]. Cell-derived oligomers were shown to interfere primarily with the induction of LTP, but not its expression, once the signaling cascades responsible for LTP were initiated [128]. These results suggested that cell-derived Aβ oligomeric assemblies altered certain aspects of hippocampal synaptic plasticity both in vivo and in vitro (reviewed in [131]).
The validity of LTP as an electrophysiological paradigm for learning and memory composition has been debated [132], To test the effect of the "natural Aβ oligomers" in a non-LTP paradigm, Cleary et al. used an in vivo behavioral model in rats that were injected with cell-conditioned media containing Aβ assemblies into the dorsal lateral cerebral ventricles. The treatment was found to cause a transient interruption of pre-learned behaviors [133]. This was attributed to Aβ oligomers because SEC fractions containing oligomers caused the deficits, whereas monomer-containing fractions were ineffective [133].
Actin-based cytoskeletal network dynamics is critical for the regulation of neuronal spine morphology and function [134]. Alterations in spine morphology and actin-regulatory mechanisms recently have emerged as a sensitive measure of early neuronal functional deficits and neurotoxicity [135–137], Because loss of synaptic termini strongly correlates with the severity of dementia, Shankar et al. assessed the effect of cell-derived soluble oligomers on synapses [138]. They showed that the density of dendritic spines decreased substantially in neurons treated with sub-nanomolar levels of cell-derived Aβ oligomers [138]. This effect was shown to be Aβ-oligomer-specific, i.e., SEC monomer fractions alone were ineffective. The decrease in spine density was reverted by monoclonal immunodepletion of Aβ, by transfer of cells back into the control media, and by scyllo-inositol [138], a molecule thought to stabilize synthetic Aβ as nontoxic species, possibly preventing Aβ interaction with neuronal target proteins [139, 140].
In cultures of dissociated cortical neurons, synthetic Aβ was shown to activate nicotinic acetylcholine receptors (nAchRs) and trigger internalization of N-methyl-D-aspartate (NMDA) receptors (NMDARs) [141]. However, Shankar et al. found that an irreversible nAchR antagonist did not affect Aβ-oligomer-mediated spine loss, indicating that nAchR activity was unnecessary for this effect [138]. Signaling cascades involving NMDARs, cofilin, and calcineurin were found to be involved in Aβ-induced spine loss as determined by inhibition studies [138]. Together these data demonstrated that cell-derived low-order Aβ oligomers could cause reduction of synapse density and loss of electro-physiologically active synapses in hippocampal pyramidal neurons, suggesting that their deleterious effects may be important mechanistic contributors to synaptic dysfunction in AD in vivo [131].
Aβ-DERIVED DIFFUSIBLE LIGANDS (ADDLS)
Structural Characterization
ADDLs are exclusively Aβ42-derived soluble pre-fibrillar assemblies that morphologically appear as 3–8-nm globules by AFM [26, 121] and have estimated masses between 17–42 kDa [26] (reviewed in [142]). It was first observed that apolipoprotein J (apoJ, also called clusterin, is a ubiquitous multifunctional glycoprotein co-localizing with fibrillar deposits in systemic/localized amyloid disorders [143]) partially inhibited Aβ aggregation and caused formation of "slowly-precipitating" Aβ42 complexes of >200 kDa [144]. Follow-up studies showed that ADDLs with the same biochemical and neurotoxic characteristics could be produced in clusterin-free solutions by incubating aggregate-free Aβ42 in phenol-red-free F-12 medium at 4–8°C for 24 h, in clusterin-free brain-slice culture media at 37°C for 24 h [26, 142], or even in phosphate-buffered saline [145].
By SEC, ADDL preparations contained two distinct peaks-an early-eluting high-mass component, which exhibited punctate binding to primary neurons, and a late-eluting low-mass component (13 kDa), which lacked this property [121]. These peaks are similar to those reported by Walsh et al. for protofibrillar and LMW preparations of Aβ, respectively (see below) [30]. However, Walsh et al. showed by TEM that the early-eluting peak contained abundant PF whereas the late-eluting peak was reported later to contain a mixture of monomer and small oligomers detected using PICUP [74]. In a recent study of ADDLs, when SEC coupled with MALLS and AU was used to determine the size distribution of ADDLs, the SEC peaks corresponding to 75 and 13 kDa showed oligomer masses ranging from 150–1,000 kDa, and a monomeric component of 4.5 kDa by MALLS [70]. This study suggested that previous reports identifying low-molecular-mass components as a composite of low-number oligomers were misrepresentations of what may actually be a monomeric Aβ42 fraction [70], ADDLs found in the early-eluting peak were shown to be in a dynamic equilibrium comprising a polydisperse population of oligomers [70]. Multiple parameters such as peptide concentration, temperature, pH, storage duration, and excipient addition were shown to affect this equilibrium dramatically [146].
Importantly, Aβ40 does not form ADDLs [70]. NMR studies have shown that the C-terminus of Aβ42 is more rigid compared to that of Aβ40 [60–62] potentially due to the extended hydrophobic C-terminus of Aβ42. This C-terminal difference and potentially the different monomeric Aβ42 conformers generated due to this structural difference may account for the increased toxicity and plaque competence of Aβ42 compared to Aβ40 [60, 61]. Indeed, some oligomeric moieties (ADDLs, paranuclei, and globulomers, see below) were shown to form by Aβ42 only. In fact, the exclusive Aβ42 derivation of ADDLs and paranuclei, and the fact that ADDLs and paranuclei are indistinguishable morphologically (compare [26, 121] with [33]), suggests that ADDLs and paranuclei may be related to each other or even be the same species obtained under different conditions [26, 33].
ADDLs have been shown to resist dissociation by low SDS concentrations (0.01%) [144]. However, when supramicellar SDS concentrations were used, ADDLs and fibrils migrated with the same electrophoretic profile yielding monomeric, trimeric and tetrameric moieties [70]. A similar profile was observed for LMW Aβ42 [33] produced by SEC or filtration [77]. At submicellar concentrations of SDS, oligomers were detected both by denaturing electrophoresis and SEC [70], When Aβ42 was electrophoresed in the presence of submicellar or supramicellar concentrations of SDS, high-molecular-mass aggregates and intermediate-sized assemblies formed, respectively [57]. During SDS-PAGE, these aggregates may partially dissociate as diffuse Aβ42 trimer/tetramer components [57] as observed for ADDLs [70], These observations re-emphasize that visualization of SDS-stable oligomeric Aβ may be misrepresenting the actual assembly state.
Biological Activity
Initial ApoJ-induced ADDL preparations (0.34 mg/mL) were neurotoxic [144], Later studies showed that ADDLs selectively targeted the principal neurons in the hippocampal strata pyramidale and granulosum in organotypic murine brain slices [26] and inhibited LTP in rat hippocampal brain slices [147, 148]. ADDLs also were shown to augment the negative synaptic plasticity of long-term depression (LTD) [149]. Prolonged maintenance of LTD along with LTP inhibition leads to an overall synapse-inhibitory effect (reviewed in [53]).
When cultured hippocampal neurons were incubated with synthetic ADDLs, F-12-prepared soluble brain extracts, or crude human CSF, and probed with ADDL-specific antibodies a punctate binding pattern reminiscent of synaptic termini was observed [150] (Fig. (2)). The antibodies used were shown to be 100-fold more sensitive to ADDLs (fmol levels) than to monomeric Aβ [27, 136, 150–153]. The ADDL-binding sites were demonstrated to coincide with dendritic spines at postsynaptic termini of excitatory synapses [150]. ADDL binding also overlapped with NMDAR subunit NR1 on highly arborized neurons positive for α calcium-calmodulin kinase II [150] which accumulates in postsynaptic termini of neurons involved in memory function [150]. In addition, ADDLs specifically bound to excitatory pyramidal, but not GABAergic, neurons [136], and to neurons positive for NMDAR subunits NR1 and NR2B [136]. Similarly, ADDLs did not bind astrocytes or inhibitory neurons expressing glutamic acid decarboxylase [136]. Preferential binding of ADDLs to excitatory synapses at postsynaptic sites was consistent with the inhibitory impact of ADDLs on NMDAR-dependent LTP [26, 149] and NMDAR-mediated phosphorylation of cAMP response-element binding-protein [154], demonstrating that ADDLs could impact crucial receptors involved in synaptic plasticity.
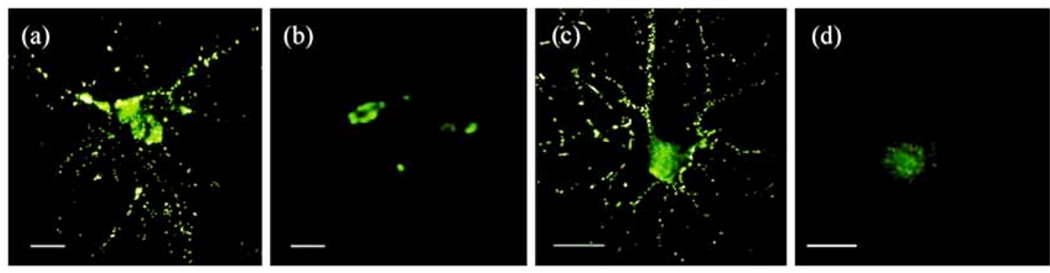
Fig. 2. Punctate ADDL-binding to neurons.
ADDLs isolated from AD brain or prepared in vitro show identical punctate binding to neuronal cell-surface proteins. Cultured hippocampal neurons were incubated with soluble extracts of human brain or synthetic ADDLs. Immunoreactivity against ADDLs was visualized by microscopy using M93 antibody. Soluble AD-brain proteins (a), soluble control-brain proteins (b), synthetic ADDLs (c), and synthetic ADDLs pre-treated (1 h) with oligomer-specific antibody M71 (d) are shown. Small puncta distributed along neurites, are evident for AD extracts and synthetic ADDLs, but not for control extracts or antibody-preadsorbed ADDLs (Scale bar = 10 µm). Adopted with permission from [27].
Defective neuronal actin-regulatory machinery is an underlying factor in dendritic and synaptic dysfunctions in many neurological disorders accompanied by cognitive deficits, including AD and Down syndrome (reviewed in [134]). ADDLs were shown to affect spine shape, which, like receptor expression is a facet of spine cell biology with ramifications for signaling and plasticity [155, 156]. ADDL-induced alterations of spine morphology resembled the morphology of immature and diseased spines associated with mental retardation and prionoses [157].
ADDL binding to neuritic spines was reported to induce expression of the activity-regulated cytoskeleton-associated protein (Arc), a synaptic immediate-early gene [150, 158]. Proper expression of Arc is essential for LTP but its ectopic and aberrant expression causes failure of long-term memory formation [159]. ADDLs generated a rapid and sustained increase in synaptic Arc protein expression [150], which interfered with long-term memory formation [159] and was hypothesized to lead to synapse failure and memory loss. De Felice et al. showed that ADDL treatment of mature primary hippocampal neurons led to reproducible and dose-dependent generation of reactive oxygen species (ROS) in the vicinity of synaptic ADDL-binding sites [160]. It was shown that ADDL binding to the NR1 subunit of NMDAR and NMDA-mediated Ca2+ influx led to ROS generation, further delineating the mechanisms of ADDL neurotoxicity [160]. Interestingly, ADDL treatment was shown to induce τ hyperphosphorylation in neuroblastoma cells and rat hippocampal primary neurons before neuronal death occurred [145]. Intrahippocampal injection of an oligomer-specific antibody was sufficient to reverse the effect of amyloid and τ pathologies, providing an additional insight into ADDL-mediated neurotoxicity [161].
Although in vitro experiments demonstrated that ADDLs interfered specifically with memory-associated experimental phenomena, a crucial question is whether ADDLs exist, and cause the same toxic effects, in vivo. Conformation-specific polyclonal [152] and monoclonal antibodies [162] shown to discriminate between ADDLs and Aβ monomers have been used to address this question. Dot-blot assays have detected ADDL immunoreactivity in transgenic mice and in AD brains which were extracted without detergents or harsh chemicals precluding extraction-induced alterations of the assembly structures [27, 153]. ADDL concentrations were 70-fold higher in AD brains compared to controls [27]. In the nontransgenic mouse brain, no ADDLs (detection limit <10 fmol/µg) were detected by dot-blotting brain extracts using an ADDL-specific antibody [153]. However, in brains of transgenic mice, ADDL concentrations varied from ~20–250 fmol/µg depending on the brain region tested [153]. Soluble brain proteins extracted in F-12 culture medium by ultracentrifugation contained ADDL immunoreactivity that correlated with presence of AD [27, 150] (reviewed in [53]). These findings support the hypothesis that ADDLs may be an important component in the amyloid cascade, as opposed to the poor correlation between insoluble amyloid deposits and cognitive impairment [53].
PROTOFIBRILS
Structural Characterization
PF are Aβ40- and Aβ42-derived curvilinear, soluble assemblies, which were described originally by Walsh et al. [30] and Harper et al. [28]. Walsh et al. reported studies using SEC, DLS, and TEM examining initial stages of Aβ oligomerization and characterizing Aβ intermediates during fibrillogenesis. By SEC, PF had apparent mass >100 kDa. They predominantly comprised curved, fibril-like structures of 6–8 nm in diameter and ≤200 nm long as observed by TEM [30]. Harper et al. detected the same protofibrillar assemblies of Aβ during polymerization by AFM [28]. They noted that Aβ40 PF, which appeared during the first week of incubation, had diameters of 3.1 nm and were 20–70 nm long [28]. The PF showed a periodic diametrical variation every 20 nm. In contrast, Aβ42 PF formed within the first day of incubation and had larger diameters (4.2 nm) than those of Aβ40. Aβ42 PF elongated overtime with diametrical periodicity similar to Aβ40 [28].
TEM examination of Aβ PF with rotary shadowing [31] demonstrated flexible rods up to ~200 nm long, with larger diameters than those observed by regular TEM or AFM. PF appeared more beaded with a periodicity of 3–6 nm, and the proportion of small PF (<10 nm) was higher, suggesting that these smaller structures might have been overlooked using TEM with routine negative staining or that the preparations were simply different [31]. The beaded structures were shown to be typical of early PF, whereas at later time points, PF appeared smoother [163]. High-resolution AFM studies have demonstrated that Aβ40 PF had a diameter of ~4.3 nm, with periodicity of ~20 nm, and coexisted with spherical species of the same diameter [112]. Spheres similar to those have been hypothesized by us and others to be precursors which join together to form PF [33, 110].
