Abstract
Obesity plays a central role in the development of insulin resistance and type 2 diabetes. We therefore examined the effects of a modified form of ciliary neurotrophic factor [Axokine, which is hereafter referred to as ciliary neurotrophic factor (CNTF)Ax15], which uses a leptin-like mechanism to reduce body weight, in the db/db murine model of type 2 diabetes. In previous studies, weight loss produced by CNTF treatment could largely be attributed to its effects on food intake. In contrast, CNTFAx15 treatment of db/db mice caused significantly greater weight loss and marked improvements in diabetic parameters (e.g., levels of glucose, insulin, triglyceride, cholesterol, and nonesterified free fatty acids) than could be accounted for by reduced caloric intake alone. These beneficial effects, above and beyond those seen in animals controlled for either food restriction or body weight, correlated with the ability of CNTFAx15 to increase metabolic rate and energy expenditure and reduce hepatic steatosis while enhancing hepatic responsiveness to insulin. The hepatic effects were linked to rapid alterations in hepatic gene expression, most notably reduced expression of stearoyl-CoA desaturase 1, a rate-limiting enzyme in the synthesis of complex lipids that is also markedly suppressed by leptin in ob/ob mice. These observations further link the mechanisms of CNTF and leptin action, and they suggest important, beneficial effects for CNTF in diabetes that may be distinct from its ability to decrease food intake; instead, these effects may be more related to its influence on energy expenditure and hepatic gene expression.
Like leptin, systemic administration of ciliary neurotrophic factor (CNTF) decreases food intake in a dose-dependent manner in obese, leptin-deficient (ob/ob) mice, resulting in reduced body weight and adiposity (1, 2). These similarities in leptin and CNTF action have been attributed to the fact that the leptin receptor ObR and the α subunit of the CNTF receptor are similarly distributed in the hypothalamus (1, 3-5), particularly in regions involved in the regulation of food intake and body weight (6-8). Moreover, the glycoprotein gp130β, a component of the tripartite CNTF receptor complex, exhibits structural homologies to ObR (9), such that binding of leptin or CNTF to their respective receptors activates similar signal transduction pathways (1, 2, 10-12), most notably the signal transducer and activator of transcription 3. Consequently, systemic administration of CNTF or leptin activates receptors in the arcuate nucleus of the hypothalamus and suppresses the expression of orexigenic peptides, such as neuropeptide Y and agouti-related peptide (2, 13-16). CNTF also effectively reduces food intake and body weight in ”leptin-resistant” forms of obesity, such as diet-induced obesity (1, 2).
Obesity plays a central role in the development of insulin resistance and other features of the metabolic syndrome. Therefore, the development of therapies that reduce appetite and/or maintain energy expenditure during periods of caloric restriction should also prove to be of significant utility in preventing or treating type 2 diabetes (17, 18). Leptin and CNTF not only reduce body weight in ob/ob mice, but also correct associated hyperinsulinemia and hyperlipidemia. Although it has been shown that CNTF effectively reduces body weight and normalizes serum insulin and lipid levels in a variety of animal models of obesity that are resistant to the actions of leptin (1, 2), published studies have not conclusively demonstrated the extent to which the improvements produced by CNTF treatment are secondary or more directly related to the observed reductions in food intake and body weight. To further explore the effects of CNTF on glucose and lipid metabolism, we conducted studies in C57BL/KS-Lepdb (db/db) mice, a model of type 2 diabetes that results from the loss of functional ObRs. In this strain of mice, metabolic abnormalities manifest early during development and are quite severe in young adult animals. Moreover, once established, these metabolic changes are resistant to modulation by caloric restriction or weight reduction compared with other mouse models of obesity-associated insulin resistance and dyslipidemia (19).
In contrast to previous studies with obese mice, in which weight loss caused by leptin and CNTF could largely be attributed to their effects on food intake, we report that CNTFAx15 treatment of db/db mice causes significantly more weight loss than can be accounted for by reduced food intake alone and, instead, correlates with marked increases in metabolic rate and energy expenditure. Moreover, administration of CNTFAx15 produces marked improvements in diabetic parameters [e.g., levels of glucose, insulin, triglyceride, cholesterol, and nonesterified free fatty acids (NEFA)], beyond that achieved by either equivalent weight loss or equivalent food restriction. We report that these beneficial effects on diabetic parameters not only correlate with increases in metabolic rate, but are also linked with the ability of CNTFAx15 to rapidly reduce hepatic steatosis while enhancing liver responsiveness to insulin. These effects are accompanied by rapid alterations in hepatic gene expression, most notably the reduced expression of stearoyl-CoA desaturase 1 (SCD-1), a rate-limiting enzyme in the synthesis of complex lipids. These observations further link the mechanisms of CNTF and leptin action and demonstrate that CNTF also exerts important, beneficial effects on glucose and lipid homeostasis in diabetes that are not secondary to its ability to decrease food intake but reflect its specific effects on energy expenditure and hepatic gene expression.
