Abstract
Background
Some individuals with autism spectrum disorders (ASD) experience linguistic difficulties similar to those found in individuals with specific language impairment (SLI). Whether these behaviours are indicative of a common underlying genetic cause or a superficial similarity is unclear.
Methods
Standardised language assessments were administered to three participant groups: parents of children with ASD (Par-A), parents of children with specific language/literacy impairment (Par-L) and parents of typically developing children (Par-T) (n = 30, in each group). Additionally, the Autism-Spectrum Quotient (AQ) was used to assess autism-like tendencies, in particular, social language use.
Results
The Par-A group performed better than the Par-L group (and identical to the Par-T group) on all language tests. Conversely, the Par-A group was characterised by higher levels of pragmatic difficulties than the other two groups, as measured by the communication subscale of the AQ.
Conclusions
No evidence was found for a shared phenotype in parents of children with ASD and SLI. A model is presented describing the relation between SLI and ASD.
Keywords: Autism spectrum disorder, specific language impairment, broad phenotype, genetics
Broader autism phenotype
It is well established that there is a strong genetic component to autism spectrum disorder (ASD). Siblings of those with ASD are at a greater risk of developing the disorder than the general population and monozygotic twins of affected children are at an even greater risk (Bailey et al., 1995). Further evidence for the genetic basis of the condition has emerged through investigations of non-affected family members of individuals with ASD, many of whom have social, communicative or imagination impairments similar to those seen in ASD but in much milder form (Bailey, Palferman, Heavey, & Le Couteur, 1998). This has led to the idea that the same genetic risk factors responsible for ASD may also be responsible for the more moderate difficulties observed in relatives (Bailey et al., 1998). Ascertaining which autistic-like behaviours are observed in non-affected family members will help reveal which deficits are genetically transmitted in ASD.
Understanding the communication deficit(s) in ASD: comparisons with SLI
Communication deficits are central to ASD. Individuals with ASD demonstrate repetitive use of language, limitations in their ability to initiate and sustain conversations, and a delay in the development of spoken language (APA, 1994). However, while pragmatic impairments – deficits in the use of language – are pervasive in this population (Bishop & Norbury, 2002), there is much variability in the linguistic difficulties affecting individuals with ASD (Kjelgaard & Tager-Flusberg, 2001). This variability has made it difficult, first, to understand the nature of the communication impairment seen in ASD, and second, to determine which communication deficit(s), if any, form part of the heritable phenotype of ASD.
Interest in the variable linguistic skills of individuals with ASD has been buoyed by comparisons with specific language impairment (SLI). SLI is recognised when a child fails to develop language in the normal timeframe for no apparent reason. As with ASD, family studies have suggested that there is a strong genetic component to SLI (Tomblin, 1989).
‘Textbook’ cases of ASD and SLI are easy enough to recognise and differentiate. The broad communicative difficulties of individuals with ASD (affecting both verbal and nonverbal communication) contrast with SLI, in which there is a relatively specific deficit in the development of linguistic ability. However, the sharp division between these diagnostic categories has come under question. First, many children have characteristics that are intermediate between ASD and SLI (Bishop & Norbury, 2002). Such cases have spawned the diagnostic category pragmatic language impairment (PLI), a disorder characterised by abnormal language use without additional autistic symptomatology (Bishop, 2000). Second, the diagnosis of SLI can be unstable over time, such that young children with a diagnosis of SLI may show symptoms more characteristic of ASD at a later date (Conti-Ramsden, Simkin, & Botting, 2006; Mawhood, Howlin, & Rutter, 2000). Third, a substantial proportion of individuals with ASD do poorly on the kinds of language task used to diagnose SLI (Kjelgaard & Tager-Flusberg, 2001). In particular, many children with ASD perform poorly on nonword repetition tests, in which they are required repeat nonsense words, such as ‘blonterstaping’ or ‘perplisteronk’ (Kjelgaard & Tager-Flusberg, 2001; Bishop et al., 2004). The task is of particular interest, because it appears to be a reliable behavioural marker of heritable SLI (Barry, Yasin, & Bishop, 2007; Bishop, North, & Donlan, 1996). Children with SLI typically repeat short nonwords accurately, but do much more poorly than control children with nonwords of three or more syllables (Bishop et al., 1996; Gathercole & Baddeley, 1990). This suggests a difficulty with holding novel phonological material in memory, rather than problems with perception or production.
The similarities between ASD and SLI have raised the suggestion that there is a subgroup of individuals with ASD – those with impaired linguistic ability – who inherit the same genetic risk factor as those with SLI (Kjelgaard & Tager-Flusberg, 2001). An alternative position is that the linguistic similarities between ASD and SLI are superficial and do not indicate any common underlying cause(s). Ascertaining the nature of the genetic relation between ASD and SLI has implications for our conceptualisation of each disorder. If there is genetic overlap between the two disorders, then the syndrome of a significant proportion of children with ASD may be better viewed as a kind of ‘SLI plus’, in which these individuals inherit the same genetic risk factor(s) as SLI but also inherit risk factor(s) associated with the additional pragmatic and nonlinguistic features of ASD (social and imagination impairments) (Bishop, 2003a). If, however, there is no overlap between the genetic risk factor(s) inherited by ASD and SLI, then diagnostic distinction between the two disorders should be maintained.