PF have a high β-sheet content, a characteristic similar to that of mature amyloid fibrils [31] and are recognized by a conformation-sensitive antibody, WO1, which reacts with the fibrillar form of various amyloid proteins [164]. Thus, PF are the latest precursors on the pathway of fibril formation described to date. Nevertheless, apparently a substantial conformational rearrangement occurs upon maturation of PF into fibrils as evidenced by the observations that PF can be readily disassembled into LMW Aβ, whereas mature fibrils do not typically disaggregate back into PF [31]. Proline substitution experiments showed that Aβ40 PF were "less structured" in the region Glu22–Gly29 compared to mature fibrils [164]. Hydrogen–deuterium exchange data demonstrated that the C-terminal Met35–Val40 and the N-terminal Asp1–Phe19 regions of Aβ40 were highly exposed to solvent both in fibrils and PF. In contrast, the Phe20–Leu34 segment was highly protected from hydrogen–deuterium exchange in fibrils but much less so in PF [67]. The β-structure (β-sheet and β-turn) content of PF was similar to that of fibrils as assessed by CD studies [30]. Collectively, these data suggested that the β-sheet elements comprising the amyloid fibrils were already present in PF. These elements could be expanded into adjacent residues and other elements, such as lateral association of filaments may contribute to the maturation of amyloid fibrils.
Biological Activity
Initial studies to assess the biological activity of PF were performed in cultured primary rat cortical neurons over a time scale of minutes to hours, presumably before PF convert to fibrils [31]. In these experiments, compromise of cell viability by SEC-isolated PF was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay [165]. It was found that PF and fibrils altered the normal physiology of cultured neurons, whereas LMW Aβ did not [31], To explore and compare the toxic effects of LMW Aβ, PF and fibrils further, Hartley et al. used rat cerebral primary mixed cultures (containing neurons, astroglia and microglia) and showed that PF caused neuronal injury and altered electrophysiological activities, ultimately causing cell death [29]. Although LMW Aβ caused a rapid but transient increase in excitatory post-synaptic currents (EPSCs), PF or fibrils (~3 µM) invariably produced rapid and sustained increases in electrical activity, six-fold greater than that induced by LMW Aβ [29]. Similarly, PF and fibrils significantly increased the frequency of action potentials and augmented the frequency and size of membrane depolarization compared to LMW Aβ preparation [29]. Substantial neuronal loss (80% as opposed to 10% non-treated cells) was observed consistently using the lactate dehydrogenase assay [166] and immunostaining against neuron-specific, microtubule-associated protein-2, after 5 days exposure to LMW and protofibrillar Aβ [29]. It was hypothesized that LMW Aβ could either convert to PF that cause the neurotoxicity or it could induce neurotoxicity by a mechanism independent of PF [29].
Important insight into the clinical relevance of PF came from investigation of a family in Northern Sweden, members of which carry a mutation in the app gene that leads to a single amino acid substitution in the Aβ region, E22G (dubbed the Arctic mutation) [167]. Surprisingly, even though carriers of this mutation have decreased plasma levels of Aβ40 and Aβ42 compared to non-carriers, they develop early-onset AD [167]. In vitro fibrillization studies showed that Aβ containing the E22G substitution formed PF faster and in larger quantities than wild-type Aβ, suggesting that PF may be the main disease-causing agents in carriers of the Arctic mutation [167]. These findings, along with the observations of PF formation by most other amyloidogenic proteins, have positioned PF as a likely primary pathogenic assembly state and an important target for drug development efforts for amyloidosis [168].
Concentrations of 4-hydroxy-2-nonenal (HNE), a metabolite of oxidative stress resulting from fatty-acid peroxidation, and of HNE-derived lipid peroxidation products, have been shown to increase in AD [169]. Recently, HNE has been shown to modify Aβ by 1,4 conjugation to primary amino groups and Schiff base formation, which could result in putative covalent intermolecular cross-linking of Aβ monomers [170]. Aβ40 prepared after a 285-h incubation with HNE predominantly had a PF-like, curved morphology when examined by AFM, whereas preparations formed in the absence of HNE comprised straight fibrils predominantly [170]. Both the long, straight fibrils formed in the absence of HNE and the curved fibrillar aggregates formed in the presence of HNE were rich in β-sheet structure, based on their CD spectra. In the presence of HNE, accelerated formation of protofibrillar Aβ40 species was observed, whereas generation of mature straight fibrils even upon extended incubations was inhibited [170]. These data suggested that HNE could cause accumulation of toxic Aβ PF by preventing their maturation to the less toxic fibrils [169]. Similarly, docosahexaenoic acid was shown to stabilize soluble Aβ42 PF and hinder their conversion to insoluble fibrils, thus leading to a sustained Aβ-induced neurotoxicity measured using cultured PC12 cells [171]. These observations suggest that toxic effects of PF could be promoted by molecular interactions that prevented downstream fibril formation.
Aβ*56
Lesné and colleagues have identified an oligomeric Aβ species, termed Aβ*56, that caused cognitive deficits in middle-aged transgenic Tg(APPSWE)2576Kahs (Tg2576) mice [32]. The Tg2576 mice express high levels of a human APP variant, which carries a familial AD-linked double mutation originating in a Swedish lineage (K670N, M671L) [172, 173]. Aβ concentrations in these mice increase rapidly at 6 months of age, and abundant amyloid plaques are apparent between 9–12 months [172]. Tg2576 mice recapitulate many other neurological features of AD, including neuroinflammation and oxidative stresses, dystrophic neurites, and significant cognitive deficits (reviewed in [172]). However, other important features, such as neurofibrillary tangles and significant neuronal loss are not found in this model [174].
Because in these and other APP transgenic mice administration of Aβ-specific antibodies has been shown to rapidly ameliorate memory decline, and because memory deficits were thought to precede plaques, Lesné et al. reasoned that a particular Aβ species could promote cognitive decline preceding plaque maturation [32]. They found that insoluble Aβ accumulated over 7 months without noticeable spatial memory decline. In contrast, certain soluble and extracellular Aβ species, which migrated as LMW bands in SDS-PAGE/Western blots, correlated strongly with memory deficits at 6 months of age, suggesting that these oligomers, particularly those with apparent gel mobility of Aβ dodecamers, were important neurotoxins in this model. Based on these data, it was proposed that similar species could be causing AD [32]. In support of this hypothesis, when pathophysiologically relevant concentrations of Aβ*56 (8.5 pmol) were administered into the lateral cerebral ventricles of healthy young rats pre-trained in the Morris water maze, the rats developed defective long-term spatial memory. These are important findings supporting the central role of oligomeric Aβ in the disease mechanism of AD.
The structural characterization of Aβ*56 as a putative dodecamer should be interpreted cautiously because the apparent electrophoretic migration of this oligomer, corresponding to 56 kDa, may not represent accurately its in vivo mass given the artifactual effects of SDS [57], which was included in the initial steps of the extraction protocol. Of note, Jacobsen et al. have found that cognitive deficits in Tg2756 mice occurred before the reported time of appearance of Aβ*56 [175].
Recently, longitudinal water-maze spatial training was reported to reduce Aβ and τ neuropathology transiently but significantly, and improve later learning performance in a triple transgenic (3×Tg) murine model of AD [176]. The 3×Tg-AD mice harbor human APP containing the Swedish mutations (KM670/671NL), human τ containing the AD-associated mutation, P301L, and a human presenilin 1 gene (PSEN1) containing the AD-linked mutation, PS1M146V [176]. The improvement in performance in 3×Tg-AD mice occurred at 6–12 months and depended strongly on spatial training [176]. To achieve this effect, pre-training was required before development of overt neuropathology, presumably because it delayed Aβ redistribution to extracellular plaques and reduced the concentration of Aβ oligomers, including one with an apparent SDS-PAGE mobility similar to Aβ*56 [176]. These findings suggest that Aβ*56 is a neurotoxic form of Aβ that may be important in the etiology of AD. Currently, the structural relationships of this species to PF and ADDLs are unknown, although under certain conditions, ADDLs also display electrophoretic migration corresponding to a dodecamer [27].
Aβ PORES
Aβ pores are channel-like structures believed to disrupt cell membranes and cellular ionic homeostasis [177]. In lipid bilayers in vitro, Aβ was shown to form uniform pore-like structures with 8–12 nm outer and 2 nm inner diameters [177, 178]. These are thought to serve as Ca2+ channels and thus have been hypothesized to cause excitotoxicity and mediate Aβ-induced neurotoxicity in AD [179, 180]. Reports of various models including artificial phospholipid membrane bilayers, excised neuronal membrane patches, whole-cell patch-clamp experiments, and phospholipid vesicles support a channel-forming property of Aβ [177, 180–192]. In these studies, imaging techniques [177, 184, 186, 187], electro-physiological experiments [180, 181, 183–185, 187, 188, 191, 192] or cation-sensitive dyes [187] were used to assess channel-like properties of Aβ. However, other studies have reported general disruption of the plasma membrane homeostasis without channel formation [193–195]. In a study by Kayed et al. the effect of spherical Aβ42 oligomers on membrane conductivity was assessed using planar lipid bilayers [195]. It was found that these Aβ42 oligomers specifically increased the conductance of the bilayer in a concentration-dependent manner whereas no increase in conductance was observed for LMW Aβ species (monomer or dimer) or for Aβ fibrils [195]. The increase in membrane conductance in response to spherical oligomers occurred in the absence of evidence for discrete ion-channel or pore formation [195]. It was postulated that soluble oligomers enhanced movement of ions through the lipid bilayer by a channel-independent mechanism [195]. High sensitivity recording has indicated that there was little change in the noise level of the current trace as the current increased from 0 to ~100 pA after oligomer addition.
Aβ42 was reported to be more prone to forming channels than Aβ40 [18]. High-resolution examination of individual Aβ42 channel-like structures revealed two subunit arrangements: rectangular and hexagonal structures, putatively comprising tetramers and hexamers, respectively [177]. The disease-associated mutant E22G form of Aβ40 was shown to form pore-like structures akin to those formed by Aβ42 [37, 196]. Treatment of the hypothalamic neuronal cells GT1-7 with Aβ40 has led to simultaneous formation of Ca2+ channels and increased intracellular Ca2+ concentration ([Ca2+]i) as determined by fluorometric measurements, suggesting that Aβ40 also could disrupt biological and artificial membranes, possibly via formation of pores [182, 197].
PARANUCLEI
Using PICUP followed by SDS-PAGE analysis, Aβ40 and Aβ42 were shown to form distinct oligomer size distributions, suggesting that the two Aβ alloforms oligomerized through distinct pathways [33]. In those experiments, Aβ42 preferentially formed pentamer/hexamer units termed paranuclei, which self-associated into larger assemblies, including dodecamers and octadecamers [33]. In contrast, Aβ40 formed a roughly equimolar, quasi-equilibrium mixture of monomers, dimers, trimers, and tetramers [74].
A systematic study using PICUP assessed oligomerization of 34 Aβ alloforms [35], including those containing familial AD-linked amino acid substitutions, N-terminal truncations found in AD plaques, and modifications that altered the charge, hydrophobicity, or conformation of Aβ [35]. C-terminal length was found to be the most important structural determinant in early oligomerization, and the side-chain of Ile41 in Aβ42 was found to be important both for effective formation of paranuclei and for their self-association [35]. Thus, Aβ41 and longer alloforms formed abundant paranuclei whereas Aβ40 and shorter alloforms did not [33]. The side-chain of Ala42, and the C-terminal carboxyl group, affected paranucleus self-association [35]. In a related study, oxidation of Met35 in Aβ42 was found to preclude paranucleus formation and led to generation of oligomers indistinguishable from those produced by Aβ40 [34]. These data demonstrated that modification of even a single atom could induce dramatic effects on Aβ paranucleus formation and downstream assembly, providing important insights into mechanisms of Aβ assembly into neurotoxic oligomers potentially relevant to AD pathogenesis. As discussed above, the difference in toxicity between Aβ40 and Aβ42 [198] correlates with observations that certain oligomeric Aβ forms, such as paranuclei and ADDLs, are produced by Aβ42 only, emphasizing strong correlation of the latter to the pathogenic process of AD.
βAMY BALLS
βamy balls are Aβ40-derived structures that form spontaneously when high concentrations of Aβ40 (60–600 µM) are incubated in phosphate-buffered saline at 30°C for 8–13 days [39]. βamy balls, have diameters of 20–200 µm and were shown to be composed of birefringent 6–10-nm diameter Aβ fibrils with random orientation [39]. Although such high Aβ concentrations used to generate βamy balls are unlikely to occur in vivo [22, 199], it was argued that these concentrations could possibly occur locally at microfoci circumscribing the amyloid plaques in AD brain [39]. Interestingly, in vivo extracellular retinal deposits called drusen have an apparent similarity to βamy balls. Drusen are Aβ-containing macromolecular assemblies and are a pathologic sign in age-related macular degeneration [200]. However, these structures have larger diameters and unlike βamy balls, which are produced from synthetic Aβ40 in vitro in the absence of other proteins, the retinal deposits contain other Aβ-binding proteins [200].
AMYLOSPHEROIDS
Amylospheroids, were described as Aβ40- and Aβ42-derived assemblies with 10–15 nm diameters [36]. Aβ40 amylospheroids formed by incubating 350 µM of the peptide in phosphate-buffered saline under slow rotation for 5–7 days at 37°C [36]. Aβ42 amylospheroids were produced by incubating 0.01–1 µM peptide in the same buffer for 8–10 h at 4°C [36]. Indeed, it was observed that Aβ42 amylospheroids, which formed faster and at substantially lower concentration than those of Aβ40, were also more toxic than Aβ40 amylospheroids [36], correlating with the higher toxicity and pathogenicity of Aβ42 in AD compared to Aβ40. Although these Aβ42 assemblies are spherical [36], they are morphologically distinct from ADDLs in their diameter (2.5 nm by AFM [70] vs. 10 nm by TEM, respectively).
