Abstract
Plant stomata limit both carbon dioxide uptake and water loss; hence, stomatal aperture is carefully set as the environment fluctuates. Aperture area is known to be regulated in part by ion transport, but few of the transporters have been characterized. Here we report that AtALMT12 (At4g17970), a homolog of the aluminum-activated malate transporter (ALMT) of wheat, is expressed in guard cells of Arabidopsis thaliana. Loss-of-function mutations in AtALMT12 impair stomatal closure induced by ABA, calcium and darkness, but do not abolish either the rapidly activated or the slowly activated anion currents previously identified as being important for stomatal closure. Expressed in Xenopus oocytes, AtALMT12 facilitates chloride and nitrate currents, but not those of organic solutes. Therefore, we conclude that AtALMT12 is a novel class of anion transporter involved in stomatal closure.
Keywords: ALMT family protein, Anion transporter, AtALMT12, Stomatal closure
Introduction
Stomatal movement is driven by turgor pressure changes in guard cells, changes predominantly achieved by solute transport through multiple ion channels (Ward et al. 2009). Several of the relevant cation channels, such as for calcium and potassium, have been reasonably well studied (Pilot et al. 2001, Hosy et al. 2003); however, much less is known about the anion channels.
To date, four types of anion channels or transporters have been implicated in stomatal movements. First, an ABC-class transporter, AtABCB14, was identified as a malate importer, modulating stomatal movement by increasing osmotic pressure in guard cells (Lee et al. 2008). Secondly, a nitrate transporter, AtNRT1.1/CHL1, functions in stomatal opening in the presence of nitrate (Guo et al. 2003). The third and fourth type of anion channel activity mediate the rapidly activated (R-type) and the slowly activated (S-type) anion currents, which are implicated in being involved in stomatal closure (Hedrich et al. 1990, Schroeder and Keller 1992, Schmidt et al. 1995, Pei et al. 1997).
Although genes encoding R-type anion currents have yet to be identified, S-type anion currents have recently been discovered to require a gene named SLOW ANION CHANNEL-ASSOCIATED 1 (SLAC1; At1g12480; Negi et al. 2008, Saji et al. 2008, Vahisalu et al. 2008). This gene was isolated in screens for mutants insensitive to carbon dioxide or hypersensitive to ozone, and appears to be a distant homolog of bacterial and fungal dicarboxylate transporters. SLAC1 is localized at the plasma membrane and is essential for stomatal closure in response to carbon dioxide, ABA and ozone (Negi et al. 2008, Vahisalu et al. 2008). However, it is not known whether additional types of anion currents must flow for stomatal aperture control.
Anion channels have also been characterized in connection with aluminum toxicity, which is a major limiting factor of plant growth in acidic soils, because an important resistance pathway involves the secretion of organic anions that chelate aluminum, such as citrate or malate (Ma et al. 2001, Kochian et al. 2004, Delhaize et al. 2007). Indeed, Triticum aestivum (wheat) has an aluminum-activated malate-permeable channel, ALMT1 (also known as TaALMT1), that is localized to the plasma membrane, and confers resistance to aluminum (Sasaki et al. 2004, Yamaguchi et al. 2005, Zhang et al. 2008). Genes with apparent homology to TaALMT1 are plant specific. Among 13 Arabidopsis thaliana genes similar to TaALMT1, AtALMT1 is likewise an aluminum-activated malate transporter, expressed in roots and related to aluminum resistance (Hoekenga et al. 2006). In contrast, AtALMT9 encodes a vacuolar malate channel, unrelated to aluminum but apparently involved in malate homeostasis, and is mainly expressed in leaf mesophyll (Kovermann et al. 2007). Also independent of aluminum resistance, a maize homolog, ZmALMT1, transports little if any malate and instead transports inorganic anions (Piñeros et al. 2008b). Therefore, ALMT-type anion channels have multiple functions in anion homeostasis, contributing to the regulation of growth and response to the environment.
Here, we show that AtALMT12 is an anion transporter, particularly permeable to chloride and nitrate, and a key regulator of stomatal closure.
Results
The role of AtALMT12 in stomatal closure
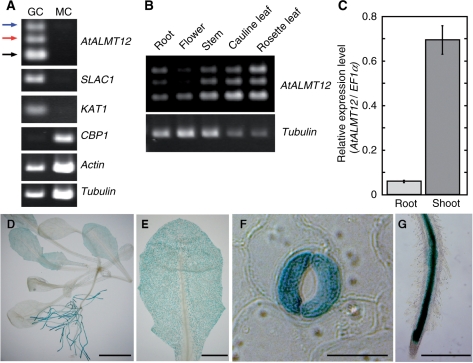
To investigate the function of the ALMT gene family in stomata, we first compared the expression of each gene in A. thaliana guard cells and mesophyll. Among the 13 genes, AtALMT12 was predominantly expressed in guard cells rather than mesophyll cells (Fig. 1A). This is similar to expression of two known guard cell channel genes, SLAC1 and KAT1, and distinct from that of the gene for the mesophyll cell marker protein calmodulin-binding family protein (CBP). Interestingly, three transcripts (249, 335 and 412 bp) were detected by reverse transcription–PCR (RT–PCR) analysis using a pair of primers amplifying between exons 4 and 6 (Fig. 2A and Supplementary Fig. S1). Sequencing of these PCR products showed that they appeared to be splicing variants, produced by retaining intron 4, or both introns 4 and 5 (Reddy 2007). The predicted translation products give rise to truncated peptides [277 amino acids without exons 5 and 6, 324 amino acids without exon 6, compared with 560 amino acids for the complete open reading frame (ORF)] (Supplementary Fig. S1B, C).