Comparing the broader phenotype of ASD and SLI
One way to reveal more about the heritable deficits of ASD is through cognitive and behavioural investigations of first-degree relatives. Two studies that considered whether relatives of people with ASD are at a higher risk for language impairments concluded that they are not (Bishop et al., 2004; Pilowsky, Yirmiya, Shalev, & Gross-Tsur, 2003).
In the current investigation, we made a direct comparison between the linguistic and pragmatic abilities of parents of children with ASD and parents of children with SLI. This study builds on work by Barry et al. (2007) who found that parents of children with SLI performed significantly worse than parents of matched controls on a number of language and short-term memory tasks. Here, we extended this study to consider performance of parents of ASD probands relative to these two groups, and also gathered data from the two clinical groups on a self-report measure that provided an index of pragmatic aspects of communication.
Method
Participants
Three groups of participants were recruited. The first group comprised parents of children with ASD (Par-A). Each participant in this group had at least one biological child (the proband) with a DSM-IV-based diagnosis of ASD (APA, 1994), which was confirmed using the Autism Diagnostic Observation Schedule-Generic (Lord et al., 2000). All probands had a nonverbal IQ of 85 or above, as determined by the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler & Chen, 1999). Parents of probands with a language/literacy impairment made up the second group (Par-L). Participants were included in this group if they had a biological child who performed below the 10th percentile on at least two standardised language tests. See Barry et al. (2007) for further detail of the diagnostic procedure. The Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003) was used to exclude ASD in any children where there was diagnostic uncertainty. The third participant group (Par-T) comprised parents of typically-developing children. The child probands of these participants had verbal and nonverbal ability within normal limits, as assessed by the WASI and the standardised language tests described by Barry et al. All participants had English as a native language, hearing within normal limits and no reported neurological disorder. The three parent groups were matched well on chronological age (p = .7), nonverbal IQ (p = .41), educational level attained (p = .68) and sex ratio (Table 1).
Table 1.
Participant details
| Par-A (n = 30) |
Par-L (n = 30) |
Par-T (n = 30) |
||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Number of participants | 20 | 10 | 22 | 8 | 23 | 7 |
| Chronological age | ||||||
| M (years;months) | 41;6 | 44;1 | 43;5 | 43.9 | 41;6 | 49;2 |
| SD (years;months) | 6;7 | 5;11 | 5;7 | 5;5 | 3;3 | 6;4 |
| Range (years;months) | 34;6 – 57;2 | 35;3 – 54;2 | 35;3 – 56;2 | 34;0 – 53;1 | 34;9 – 47;8 | 40;6 – 56;4 |
| NVIQ | ||||||
| M | 115.1 | 115.6 | 110.27 | 119.5 | 110.13 | 115.71 |
| SD | 8.58 | 9.38 | 12.71 | 15.15 | 9.7 | 14.15 |
| Range | 99 – 136 | 100 – 134 | 85 – 130 | 90 – 138 | 91 – 129 | 97 – 141 |
| Age at leaving full-time education (median years) | 18 | 19.5 | 18 | 18 | 18 | 20 |
Descriptive data for the child probands of the three parent-groups are included in Table 2. A one-way ANOVA revealed a significant group-difference in the chronological age of these children, F (2,102) = 6.79, p < .01. The child probands of the Par-A group were significantly younger than the child probands of the Par-L group (p < .01). There were no other group differences for chronological age. Nonverbal IQ was not statistically difference for the three child-proband groups, F (2,102) = 1.2, p = .32. On the criteria used to determine language impairment for the child probands with SLI, seven of the 25 children with ASD had significant difficulties with structural language.
Table 2.
Details of the child probands for the three parent groups
| Autism (n = 25) |
Language (n = 42) |
Typical (n = 38) |
|
|---|---|---|---|
| Sex | 0F,25M | 13F,29M | 15F,23M |
| Chronological age | |||
| M (years; months) | 10;4 | 12;7 | 11;6 |
| SD (years; months) | 2;6 | 2;8 | 2;2 |
| Range (years; months) | 7;2 – 15;7 | 7;11 – 16;3 | 6;10 – 15;1 |
| NVIQ | |||
| M | 109.48 | 101.35 | 105.06 |
| SD | 14.58 | 13.21 | 14.05 |
| Range | 88 – 137 | 85 – 137 | 92 – 137 |
The Par-A group contained six ‘couples’ (i.e., both biological parents of a proband) and the Par-L group contained four couples. There were no couples in the Par-T group. In the Par-A group, one couple had two children with ASD. The four couples in the Par-L group each had two children with SLI. Among the other participants in the Par-L group, 9 had two children with language/literacy impairment and one had three children with language/literacy impairment. Eight participants in the Par-T group had two children who were tested, and one participant had three children who were tested.