Methods
Animals and Experimental Procedures. Male C57BL/KS-Lepdb (db/db) and nondiabetic littermate mice (The Jackson Laboratory) were obtained at 7-8 weeks of age and housed in 12 hr of light per day at 21-23°C and 40-60% humidity. All experiments began at 10 weeks of age, and all animal procedures were conducted in compliance with protocols approved by the Institutional Animal Care and Use Committee. CNTFAx15, which is our designation for a recombinant variant of human CNTF (Axokine), was administered by s.c. injection as described (2). For glucose tolerance testing, all animals were fasted for 16-18 hr before gavaging with a standard glucose bolus, as outlined (19). For assessment of insulin activation of signaling molecules, animals were anesthetized and a bolus of insulin (1 unit) was administered through the jugular vein; 2 or 10 min later, the liver was rapidly removed and frozen at -80°C until processed.
Serum Chemistry and Tissue Analysis. Serum samples were taken between 1000 and 1200 hours and analyzed for glucose, triglycerides, and cholesterol with the Monarch blood chemistry analyzer (Instrumentation Laboratory, Lexington, MA). NEFA were analyzed with a diagnostic kit (Wako Chemical, Osaka) and insulin levels by ELISA (Linco Research Immunoassay, St. Charles, MO). Tissue samples for histological analysis were taken at the conclusion of the experiments and fixed overnight in 10% buffered formalin. For hematoxylin/eosin staining, tissue was embedded in paraffin and sections were cut at 6 μm, placed onto glass slides, and deparaffinized with xylene. For analysis of endogenous lipids, frozen sections of liver were mounted on glass slides and stained with oil red O. Liver glycogen was measured from frozen tissue by assaying for glucose after amyloglucosidase digestion with a correction for nonglycogen glucose (19).
Tissue Lysates and SDS/PAGE. Liver samples were separately homogenized on ice in buffer A (1% Nonidet P-40 buffer/50 mM Hepes, pH 7.4/150 mM NaCl/1 mM EDTA/30 mM sodium pyrophosphate/50 mM sodium fluoride/0.5 mM sodium orthovanadate/5 μg/ml aprotinin/5 μg/ml leupeptin/1 mM PMSF) and centrifuged for 10 min at 14,000 × g. The supernatant was taken and the protein level was quantified by using a bicinchoninic acid protein assay (BCA; Pierce). Samples were used for immunoprecipitation (1 mg), or equal amounts of protein were resolved by SDS/PAGE (8% precast gels; NOVEX, San Diego). Proteins were transferred to nitrocellulose membranes to be blocked and then immunoblotted with anti-phosphotyrosine (Upstate Biotechnologies, Lake Placid, NY), anti-αp85 subunit (Santa Cruz Biotechnology), or phosphospecific Akt (Ser-473) polyclonal antibodies (Cell Signaling Technology, Beverly, MA). After secondary antibody incubation (goat anti-rabbit- or anti-mouse horseradish peroxidase-conjugated; Roche Molecular Biochemicals) detection was by the Renaissance enhanced chemiluminescence detection system (Dupont/NEN).
Real-Time PCR and Northern Blotting. Tissues were rapidly dissected and immediately frozen at -80°C. RNA was isolated by using Tri reagent (Invitrogen). Tissue specific expression was analyzed in separate reactions with the TaqMan real-time PCR chemistry and detection system [Applied Biosystems for SCD-1, glycerolpalmitoyl acyl-transferase (GPAT), carnitine palmitoyl-CoA transferase 1 (CPT-1), peroxisomal proliferation-activated receptor (PPAR)α, PPARγ, uncoupling protein (UCP)-2]. Control genomic DNA was used as a standard to estimate copies of molecule per cell, and all probes were run with a no-reverse transcriptase control for assessment of genomic DNA contamination. Samples were done in duplicate from individual animal tissues. Results are expressed as fold change from vehicle-treated db/db levels. Northern blots were done on samples from pools of three mice as described (2).
Indirect Calorimetry. Metabolic measurements were obtained by using an Oxymax open circuit indirect calorimetry system (Columbus Instruments, Columbus, OH). The system was calibrated against a standard gas mixture to measure O2 consumed (VO2, ml/kg per hr) and CO2 generated (VCO2, ml/kg per hr). These measurements were taken on animals that had received 9 days of CNTF or vehicle treatment. The first 2 hr were a period of adaptation for the animals, and then metabolic rate (VO2), respiratory quotient (ratio of VCO2/VO2) and activity (counts) were evaluated for a 24-hr period. Energy expenditure (or heat) was calculated as the product of the calorific value of oxygen (3.815 + 1.232 × respiratory quotient) and the volume of O2 consumed.
Statistical Analyses. Data are expressed as mean ± SEM, and ANOVA was conducted by using the program STATVIEW (Abacus Concepts, Berkeley, CA). When a significant F ratio was obtained (P < 0.05), a post hoc analysis was conducted between groups by using a multiple comparison procedure with a Bonferroni/Dunn correction of means (ANOVA) or a Dunnett's post hoc comparison. P values <0.05 were considered significant.