Fig. 1.
AtALMT12 is expressed in guard cells. (A) The expression of AtALMT12 (At4g17970), SLAC1 (At1g12480), KAT1 (At5g46240), CBP (At4g33050), actin (At5g09810) and β-tubulin genes (AT1g75780, AT5g62690, AT5g62700 and AT5g44340) in guard cells (GC) and mesophyll cells (MC) was detected by RT–PCR. Note that three amplification products are detected for AtALMT12 (249 bp, black arrowhead; 335 bp, red arrowhead; 412 bp, blue arrowhead). (B) Comparison of the expression level of AtALMT12 among plant organs. (C) Expression of AtALMT12 determined by real-time PCR using primer set #2 (see Fig. 2A). Plants (Columbia) were grown in hydroponic medium and RNA was isolated from whole seedlings. Relative expression levels were normalized against the values of the EF1α transcript (At5g60390). Bars show the mean ± SEM (n = 3). (D–G) GUS reporter expression in seedling (D), leaf (E), guard cell (F) and root (G). GUS is driven by the region 3,157 bp upstream of the start codon. Bars = 5 mm (D), 1 mm (E), 20 μm (F) and 500 μm (G).
Fig. 2.
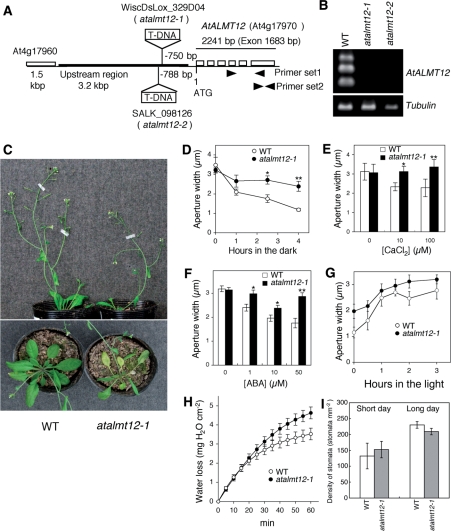
Knockdown mutation of AtALMT12 impairs stomatal responses and has a wilty phenotype. (A) Schematic of the AtALMT12 locus (At4g17970) showing T-DNA insertion sites and primer locations (arrowheads) for RT–PCR analysis. (B) RT–PCR analysis using primer set #1 (or β-tubulin primers). (C) One-week-old wild-type (WT) and atalmt12-1 plants were subjected to water withholding for a further 2 weeks. Photographs of representative plants from three independent replicates were taken from the side and top. (D) Dark-induced stomatal closure (n = 4 experiments). (E) Calcium-induced stomatal closure (n = 4 experiments). (F) ABA-induced stomatal closure (n = 10 experiments for 0, 1 and 10 μM ABA; n = 4 experiments for 50 μM ABA). For D–F, 20 stomata were measured for each genotype in each experiment, and symbols or bars plot the mean ± SEM. (G) Light-induced stomatal opening in the atalmt12-1 mutant (n = 5 experiments). (H) Water loss of detached leaves (n = 3). Symbols plot the mean ± SEM. (I) Stomatal density. Plants were grown under short days (8L:16D) for 3 months or long days (16L:8D) for 3 weeks. Data are presented as the mean ± SD (n = 5 leaves). Differences from WT values were significant at *P < 0.05 and **P < 0.01, respectively.
To investigate expression further, we first used RT–PCR, which revealed a broad expression pattern throughout the plant (Fig. 1B). However, quantitative real-time RT–PCR showed that AtALMT12 transcripts were 10-fold higher in shoots than in roots (Fig. 1C). Analysis of transgenic plants expressing the reporter gene, β-glucuronidase (GUS), under the control of the putative AtALMT12 promoter (3,157 bp upstream of the first ATG) showed that roots were stained largely in the vascular stele but that leaves were stained principally in guard cells (Fig. 1D–G).