Procedure
Ethical approval for this study was attained from the Central University Research Ethics Committee of Oxford University. Participants received written information concerning the aims and nature of the study. All participants gave written consent to participate in this study.
Psychometric battery
Each participant was administered a battery of nine psychometric assessments, selected to be sensitive to language deficits in SLI. For further information on the tests described below, see Barry et al. (2007).
Nonverbal reasoning was assessed via the Block Design and Matrix Reasoning tasks from the WASI (Wechsler & Chen, 1999). Receptive grammatical knowledge was assessed with the use of Test for Reception of Grammar-2 (TROG-2; Bishop, 2003b).
Reading skills were assessed with two subtests from the Test of Word Reading Efficiency (Torgesen et al., 1999): Sight Word Efficiency, containing real words, and Phonemic Decoding Efficiency subtest, containing nonsense words.
Short-term memory was assessed with a modified version of the Digit Span task from the Wechsler Intelligence Scale for Children – Revised (Wechsler, 1974).
Two tasks from the NEPSY test battery (Korkman, et al., 1998) were used: Oromotor Sequences and Repetition of Nonsense Words.
Spelling ability was assessed with a speeded dictation task, developed by our research group (Barry et al., 2007).
Autism quotient
Participants in the Par-A and Par-L group completed the Autism Quotient (AQ; Baron-Cohen, Wheelwright, Skinner, Martin, & Clubley, 2001), a self-administered instrument designed to assess the broader autism phenotype in adults who have an IQ within normal limits. Respondents are provided with 50 statements and asked to indicate on a 4-point scale how well that statement applies to them (strongly agree, agree, disagree, strongly disagree). The statements are divided into five subscales: social skills (‘I prefer to do things with others, rather than on my own’), communication (‘people often tell me that I keep going on and on about the same thing’), imagination (‘I don’t particularly enjoy reading fiction’), attention to detail (‘I am fascinated by numbers’), and attention switching (‘I enjoy doing things spontaneously’). Importantly, the communication subscale assesses one’s self-report of language use in a social context, rather than verbal ability. Previous research has taken a summed score on the communication and social subscales of 11 or above to be an indicator of the broader autism phenotype (Bishop et al., 2004).
Results
Sex effects
First, we investigated if there were sex differences in the performance of the three parent groups on the behavioural tasks. A separate MANOVA was conducted for each group. Scores from the eight behavioural tasks (i.e., excluding the AQ) were included in each MANOVA as dependent variables. Sex served as the independent variable. There was no effect of sex in the Par-A group, F (8,21) = 1.21, p = .34, Wilks’ Lambda = .68, partial eta2 = .32, or the Par-T group, F (8,21) = 1.4, p = .25, Wilks’ Lambda = .65, partial eta2 = .35. There was, however, a main effect of sex in the Par-L group, F (8,21) = 5.20, p < .01, Wilks’ Lambda = .34, partial eta2 = .66. Due to this difference, sex was included as a = second independent variable (along with group) in the ensuing analyses.
Language impairment as assessed by the behavioural test battery
There were substantial ceiling effects on both the TROG-2 and the spelling test, indicating that a large proportion of participants had adequate receptive grammatical knowledge and spelling ability. For each task we calculated, firstly, how many participants in each group scored below the 10th centile, and secondly, how many participants in each group scored at ceiling. To investigate whether there were between-group differences on the proportion of participants scoring at either end of the data distributions, a separate chi-square analysis was conducted for each task. The proportion of each group scoring in the two tails of the data distribution were similar for both the TROG-2 (χ2 = 1.93, df = 2, p = .38) and spelling task (χ2 = .84, df = 2, p = .66). A small number of participants scored below the 10th centile on the TROG-2 (Par-A: 2 females, 1 male; Par-L: 2 females, 2 males; Par-T: 1 female, 0 females) and spelling tasks (Par-A: 1 female, 2 males; Par-L: 4 females, 1 male; Par-T: 3 females 0 males), while a larger number of participants scored at ceiling on these tests (TROG-2 – Par-A: 18 males, 5 males; Par-L: 19 females, 5 males; Par-T: 20 females, 7 males; Spelling – Par-A: 6 females, 1 male; Par-L: 4 females, 0 males; Par-T: 6 females 0 males).
To investigate sex effects on scores from the TROG-2 and spelling tests, we conducted a MANOVA including group and sex as independent variables. The interaction between group and sex fell short of significance, F (4,166) = 2.32, p = .06, Wilks’ Lambda = .9.