GLOBULOMERS
Globulomers are Aβ42 oligomers produced by incubating 400 µM Aβ42 in phosphate-buffered saline in the presence of 0.2% SDS at 37°C for 6 h [201]. These species also have been produced by incubation of Aβ42 with lauric, oleic, or arachidonic acids [201], suggesting that they are generated by interaction of Aβ42 with micelles of SDS or fatty acids. Aβ42 globulomers were neurotoxic to rat brain slices and antibodies generated against globulomers detected immunoreactive epitopes in tissue sections [201]. Similar globular structures of Aβ40 were reported to form after 18 h incubation in 25 mM 2-morpholinoethanesulfonic acid buffer (pH 4.5) in a "hanging-drop" environment [202]. Hanging-drop environment is used extensively for protein crystallization and provides a static, low-convection environment with a markedly increased hydrophobic air-buffer interfacial area compared to that of the microfuge-tube environment [202].
OLIGOMERS OF DISEASE-RELATED AMYLOIDOGENIC PROTEINS OTHER THAN Aβ
Over twenty human amyloidoses are caused by aberrant protein folding and aggregation [203–205]. As Aβ often is considered an archetypal protein in studies of these diseases, the discoveries of toxic pre-fibrillar Aβ assemblies and their centrality in AD have led to a search for similar assemblies of other amyloidosis-related proteins. To date, at least 24 different proteins have been identified as causative agents of amyloidoses [206], In essentially all cases, such assemblies have been found and had adverse biological effects similar to those of Aβ oligomers [5, 7, 11, 207]. Because this review focuses on pre-fibrillar assemblies of Aβ, we do not intend to cover assemblies of other amyloidogenic proteins in detail but rather to highlight a few examples and discuss current features that are common to all or most of these assemblies.
The structures reported for amyloidogenic protein oligomers, in general have been similar to those described for Aβ, namely PF, annular (pore-like) PF, and spherical oligomers. In several cases, annular PF have been the predominant structures found. However, as discussed elsewhere [57], it is important to note that in many cases the term PF has been used even though the morphologies of the assemblies under study were distinct from those originally defined as PF.
One of the most studied amyloidogenic proteins is α-synuclein, the function of which is not clear although it is believed to be part of the ubiquitin system that marks proteins for proteasomal degradation [208, 209], α-synuclein is the predominant component in Lewy bodies, the pathological hallmarks in Parkinson's disease brain, and has been implicated in other degenerative disorders (synucleinopathies), including dementia with Lewy bodies and multiple system atrophy [210]. Similar to Aβ, α-synuclein belongs to a growing family of "intrinsically unstructured" proteins [211, 212], a characteristic that perhaps renders these proteins more prone to undergoing amyloidogenic assembly. Mutant α-synuclein alloforms linked to familial Parkinson's disease were found to oligomerize faster than the wild-type protein, whereas the rate of fibril formation did not correlate with the presence of disease-causing mutations [213]. Pre-fibrillar assemblies of both wild-type and mutant α-synuclein included spherical oligomers, protofibrillar structures, and most abundantly, annular PF [196, 214]. The latter morphology suggested that the mechanism by which α-synuclein induces toxicity is pore formation in cell membranes. In agreement with this idea, protofibrillar α-synuclein was found to permeabilize synthetic vesicles [215]. Interestingly, this effect was increased by the familial PD-linked mutants A30P and A53T [216] but not by the mutant E46K [217]. Thus, although pore formation may be involved in α-synuclein-induced toxicity, other mechanisms also have been implicated, but these are not well understood [218].
Two amyloidogenic proteins involved in sugar metabolism are insulin and islet amyloid polypeptide (IAPP, also called amylin). Insulin aggregation is not associated with disease but has been studied by multiple groups as a convenient in vitro model [219–222]. Biophysical investigation of insulin fibrillogenesis has identified oligomeric populations with conformations distinct from those of natively folded insulin dimer and hexamer [223]. Taking advantage of the relative stability of insulin oligomers and using special instrumentation, Robinson and co-workers have provided one of the first examples of mass spectrometric investigation of amyloidogenic protein oligomers, and demonstrated the power of this experimental approach for studying the effects of pH and metal ion binding on oligomerization [224]. In a recent study combining structural characterization and cytotoxicity experiments, Grudzielanek et al. found no toxicity for low-order insulin oligomers whereas substantial toxicity was measured for high-order, β-sheet-rich aggregates that displayed either fibrillar or amorphous morphology [225].
In contrast to insulin, IAPP aggregation is believed to be causative in T2D. IAPP is a 37-residue peptide hormone produced in pancreatic β-cells and co-secreted with insulin. Early stages of T2D are characterized by insulin resistance followed by increased insulin and IAPP secretion. Elevated IAPP concentrations lead to its assembly into toxic oligomers and insoluble aggregates [14]. Oligomeric and protofibrillar IAPP were shown to interact with synthetic membranes [226], a characteristic that decreases with further aggregation, providing a clue for the mechanism of IAPP toxicity [227]. The interaction with biological membranes may induce a transient α-helical conformation in IAPP, presumably facilitating penetration of the oligomers into the membrane resulting in solute leakage across the membrane [228, 229]. Strong evidence for the neurotoxic role of IAPP oligomers in T2D was given in a study in which rifampicin, an inhibitor of IAPP fibril, but not oligomer formation, did not protect pancreatic β-cells against apoptosis induced by either endogenously expressed or externally applied IAPP [230]. More recent data have suggested that in vivo, toxic IAPP oligomers are formed intracellularly and therefore, oligomer-specific antibodies do not prevent cell death in vitro and in vivo [231].
Numerous other examples have demonstrated the important role of oligomers of amyloidogenic proteins as disease-causing agents. Before the focus in the amyloid field shifted from fibrils to oligomers, it had been known that although no sequence similarity was found among amyloidogenic proteins, the fibril structures of all were highly similar, characterized by fibrillar morphology with periodic helical twist and cross-β structures [232, 233]. The realization that the precursor oligomers may be the proximate disease-causing agents in the amyloidoses related to these proteins raised the question whether oligomer structures also were similar. High-resolution microscopic studies of oligomeric structures, mostly by TEM and AFM, have demonstrated that in most cases the morphologies observed were spherical, annular, or protofibrillar (worm-like). Conformational studies of oligomers have been difficult because the oligomers typically are metastable and exist in mixtures. Structural insight has been offered by Glabe and co-workers who developed antibodies that showed specificity for oligomers of proteins with unrelated sequences but did not bind the monomeric or fibrillar forms of these proteins [234]. The first polyclonal antibody, A11, and similar antibodies developed in follow-up studies showed remarkable ability to bind to oligomers formed by proteins as diverse as Aβ, α-synulein, IAPP, lysozyme, insulin, poly Q, and prion fragments [234]. These observations strongly suggested that a predominant mechanism by which these oligomers injure cells is through permeabilization of the plasma membrane because toxic oligomers sharing a common structure formed by both intracellular and extracellular proteins [194, 195]. As discussed above, oligomers may interact with membranes by several mechanisms, including pore-formation and shallow penetration under the surface resulting in thinning of the membrane and a net increase in its permeability. Further delineation of the specific mechanisms governing these interactions requires additional studies and will be highly important for designing reagents that block them.
PRE-FIBRILLAR ASSEMBLIES OF DISEASE-UNRELATED PROTEINS
In his 1972 Nobel-Prize acceptance speech, Anfinsen stated that "the native conformation [of proteins] is determined by the totality of inter-atomic interactions and hence by the amino acid sequence, in a given environment" which does not always favor the normally functional and folded state of proteins. Consistent with Anfinsen's theory, the conformational-change hypothesis postulates that one of 17 normally soluble and functional human proteins could undergo structural alterations under partially denaturing conditions leading to self-assembly and amyloid fibril formation [235]. Besides disease-associated amyloid-forming proteins, and proteins that naturally form non-pathological, functional amyloid-like fibrils (reviewed in [236]), disease-unrelated proteins [237] and artificially designed peptides [238–240] have been found to form amyloid under particular non-native conditions. To the best of our knowledge, the ability of disease-unrelated peptides and proteins to form amyloid fibrils was first reported by Guijarro et al. [241] and Litvinovich et al. [242]. The src-homology 3 (SH3) domain of bovine phosphatidyl inositol 3-kinase (PI3K), an 85-residue, β-structured protein, was shown to aggregate slowly and form amyloid fibrils under acidic pH [241]. Thenceforth, the disease-unrelated SH3 domain has served as an excellent model for systematic studies examining structural properties of amyloid fibrils and molecular mechanisms of amyloid formation [243–246]. The PI3K-SH3 was shown to adopt a compact denatured state under acidic conditions before formation of amyloid fibrils [247]. Limited proteolysis studies showed that PI3K-SH3 at low pH had a partially folded conformation [247] and progressively displayed enhanced susceptibility to proteolysis, suggesting that the protein became more unfolded in the early stages of aggregation [247]. In contrast, the amyloid fibrils that formed over longer periods of time were resistant to proteolysis [247]. It was suggested that the protein aggregates formed initially were relatively dynamic species and this flexibility allowed dynamic species and this flexibility allowed for the particular interactions leading to formation of the highly ordered fibrils [247].
After Litvinovich et al. demonstrated formation of amyloid-like fibrils by self-association of murine fibronectin type III module [242], others reported that similar conversions in a number of disease-unrelated proteins could be induced in vitro by a deliberate, rational choice of excipient conditions [248, 249], Examples (reviewed in [237]) include human apolipoprotein CII, ADA2H, amphoterin, stefin B and endostatin, murine VI domain, equine acylphosphatase (AcP) and apomyoglobin, monellin (Dioscoreophyllum camminsii), and yeast phosphoglycerate kinase. Fezoui et al. reported de novo design of a monomeric α-helix-turn-α-helix peptide (αtα) which converted to β-sheet-rich amyloid-type, protease-resistant, 6–10-nm fibrils at 37°C in a neutral aqueous buffer [238]. Formation of fibrils from full-length proteins requires solution conditions that partially or completely disrupt the native structure of the protein but not completely disturb hydrogen bonds [248]. It was observed that proteins with as few as four residues, and amino-acid homopolymers that are unable to fold into stable globular structures, form fibrils readily [237, 250, 251]. Therefore, it has been suggested that the ability to form amyloid fibrils could be a generic property of polypeptide chains [237].
In one study of pre-fibrillar assemblies of disease-unrelated proteins, tapping-mode AFM was used to follow the process of HypF-N aggregation which was induced by incubating the protein in the presence of trifluoroethanol [252], HypF-N was shown to aggregate hierarchically through a number of distinct steps with morphologically different intermediates [252]. Initially, globular assemblies appeared, which subsequently self-assembled into beaded chains, similar to those found for amyloidogenic proteins [253–255]. Subsequently, these organized into crescents, large annular and ribbon-like structures (Fig. (3)), and eventually assembled into mature fibrils of different sizes [252]. The globule height was measured to be 2.8–3.0 nm [252]. Although HypF-N and AcP are similarly prone to conversion from a predominantly α-helical conformation to one rich in β-sheet, HypF-N aggregation rate was found to be dramatically higher (~1,000-fold) than AcP, possibly due to the higher hydrophobicity and lower net charge of HypF-N compared to AcP [256].
Fig. 3. Hierarchical aggregation process of HypF-N.
(a) Tapping AFM images taken under liquid (height data, scan size 670 nm, Z range 20 nm) of HypF-N globular aggregates observed a few hours after the onset of the aggregation process in the presence of 30% trifluoroethanol (TFE). Scale bar = 100 nm. Inset, STM image (height data, scan size 42 nm, Z range 15 nm) of globular aggregates obtained under the same conditions, but diluted 1:50 prior to deposition onto the substrate; the globules are apparently asymmetric and tend to form a defined mutual orientation. Scale bar = 10 nm. (b) HypF-N crescents and a ring observed after 3 days of incubation in 30% TFE (scan size 5.3 µm, Z range 45 nm). Inset, high resolution image of a ring (scan sizel.9 µm, Z range 120 nm), revealing its globular components, taken after 5 days of incubation in 30% TFE. Scale bars = 400 nm. (c) Tapping-mode AFM image taken in air (height data) after three days of incubation in 30% TFE showing the co-existence of annular structures with thin and wide ribbon-like fibrils. Scan size 4.8 µm, Z range 40 nm. Scale = 500 nm. (d) Tapping-mode AFM images taken in air (height data) of mature fibrils obtained in 30% TFE showing supercoiled fibrils after eight days (scan size 3.6 µm, Z range 80 nm). Adopted with permission from [252].
In contrast to fibrils of disease-causing amyloidogenic proteins, those formed by disease-unrelated proteins did not cause cytotoxicity in cell-culture experiments. For example, fibrils formed by the aforementioned αtα peptide displayed no neurotoxicity, even though they were morphologically indistinguishable from Aβ and IAPP fibrils, which were toxic [238]. It was therefore unexpected that the pre-fibrillar assemblies of PI3K-SH3 and HypF-N were shown to be highly toxic to PC12 cells and murine fibroblasts in vitro [257]. The extent of cellular injury caused by the cytotoxic oligomers was comparable to that of Aβ42 oligomers, whereas the corresponding fibrils of both PI3K-SH3 and HypF-N were benign.
Early pre-fibrillar HypF-N assemblies were shown to permeabilize artificial phospholipid membranes more efficiently than mature fibrils, indicating that this disease-unrelated protein displayed the same toxic properties as pre-fibrillar assemblies of pathological peptides and proteins [252]. Further investigation of the cellular effects of HypF-N oligomers revealed that they entered the cytoplasm and caused an acute rise in ROS levels and [Ca2+]i, leading to cell death [258]. In a study where murine fibroblasts and endothelial cells were treated with pre-fibrillar HypF-N assemblies, the two cell types underwent two different death mechanisms—fibroblasts exposed for 24 h to 10 µM HypF-N oligomers underwent necrosis, whereas endothelial cells treated similarly sustained apoptosis [259]. A similar study comparing cytotoxic effects of pre-fibrillar and fibrillar HypF-N assemblies using a panel of normal and pathological cell-lines showed that cells were variably affected by the same amount of pre-fibrillar aggregates, whereas mature fibrils showed little or no toxicity [260]. This difference in the extent of compromise of cell viability was significantly related to the cell-membrane cholesterol content and to different cellular Ca2+-buffering and antioxidant capacities of the various cell types [260]. Recently, it has been shown that microinjection into rat brain nucleus basalis magnocellularis of PI3K-SH3 or HypF-N assemblies, but not the corresponding mature fibrils, compromised neuronal viability dose-dependently [8]. Taken together, these data clearly demonstrate that the pre-fibrillar assemblies of disease-unrelated proteins are highly toxic whereas the corresponding mature fibrils are not [8]. The toxic effect of the oligomers may arise when these assemblies assume a "misfolded" conformation which may expose hydrophobic residues that are natively entombed within the core structure. Such aggregation-prone regions may interact with membranes and other cellular components modifying their structural/functional homeostasis.