Results
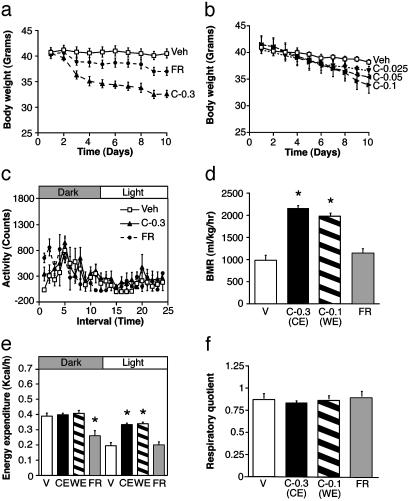
CNTFAx15 Markedly Increases Metabolic Rate in db/db Mice. CNTFAx15 treatment (0.3 mg/kg per day) consistently produced greater weight loss in diabetic db/db mice than was evident in control db/db mice given the same amount of food [food restricted (FR) group] (Fig. 1a). Indeed, a 3-fold lower dose of CNTFAx15 (0.1 mg/kg), which results in a milder reduction in food intake, was required to match the weight loss achieved by the FR group (Fig. 1b). This reduction is in contrast to the situation in ob/ob mice or diet-induced obesity in AKR/J mice, in which most of the weight loss induced by CNTFAx15 can be attributed to decreased food intake, because the amount of weight loss in these models can be mimicked by simply restricting food intake to the same degree it is suppressed by CNTFAx15 (1, 2).
Fig. 1.
CNTFAx15 treatment causes greater weight loss than food restriction in db/db mice because of increases in metabolic rate. (a) Weight loss in groups of db/db mice receiving daily s.c. injection of vehicle or CNTFAx15 at 0.3 mg/kg per day (C-0.3) compared with the weight loss in FR db/db mice forced to reduce their food intake to the same degree as the C-0.3 mice (note less weight loss in the FR group, although they consumed the same number of calories as the C-0.3 group). (b) Weight loss in groups of db/db mice treated with CNTFAx15 at 0.1, 0.05, or 0.025 mg/kg per day (C-0.1, C-0.05, or C-0.025). Different amounts of CNTFAx15 were used to find a dose (C-0.1) that matches the degree of weight loss seen in the FR group from a. All data are expressed as mean ± SEM (n = 4-6). On the 10th day, indirect calorimetry was performed for assessment of locomotor activity (counts; each interval ≈1 hr) (c), basal metabolic rate (BMR) during the light period (VO2; ml/kg per hr) (d), energy expenditure in kcal/hr (e), and respiratory quotient (ratio of VCO2/VO2) (f). The shaded portions of c and e indicate the dark period, and each point represents mean ± SEM (n = 4-6). P < 0.001 - the difference from ad lib-fed db/db vehicle (V) by Dunnett's post hoc test. CE, caloric equivalent; WE, weight equivalent.
The disparity in weight loss caused by CNTFAx15 compared with equivalent caloric restriction in db/db mice suggested that CNTFAx15 might also increase metabolic rate or energy expenditure. To test this possibility, CNTFAx15-treated db/db mice were compared with FR db/db mice by using indirect calorimetry; comparisons were made between FR db/db mice and those treated with doses of CNTFAx15 that were matched for equivalent caloric intake [caloric equivalent (CE) group, CNTFAx15 at 0.3 mg/kg per day] or for the weight equivalence [weight equivalent (WE) group, CNTFAx15 at 0.1 mg/kg per day]. Regardless of the dose, CNTFAx15 treatment elevated basal metabolic rate (BMR; VO2 during the light period only) (Fig. 1d) and increased energy expenditure [kcal/hr (1 kcal = 4.18 kJ)] (Fig. 1e) above that seen in both vehicle and FR controls. However, the levels and circadian periodicity of locomotor activity were not altered in CNTFAx15-treated mice relative to vehicle controls (Fig. 1c), and no switch in metabolic substrate was observed as evidenced by similar respiratory quotients (Fig. 1f). These observations show that in diabetic mice, CNTFAx15 treatment can produce a much more profound decrease in body weight than comparable caloric restriction and that this differential weight loss can be attributed to marked increases in BMR and energy expenditure.