Data from the remaining behavioural tasks were then compared among groups. Univariate normality was tested for the three groups’ performance on each test (Kolmogorov-Smirnov test of normality, p < .05). Scores on all tasks were normally distributed. Language test scores were entered into a MANOVA as dependent variables. Group and sex served as the independent variables. The analysis indicated a main effect of group, F (10,160) = 271, p < .01, Wilks’ Lambda = .73, but no main effect of sex, F (580) = 1.86, p = .11, Wilks’ Lambda = .9, nor an interaction between group and sex, F (10,160) = 1.26, p = .26, Wilks’ Lambda = .86. Between-group differences were found in the performance on tasks of Digit Span, Oromotor Sequences, Repetition of Nonsense Words and Phonemic Decoding Efficiency but not on Sight Word Efficiency (Table 3).
Table 3.
Mean performance of the three groups on part of the battery of language tests (SDs and ranges are in parentheses). Bonferroni post-hoc tests are for group differences
| Par-A | Par-L | Par-T | Bonferroni post-hoc tests | Partial eta2 | Follow up comparisons | |
|---|---|---|---|---|---|---|
| Digit Span (raw score) | 10.97 (2.27, 6–16) | 9.47 (2.14, 5–14) | 11.3 (2.25, 7–16) | F (2,90) = 5.81, p < .01 | .12 | Par-A = Par-T Par-A > Par-L |
| Female | 10.65 (1.9, 6–13) | 9.68 (2.1, 6–14) | 11.17 (2.40, 7–16) | |||
| Male | 11.6 (2.87, 7–16) | 8.87 (2.29, 5–12) | 11.71 (1.7, 9–14) | |||
| Oromotor Sequences (raw score) | 61.93 (5.55, 42–69) | 59.03 (5.03, 45–70) | 64.17 (4.56, 54–70) | F (2,90) = 7.75, p < .01 | .15 | Par-A = Par-T Par-A > Par-L |
| Female | 61.5 (5.73, 42–69) | 60.09 (4.48, 53–70)* | 65.17 (4.1, 55–70) | |||
| Male | 62.8 (5.37, 56–69) | 56.13 (5.62, 45–61) | 60.86 (4.71, 54–69) | |||
| Sight Word Efficiency (standard score) | 92.4 (12.63, 72–103) | 89.63 (16.59, 55–115) | 92.8 (13.28, 72–115) | F (2,90) = .51, p = .6 | .01 | No group difference |
| Female | 91.45 (12.11, 73–113) | 91.9 (15.13, 55–115)* | 93.35 (14.54, 72–115) | |||
| Male | 94.3 (14.09,72–113) | 83.25 (19.77, 56–115) | 91 (8.5, 77–100) | |||
| Phonemic Decoding Efficiency (standard score) |
101.1 (15.18, 65–120) | 91.23 (16.22, 55–120) | 97.8 (12.04, 77–120) | F (2,90) = 3.23, p < .05 | .07 | Par-A = Par-T Par-A > Par-L |
| Female | 102 (15.39, 65–120) | 94.82 (14.73, 55–122)* | 98.39 (11.96, 77–120) | |||
| Male | 99.3 (15.41, 76–120) | 81.37 (16.94, 55–98) | 95.86 (13.06, 77–112) | |||
| Repetition of Nonsense Words (raw score) |
40.53 (4.26, 27–45) | 36.6 (4.52, 25–44) | 40.7 (3.62 34–46) | F (2,90) = 9.36, p < .01 | .18 | Par-A = Par-T Par-A > Par-L |
| Female | 40.55 (3.98, 28–45) | 37.45 (3.98, 26–44) | 40.39 (3.61, 34–46) | |||
| Male | 40.5 (5.02, 27–44) | 34.25 (25–40) | 41.71 (3.73, 34–45) |
Significant gender difference at the p < .05 level.
To further examine the performance of the parents of ASD probands, planned comparisons were conducted between the Par-A group and the other two groups (see Barry et al. (2007) for comparisons between the Par-L and the Par-T group). For the first follow-up analysis, MANOVA was used to compare the performance of the Par-A and Par-T groups on the tasks of Digit Span, Oromotor Sequences, Phonemic Decoding Efficiency, Sight Word Efficiency and Repetition of Nonsense Words. The independent variables were group and sex. No significant effects emerged from the analysis (for all comparisons: p > .2, partial eta2 < .01).