Dobson and co-workers have proposed that evolutionary mechanisms may have been in force to ensure propagation of proteins that resist aggregation for efficient function [261]. However, genetic, environmental and metabolic factors that decrease the solubility or increase the concentration of susceptible proteins in vivo may act against those forces and induce protein misfolding disorders including neurodegenerative diseases [261].
The fact that aggregates of some disease-unrelated proteins could function similarly to those formed by amyloidogenic, disease-related peptides and proteins, has profound implications for understanding the mechanistic fundamentals of abnormal protein deposition in amyloidoses. These observations facilitate investigation and discovery of the general mechanistic features underlying protein misfolding and aggregation [237] and help defining likely targets for drug design.
CONCLUSION
Since the amyloid-cascade hypothesis was formulated [1], intensive research has led to an exponential accumulation of data elucidating the pathogenic mechanisms of AD. In the end of August, 2007, PubMed database searches, using the queries "Alzheimer's disease" or "amyloid" returned 53,767 and 32,576 hits, respectively. Importantly, as our understanding of the devastating morbus Alzheimer, and other neurodegenerative and protein misfolding diseases has been growing, an alternative, encompassing paradigm has emerged. This paradigm postulates that pre-fibrillar protein assemblies rather than mature amyloidogenic fibrils likely are the key neurotoxins responsible for most of the pathogenic mechanisms in protein-misfolding and neurodegenerative diseases, including AD. Accordingly, oligomeric species with different degrees of structural order are thought to mediate various pathogenic mechanisms that may lead to cytotoxicity and cell loss eventuating in organic and systemic morbidity. With the progressive use of classical techniques, and the advent of novel, sophisticated methodologies, several different forms of pre-fibrillar assemblies of Aβ and those of other amyloidogenic proteins have been described. This has led to considerable progress in the elucidation of structural and functional features and fundamentals of the assembly of these proteins at the molecular level. Overall, it is postulated that the pre-fibrillar amyloidogenic proteins are on path to fibrillogenesis. The resulting protein fibrils are thought to be the end-stage sinks for the toxic pre-fibrillar species. Fibrillar assemblies accumulate progressively into intra- and/or extracellular proteinaceous amyloid aggregates generating the disease-specific lesions in vivo.
In AD research, various forms of Aβ pre-fibrillar assemblies, including activated monomeric conformers, ADDLs, PF, cell-derived dimers and trimers, and annular assemblies have been described (Table 1). In many cases, the structural and functional interrelationships amongst these assemblies are still elusive. Nevertheless, some have been shown to be pathogenic through one or several common pathways, suggesting that they may share structural features and possibly mechanisms of action. Understanding these intricate structure-function correlations will decipher a complex and interconnected array of pathogenic mechanisms.
Global research efforts have established a framework for understanding the fundamentals of Aβ assembly [7, 10, 11]. A remaining challenge is to assess how these fundamental structural principles are linked to cellular and tissular microenvironments during progression of AD. Many experimental conditions have been used to study the structure and function of pre-fibrillar assemblies, but it is difficult to regenerate and scrutinize the actual in vivo milieus and conditions in which protein assembly, oligomerization, fibrillization, and deposition occur. Similarly, it is extremely difficult to assess all the possible interactions these assemblies may have with various cellular components and organelles. A multitude of detrimental mechanisms, including disruption of cellular metabolism, synapse structure and function deregulation, membrane damage, ionic imbalance, oxidative/inflammatory stress, apoptotic, and other cytotoxic effects, have been shown to be mediated by pre-fibrillar Aβ assemblies, emphasizing that a single therapeutic approach likely will be insufficient to prevent or treat the progression of AD. By inference, this likely is true also for other amyloidoses. The intricacy of the pathogenic mechanisms in these diseases calls for rational diagnostic and therapeutic approaches that would target not only a single assembly or a single mechanism but a multitude of assemblies and mechanisms. It is important to design and discover therapeutic agents that could target various assemblies that potentially become active early or are continuously active throughout the course of disease. Successful targeting of pre-fibrillar assemblies in vitro and in animal models could have crucial diagnostic and prognostic implications for amyloid-related diseases.
ACKNOWLEDGEMENTS
We acknowledge Mr. Sean M. Spring for conducting the experiment described in Fig. (1). This work was supported by grants AG027818 from NIH/NIA and 20052E from the Larry L. Hillblom Foundation, and by a generous gift from the Turken family.
ABBREVIATIONS
- αtα
α-helix-turn-α-helix
- AcP
Acylphosphatase
- AD
Alzheimer’s disease
- ADDLs
Aβ-derived diffusible ligands
- AFM
Atomic-force microscopy
- apoJ
Apolipoprotein J
- APP
Amyloid β-protein precursor
- Arc
Activity-regulated cytoskeleton-associated protein
- AU
Analytical ultracentrifugation
- Aβ
Amyloid β-protein
- [Ca2+]i
Intracellular Ca2+ concentration
- CD
Circular dichroism
- CSF
Cerebrospinal fluid
- DLS
Dynamic light scattering
- EPSC
Excitatory post-synaptic current
- FTIR
Fourier-transform infrared spectroscopy
- HFIP
1,1,1,3,3,3-hexafluoro-2-propanol
- HNE
4-hydroxy-2-nonenal
- HPLC
High-performance liquid chromatography
- HypF-N
Hydrogenase maturation-factor
- IMS-MS
Ion-mobility spectrometry-mass spectrometry
- LMW
Low-molecular weight
- LTD
Long-term depression
- LTP
Long-term potentiation
- MALDI
Matrix-assisted laser-desorption ionization
- MALLS
Multi-angle laser light scattering
- MPL
Mass-per-length
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- nAchR
Nicotinic acetylcholine receptor
- NMDA
N-methyl-D-aspartate
- NMDAR(s)
NMDA receptor(s)
- PF
Protofibrils
- PI3K
Phosphatidyl inositol 3-kinase
- PICUP
Photo-induced cross-linking of unmodified proteins
- ROS
Reactive oxygen species
- SDS
Sodium dodecyl sulfate
- SEC
Size-exclusion chromatography
- SH3
Src-homology 3
- SPPS
Solid-phase peptide synthesis
- STEM
Scanning transmission electron microscopy
- STM
Scanning tunneling microscopy
- T2D
Type-2 diabetes mellitus
- TEM
Transmission electron microscopy
- TFE
Trifluoroethanol
REFERENCES
- 1.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 2.Terry RD, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, et al. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 3.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, et al. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein WL, Krafft GA, Finch CE. Targeting small Aβ oligomers: the solution to an Alzheimer's disease conundrum? Trends Neurosci. 2001;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- 5.Kirkitadze MD, Bitan G, Teplow DB. Paradigm shifts in Alzheimer's disease and other neurodegenerative disorders: the emerging role of oligomeric assemblies. J Neurosci Res. 2002;69:567–577. doi: 10.1002/jnr.10328. [DOI] [PubMed] [Google Scholar]
- 6.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 7.Caughey B, Lansbury PT. Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci. 2003;26:267–298. doi: 10.1146/annurev.neuro.26.010302.081142. [DOI] [PubMed] [Google Scholar]
- 8.Baglioni S, Casamenti F, Bucciantini M, Luheshi LM, Taddei N, Chiti F, et al. Prefibrillar amyloid aggregates could be generic toxins in higher organisms. J Neurosci. 2006;26:8160–8167. doi: 10.1523/JNEUROSCI.4809-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lansbury PT, Lashuel HA. A century-old debate on protein aggrgation and neurodegeneration enters the clinic. Nature. 2006;443:774–779. doi: 10.1038/nature05290. [DOI] [PubMed] [Google Scholar]
- 10.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira ST, Vieira MN, De Felice FG. Soluble protein oligomers as emerging toxins in alzheimer's and other amyloid diseases. IUBMB Life. 2007;59:332–345. doi: 10.1080/15216540701283882. [DOI] [PubMed] [Google Scholar]
- 12.Fleming Outeiro T, Tetzlaff J. Mechanisms of disease II: cellular protein quality control. Semin Pediatr Neurol. 2007;14:15–25. doi: 10.1016/j.spen.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Agorogiannis El, Agorogiannis GI, Papadimitriou A, Hadjigeorgiou GM. Protein misfolding in neurodegenerative diseases. Neuropathol Appl Neurobiol. 2004;30:215–224. doi: 10.1111/j.1365-2990.2004.00558.x. [DOI] [PubMed] [Google Scholar]
- 14.Marzban L, Park K, Verchere CB. Islet amyloid polypeptide and type 2 diabetes. Exp Gerontol. 2003;38:347–351. doi: 10.1016/s0531-5565(03)00004-4. [DOI] [PubMed] [Google Scholar]
- 15.Lachmann HJ, Hawkins PN. Systemic amyloidosis. Curr Opin Pharmacol. 2006;6:214–220. doi: 10.1016/j.coph.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Finder VH, Glockshuber R. Amyloid-β aggregation. Neurodegener Dis. 2007;4:13–27. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 17.Teplow DB. Structural and kinetic features of amyloid β-protein fibrillogenesis. Amyloid. 1998;5:121–142. doi: 10.3109/13506129808995290. [DOI] [PubMed] [Google Scholar]
- 18.Lashuel HA, Lansbury PT., Jr Are amyloid diseases caused by protein aggregates that mimic bacterial pore-forming toxins? Q Rev Biophy. 2006;39:167–201. doi: 10.1017/S0033583506004422. [DOI] [PubMed] [Google Scholar]
- 19.Taylor BM, Sarver RW, Fici G, Poorman RA, Lutzke BS, Molinari A, et al. Spontaneous aggregation and cytotoxicity of the β-amyloid Aβ1–40: a kinetic model. J Protein Chem. 2003;22:31–40. doi: 10.1023/a:1023063626770. [DOI] [PubMed] [Google Scholar]
- 20.Roher AE, Palmer KC, Yurewicz EC, Ball MJ, Greenberg BD. Morphological and biochemical analyses of amyloid plaque core proteins purified from Alzheimer disease brain tissue. J Neurochem. 1993;61:1916–1926. doi: 10.1111/j.1471-4159.1993.tb09834.x. [DOI] [PubMed] [Google Scholar]
- 21.Enya M, Morishima-Kawashima M, Yoshimura M, Shinkai Y, Kusui K, Khan K, et al. Appearance of sodium dodecyl sulfatestable amyloid β-protein (Aβ) dimer in the cortex during aging. Am J Pathol. 1999;154:271–279. doi: 10.1016/s0002-9440(10)65273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vigo-Pelfrey C, Lee D, Keim P, Lieberburg I, Schenk DB. Characterization of β-amyloid peptide from human cerebrospinal fluid. J Neurochem. 1993;61:1965–1968. doi: 10.1111/j.1471-4159.1993.tb09841.x. [DOI] [PubMed] [Google Scholar]
- 23.Podlisny MB, Ostaszewski BL, Squazzo SL, Koo EH, Rydell RE, Teplow DB, et al. Aggregation of secreted amyloid β-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995;270:9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- 24.Walsh DM, Tseng BP, Rydel RE, Podlisny MB, Selkoe DJ. The oligomerization of amyloid β-protein begins intracellularly in cells derived from human brain. Biochemistry. 2000;39:10831–10839. doi: 10.1021/bi001048s. [DOI] [PubMed] [Google Scholar]
- 25.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 26.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, et al. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, Chang L, Viola KL, Lacor PN, Lambert MP, Finch CE, et al. Alzheimer's disease-affected brain: presence of oligomeric Aβ ligands (ADDLs) suggests a molecular basis for reversible memory loss. Proc Natl Acad Sci USA. 2003;100:10417–10422. doi: 10.1073/pnas.1834302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper JD, Wong SS, Lieber CM, Lansbury PT. Observation of metastable Aβ amyloid protofibrils by atomic force microscopy. Chem Biol. 1997;4:119–125. doi: 10.1016/s1074-5521(97)90255-6. [DOI] [PubMed] [Google Scholar]
- 29.Hartley DM, Walsh DM, Ye CP, Diehl T, Vasquez S, Vassilev PM, et al. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh DM, Lomakin A, Benedek GB, Condron MM, Teplow DB. Amyloid β-protein fibrillogenesis. Detection of a protofibrillar intermediate. J Biol Chem. 1997;272:22364–22372. doi: 10.1074/jbc.272.35.22364. [DOI] [PubMed] [Google Scholar]
- 31.Walsh DM, Hartley DM, Kusumoto Y, Fezoui Y, Condron MM, Lomakin A, et al. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 32.Lesne S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, et al. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352–357. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- 33.Bitan G, Kirkitadze MD, Lomakin A, Vollers SS, Benedek GB, Teplow DB. Amyloid β-protein (Aβ) assembly: Aβ40 and Aβ42 oligomerize through distinct pathways. Proc Natl Acad Sci USA. 2003;100:330–335. doi: 10.1073/pnas.222681699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bitan G, Tarus B, Vollers SS, Lashuel HA, Condron MM, Straub JE, et al. A molecular switch in amyloid assembly: Met35 and amyloid β-protein oligomerization. J Am Chem Soc. 2003;125:15359–15365. doi: 10.1021/ja0349296. [DOI] [PubMed] [Google Scholar]