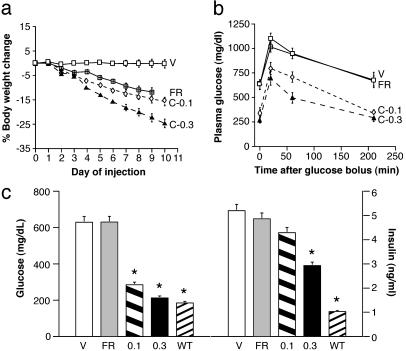
CNTFAx15 Improves Diabetic Parameters Beyond That Achieved by Food Restriction Alone. Vehicle-treated db/db mice exhibited fasting hyperglycemia (630 ± 50 mg/dl), hyperinsulinemia (5.2 ± 0.75 ng/ml), and impaired glucose tolerance characteristic of this strain (20, 21). Fasting plasma glucose and insulin levels were significantly reduced in mice treated with CNTFAx15 (Fig. 2b), and oral glucose tolerance was also markedly improved (Fig. 2c; ANOVA for group and interaction; P < 0.001). Similar improvements in glucose and insulin homeostasis were not seen in FR control mice, whether compared with CNTFAx15-treated mice matched for caloric intake (group C-0.3) or body weight (group C-0.1). Serum NEFA and triglycerides were also significantly reduced by CNTFAx15 relative to levels evident in both vehicle and FR control mice (Table 1). In a time course experiment conducted on a separate cohort of mice, the effect of CNTFAx15 (0.3 mg/kg per day) on nonfasting serum glucose levels was apparent within 2 days of the initiation of treatment, with a 50% reduction evident by day 4 (P < 0.05; data not shown). Fasting glucose levels approached nondiabetic control levels by day 10 of treatment (213 ± 23; not significantly different from lean db/? controls, 185 ± 8 mg/dl). At no time were any of the CNTFAx15-treated db/db mice hypoglycemic. Caloric restriction did produce a modest (≈25%) reduction in serum cholesterol levels similar to that observed with CNTFAx15 treatment. However, food restriction alone again failed to improve glycemic control in db/db mice. This observation is particularly significant, because rigorous control of hyperglycemia in diabetic humans can significantly attenuate the development of chronic complications associated with type 2 diabetes, such as retinopathy and nephropathy (22, 23).
Fig. 2.
Treatment with CNTFAx15 decreases body weight and improves glucose tolerance. Male db/db mice treated with either vehicle (V), CNTFAx15 at 0.1 or 0.3 mg/kg per day (C-0.1 or C-0.3), or pair-fed to the C-0.3 treatment group (FR). (a) Daily body weight changes are shown as the percent difference from the first day of injection. (b) An oral glucose tolerance test was conducted 24 hr after the last treatment, and serum glucose was measured at the indicated times after oral gavage. All data are expressed as mean (n ≥ 6) ± SEM. (c) Fasting serum glucose (mg/dl) and insulin (ng/ml) levels (mean ± SEM, n = 6-10) for each group and from lean, nondiabetic littermates (WT). Tolerance test, C-0.1 vs. vehicle (F1,3 = 114.8, P < 0.001) and C-0.3 vs. vehicle (F1,3 = 78.8, P < 0.001). For glucose P < 0.001 and for insulin P < 0.05. * indicates the difference between ad lib-fed db/db vehicle and FR controls by Dunnett's post hoc test.
Table 1. Effects of treatment with CNTFAx15 (0.1 or 0.3 mg/kg per day s.c. for 10 days; C-0.1 or C-0.3) or pair feeding (FR) on body weight and serum levels in male db/db mice.
| Genotype | Treatment | No. | Body weight, g | Insulin, ng/ml | Glucose, mg/dl | NEFA, mmol/liter | Triglyceride, mg/dl | Cholesterol, mg/dl |
|---|---|---|---|---|---|---|---|---|
| db/? | 24 | 26.4 ± 0.4* | 1.0 ± 0.2* | 185 ± 8* | 0.88 ± 0.05* | 69 ± 4* | 91 ± 5 | |
| db/db | Vehicle | 12 | 40.0 ± 0.6 | 5.2 ± 0.8 | 630 ± 50 | 1.53 ± 0.09 | 92 ± 10 | 121 ± 14 |
| C-0.1 | 24 | 35.3 ± 0.4* | 4.3 ± 0.4* | 285 ± 32* | 1.00 ± 0.1* | 70 ± 6 | 115 ± 5 | |
| C-0.3 | 24 | 31.5 ± 0.6* | 2.9 ± 0.4 | 213 ± 23* | 1.05 ± 0.07* | 57 ± 3* | 89 ± 7* | |
| FR | 18 | 35.7 ± 0.5* | 4.9 ± 0.4 | 631 ± 44 | 1.22 ± 0.04 | 73 ± 3 | 92 ± 3* |
Mean ± SEM for body weight and serum levels of insulin, glucose, NEFA, triglycerides, and cholesterol are shown. *, P < 0.05 versus the vehicle-treated mice.