In the second follow-up analysis, MANOVA compared the performance of the Par-A and Par-L groups on the same tasks. There was a main effect of group, F (5,52) = 3.01, p < .05, Wilks’ Lambda = .77. Follow up analyses revealed that the Par-A group performed significantly better than the Par-L group on the tasks of Digit Span (partial eta2 = .14), Oromotor Sequences (.12), Phonemic Decoding Efficiency (.09), Sight Word Efficiency (.12) and Repetition of Nonsense Words (.2), at the p < .05 level. There were also group by sex interactions for the participants’ performance on the tasks of Phonemic Decoding Efficiency, F (1,60) = 2.6, p < .05, partial eta2 = .05, Sight Word Efficiency, F (1,60) = 3.4, p < .05, partial eta2 = .06, and Oromotor Sequences, F (1,60) = 3.18, p < .05, partial eta2 .05. Independent samples t-tests revealed that females in the Par-L group performed significantly better than males on all three tasks (all comparisons: p < .05). In contrast, there were no sex differences in the performance of the Par-A group on these tasks (all comparisons: p > .5, Table 3).
A subgroup of individuals with ASD resembling SLI?
Further analyses were undertaken to determine whether only a subgroup of children with ASD inherit a language phenotype similar to SLI. Participants in the Par-A group were subdivided according to their child’s performance on the Repetition of Nonsense Words test, with a cutoff standard score of 8. Nine parents had a child with a standard score of 8 or less, and 21 had a child scoring above this level. Chisquare analyses found no difference between the proportions of the two subgroups scoring at either end of the data distribution on the TROG-2 (χ2 = .01, df = 1 p = 1) and spelling tasks (χ2 = .48, df = 1, p = 1). MANOVA indicated that performance of the two subgroups of parents on the remaining language tasks did not differ, F (4,25) = .58, p = .68, Pillai’s Trace = .08.
We defined language/literacy impairment as having scores below the 10th centile on two or more standardised language tests. On this criterion, the Par-A and Par-T control groups each contained one male and one female with impairment. In the Par-L group, four males and four females fulfilled this criterion. The difference in proportions between the Par-A and Par-L groups was statistically significant (Fisher’s exact test, p < .05, one-tailed). Both participants in the Par-A group who met the criteria for language impairment (n = 2, 1 child each) had children who had significant language difficulties (i.e., both children had standard scores on the TROG-2 and Repetition of Nonsense Words, at least one SD below the mean).
Autism quotient
AQ data were missing for five participants in the Par-L group. MANOVA showed that the chronological age, nonverbal IQ and performance on the behavioural tasks of these participants did not differ from the other participants in the Par-L group (p = .5). The total AQ scores for the Par-A (n = 30) and the Par-L group (n = 25) were compared on a two-way ANOVA (group and sex were independent variables). Par-A group had a significantly higher AQ than the Par-L group, indicating greater autistic symptomatology in the parents of the children with ASD (Table 4). There was no effect of sex and no interaction between group and sex (both comparisons: F < 1).
Table 4.
Mean scores of the Par-A and Par-L groups on the Autism-Spectrum Quotient (AQ) and its five subscales. SDs and ranges are presented in parentheses
| Par-A (n = 30) | Par-L (n = 25) | Comparisons | Partial eta2 | |
|---|---|---|---|---|
| AQ (overall) | 18.06 (7.41, 6–31) | 13.44 (6.99, 5–27) | F (1,51) = 4.65, p < .05 | .08 |
| Social skill | 3.17 (2.07, 1–8) | 1.8 (1.96, 0–7) | F (1,51) = 3.53, p = .06 | .01 |
| Attention switching | 4.63 (2.12, 1–9) | 3.2 (2.1, 0–7) | F (1,51) = 5.59, p < .05 | .01 |
| Attention to detail | 4.33 (1.07, 1–8) | 4.12 (1.99, 1–8) | F (1,51) = .01, p = .91 | 0 |
| Communication | 3.37 (2.01, 0–7) | 2.28 (1.24, 0–5) | F (1,51) = 5.63, p < .05 | .07 |
| Imagination | 2.9 (1.9, 0–7) | 2.04 (2.28, 0–7) | F (1,51) = 2.52, p = .12 | .05 |
Analyses then focused on the five AQ subscales (Table 4). These subscales had high internal consistency (Cronbach’s alpha = .78). The components of the AQ were entered into a MANOVA as dependent variables, while group (Par-A vs. Par-L) and sex served as independent variables. No effect involving sex reached significance. Although there was no overall main effect of group, F (5,47) = 1.81, p = .13, the Wilks’ Lambda = .84, differences between two groups on several subscales reached significance. The Par-A group scored significantly higher than the Par-L group on the communication subscale and the attention switching subscale, while scores on the social skills subscales demonstrated a trend in this direction. There were no differences between the two groups on either the attention to detail or imagination subscales. Six participants in the Par-A group (5 female, 1 male) had an AQ composite score indicative of the broader autism phenotype. In comparison, only 1 (female) of 22 participants in the Par-L group met this criterion. This difference was not, however, statistically significant in this small sample (Fisher exact test, one-tailed p = .11).
Discussion
The Par-A and Par-L groups showed a double dissociation between linguistic performance (intact in Par-A and impaired in Par-L) and social communication ability (intact in Par-L and impaired in Par-A). This suggests that impairments in social communication, but not structural language, are part of the heritable communication deficit in ASD.