- 35.Bitan G, Vollers SS, Teplow DB. Elucidation of primary structure elements controlling early amyloid β-protein oligomerization. J Biol Chem. 2003;278:34882–34889. doi: 10.1074/jbc.M300825200. [DOI] [PubMed] [Google Scholar]
- 36.Hoshi M, Sato M, Matsumoto S, Noguchi A, Yasutake K, Yoshida N, et al. Spherical aggregates of β-amyloid (amylospheroid) show high neurotoxicity and activate tau protein kinase I/glycogen synthase kinase-3β. Proc Natl Acad Sci USA. 2003;100:6370–6375. doi: 10.1073/pnas.1237107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lashuel HA, Hartley D, Petre BM, Walz T, Lansbury PT., Jr Neurodegenerative disease: amyloid pores from pathogenic mutations. Nature. 2002;418:291. doi: 10.1038/418291a. [DOI] [PubMed] [Google Scholar]
- 38.Lashuel HA, Hartley DM, Petre BM, Wall JS, Simon MN, Walz T, et al. Mixtures of wild-type and a pathogenic (E22G) form of Aβ40 in vitro accumulate protofibrils, including amyloid pores. J Mol Biol. 2003;332:795–808. doi: 10.1016/s0022-2836(03)00927-6. [DOI] [PubMed] [Google Scholar]
- 39.Westlind-Danielsson A, Arnerup G. Spontaneous in vitro formation of supramolecular β-amyloid structures, "βamy balls", by β-amyloid 1–40 peptide. Biochemistry. 2001;40:14736–14743. doi: 10.1021/bi010375c. [DOI] [PubMed] [Google Scholar]
- 40.Merrifield B. Concept and early development of solid-phase peptide synthesis. Methods Enzymol. 1997;289:3–13. doi: 10.1016/s0076-6879(97)89040-4. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Moss JA, Janda KD. Biological tuning of synthetic tactics in solid-phase synthesis: application to Aβ(1–42) J Org Chem. 2004;69:7776–7778. doi: 10.1021/jo048922y. [DOI] [PubMed] [Google Scholar]
- 42.Sharpe S, Yau WM, Tycko R. Expression and purification of a recombinant peptide from the Alzheimer's β-amyloid protein for solid-state NMR. Protein Expr Purif. 2005;42:200–210. doi: 10.1016/j.pep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Lee EK, Hwang JH, Shin DY, Kim DI, Yoo YJ. Production of recombinant amyloid-β peptide 42 as an ubiquitin extension. Protein Expr Purif. 2005;40:183–189. doi: 10.1016/j.pep.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Dobeli H, Draeger N, Huber G, Jakob P, Schmidt D, Seilheimer B, et al. A biotechnological method provides access to aggregation competent monomeric Alzheimer's 1–42 residue amyloid peptide. Nat Biotechnol. 1995;13:988–993. doi: 10.1038/nbt0995-988. [DOI] [PubMed] [Google Scholar]
- 45.Carrotta R, Di Carlo M, Manno M, Montana G, Picone P, Romancino D, et al. Toxicity of recombinant β-amyloid prefibrillar oligomers on the morphogenesis of the sea urchin Paracentrotus lividus. FASEB J. 2006;20:1916–1917. doi: 10.1096/fj.06-5716fje. [DOI] [PubMed] [Google Scholar]
- 46.Tickler AK, Barrow CJ, Wade JD. Improved preparation of amyloid-β peptides using DBU as Nalpha-Fmoc deprotection reagent. J Pept Sci. 2001;7:488–494. doi: 10.1002/psc.342. [DOI] [PubMed] [Google Scholar]
- 47.Zarandi M, Soos K, Fulop L, Bozso Z, Datki Z, Toth GK, et al. Synthesis of Aβ(1–42) and its derivatives with improved efficiency. J Pept Sci. 2007;13:94–99. doi: 10.1002/psc.801. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Martin F, Quintanar-Audelo M, Garcia-Ramos Y, Cruz LJ, Gravel C, Furic R, et al. ChemMatrix, a poly(ethylene glycol)-based support for the solid-phase synthesis of complex peptides. J Comb Chem. 2006;8:213–220. doi: 10.1021/cc0600019. [DOI] [PubMed] [Google Scholar]
- 49.Sohma Y, Sasaki M, Hayashi Y, Kimura T, Kiso Y. Design and synthesis of a novel water-soluble Aβ1–42 isopeptide: an efficient strategy for the preparation of Alzheimer's disease-related peptide, Aβ1–42, via O-N intramolecular acyl migration reaction. Tetrahedron Lett. 2004;45:5965–5968. [Google Scholar]
- 50.Sohma Y, Taniguchi A, Yoshiya T, Chiyomori Y, Fukao F, Nakamura S, et al. 'Click peptide': a novel 'O-acyl isopeptide method' for peptide synthesis and chemical biology-oriented synthesis of amyloid β peptide analogues. J Pept Sci. 2006;12:823–828. doi: 10.1002/psc.817. [DOI] [PubMed] [Google Scholar]
- 51.Cornista JC, Koga Y, Takano K, Kanaya S. Amyloidogenecity and pitrilysin sensitivity of a lysine-free derivative of amyloid β-peptide cleaved from a recombinant fusion protein. J Biotechnol. 2006;122:186–197. doi: 10.1016/j.jbiotec.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Teplow DB. Preparation of amyloid β-protein for structural and functional studies. Methods Enzymol. 2006;413:20–33. doi: 10.1016/S0076-6879(06)13002-5. [DOI] [PubMed] [Google Scholar]
- 53.Klein WL, Stine WB, Jr, Teplow DB. Small assemblies of unmodified amyloid β-protein are the proximate neurotoxin in Alzheimer's disease. Neurobiol Aging. 2004;25:569–580. doi: 10.1016/j.neurobiolaging.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 54.Howlett DR, Jennings KH, Lee DC, Clark MS, Brown F, Wetzel R, et al. Aggregation state and neurotoxic properties of Alzheimer β-amyloid peptide. Neurodegeneration. 1995;4:23–32. doi: 10.1006/neur.1995.0003. [DOI] [PubMed] [Google Scholar]
- 55.Simmons LK, May PC, Tomaselli KJ, Rydel RE, Fuson KS, Brigham EF, et al. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Mol Pharmacol. 1994;45:373–379. [PubMed] [Google Scholar]
- 56.Soto C, Castano EM, Kumar RA, Beavis RC, Frangione B. Fibrillogenesis of synthetic amyloid-β peptides is dependent on their initial secondary structure. Neurosci Lett. 1995;200:105–108. doi: 10.1016/0304-3940(95)12089-m. [DOI] [PubMed] [Google Scholar]
- 57.Bitan G, Fradinger EA, Spring SM, Teplow DB. Neurotoxic protein oligomers—what you see is not always what you get. Amyloid. 2005;12:88–95. doi: 10.1080/13506120500106958. [DOI] [PubMed] [Google Scholar]
- 58.Lee JP, Stimson ER, Ghilardi JR, Mantyh PW, Lu YA, Felix AM, et al. 1H NMR of Aβ amyloid peptide congeners in water solution. Conformational changes correlate with plaque competence. Biochemistry. 1995;34:5191–5200. doi: 10.1021/bi00015a033. [DOI] [PubMed] [Google Scholar]
- 59.Zhang S, Iwata K, Lachenmann MJ, Peng JW, Li S, Stimson ER, et al. The Alzheimer's peptide Aβ adopts a collapsed coil structure in water. J Struct Biol. 2000;130:130–141. doi: 10.1006/jsbi.2000.4288. [DOI] [PubMed] [Google Scholar]
- 60.Yan Y, Wang C. Aβ42 is more rigid than Aβ40 at the C terminus: implications for Aβ aggregation and toxicity. J Mol Biol. 2006;364:853–862. doi: 10.1016/j.jmb.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 61.Riek R, Guntert P, Dobeli H, Wipf B, Wuthrich K. NMR studies in aqueous solution fail to identify significant conformational differences between the monomeric forms of two Alzheimer peptides with widely different plaque-competence, Aβ(1–40)OX and Aβ(1–42)ox. Eur J Biochem. 2001;268:5930–5936. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
- 62.Hou L, Shao H, Zhang Y, Li H, Menon NK, Neuhaus EB, et al. Solution NMR studies of the Aβ(1–40) and Aβ(1–42) peptides establish that the Met35 oxidation state affects the mechanism of amyloid formation. J Am Chem Soc. 2004;126:1992–2005. doi: 10.1021/ja036813f. [DOI] [PubMed] [Google Scholar]
- 63.Sgourakis NG, Yan Y, McCallum SA, Wang C, Garcia AE. The Alzheimer's peptides Aβ40 and 42 adopt distinct conformations in water: a combined MD/NMR study. J Mol Biol. 2007;368:1448–1457. doi: 10.1016/j.jmb.2007.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lazo ND, Grant MA, Condron MC, Rigby AC, Teplow DB. On the nucleation of amyloid β-protein monomer folding. Protein Sci. 2005;14:1581–1596. doi: 10.1110/ps.041292205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.MacArthur MW, Driscoll PC, Thornton JM. NMR and crystallography—complementary approaches to structure determination. Trends Biotechnol. 1994;12:149–153. doi: 10.1016/0167-7799(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 66.Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447:453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 67.Kheterpal I, Chen M, Cook KD, Wetzel R. Structural differences in Aβ amyloid protofibrils and fibrils mapped by hydrogen exchange–mass spectrometry with on-line proteolytic fragmentation. J Mol Biol. 2006;361:785–795. doi: 10.1016/j.jmb.2006.06.066. [DOI] [PubMed] [Google Scholar]
- 68.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 69.Mori H, Takio K, Ogawara M, Selkoe DJ. Mass spectrometry of purified amyloid β protein in Alzheimer's disease. J Biol Chem. 1992;267:17082–17086. [PubMed] [Google Scholar]
- 70.Hepler RW, Grimm KM, Nahas DD, Breese R, Dodson EC, Acton P, et al. Solution state characterization of amyloid β-derived diffusible ligands. Biochemistry. 2006;45:15157–15167. doi: 10.1021/bi061850f. [DOI] [PubMed] [Google Scholar]
- 71.Fancy DA, Kodadek T. Chemistry for the analysis of protein-protein interactions: rapid and efficient cross-linking triggered by long wavelength light. Proc Natl Acad Sci USA. 1999;96:6020–6024. doi: 10.1073/pnas.96.11.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bitan G, Teplow DB. Rapid photochemical cross-linking—a new tool for studies of metastable, amyloidogenic protein assemblies. Acc Chem Res. 2004;37:357–364. doi: 10.1021/ar000214l. [DOI] [PubMed] [Google Scholar]
- 73.Bitan G. Structural study of metastable amyloidogenic protein oligomers by photo-induced cross-linking of unmodified proteins. Methods Enzymol. 2006;413:217–236. doi: 10.1016/S0076-6879(06)13012-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bitan G, Lomakin A, Teplow DB. Amyloid β-protein oligomerization: prenucleation interactions revealed by photo-induced cross-linking of unmodified proteins. J Biol Chem. 2001;276:35176–35184. doi: 10.1074/jbc.M102223200. [DOI] [PubMed] [Google Scholar]
- 75.Nichols MR, Moss MA, Reed DK, Lin WL, Mukhopadhyay R, Hoh JH, et al. Growth of β-amyloid(1–40) protofibrils by monomer elongation and lateral association. Characterization of distinct products by light scattering and atomic force microscopy. Biochemistry. 2002;41:6115–6127. doi: 10.1021/bi015985r. [DOI] [PubMed] [Google Scholar]
- 76.Kheterpal I, Lashuel HA, Hartley DM, Walz T, Lansbury PT, Jr, Wetzel R. Aβ protofibrils possess a stable core structure resistant to hydrogen exchange. Biochemistry. 2003;42:14092–14098. doi: 10.1021/bi0357816. [DOI] [PubMed] [Google Scholar]
- 77.Bitan G, Teplow DB. Preparation of aggregate-free, low molecular weight amyloid-β for assembly and toxicity assays. Methods Mol Biol. 2005;299:3–9. doi: 10.1385/1-59259-874-9:003. [DOI] [PubMed] [Google Scholar]
- 78.Urbanke C, Witte G, Curth U. Sedimentation velocity method in the analytical ultracentrifuge for the study of protein-protein interactions. Methods Mol Biol. 2005;305:101–114. doi: 10.1385/1-59259-912-5:101. [DOI] [PubMed] [Google Scholar]
- 79.Mok YF, Howlett GJ. Sedimentation velocity analysis of amyloid oligomers and fibrils. Methods Enzymol. 2006;413:199–217. doi: 10.1016/S0076-6879(06)13011-6. [DOI] [PubMed] [Google Scholar]
- 80.Lebowitz J, Lewis MS, Schuck P. Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 2002;11:2067–2079. doi: 10.1110/ps.0207702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang TH, Yang DS, Plaskos NP, Go S, Yip CM, Fraser PE, et al. Structural studies of soluble oligomers of the Alzheimer β-amyloid peptide. J Mol Biol. 2000;297:73–87. doi: 10.1006/jmbi.2000.3559. [DOI] [PubMed] [Google Scholar]
- 82.Lomakin A, Benedek GB, Teplow DB. Monitoring protein assembly using quasielastic light scattering spectroscopy. Methods Enzymol. 1999;309:429–459. doi: 10.1016/s0076-6879(99)09029-1. [DOI] [PubMed] [Google Scholar]
- 83.Tomski SJ, Murphy RM. Kinetics of aggregation of synthetic β-amyloid peptide. Arch Biochem Biophys. 1992;294:630–638. doi: 10.1016/0003-9861(92)90735-f. [DOI] [PubMed] [Google Scholar]
- 84.Shen CL, Scott GL, Merchant F, Murphy RM. Light scattering analysis of fibril growth from the amino-terminal fragment β(1–28) of β-amyloid peptide. Biophys J. 1993;65:2383–2395. doi: 10.1016/S0006-3495(93)81312-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lomakin A, Chung DS, Benedek GB, Kirschner DA, Teplow DB. On the nucleation and growth of amyloid β-protein fibrils: detection of nuclei and quantitation of rate constants. Proc Natl Acad Sci USA. 1996;93:1125–1129. doi: 10.1073/pnas.93.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Murphy RM, Pallitto MM. Probing the kinetics of β-amyloid self-association. J Struct Biol. 2000;130:109–122. doi: 10.1006/jsbi.2000.4253. [DOI] [PubMed] [Google Scholar]
- 87.Ward RV, Jennings KH, Jepras R, Neville W, Owen DE, Hawkins J, et al. Fractionation and characterization of oligomeric, protofibrillar and fibrillar forms of β-amyloid peptide. Biochem J. 2000;348(Pt 1):137–144. [PMC free article] [PubMed] [Google Scholar]
- 88.Oliva A, Llabres M, Farina JB. Applications of multi-angle laser light-scattering detection in the analysis of peptides and proteins. Curr Drug Discov Technol. 2004;1:229–242. doi: 10.2174/1570163043334938. [DOI] [PubMed] [Google Scholar]