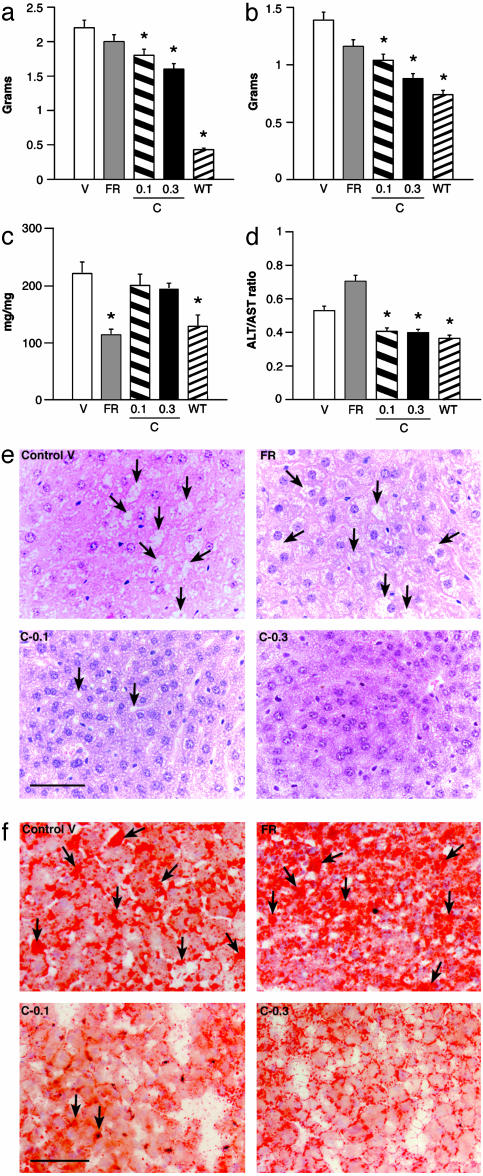
Because white adipose tissue plays a prominent role in energy homeostasis, we examined the effect of CNTFAx15 treatment on epididymal white adipose tissue (EWAT). The mass of this fat depot was reduced significantly in mice treated with CNTFAx15 (Fig. 3a) compared with mice injected with vehicle. Food restriction tended to reduce EWAT weight, although not to the same degree as that seen in CNTFAx15-treated mice. Microscopic evaluation revealed no obvious changes in adipocyte cell morphology in the EWAT of CNTFAx15-treated mice relative to FR controls, and there was no evidence of multilocular brown adipocytes (data not shown).
Fig. 3.
CNTFAx15 treatment decreases adiposity and improves hepatic function. Male db/db mice treated with CNTFAx15 at 0.1 or 0.3 mg/kg per day for 10 days had reduced EWAT (a) compared with FR or vehicle and reduced total liver weight (b). Liver glycogen content (c) was significantly reduced by pair feeding (FR), whereas liver function (d), as assessed by the ratio of serum ALT/AST, was significantly improved only by CNTFAx15 treatment. Each bar represents mean ± SEM (n = 6-8). (e) Hematoxylin/eosin (H&E) staining of liver sections revealed reduced lipid vacuoles (arrows) (scale bar = 100 μm), which was confirmed by oil red O staining (f). EWAT, P < 0.001; liver, P < 0.001; glycogen, P < 0.001. * indicates the difference from ad lib-fed db/db vehicle control by Dunnett's post hoc test.
CNTFAx15 Improves Hepatic Adiposity, Function, and Insulin Sensitivity Beyond That Achieved by Simple Food Restriction or Weight Reduction. Serum and tissue markers of hepatic function also were measured. Characteristically, db/db mice present with moderate hepatomegaly (Fig. 3b) and impaired liver function as indicated by an elevation in the serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (Fig. 3d). These changes are thought to be secondary to the hepatic accumulation of fat, which is evident in hematoxylin and eosin (Fig. 3e) and oil red O-stained sections of the livers of control db/db mice (Fig. 3f). Treatment with CNTFAx15 reduced the liver weight (Fig. 3b) and normalized the serum ALT/AST (Fig. 3d). Lipid deposition in liver was also markedly reduced (Fig. 3f). In contrast, food restriction produced only a small, insignificant reduction in liver weight, which was accompanied by a marked depletion of hepatic glycogen stores (Fig. 3c). Food restriction also did not improve hepatic function (ALT/AST) or decrease lipid deposition in the liver. The combined effect of CNTFAx15 to reduce serum glucose and lipid levels, preserve liver glycogen, reduce the ALT/AST ratio, and reduce the deposition of neutral lipid in the liver are indicative of a marked improvement in obesity-related fatty liver that is distinct from the effects of equivalent caloric restriction or weight reduction alone.
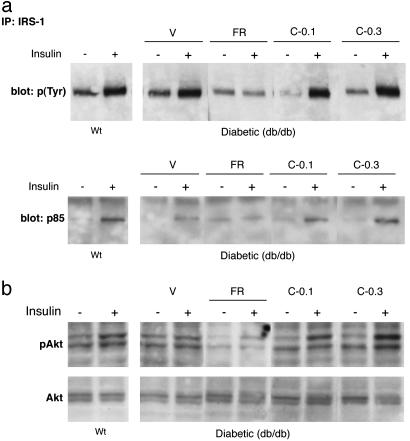
The differential improvements in liver function and glycemic control produced by CNTFAx15 treatment could be the consequence of improved hepatic insulin action. To explore this possibility, we compared the activation of insulin-specific intracellular signaling cascades in control and treated mice. In lean mice, injection of insulin produced rapid tyrosine phosphorylation of insulin receptor substrate 1 (IRS-1); recruitment of p85, the regulatory subunit of phosphatidylinositol 3-kinase; and phosphorylation of the downstream signaling molecule Akt (Fig. 4). Although the insulin-induced phosphorylation of IRS-1 did not appear to be blunted in diabetic db/db mice, phosphatidylinositol 3-kinase recruitment and activation of Akt kinase were compromised (Fig. 4 a and b). These findings are consistent with previously published reports (24). CNTFAx15 treatment increased hepatic insulin sensitivity as evidenced by the robust phosphorylation of IRS-1, improved recruitment of p85, and restored activation of Akt kinase (groups C-0.1 and C-0.3). However, food restriction alone failed to improve hepatic insulin sensitivity (Fig. 4 a and b).