Before we discuss the broader implications of this finding, it is important to address two issues concerning the participant sample. First, males in the Par-L group performed significantly worse than females on a number of tasks. In contrast, there were no sex differences in the performance of the Par-A group. Although the two groups were matched on sex ratio (i.e., far fewer males than females), it was substantially harder to recruit fathers of language/literacy impaired probands than fathers of ASD probands. It is possible that we overestimated the language difficulties of males in the Par-L group if a general reluctance of these individuals to participate was overcome by interest in the study of those with a personal history of language impairment. For this reason, we urge caution when comparing sex differences in performance between the Par-A and Par-L groups.
Second, the Par-A and Par-L groups were not matched for the number of participants from multiple incident families (18 individuals in the Par-L group and two individuals in the Par-A group). The genetic loading of ‘autism’ in multiplex families is most likely different from the genetic loading of one-child families (Piven & Folstein, 1994). Note, however, that the risk for SLI in relatives of SLI probands is greater than the risk for ASD in relatives of ASD probands (Tomblin, 1989); thus, it is inevitable that there are more multiplex cases in an SLI sample. If we had matched the two groups for the number of individuals from multiple incident families, one of the groups would have constituted an atypical sample.
The aetiology of SLI and ASD
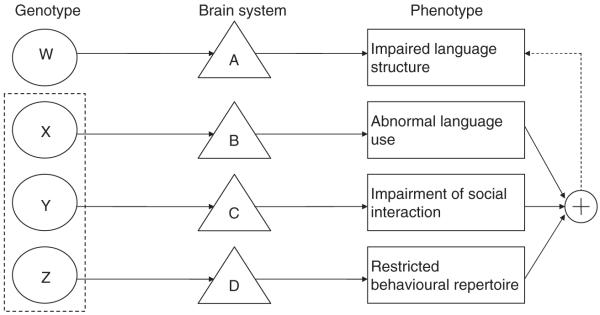
We found no evidence for aetiological overlap between ASD and SLI; whereas the language phenotype transmitted in SLI is characterised by problems with grammatical structure and aspects of verbal memory (Bishop et al., 1996), the heritable language impairment of ASD is characterised by abnormal language use (Bailey et al., 1995). A significant proportion of children with ASD perform poorly on language tests sensitive to SLI (Kjelgaard & Tager-Flusberg, 2001); however, the present study suggests that these difficulties arise for different reasons, rather than being indicative of a common aetiology. This position is consistent with studies that found no evidence for genetic overlap between SLI and ASD (Wassink et al., 2002). By integrating the current findings with previous research, we present one possible model for the aetiological relation between ASD and SLI (Figure 1).
Figure 1.
A proposed model for the relation between ASD and SLI. ASD and SLI result from separate genetic risk factors. Children with SLI inherit a genetic risk factor W, which affects brain system A and leads to structural language impairments. ASD is the result of the inheritance of three separable genetic risk factor (X,Y,Z), indicated by the dotted box, each leading to one part of the autistic triad. The resulting ASD phenotype, which is a combination of these three behaviours (+), has the potential to limit linguistic development, including phonological short-term memory (dotted path)
Genetic research suggests that the aetiology of SLI is complex, and involves polygenic inheritance, with several independent genes combining with environmental risk factors to determine whether a particular behavioural pattern is likely to develop (Bishop, 2006). In Figure 1 this is shown as a genotype (W) that disrupts neural systems implicated in linguistic development (A).
Genetic studies also indicate that ASD is inherited through a combination of adverse risk genes. On the basis of twin data, Happé, Ronald, and Plomin (2006) argued that ASD consists of a constellation of genetically transmitted impairments. From this perspective, if a single risk gene is inherited, only mild autism-like difficulties will result. Recent evidence that members of the general population demonstrate autistic-like traits (e.g., restricted interests) independent of other autism-like traits (e.g., communication or social deficits) speaks to this point (Ronald et al., 2006). Figure 1 reflects this view in depicting three independently inherited risk genes (X,Y,Z) that affect three separate brain systems (B,C,D), each leading to one part of the autistic triad.
Although the findings of the current study suggest that linguistic impairment is not part of the heritable deficits transmitted in ASD, we suggest that some children with ASD still inherit a genetic risk factor for structural language difficulties (e.g., W). For example, the two parents in the Par-A group who demonstrated linguistic impairment had children with significant language and literacy difficulties. Similarly, Bishop et al. (2006) found that a minority of siblings of children with ASD had evidence of language impairments resembling SLI. Importantly, however, evidence from the current study and those of previous studies (Pilowsky et al., 2003) indicate that the rate of heritable linguistic deficits within the ASD population is similar to that of the general population (i.e., between 7 and 12%; Tomblin et al., 1997).