- 89.Lomakin A, Teplow DB. Quasielastic light scattering study of amyloid β-protein fibril formation. Protein Pept Lett. 2006;13:247–254. doi: 10.2174/092986606775338515. [DOI] [PubMed] [Google Scholar]
- 90.Lomakin A, Teplow DB, Benedek GB. Quasielastic light scattering for protein assembly studies. Methods Mol Biol. 2005;299:153–174. doi: 10.1385/1-59259-874-9:153. [DOI] [PubMed] [Google Scholar]
- 91.Bernstein SL, Wyttenbach T, Baumketner A, Shea JE, Bitan G, Teplow DB, et al. Amyloid β-protein: monomer structure and early aggregation states of Aβ42 and its Pro19 alloform. J Am Chem Soc. 2005;127:2075–2084. doi: 10.1021/ja044531p. [DOI] [PubMed] [Google Scholar]
- 92.Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid β-protein fibrillogenesis. J Mol Biol. 2001;312:1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 93.Gursky O, Aleshkov S. Temperature-dependent β-sheet formation in β-amyloid Aβ1–40 peptide in water: uncoupling β-structure folding from aggregation. Biochim Biophys Acta. 2000;1476:93–102. doi: 10.1016/s0167-4838(99)00228-9. [DOI] [PubMed] [Google Scholar]
- 94.Lim KH, Collver HH, Le YT, Nagchowdhuri P, Kenney JM. Characterizations of distinct amyloidogenic conformations of the Aβ (1–40) and (1–42) peptides. Biochem Biophys Res Commun. 2007;353:443–449. doi: 10.1016/j.bbrc.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 95.Hou L, Kang I, Marchant RE, Zagorski MG. Methionine 35 oxidation reduces fibril assembly of the amyloid Aβ-(1–42) peptide of Alzheimer's disease. J Biol Chem. 2002;277:40173–40176. doi: 10.1074/jbc.C200338200. [DOI] [PubMed] [Google Scholar]
- 96.Ahting U, Thieffry M, Engelhardt H, Hegerl R, Neupert W, Nussberger S. Tom40, the pore=forming component of the protein-conducting TOM channel in the outer membrane of mitochondria. J Cell Biol. 2001;153:1151–1160. doi: 10.1083/jcb.153.6.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hetenyi C, Szabo Z, Klement E, Datki Z, Kortvelyesi T, Zarandi M, et al. Pentapeptide amides interfere with the aggregation of β-amyloid peptide of Alzheimer's disease. Biochem Biophys Res Commun. 2002;292:931–936. doi: 10.1006/bbrc.2002.6745. [DOI] [PubMed] [Google Scholar]
- 98.Lin SY, Chu HL. Fourier transform infrared spectroscopy used to evidence the prevention of β-sheet formation of amyloid β(1–40) peptide by a short amyloid fragment. Int J Biol Macromol. 2003;32:173–177. doi: 10.1016/s0141-8130(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 99.Kakio A, Yano Y, Takai D, Kuroda Y, Matsumoto O, Kozutsumi Y, et al. Interaction between amyloid β-protein aggregates and membranes. J Pept Sci. 2004;10:612–621. doi: 10.1002/psc.570. [DOI] [PubMed] [Google Scholar]
- 100.Brzyska M, Trzesniewska K, Gers T, Elbaum D. Discrete conformational changes as regulators of the hydrolytic properties of beta-amyloid (1–40) FEBS J. 2006;273:5598–5611. doi: 10.1111/j.1742-4658.2006.05549.x. [DOI] [PubMed] [Google Scholar]
- 101.Mastrangelo IA, Ahmed M, Sato T, Liu W, Wang C, Hough P, et al. High-resolution atomic force microscopy of soluble Aβ42 oligomers. J Mol Biol. 2006;358:106–119. doi: 10.1016/j.jmb.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 102.Nybo M, Svehag SE, Holm Nielsen E. An ultrastructural study of amyloid intermediates in Aβ1–42 fibrillogenesis. Scand J Immunol. 1999;49:219–223. doi: 10.1046/j.1365-3083.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 103.Modler AJ, Gast K, Lutsch G, Damaschun G. Assembly of amyloid protofibrils via critical oligomers—a novel pathway of amyloid formation. J Mol Biol. 2003;325:135–148. doi: 10.1016/s0022-2836(02)01175-0. [DOI] [PubMed] [Google Scholar]
- 104.Roher AE, Chaney MO, Kuo YM, Webster SD, Stine WB, Haverkamp LJ, et al. Morphology and toxicity of Aβ-(1–42) dimer derived from neuritic and vascular amyloid deposits of Alzheimer's disease. J Biol Chem. 1996;271:20631–20635. doi: 10.1074/jbc.271.34.20631. [DOI] [PubMed] [Google Scholar]
- 105.Selenica ML, Wang X, Ostergaard-Pedersen L, Westlind-Danielsson A, Grubb A. Cystatin C reduces the in vitro formation of soluble Aβ1–42 oligomers and protofibrils. Scand J Clin Lab Invest. 2007;67:179–190. doi: 10.1080/00365510601009738. [DOI] [PubMed] [Google Scholar]
- 106.Engel A, Colliex C. Application of scanning transmission electron microscopy to the study of biological structure. Curr Opin Biotechnol. 1993;4:403–411. doi: 10.1016/0958-1669(93)90005-h. [DOI] [PubMed] [Google Scholar]
- 107.Goldsbury C, Frey P, Olivieri V, Aebi U, Muller SA. Multiple assembly pathways underlie amyloid-β fibril polymorphisms. J Mol Biol. 2005;352:282–298. doi: 10.1016/j.jmb.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 108.Goldsbury CS, Wirtz S, Muller SA, Sunderji S, Wicki P, Aebi U, et al. Studies on the in vitro assembly of Aβ 1–40: implications for the search for a β fibril formation inhibitors. J Struct Biol. 2000;130:217–231. doi: 10.1006/jsbi.2000.4259. [DOI] [PubMed] [Google Scholar]
- 109.Petkova AT, Leapman RD, Guo Z, Yau WM, Mattson MP, Tycko R. Self-propagating, molecular-level polymorphism in Alzheimer's β-amyloid fibrils. Science. 2005;307:262–265. doi: 10.1126/science.1105850. [DOI] [PubMed] [Google Scholar]
- 110.Blackley HK, Sanders GH, Davies MC, Roberts CJ, Tendler SJ, Wilkinson MJ. In-situ atomic force microscopy study of β-amyloid fibrillization. J Mol Biol. 2000;298:833–840. doi: 10.1006/jmbi.2000.3711. [DOI] [PubMed] [Google Scholar]
- 111.Kowalewski T, Holtzman DM. In situ atomic force microscopy study of Alzheimer's β-amyloid peptide on different substrates: new insights into mechanism of β-sheet formation. Proc Natl Acad Sci USA. 1999;96:3688–3693. doi: 10.1073/pnas.96.7.3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harper JD, Wong SS, Lieber CM, Lansbury PT., Jr Assembly of Aβ amyloid protofibrils: an in vitro model for a possible early event in Alzheimer's disease. Biochemistry. 1999;38:8972–8980. doi: 10.1021/bi9904149. [DOI] [PubMed] [Google Scholar]
- 113.Arimon M, Diez-Perez I, Kogan MJ, Durany N, Giralt E, Sanz F, et al. Fine structure study of Aβ1–42 fibrillogenesis with atomic force microscopy. FASEB J. 2005;19:1344–1346. doi: 10.1096/fj.04-3137fje. [DOI] [PubMed] [Google Scholar]
- 114.Wang Z, Zhou C, Wang C, Wan L, Fang X, Bai C. AFM and STM study of β-amyloid aggregation on graphite. Ultramicroscopy. 2003;97:73–79. doi: 10.1016/S0304-3991(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 115.Losic D, Martin LL, Mechler A, Aguilar MI, Small DH. High resolution scanning tunnelling microscopy of the β-amyloid protein (Aβ1–40)of Alzheimer's disease suggests a novel mechanism of oligomer assembly. J Struct Biol. 2006;155:104–110. doi: 10.1016/j.jsb.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 116.Cooper JA, Buhle EL, Jr, Walker SB, Tsong TY, Pollard TD. Kinetic evidence for a monomer activation step in actin polymerization. Biochemistry. 1983;22:2193–2202. doi: 10.1021/bi00278a021. [DOI] [PubMed] [Google Scholar]
- 117.Stratman NC, Castle CK, Taylor BM, Epps DE, Melchior GW, Carter DB. Isoform-specific interactions of human apolipoprotein E to an intermediate conformation of human Alzheimer amyloid-β peptide. Chem Phys Lipids. 2005;137:52–61. doi: 10.1016/j.chemphyslip.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 118.Shen CL, Murphy RM. Solvent effects on self-assembly of β-amyloid peptide. Biophys J. 1995;69:640–651. doi: 10.1016/S0006-3495(95)79940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lee S, Fernandez EJ, Good TA. Role of aggregation conditions in structure, stability, toxicity of intermediates in the Aβ fibril formation pathway. Protein Sci. 2007;16:723–732. doi: 10.1110/ps.062514807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chimon S, Ishii Y. Capturing intermediate structures of Alzheimer's β-amyloid, Aβ(1–40), by solid-state NMR spectroscopy. J Am Chem Soc. 2005;127:13472–13473. doi: 10.1021/ja054039l. [DOI] [PubMed] [Google Scholar]
- 121.Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, et al. Self-assembly of Aβ(1–42) into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- 122.Sun XD, Mo ZL, Taylor BM, Epps DE. A slowly formed transient conformer of Aβ(1–40) is toxic to inward channels of dissociated hippocampal and cortical neurons of rats. Neurobiol Dis. 2003;14:567–578. doi: 10.1016/j.nbd.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 123.Koudinov AR, Koudinova NV, Kumar A, Beavis RC, Ghiso J. Biochemical characterization of Alzheimer's soluble amyloid beta protein in human cerebrospinal fluid: association with high density lipoproteins. Biochem Biophys Res Commun. 1996;223:592–597. doi: 10.1006/bbrc.1996.0940. [DOI] [PubMed] [Google Scholar]
- 124.Koudinov AR, Berezov TT, Koudinova NV. The levels of soluble amyloid beta in different high density lipoprotein subfractions distinguish Alzheimer's and normal aging cerebrospinal fluid: implication for brain cholesterol pathology? Neurosci Lett. 2001;314:115–118. doi: 10.1016/s0304-3940(01)02263-7. [DOI] [PubMed] [Google Scholar]
- 125.Kawarabayashi T, Shoji M, Younkin LH, Wen-Lang L, Dickson DW, Murakami T, et al. Dimeric amyloid β protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2004;24:3801–3809. doi: 10.1523/JNEUROSCI.5543-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, et al. Enhanced production and oligomerization of the 42-residue amyloid β-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem. 1997;272:7977–7982. doi: 10.1074/jbc.272.12.7977. [DOI] [PubMed] [Google Scholar]
- 127.Morishima-Kawashima M, Ihara Y. The presence of amyloid β-protein in the detergent-insoluble membrane compartment of human neuroblastoma cells. Biochemistry. 1998;37:15247–15253. doi: 10.1021/bi981843u. [DOI] [PubMed] [Google Scholar]
- 128.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid β-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Walsh DM, Klyubin I, Fadeeva JV, Rowan MJ, Selkoe DJ. Amyloid-β oligomers: their production, toxicity and therapeutic inhibition. Biochem Soc Trans. 2002;30:552–557. doi: 10.1042/bst0300552. [DOI] [PubMed] [Google Scholar]
- 130.Walsh DM, Townsend M, Podlisny MB, Shankar GM, Fadeeva JV, El Agnaf O, et al. Certain inhibitors of synthetic amyloid β-peptide (Aβ) fibrillogenesis block oligomerization of natural Aβ and thereby rescue long-term potentiation. J Neurosci. 2005;25:2455–2462. doi: 10.1523/JNEUROSCI.4391-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Walsh DM, Selkoe DJ. Aβ Oligomers – a decade of discovery. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 132.Dudai Y. Molecular bases of long-term memories: a question of persistence. Curr Opin Neurobiol. 2002;12:211–216. doi: 10.1016/s0959-4388(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 133.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, et al. Natural oligomers of the amyloid-β protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 134.Kojima N, Shirao T. Synaptic dysfunction and disruption of post-synaptic drebrin-actin complex: a study of neurological disorders accompanied by cognitive deficits. Neurosci Res. 2007;58:1–5. doi: 10.1016/j.neures.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 135.Calabrese B, Shaked GM, Tabarean IV, Braga J, Koo EH, Halpain S. Rapid, concurrent alterations in pre- and postsynaptic structure induced by naturally-secreted amyloid-β protein. Mol Cell Neurosci. 2007;35:183–193. doi: 10.1016/j.mcn.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lacor PN, Buniel MC, Furlow PW, Clemente AS, Velasco PT, Wood M, et al. Aβ oligomer-induced aberrations in synapse composition, shape, density provide a molecular basis for loss of connectivity in Alzheimer's disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shrestha BR, Vitolo OV, Joshi P, Lordkipanidze T, Shelanski M, Dunaevsky A. Amyloid β peptide adversely affects spine number and motility in hippocampal neurons. Mol Cell Neurosci. 2006;33:274–282. doi: 10.1016/j.mcn.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 138.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McLaurin J, Golomb R, Jurewicz A, Antel JP, Fraser PE. Inositol stereoisomers stabilize an oligomeric aggregate of Alzheimer amyloid β peptide and inhibit Aβ-induced toxicity. J Biol Chem. 2000;275:18495–18502. doi: 10.1074/jbc.M906994199. [DOI] [PubMed] [Google Scholar]
- 140.Townsend M, Cleary JP, Mehta T, Hofmeister J, Lesne S, O'Hare E, et al. Orally available compound prevents deficits in memory caused by the Alzheimer amyloid-β oligomers. Ann Neurol. 2006;60:668–676. doi: 10.1002/ana.21051. [DOI] [PubMed] [Google Scholar]
- 141.Snyder EM, Nong Y, Almeida CG, Paul S, Moran T, Choi EY, et al. Regulation of NMDA receptor trafficking by amyloid-β. Nat Neurosci. 2005;8:1051–1058. doi: 10.1038/nn1503. [DOI] [PubMed] [Google Scholar]
- 142.Klein WL. Aβ toxicity in Alzheimer's disease: globular oligomers (ADDLs) as new vaccine and drug targets. Neurochem Int. 2002;41:345–352. doi: 10.1016/s0197-0186(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 143.Calero M, Rostagno A, Matsubara E, Zlokovic B, Frangione B, Ghiso J. Apolipoprotein J (clusterin) and Alzheimer's disease. Microsc Res Tech. 2002;50:305–315. doi: 10.1002/1097-0029(20000815)50:4<305::AID-JEMT10>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 144.Oda T, Wals P, Osterburg HH, Johnson SA, Pasinetti GM, Morgan TE, et al. Clusterin (apoJ) alters the aggregation of amyloid β-peptide (Aβ1–42) and forms slowly sedimenting Aβ complexes that cause oxidative stress. Exp Neurol. 1995;136:22–31. doi: 10.1006/exnr.1995.1080. [DOI] [PubMed] [Google Scholar]