Fig. 4.
CNTFAx15 treatment improves insulin-stimulated signaling in the liver of diabetic mice. After 10 days of CNTFAx15 treatment, mice were given a bolus of insulin (+) or vehicle (-) and assessed for IRS-1 phosphorylation status and recruitment of the p85 subunit of phosphatidylinositol 3-kinase in lean, nondiabetic mice (WT) and treated, db/db mice [vehicle (V), FR, C-0.1, and C-0.3] (a). A robust tyrosine phosphorylation [p(Tyr)] of IRS-1 and subsequent p85 recruitment was seen consistently in both groups of CNTFAx15-treated mice (C-0.1 and C-0.3) but not in FR mice. (b) Activation of the downstream signaling molecule Akt kinase was assessed in similarly treated mice 10 min after insulin administration. A consistent increase in phospho-Akt (pAKt) was observed in lean control mice but not in diabetic FR mice and diabetic mice that were injected with vehicle. In contrast, the insulin-stimulated increase in pAkt was at least as robust in CNTFAx15-treated db/db mice as in lean, nondiabetic littermates. Moreover, a clear decrease in basal levels of phospho-Akt was evident in FR mice, and this was also corrected by administration of CNTFAx15.
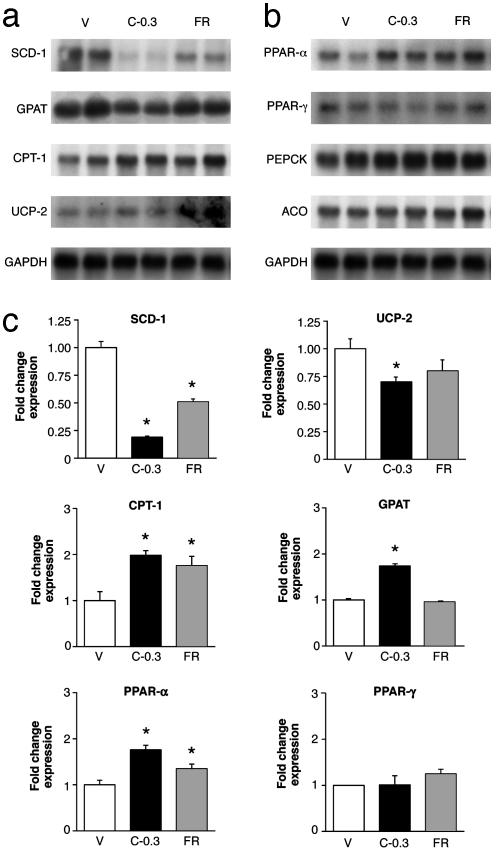
CNTFAx15-Induced Changes in Liver Correlate with Rapid Reduction in mRNA Levels of SCD-1 and Other Genes Associated with Fatty Acid Synthesis. To elucidate the molecular mechanisms by which CNTF induces physiological changes, we probed for differential changes in gene expression by Northern blot analysis and real-time PCR assays in hepatic tissue taken 4 days after the initiation of CNTFAx15 treatment or food restriction (Fig. 5). One of the most striking features of CNTFAx15 treatment was the rapid and specific decrease in the expression of genes associated with triacylglycerol synthesis and uptake in the liver, such as GPAT and SCD-1. The change in SCD-1, in particular, was marked in CNTFAx15-treated mice as compared with a more modest change in FR animals. Associated with treatment, but also evident in the FR group, PPARα mRNA was increased < 2-fold (Fig. 5 b and c) with its target enzyme of fatty acid oxidation, CPT-1. No change was detected in Acyl Co-A oxidase mRNA. The net effect of the changes observed in CNTFAx15-treated mice would be to reduce fatty acid biosynthesis and decrease hepatic lipid content, consistent with the above histological and serum chemistry results. The expression of several genes important in carbohydrate metabolism and known to be regulated by leptin (25), such as phosphoenolpyruvate carboxylase (Fig. 5b), glucose-6-phosphatase, fructose bisphosphatase, and hexokinase, were not altered by this brief CNTFAx15 treatment (data not shown). Analysis of EWAT showed that CNTFAx15 administration produced a rapid, 2-fold decrease in mRNA for fatty acid synthase, but no changes in expression could be detected in EWAT by real-time PCR in 1-aminocyclopropane-1-carboxylate, GPAT, PPARγ, UCP-1, UCP-2, UCP-3, glucose transporters GLUT4 and GLUT1, and CPT-1 (data not shown).
Fig. 5.