If only a relatively small percentage of children with ASD inherit linguistic deficits, why does a large proportion of the ASD population have poor linguistic ability? One suggestion is that linguistic impairments may be secondary to the heritable behavioural and/or cognitive deficits of this population. When a single domain is impaired (as in some relatives) language development is unaffected, but the presence of two or more deficits (e.g., poor joint attention plus restricted interests) creates a risk of language impairment. Figure 1 depicts this synergistic interaction between deficits with a dotted path between the resulting ASD phenotype (+) and the box depicting structural language deficits.
This point is especially relevant to the performance of children with ASD and SLI on tasks of nonword repetition. Poor nonword repetition, which is indicative of limitations of phonological short-term memory and known to be closely related to deficits in language learning (Gathercole & Baddeley, 1990), has been shown to be highly heritable in individuals with SLI (Bishop et al., 1996). In contrast, although poor nonword repetition is also observed in many children with ASD (Kjelgaard & Tager-Flusberg, 2001), the current study replicates previous findings (Bishop et al., 2004) in demonstrating that this deficit is not an endophenotype of ASD. Poor nonword repetition may arise for different reasons in children with ASD and those with SLI. For instance, poor skills in imitation coupled with lack of social interest might underlie the difficulties in children with ASD, rather than limitations of phonological short-term memory. From this perspective, detailed analysis of the errors made by children with ASD and SLI on tasks of nonword repetition may reveal qualitative differences between the populations.
Given that we are suggesting that ASD and SLI do not share a common aetiology, we must ask why a significant minority of children with SLI appear develop more ASD-like difficulties in adolescence or adulthood (Conti-Ramsden et al., 2006). One possibility is that it is difficult to distinguish between pragmatic difficulties (of children with ASD) and SLI during childhood. Children with pragmatic difficulties often present with severe comprehension difficulties (Bishop & Rosenbloom, 1987) and the conspicuous nature of these deficits may overshadow more subtle ASD characteristics. As a child develops better formal language skills, the deficits related to the social and repetitive domains of the autistic triad may become more discernible (e.g., poor friendships for school-aged children). However, while we suggest that this explanation may account for some of the putative overlap between SLI and ASD, it cannot account for all of the evidence linking these two disorders, e.g., those with PLI, who show characteristics intermediate between ASD and SLI. Family studies of individuals with PLI have the potential to shed more light on the relation between ASD and SLI.
In summary, the results of this study suggest that language deficits in children with ASD and children with SLI have different origins. Whereas children with SLI appear to inherit a phenotype characterised by poor phonological short-term memory and a diminished capacity to carry out grammatical computations, poor social communication ability appears to be transmitted in ASD. The model proposed here is one possibility for the aetiological relation between ASD and SLI, and is intended to stimulate research leading towards a better understanding of the two developmental disorders.
Acknowledgements
The research was supported by grant from Autism Speaks to Andrew Whitehouse and Dorothy Bishop. We would like to thank Anne Robinson for help with participant recruitment. Thanks also Tracy O’Donnell, David McDonald and Liz Line for their help during the testing phase of this project. A final thanks to Emma Jaquet for her helpful comments on earlier drafts of this manuscript.
Abbreviations
- ADOS-G
Autism Diagnostic Observation Schedule-Generic
- AQ
Autism-Spectrum Quotient
- ASD
autism spectrum disorder
- Par-A
parents of children with autism spectrum disorder
- Par-L
parents of children with language/literacy impairment
- Par-T
parents of typically developing children
- SLI
specific language impairment
- TROG-2
Test for Reception of Grammar-2
- WASI
Wechsler Abbreviated Scale of Intelligence
Footnotes
Conflict of interest statement: No conflicts declared.