- 145.De Felice FG, Wu D, Lambert MP, Fernandez SJ, Velasco PT, Lacor PN, et al. Alzheimer's disease-type neuronal tau hyperphos-phorylation induced by Aβ oligomers. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stine WB, Jr, Dahlgren KN, Krafft GA, LaDu MJ. In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J Biol Chem. 2003;278:11612–11622. doi: 10.1074/jbc.M210207200. [DOI] [PubMed] [Google Scholar]
- 147.Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- 148.Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 149.Wang HW, Pasternak JF, Kuo H, Ristic H, Lambert MP, Chromy B, et al. Soluble oligomers of β amyloid (1–42) inhibit long-term potentiation but not long-term depression in rat dentate gyrus. Brain Res. 2002;924:133–140. doi: 10.1016/s0006-8993(01)03058-x. [DOI] [PubMed] [Google Scholar]
- 150.Lacor PN, Buniel MC, Chang L, Fernandez SJ, Gong Y, Viola KL, et al. Synaptic targeting by Alzheimer's-related amyloid β oligomers. J Neurosci. 2004;24:10191–10200. doi: 10.1523/JNEUROSCI.3432-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fong DK, Rao A, Crump FT, Craig AM. Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/calmodulin-dependent kinase II. J Neurosci. 2002;22:2153–2164. doi: 10.1523/JNEUROSCI.22-06-02153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lambert MP, Viola KL, Chromy BA, Chang L, Morgan TE, Yu J, et al. Vaccination with soluble Aβ oligomers generates toxicity-neutralizing antibodies. J Neurochem. 2001;79:595–605. doi: 10.1046/j.1471-4159.2001.00592.x. [DOI] [PubMed] [Google Scholar]
- 153.Chang L, Bakhos L, Wang Z, Venton DL, Klein WL. Femtomole immunodetection of synthetic and endogenous amyloid-β oligomers and its application to Alzheimer's disease drug candidate screening. J Mol Neurosci. 2003;20:305–313. doi: 10.1385/JMN:20:3:305. [DOI] [PubMed] [Google Scholar]
- 154.Tong L, Balazs R, Thornton PL, Cotman CW. β-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. J Neurosci. 2004;24:6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 156.Carlisle HJ, Kennedy MB. Spine architecture and synaptic plasticity. Trends Neurosci. 2005;28:182–187. doi: 10.1016/j.tins.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 157.Fiala JC, Spacek J, Harris KM. Dendritic spine pathology: cause or consequence of neurological disorders? Brain Res Brain Res Rev. 2002;39:29–54. doi: 10.1016/s0165-0173(02)00158-3. [DOI] [PubMed] [Google Scholar]
- 158.Tzingounis AV, Nicoll RA. Arc/Arg3.1: linking gene expression to synaptic plasticity and memory. Neuron. 2006;52:403–407. doi: 10.1016/j.neuron.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 159.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, et al. Inhibition of activity-dependent are protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, et al. Aβ oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 161.Oddo S, Caccamo A, Tran L, Lambert MP, Qlabe CG, Klein WL, et al. Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease. A link between Aβ and tau pathology. J Biol Chem. 2006;281:1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- 162.Lambert MP, Velasco PT, Chang L, Viola KL, Fernandez S, Lacor PN, et al. Monoclonal antibodies that target pathological assemblies of Aβ. J Neurochem. 2007;100:23–35. doi: 10.1111/j.1471-4159.2006.04157.x. [DOI] [PubMed] [Google Scholar]
- 163.Seilheimer B, Bohrmann B, Bondolfi L, Muller F, Stuber D, Dobeli H. The toxicity of the Alzheimer's β-amyloid peptide correlates with a distinct fiber morphology. J Struct Biol. 1997;119:59–71. doi: 10.1006/jsbi.1997.3859. [DOI] [PubMed] [Google Scholar]
- 164.Williams AD, Sega M, Chen M, Kheterpal I, Geva M, Berthelier V, et al. Structural properties of Aβ protofibrils stabilized by a small molecule. Proc Natl Acad Sci USA. 2005;102:7115–7120. doi: 10.1073/pnas.0408582102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 166.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–320. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 167.Nilsberth C, Westlind-Danielsson A, Eckman CB, Condron MM, Axelman K, Forsell C, et al. The 'Arctic' APP mutation (E693G) causes Alzheimer's disease by enhanced Aβ protofibril formation. Nat Neurosci. 2001;4:887–893. doi: 10.1038/nn0901-887. [DOI] [PubMed] [Google Scholar]
- 168.Haass C, Steiner H. Protofibrils, the unifying toxic molecule of neurodegenerative disorders? Nat Neurosci. 2001;4:859–860. doi: 10.1038/nn0901-859. [DOI] [PubMed] [Google Scholar]
- 169.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer's disease. J Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 170.Siegel SJ, Bieschke J, Powers ET, Kelly JW. The oxidative stress metabolite 4-hydroxynonenal promotes Alzheimer protofibril formation. Biochemistry. 2007;46:1503–1510. doi: 10.1021/bi061853s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Johansson AS, Garlind A, Berglind-Dehlin F, Karlsson G, Edwards K, Gellerfors P, et al. Docosahexaenoic acid stabilizes soluble amyloid-β protofibrils and sustains amyloid-β-induced neurotoxicity in vitro. FEBS J. 2007;274:990–1000. doi: 10.1111/j.1742-4658.2007.05647.x. [DOI] [PubMed] [Google Scholar]
- 172.Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- 173.Codita A, Winblad B, Mohammed AH. Of mice and men: more neurobiology in dementia. Curr Opin Psychiatry. 2006;19:555–563. doi: 10.1097/01.yco.0000245757.06374.6a. [DOI] [PubMed] [Google Scholar]
- 174.Cole GM, Frautschy SA. Alzheimer's amyloid story finds its star. Trends Mol Med. 2006;12:395–396. doi: 10.1016/j.molmed.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 175.Jacobsen JS, Wu CC, Redwine JM, Comery TA, Arias R, Bowlby M, et al. Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proc Natl Acad Sci USA. 2006;103:5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Billings LM, Green KN, McGaugh JL, LaFerla FM. Learning decreases Aβ*56 and tau pathology and ameliorates behavioral decline in 3×Tg-AD mice. J Neurosci. 2007;27:751–761. doi: 10.1523/JNEUROSCI.4800-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lin H, Bhatia R, Lal R. Amyloid β protein formsion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 2001;15:2433–2444. doi: 10.1096/fj.01-0377com. [DOI] [PubMed] [Google Scholar]
- 178.Quist A, Doudevski I, Lin H, Azimova R, Ng D, Frangione B, et al. Amyloid ion channels: a common structural link for protein-misfolding disease. Proc Natl Acad Sci USA. 2005;102:10427–10432. doi: 10.1073/pnas.0502066102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Arispe N, Pollard HB, Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid β-protein [AβP-(1–40)] in bilayer membranes. Proc Natl Acad Sci USA. 1993;90:10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid β protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kawahara M, Arispe N, Kuroda Y, Rojas E. Alzheimer's disease amyloid β-protein forms Zn2+-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophys J. 1997;73:67–75. doi: 10.1016/S0006-3495(97)78048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kawahara M, Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer's β-amyloid protein: channel formation and disruption of calcium homeostasis. Brain Res Bull. 2000;53:389–397. doi: 10.1016/s0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 183.Sanderson KL, Butler L, Ingram VM. Aggregates of a β-amyloid peptide are required to induce calcium currents in neuron-like human teratocarcinoma cells: relation to Alzheimer's disease. Brain Res. 1997;744:7–14. doi: 10.1016/s0006-8993(96)01060-8. [DOI] [PubMed] [Google Scholar]
- 184.Rhee SK, Quist AP, Lal R. Amyloid β protein-(1–42) forms calcium-permeable, Zn2+-sensitive channel. J Biol Chem. 1998;273:13379–13382. doi: 10.1074/jbc.273.22.13379. [DOI] [PubMed] [Google Scholar]
- 185.Hirakura Y, Lin MC, Kagan BL. Alzheimer amyloid Aβ1–42 channels: effects of solvent, pH, and Congo Red. J Neurosci Res. 1999;57:458–466. [PubMed] [Google Scholar]
- 186.Lin H, Zhu YJ, Lal R. Amyloid β protein (1–40) forms calcium-permeable, Zn2+-sensitive channel in reconstituted lipid vesicles. Biochemistry. 1999;38:11189–11196. doi: 10.1021/bi982997c. [DOI] [PubMed] [Google Scholar]
- 187.Bhatia R, Lin H, Lal R. Fresh and globular amyloid β protein (1–42) induces rapid cellular degeneration: evidence for AβP channel-mediated cellular toxicity. FASEB J. 2000;14:1233–1243. doi: 10.1096/fasebj.14.9.1233. [DOI] [PubMed] [Google Scholar]
- 188.Kourie JI, Henry CL, Farrelly P. Diversity of amyloid β protein fragment [1–40]-formed channels. Cell Mol Neurobiol. 2001;21:255–284. doi: 10.1023/A:1010995121153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kagan BL, Hirakura Y, Azimov R, Azimova R, Lin MC. The channel hypothesis of Alzheimer's disease: current status. Peptides. 2002;23:1311–1315. doi: 10.1016/s0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 190.Lin MC, Kagan BL. Electrophysiologic properties of channels induced by Aβ25–35 in planar lipid bilayers. Peptides. 2002;23:1215–1228. doi: 10.1016/s0196-9781(02)00057-8. [DOI] [PubMed] [Google Scholar]
- 191.Bahadi R, Farrelly PV, Kenna BL, Curtain CC, Masters CL, Cappai R, et al. Cu 2+-induced modification of the kinetics of Aβ(1–42) channels. Am J Physiol Cell Physiol. 2003;285:C873–C880. doi: 10.1152/ajpcell.00147.2003. [DOI] [PubMed] [Google Scholar]
- 192.Alarcon JM, Brito JA, Hermosilla T, Atwater I, Mears D, Rojas E. Ion channel formation by Alzheimer's disease amyloid β-peptide (Aβ40) in unilamellar liposomes is determined by anionic phospholipids. Peptides. 2006;27:95–104. doi: 10.1016/j.peptides.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 193.Sokolov Y, Kozak JA, Kayed R, Chanturiya A, Glabe C, Hall JE. Soluble amyloid oligomers increase bilayer conductance by altering dielectric structure. J Gen Physiol. 2006;128:637–647. doi: 10.1085/jgp.200609533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 195.Kayed R, Sokolov Y, Edmonds B, McIntire TM, Milton SC, Hall JE, et al. Permeabilization of lipid bilayers is a common conformation-dependent activity of soluble amyloid oligomers in protein misfolding diseases. J Biol Chem. 2004;279:46363–46366. doi: 10.1074/jbc.C400260200. [DOI] [PubMed] [Google Scholar]
- 196.Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, et al. α-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. J Mol Biol. 2002;322:1089–1102. doi: 10.1016/s0022-2836(02)00735-0. [DOI] [PubMed] [Google Scholar]
- 197.Kawahara M, Kuroda Y. Intracellular calcium changes in neuronal cells induced by Alzheimer's β-amyloid protein are blocked by estradiol and cholesterol. Cell Mol Neurobiol. 2001;21:1–13. doi: 10.1023/A:1007168910582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dahlgren KN, Manelli AM, Stine WB, Jr, Baker LK, Krafft GA, LaDu MJ. Oligomeric and fibrillar species of amyloid-β peptides differentially affect neuronal viability. J Biol Chem. 2002;277:32046–32053. doi: 10.1074/jbc.M201750200. [DOI] [PubMed] [Google Scholar]
- 199.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 200.Anderson DH, Talaga KC, Rivest AJ, Barron E, Hageman GS, Johnson LV. Characterization of β amyloid assemblies in drusen: the deposits associated with aging and age-related macular degeneration. Exp Eye Res. 2004;78:243–256. doi: 10.1016/j.exer.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 201.Barghorn S, Nimmrich V, Striebinger A, Krantz C, Keller P, Janson B, et al. Globular amyloid β-peptide1–42 oligomer – a homogenous and stable neuropathological protein in Alzheimer's disease. J Neurochem. 2005;95:834–847. doi: 10.1111/j.1471-4159.2005.03407.x. [DOI] [PubMed] [Google Scholar]
- 202.Moore RA, Hayes SF, Fischer ER, Priola SA. Amyloid formation via supramolecular peptide assemblies. Biochemistry. 2007;46:7079–7087. doi: 10.1021/bi700247y. [DOI] [PubMed] [Google Scholar]
- 203.Buxbaum JN. The amyloidoses. Mt Sinai J Med. 1996;63:16–23. [PubMed] [Google Scholar]
- 204.Buxbaum JN. The systemic amyloidoses. Curr Opin Rheumatol. 2004;16:67–75. doi: 10.1097/00002281-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 205.Gambetti P, Russo C. Human brain amyloidoses. Nephrol Dial Transplant. 1998;13 Suppl 7:33–40. doi: 10.1093/ndt/13.suppl_7.33. [DOI] [PubMed] [Google Scholar]
- 206.Bellotti V, Nuvolone M, Giorgetti S, Obici L, Palladini G, Russo P, et al. The workings of the amyloid diseases. Ann Med. 2007;39:200–207. doi: 10.1080/07853890701206887. [DOI] [PubMed] [Google Scholar]
- 207.Glabe CG. Common mechanisms of amyloid oligomer pathogenesis in degenerative disease. Neurobiol Aging. 2006;27:570–575. doi: 10.1016/j.neurobiolaging.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 208.Layfield R, Cavey JR, Lowe J. Role of ubiquitin-mediated proteolysis in the pathogenesis of neurodegenerative disorders. Ageing Res Rev. 2003;2:343–356. doi: 10.1016/s1568-1637(03)00025-4. [DOI] [PubMed] [Google Scholar]