CNTFAx15 treatment alters hepatic expression of genes involved in lipid metabolism. Liver tissue was collected from male db/db mice treated with CNTFAx15 (C-0.3) for 4 days and from FR or vehicle (V)-injected controls. Total RNA was prepared for assessment by Northern blots for SCD-1, GPAT, CPT-1, UCP-2 (a), PPARα, PPARγ, phosphoenolpyruvate carboxylase, and ACO (b). Each lane represents a pool of RNA from three mice. (c) The same RNA samples were analyzed by real-time PCR, and the results are expressed in bar graphs as the fold increase/decrease relative to controls that received only vehicle injections (mean ± SEM, n = 6-10). CPT-1, P < 0.001; PPARγ, P < 0.001; GPAT, P < 0.001. * indicates difference from ad lib-fed db/db vehicle control by Dunnett's post hoc test.
Conclusion
CNTF acts in a leptin-like manner by means of its receptors on hypothalamic neurons to activate pathways that are associated with decreases in food intake and body weight (2, 26). In studies reported to date, the weight loss caused by CNTF administration has largely been attributed to its effects on food intake. Herein we report that in the db/db model of diabetes, the effects of CNTFAx15 on weight loss and glucose and lipid metabolism are much more pronounced than would have been expected based on its effects on food intake. Thus, compared with db/db mice undergoing equivalent food restriction, CNTFAx15-treated animals lose significantly more weight, and this increased weight loss correlates to increased BMR and energy expenditure. In addition, CNTFAx15 treatment produced differential improvements in diabetic parameters (e.g., hyperinsulinemia, hyperglycemia, and hyperlipidemia) in db/db mice that correlate to a marked reduction in hepatic steatosis, improved liver function (as determined by serum ALT/AST), and enhanced responsiveness of the liver to insulin. These changes were accompanied by rapid alterations in hepatic gene expression, most notably reduced expression of SCD-1, a rate-limiting enzyme in the synthesis of complex lipids, and increased expression of CPT-1, which promotes lipid oxidation. Here, the specific CNTF-mediated reduction in the expression of hepatic SCD-1 is particularly noteworthy. Recently, Cohen et al. (25) reported that leptin administration dramatically reduces SCD-1 expression in the liver of ob/ob mice, further linking the mechanisms of CNTF and leptin action. Moreover, mice deficient in SCD-1 are lean and hypermetabolic, whereas ob/ob mice bearing a mutation in the SCD-1 gene are less obese and exhibit elevated levels of energy expenditure compared with control ob/ob mice.
These observations indicate that the marked improvements in glucose regulation observed in CNTFAx15-treated mice may not be attributable to a direct insulin-sensitizing action of CNTF on peripheral tissues. Instead, our observations suggest that these improvements in glucose regulation are secondary to a reduction in fatty acid synthesis and lipid accumulation in nonadipose tissues (such as liver, muscle, and pancreas), which are well established causes of insulin resistance. Therefore, in addition to producing weight loss per se, CNTFAx15 may afford additional benefits in the treatment of obesity associated with the metabolic syndrome type 2 diabetes and hepatic steatosis.
Acknowledgments
We thank the staff at Regeneron Pharmaceuticals for their support and assistance, especially V. Lan and S. Staton for artwork and K. D. Anderson for comments on and revisions to the manuscript.
Abbreviations: CNTF, ciliary neurotrophic factor; SCD-1, stearoyl-CoA desaturase 1; GPAT, glycerol-palmitoyl acyl-transferase; CPT-1, carnitine palmitoyl-CoA transferase 1; PPAR, peroxisomal proliferation-activated receptor; UCP, uncoupling protein; FR, food-restricted; CE, caloric equivalent; WE, weight equivalent; BMR, basal metabolic rate; EWAT, epididymal white adipose tissue; ALT, alanine aminotransferase; AST, aspartate aminotransferase; IRS-1, insulin receptor substrate 1.