References
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th edn American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Bailey A, Le Couteur A, Gottestman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychological Medicine. 1995;25:63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Bailey A, Palferman S, Heavey L, Le Couteur A. Autism: The phenotype in relatives. Journal of Autism and Developmental Disorders. 1998;28:369–392. doi: 10.1023/a:1026048320785. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S, Skinner R, Martin J, Clubley E. The autism spectrum quotient (AQ): Evidence from Asperger Syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- Barry JG, Yasin I, Bishop DVM. Heritable risk factors associated with language impairments. Genes, Brain and Behaviour. 2007;6:66–76. doi: 10.1111/j.1601-183X.2006.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM. Pragmatic language impairment: A correlate of SLI, a distinct subgroup, or part of the autistic continuum? In: Bishop DVM, Leonard LB, editors. Speech and language impairments in children: Causes, characteristics, intervention and outcome. Psychology Press; UK: 2000. pp. 99–113. [Google Scholar]
- Bishop DVM. Autism and specific language impairment: Categorical distinction or continuum?. In: Bock G, Goode J, editors. Autism: Neural basis and treatment possibilities; Novartis Foundation Symposium 251; Chichester: John Wiley. 2003a; pp. 213–226. [PubMed] [Google Scholar]
- Bishop DVM. Test for Reception of grammar (version 2) Psychological Corporation; London: 2003b. [Google Scholar]
- Bishop DVM. What causes specific language impairment in children? Current Directions in Psychological Science. 2006;15:217–221. doi: 10.1111/j.1467-8721.2006.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop DVM, Maybery M, Wong D, Maley A, Hallmayer J. Characteristics of the broader phenotype in autism: A study of siblings using the Children’s Communication Checklist-2. American Journal of Medical Genetics Part B (Neuropsychiatric Genetics) 2006;141B:117–122. doi: 10.1002/ajmg.b.30267. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Maybery M, Wong D, Maley A, Hill W, Hallmayer J. Are phonological processing deficits part of the broad autism phenotype? American Journal of Medical Genetics: (Neuropsychiatric Genetics) 2004;128B:54–60. doi: 10.1002/ajmg.b.30039. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Norbury CF. Exploring borderlands of autistic disorder and specific language impairment: A study using standardised diagnostic instruments. Journal of Child Psychology and Psychiatry. 2002;43:917–929. doi: 10.1111/1469-7610.00114. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, North T, Donlan C. Nonword repetition as a behavioural marker for inherited language impairment: Evidence from a twin study. Journal of Child Psychology and Psychiatry. 1996;37:391–403. doi: 10.1111/j.1469-7610.1996.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bishop DVM, Rosenbloom L. Classification of childhood language disorders. In: Yule W, Rutter M, editors. Language development and disorders. MacKeith Press; London: 1987. pp. 16–41. [Google Scholar]
- Conti-Ramsden G, Simkin Z, Botting N. The prevalence of autistic spectrum disorders in adolescents with a history of specific language impairment (SLI) Journal of Child Psychology and Psychiatry. 2006;47:621–628. doi: 10.1111/j.1469-7610.2005.01584.x. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Baddeley AD. Phonological memory deficits in language disordered children: Is there a causal connection? Journal of Memory and Language. 1990;29:336–360. [Google Scholar]
- Happé F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience. 2006;9:1218–1220. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kirk U, Kemp SI. NEPSY: A developmental neuropsychological assessment. Psychological Corporation; San Antonio, TX: 1998. [Google Scholar]
- Kjelgaard MM, Tager-Flusberg H. An investigation of language impairment in autism: Implications for genetic subgroups. Language and Cognitive Processes. 2001;16:287–308. doi: 10.1080/01690960042000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Leventhal BL, DiLavore PC, Pickles A, Rutter M. The Autism Diagnostic Observation Schedule–Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30:205–223. [PubMed] [Google Scholar]
- Mawhood L, Howlin P, Rutter M. Autism and developmental receptive language disorder – a comparative follow up in Early Adult Life. I: Cognitive and language outcomes. Journal of Child Psychology and Psychiatry. 2000;41:547–559. doi: 10.1111/1469-7610.00642. [DOI] [PubMed] [Google Scholar]
- Pilowsky T, Yirmiya N, Shalev RS, Gross-Tsur V. Language abilities of siblings of children with autism. Journal of Child Psychology and Psychiatry. 2003;44:914–925. doi: 10.1111/1469-7610.00175. [DOI] [PubMed] [Google Scholar]
- Piven J, Folstein S. The genetics of autism. In: Bauman ML, Kemper TL, editors. The neurobiology of autism. John Hopkins University Press; Baltimore: 1994. pp. 18–44. [Google Scholar]
- Ronald A, Happé F, Bolton P, Butcher LM, Price TS, Wheelwright S, Baron-Cohen S, Plomin R. Genetic heterogeneity between the three components of the autism spectrum: A twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:691–699. doi: 10.1097/01.chi.0000215325.13058.9d. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Western Psychological Services; Los Angeles: 2003. [Google Scholar]
- Tomblin JB. Familial concentration of developmental language impairment. Journal of Speech and Hearing Disorders. 1989;54:287–295. doi: 10.1044/jshd.5402.287. [DOI] [PubMed] [Google Scholar]
- Tomblin JB, Records NL, Buckwalter P, Zhang X, Smith E, O’Brien M. Prevalence of specific language impairment in kindergarten children. Journal of Speech Language Hearing Research. 1997;40:1245–1260. doi: 10.1044/jslhr.4006.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgesen JK, Wagner R, Rashotte C. Test of Word Reading Efficiency (TOWRE) Psychological Corporation; New York: 1999. [Google Scholar]
- Wassink TH, Piven J, Vieland VJ, Pietela K, Goedken RJ, Folstein SE, et al. Evaluation of FOXP2 as an autism susceptibility gene. American Journal of Medical Genetics (Neuropsychiatric Genetics) 2002;114:566–569. doi: 10.1002/ajmg.10415. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Psychological Corporation; New York: 1974. [Google Scholar]
- Wechsler D, Chen H-Y. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; New York: 1999. [Google Scholar]