- 209.Brbet R, Sherer TB, Greenamyre JT. Ubiquitin-proteasome system and Parkinson's diseases. Exp Neurol. 2005;191 Suppl 1:S17–S27. doi: 10.1016/j.expneurol.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 210.Murray IVJ, Lee VMY, Trojanowski JQ. Synucleinopathies: A pathological and molecular review. Clin Neurosci Res. 2001;1:445–455. [Google Scholar]
- 211.Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. doi: 10.1016/s0968-0004(02)02169-2. [DOI] [PubMed] [Google Scholar]
- 212.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 213.Conway KA, Lee SJ, Rochet JC, Ding TT, Williamson RE, Lansbury PT., Jr Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson's disease: implications for pathogenesis and therapy. Proc Natl Acad Sci USA. 2000;97:571–576. doi: 10.1073/pnas.97.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ding TT, Lee SJ, Rochet JC, Lansbury PT., Jr Annular α-synuclein protofibrils are produced when spherical protofibrils are incubated in solution or bound to brain-derived membranes. Biochemistry. 2002;41:10209–10217. doi: 10.1021/bi020139h. [DOI] [PubMed] [Google Scholar]
- 215.Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, et al. Vesicle permeabilization by protofibrillar α-synuclein: implications for the pathogenesis and treatment of Parkinson's disease. Biochemistry. 2001;40:7812–7819. doi: 10.1021/bi0102398. [DOI] [PubMed] [Google Scholar]
- 216.Volles MJ, Lansbury PT., Jr Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson's disease-linked mutations and occurs by a pore-like mechanism. Biochemistry. 2002;41:4595–4602. doi: 10.1021/bi0121353. [DOI] [PubMed] [Google Scholar]
- 217.Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, et al. The impact of the E46K mutation on the properties of α-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46:7107–7118. doi: 10.1021/bi7000246. [DOI] [PubMed] [Google Scholar]
- 218.Takeda A, Hasegawa T, Matsuzaki-Kobayashi M, Sugeno N, Kikuchi A, Itoyama Y, et al. Mechanisms of neuronal death in synucleinopathy. J Biomed Biotech. 2006;2006:19365. doi: 10.1155/JBB/2006/19365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Murali J, Jayakumar R. Spectroscopic studies on native and protofibrillar insulin. J Struct Biol. 2005;150:180–189. doi: 10.1016/j.jsb.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 220.Dzwolak W, Loksztejn A, Galinska-Rakoczy A, Adachi R, Goto Y, Rupnicki L. Conformational indeterminism in protein misfolding: chiral amplification on amyloidogenic pathway of insulin. J Am Chem Soc. 2007;129:7517–7522. doi: 10.1021/ja066703j. [DOI] [PubMed] [Google Scholar]
- 221.Dzwolak W, Loksztejn A, Smirnovas V. New insights into the self-assembly of insulin amyloid fibrils: an H-D exchange FT-IR study. Biochemistry. 2006;45:8143–8151. doi: 10.1021/bi060341a. [DOI] [PubMed] [Google Scholar]
- 222.Grudzielanek S, Smirnovas V, Winter R. The effects of various membrane physical-chemical properties on the aggregation kinetics of insulin. Chemistry and physics of lipids. 2007;149:28–39. doi: 10.1016/j.chemphyslip.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 223.Ahmad A, Uversky VN, Hong D, Fink AL. Early events in the fibrillation of monomeric insulin. J Biol Chem. 2005;280:42669–42675. doi: 10.1074/jbc.M504298200. [DOI] [PubMed] [Google Scholar]
- 224.Nettleton EJ, Tito P, Sunde M, Bouchard M, Dobson CM, Robinson CV. Characterization of the oligomeric states of insulin in self-assembly and amyloid fibril formation by mass spectrometry. Biophys J. 2000;79:1053–1065. doi: 10.1016/S0006-3495(00)76359-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Grudzielanek S, Velkova A, Shukla A, Smirnovas V, Tatarek-Nossol M, Rehage H, et al. Cytotoxicity of insulin within its self-assembly and amyloidogenic pathways. J Mol Biol. 2007;370:372–384. doi: 10.1016/j.jmb.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 226.Anguiano M, Nowak RJ, Lansbury PT., Jr Protofibrillar islet amyloid polypeptide permeabilizes synthetic vesicles by a pore-like mechanism that may be relevant to type II diabetes. Biochemistry. 2002;41:11338–11343. doi: 10.1021/bi020314u. [DOI] [PubMed] [Google Scholar]
- 227.Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochemistry. 2003;42:10971–10977. doi: 10.1021/bi034889i. [DOI] [PubMed] [Google Scholar]
- 228.Jayasinghe SA, Langen R. Lipid membranes modulate the structure of islet amyloid polypeptide. Biochemistry. 2005;44:12113–12119. doi: 10.1021/bi050840w. [DOI] [PubMed] [Google Scholar]
- 229.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound α-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 230.Meier JJ, Kayed R, Lin CY, Gurlo T, Haataja L, Jayasinghe S, et al. Inhibition of human IAPP fibril formation does not prevent β-cell death: evidence for distinct actions of oligomers and fibrils of human IAPP. Am J Physiol Endocrinol Metab. 2006;291:E1317–E1324. doi: 10.1152/ajpendo.00082.2006. [DOI] [PubMed] [Google Scholar]
- 231.Lin CY, Gurlo T, Kayed R, Butler AE, Haataja L, Glabe CG, et al. Toxic human islet amyloid polypeptide (h-IAPP) oligomers are intracellular, and vaccination to induce anti-toxic oligomer antibodies does not prevent h-IAPP-induced β-cell apoptosis in h-IAPP transgenic mice. Diabetes. 2007;56:1324–1332. doi: 10.2337/db06-1579. [DOI] [PubMed] [Google Scholar]
- 232.Serpell LC. Alzheimer's amyloid fibrils: structure and assembly. Biochim Biophys Acta. 2000;1502:16–30. doi: 10.1016/s0925-4439(00)00029-6. [DOI] [PubMed] [Google Scholar]
- 233.Eisenberg D, Nelson R, Sawaya MR, Balbirnie M, Sambashivan S, Ivanova MI, et al. The structural biology of protein aggregation diseases: Fundamental questions and some answers. Ace Chem Res. 2006;39:568–575. doi: 10.1021/ar0500618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kayed R, Head E, Thompson JL, McIntire TM, Milton SC, Cotman CW, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–489. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- 235.Kelly JW. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Curr Opin Struct Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 236.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 237.Stefani M, Dobson CM. Protein aggregation and aggregate toxicity: new insights into protein folding, misfolding diseases and biological evolution. J Mol Med. 2003;81:678–699. doi: 10.1007/s00109-003-0464-5. [DOI] [PubMed] [Google Scholar]
- 238.Fezoui Y, Hartley DM, Walsh DM, Selkoe DJ, Osterhout JJ, Teplow DB. A do novo designed helix-turn-helix peptide forms non-toxic amyloid fibrils. Nat Struct Biol. 2000;7:1095–1099. doi: 10.1038/81937. [DOI] [PubMed] [Google Scholar]
- 239.Wang C, Huang L, Wang L, Hong Y, Sha Y. One-dimensional self-assembly of a rational designed β-structure peptide. Biopolymers. 2007;86:23–31. doi: 10.1002/bip.20681. [DOI] [PubMed] [Google Scholar]
- 240.Kammerer RA, Steinmetz MO. Do novo design of a two-stranded coiled-coil switch peptide. J Struct Biol. 2006;155:146–153. doi: 10.1016/j.jsb.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 241.Guijarro JI, Sunde M, Jones JA, Campbell ID, Dobson CM. Amyloid fibril formation by an SH3 domain. Proc Natl Acad Sci USA. 1998;95:4224–4228. doi: 10.1073/pnas.95.8.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Litvinovich SV, Brew SA, Aota S, Akiyama SK, Haudenschild C, Ingham KC. Formation of amyloid-like fibrils by self-association of a partially unfolded fibronectin type III module. J Mol Biol. 1998;280:245–258. doi: 10.1006/jmbi.1998.1863. [DOI] [PubMed] [Google Scholar]
- 243.Jimenez JL, Guijarro JI, Orlova E, Zurdo J, Dobson CM, Sunde M, et al. Cryo-electron microscopy structure of an SH3 amyloid fibril and model of the molecular packing. EMBO J. 1999;18:815–821. doi: 10.1093/emboj/18.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zurdo J, Guijarro JI, Dobson CM. Preparation and characterization of purified amyloid fibrils. J Am Chem Soc. 2001;123:8141–8142. doi: 10.1021/ja016229b. [DOI] [PubMed] [Google Scholar]
- 245.Zurdo J, Guijarro JI, Jimenez JL, Saibil HR, Dobson CM. Dependence on solution conditions of aggregation and amyloid formation by an SH3 domain. J Mol Biol. 2001;311:325–340. doi: 10.1006/jmbi.2001.4858. [DOI] [PubMed] [Google Scholar]
- 246.Carulla N, Caddy GL, Hall DR, Zurdo J, Gairi M, Feliz M, et al. Molecular recycling within amyloid fibrils. Nature. 2005;436:554–558. doi: 10.1038/nature03986. [DOI] [PubMed] [Google Scholar]
- 247.Polverino de Laureto P, Taddei N, Frare E, Capanni C, Costantini S, Zurdo J, et al. Protein aggregation and amyloid fibril formation by an SH3 domain probed by limited proteolysis. J Mol Biol. 2003;334:129–141. doi: 10.1016/j.jmb.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 248.Chiti F, Bucciantini M, Capanni C, Taddei N, Dobson CM, Stefani M. Solution conditions can promote formation of either amyloid protofilaments or mature fibrils from the HypF N-terminal domain. Protein Sci. 2001;10:2541–2547. doi: 10.1110/ps.10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Chiti F, Webster P, Taddei N, Clark A, Stefani M, Ramponi G, et al. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc Natl Acad Sci USA. 1999;96:3590–3594. doi: 10.1073/pnas.96.7.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tjernberg L, Hosia W, Bark N, Thyberg J, Johansson J. Charge attraction and β propensity are necessary for amyloid fibril formation from tetrapeptides. J Biol Chem. 2002;277:43243–43246. doi: 10.1074/jbc.M205570200. [DOI] [PubMed] [Google Scholar]
- 251.Lopez De La Paz M, Goldie K, Zurdo J, Lacroix E, Dobson CM, Hoenger A, et al. De novo designed peptide-based amyloid fibrils. Proc Natl Acad Sci USA. 2002;99:16052–16057. doi: 10.1073/pnas.252340199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Relini A, Torrassa S, Rolandi R, Gliozzi A, Rosano C, Canale C, et al. Monitoring the process of HypF fibrillization and liposome permeabilization by protofibrils. J Mol Biol. 2004;338:943–957. doi: 10.1016/j.jmb.2004.03.054. [DOI] [PubMed] [Google Scholar]
- 253.Parbhu A, Lin H, Thimm J, Lal R. Imaging real-time aggregation of amyloid β protein (1–42) by atomic force microscopy. Peptides. 2002;23:1265–1270. doi: 10.1016/s0196-9781(02)00061-x. [DOI] [PubMed] [Google Scholar]
- 254.Poirier MA, Li H, Macosko J, Cai S, Amzel M, Ross CA. Huntingtin spheroids and protofibrils as precursors in polyglutamine fibrilization. J Biol Chem. 2002;277:41032–41037. doi: 10.1074/jbc.M205809200. [DOI] [PubMed] [Google Scholar]
- 255.Conway KA, Harper JD, Lansbury PT., Jr Fibrils formed in vitro from α-synuclein and two mutant forms linked to Parkinson's disease are typical amyloid. Biochemistry. 2000;39:2552–2563. doi: 10.1021/bi991447r. [DOI] [PubMed] [Google Scholar]
- 256.Calamai M, Taddei N, Stefani M, Ramponi G, Chiti F. Relative influence of hydrophobicity and net charge in the aggregation of two homologous proteins. Biochemistry. 2003;42:15078–15083. doi: 10.1021/bi030135s. [DOI] [PubMed] [Google Scholar]
- 257.Bucciantini M, Giannoni E, Chiti F, Baroni F, Formigli L, Zurdo J, et al. Inherent toxicity of aggregates implies a common mechanism for protein misfolding diseases. Nature. 2002;416:507–511. doi: 10.1038/416507a. [DOI] [PubMed] [Google Scholar]
- 258.Bucciantini M, Calloni G, Chiti F, Formigli L, Nosi D, Dobson CM, et al. Prefibrillar amyloid protein aggregates share common features of cytotoxicity. J Biol Chem. 2004;279:31374–31382. doi: 10.1074/jbc.M400348200. [DOI] [PubMed] [Google Scholar]
- 259.Bucciantini M, Rigacci S, Berti A, Pieri L, Cecchi C, Nosi D, et al. Patterns of cell death triggered in two different cell lines by HypF-N prefibrillar aggregates. FASEB J. 2005;19:437–439. doi: 10.1096/fj.04-3086fje. [DOI] [PubMed] [Google Scholar]
- 260.Cecchi C, Pensalfini A, Baglioni S, Fiorillo C, Caporale R, Formigli L, et al. Differing molecular mechanisms appear to underlie early toxicity of prefibrillar HypF-N aggregates to different cell types. FEBS J. 2006;273:2206–2222. doi: 10.1111/j.1742-4658.2006.05234.x. [DOI] [PubMed] [Google Scholar]
- 261.Tartaglia GG, Pechmann S, Dobson CM, Vendruscolo M. Life on the edge: a link between gene expression levels and aggregation rates of human proteins. Trends Biochem Sci. 2007;32:204–206. doi: 10.1016/j.tibs.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 262.White JA, Manelli AM, Holmberg KH, Van Eldik LJ, Ladu MJ. Differential effects of oligomeric and flbrillar amyloid-β1–42 on astrocyte-mediated inflammation. Neurobiol Dis. 2005;18:459–465. doi: 10.1016/j.nbd.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 263.Ye C, Walsh DM, Selkoe DJ, Hartley DM. Amyloid β-protein induced electrophysiological changes are dependent on aggregation state: N-methyl-D-aspartate (NMDA) versus non-NMDA receptor/channel activation. Neurosci Lett. 2004;366:320–325. doi: 10.1016/j.neulet.2004.05.060. [DOI] [PubMed] [Google Scholar]
- 264.Ye CP, Selkoe DJ, Hartley DM. Protofibrils of amyloid β-protein inhibit specific K+ currents in neocortical cultures. Neurobiol Dis. 2003;13:177–190. doi: 10.1016/s0969-9961(03)00068-8. [DOI] [PubMed] [Google Scholar]