References
- 1.Gloaguen, I., Costa, P., Demartis, A., Lazzaro, D., Marco, A. D., Graziani, R., Paonessa, G., Chen, F., Rosenblum, C. I., Van der Ploeg, L. H. T., et al. (1997) Proc. Natl. Acad. Sci. USA 94, 6456-6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lambert, P. D., Anderson, K. D., Sleeman, M. W., Wong, V., Tan, J., Hijarunguru, A., Corcoran, T. L., Murray, J. D., Thabet, K. E., Yancopoulos, G. D. & Wiegand, S. J. (2001) Proc. Natl. Acad. Sci. USA 98, 4652-4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLennan, A. J., Vinson, E. N., Marks, L., McLaurin, D. L., Pfeifer, M. & Lee, N. (1996) J. Neurosci. 16, 621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee, M.-Y., Hofmann, H.-D. & Kirsch, M. (1997) Neuroscience 77, 233-246. [DOI] [PubMed] [Google Scholar]
- 5.Sleeman, M. W., Anderson, K. D., Lambert, P. D., Yancopoulos, G. D. & Wiegand, S. J. (2000) Pharm. Acta Helv. 74, 265-272. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, C. C., Clifton, D. K. & Steiner, R. A. (1997) Endocrinology 138, 4489-4492. [DOI] [PubMed] [Google Scholar]
- 7.Elias, C. F., Aschkenasi, C., Lee, C., Kelly, J., Ahima, R. S., Bjørbæk, C., Flier, J. S., Saper, C. B. & Elmquist, J. K. (1999) Neuron 23, 775-786. [DOI] [PubMed] [Google Scholar]
- 8.Elmquist, J. K., Maratos-Flier, E., Saper, C. B. & Flier, J. S. (1998) Nat. Neurosci. 1, 445-450. [DOI] [PubMed] [Google Scholar]
- 9.Davis, S., Aldrich, T. H., Stahl, N., Pan, L., Taga, T., Kishimoto, T., Ip, N. Y. & Yancopoulos, G. D. (1993) Science 260, 1805-1808. [DOI] [PubMed] [Google Scholar]
- 10.Boulton, T. G., Stahl, N. & Yancopoulos, G. D. (1994) J. Biol. Chem. 269, 11648-11655. [PubMed] [Google Scholar]
- 11.Bjørbæk, C., Elmquist, J. K., El-Haschimi, K., Kelly, J., Ahima, R. S., Hileman, S. & Flier, J. S. (1998) Mol. Cell 1, 619-625.9660946 [Google Scholar]
- 12.Ziotopoulou, M., Erani, D. M., Hileman, S. M., Bjørbæk, C. & Mantzoros, C. S. (2000) Diabetes 49, 1890-1896. [DOI] [PubMed] [Google Scholar]
- 13.Stephens, T. W., Basinski, M., Bristow, P. K., Bue-Valleskey, J. M., Burgett, S. G., Craft, L., Hale, J., Hoffmann, J., Hsiung, H. M., Kriauciunas, A., et al. (1995) Nature 377, 530-532. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz, M. W., Seeley, R. J., Campfield, L. A., Burn, P. & Baskin, D. G. (1996) J. Clin. Invest. 98, 1101-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu, B., Dube, M. G., Kalra, P. S., Farmerie, W. G., Kaibara, A., Moldawer, L. L., Martin, D. & Kalra, S. P. (1998) Endocrinology 139, 466-473. [DOI] [PubMed] [Google Scholar]
- 16.Pu, S., Dhillon, H., Moldawer, L. L., Kalra, P. S. & Kalra, S. P. (2000) J. Neuroendocrinol. 12, 827-832. [DOI] [PubMed] [Google Scholar]
- 17.Tuomilehto, J., Lindstrom, J., Eriksson, J. G., Valle, T. T., Hamalainen, H., Ilanne-Parikka, P., Keinanen-Kiukaanniemi, S., Laakso, M., Louheranta, A., Rastas, M., et al. (2001) N. Engl. J. Med. 344, 1343-1350. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, B. C. (1999) Ann. N.Y. Acad. Sci. 892, 1-25. [DOI] [PubMed] [Google Scholar]
- 19.Tonra, J. R., Ono, M., Liu, X., Garcia, K., Jackson, C., Yancopoulos, G. D., Wiegand, S. J. & Wong, V. (1999) Diabetes 48, 588-594. [DOI] [PubMed] [Google Scholar]
- 20.Chen, H., Charlat, O., Tartaglia, L. A., Woolf, E. A., Weng, X., Ellis, S. J., Lakey, N. D., Culpepper, J., More, K. J., Breitbart, R. E., et al. (1996) Cell 84, 491-495. [DOI] [PubMed] [Google Scholar]
- 21.Tartaglia, L. A., Dembski, M., Weng, X., Deng, N., Culpepper, J., Devos, R., Richards, G. J., Campfield, L. A., Clark, F. T., Deeds, J., et al. (1995) Cell 83, 1263-1271. [DOI] [PubMed] [Google Scholar]
- 22.Colagiuri, S., Cull, C. A. & Holman, R. R. (2002) Diabetes Care 25, 1410-1417. [DOI] [PubMed] [Google Scholar]
- 23.Klein, R. & Klein, B. E. (1998) Diabetes Care 21, Suppl. 3, C39-C43. [DOI] [PubMed] [Google Scholar]
- 24.Shao, J., Yamashita, H., Qiao, L. & Friedman, J. E. (2001) J. Endocrinol. 167, 107-115. [DOI] [PubMed] [Google Scholar]
- 25.Cohen, P., Miyazaki, M., Socci, N. D., Hagge-Greenberg, A., Liedtke, W., Soukas, A. A., Sharma, R., Hudgins, L. C., Ntambi, J. M. & Friedman, J. M. (2002) Science 297, 240-243. [DOI] [PubMed] [Google Scholar]
- 26.Anderson, K. A., Lambert, P. D., Corcoran, T. L., Murray, J. D., Thabet, K. E., Yancopoulos, G. D. & Wiegand, S. J. (2003) J. Neuroendocrinol. 15, 1-12. [DOI] [PubMed] [Google Scholar]