To analyze the function of AtALMT12, we obtained two AtALMT12 knock-down lines (atalmt12-1, WiscDsLox_329D04; and atalmt12-2, SALK_098126). In these lines, the T-DNA was inserted around 750 bp upstream from the start codon (Fig. 2A) and resulted in a significant reduction of transcript in leaves (Fig. 2B). As the phenotypes of the lines were indistinguishable (Supplementary Fig. S2A), we present data here for atalmt12-1.
Intact atalmt12-1 plants had a wilty phenotype, consistent with impaired stomatal regulation (Fig. 2C). In this line, stomatal closure was suppressed in response to darkness, calcium and exogenous ABA (Fig. 2D–F). The F1 progeny from a cross of the wild type and atalmt12-1 had both wild-type morphology and sensitivity to ABA, indicating that the mutant is recessive (data not shown). Also implicating impaired stomatal closure, the average aperture in dark-adapted leaves was larger in atalmt12-1 than in the wild type (Fig. 2G, time zero). In contrast, stomata in atalmt12-1 opened in response to light with similar kinetics to those of the wild type (Fig. 2G), suggesting that AtALMT12 is required for stomatal closure but not for opening.
From excised leaves, the rate of water loss over the first 20 min was indistinguishable between the genotypes (Fig. 2H). However, over longer times, as the leaves became dehydrated, the wild type lost water more slowly than did atalmt12-1; for example, between 40 to 60 min, the rates of water loss were 19.6 ± 2.1 and 34.6 ± 2.0 μg H2O cm−2 min−1 for the wild type and atalmt12-1, respectively (with equivalence of these mean rates being rejected at P < 0.01). These results suggest that the stomata of atalmt12-1 are defective in drought-induced closure, in agreement with their relatively low sensitivity to ABA. Taken together with the comparable density of stomata on the leaves of the two genotypes (Fig. 2I), our findings imply that AtALMT12 is involved in the control of stomatal closure under darkness and water-deficient conditions.
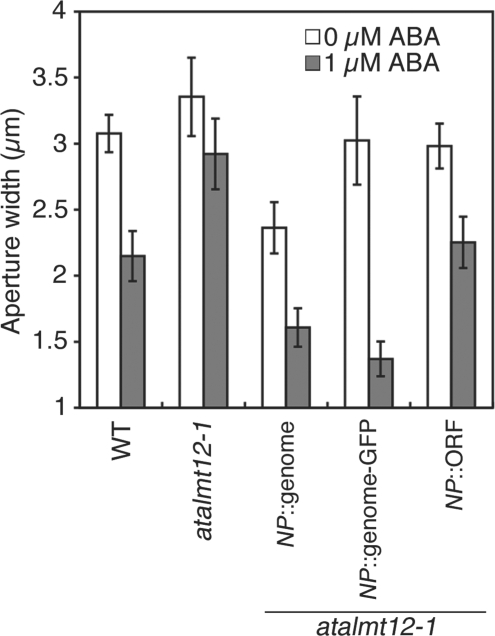
To determine whether the T-DNA insertion was responsible for the phenotypes, we stably transformed atalmt12-1 with the wild-type gene, using either a 2,241 bp genomic sequence that spans the coding regions or the same sequence fused with green fluorescent protein (GFP) at the C-terminus, or the coding sequence fused to 3,157 bp of upstream sequence (Fig. 2A and Supplementary Fig. S1). Stomata in transformants with the genomic sequences with or without GFP, or with the coding sequence had a restored sensitivity to ABA (Fig. 3 and Supplementary Fig. S3).
Fig. 3.
Complementation of the ABA-insensitive phenotype of atalmt12-1 with genomic AtALMT12 sequences. Plants were treated with or without 1 μM ABA and stomatal closure was assayed. Complementation of atalmt12-1 by the native promoter (NP)-driven genomic sequence of AtALMT12 with or without GFP fusion (NP::genome, NP::genome–GFP; n = 5–6 independent experiments), and the NP-driven coding sequence (NP::ORF, n = 8 independent experiments).
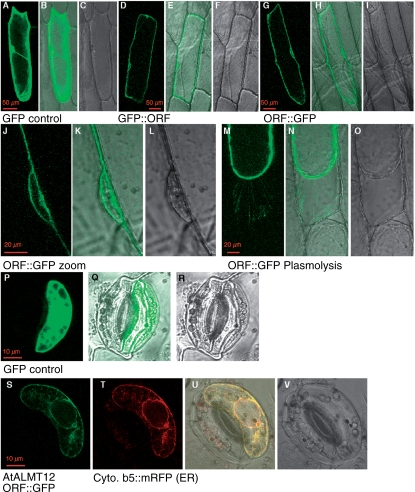
Because atalmt12-1 plants were complemented by an AtALMT12 genomic construct containing GFP (Fig. 3), we examined them to assess localization; however, GFP fluorescence was undetectable (data not shown). We also ran immunoblots to detect the protein, probing a crude microsomal fraction prepared from isolated guard cells with antisera against either GFP or an AtALMT12 peptide; again, AtALMT12 was undetectable, in both the wild type and the transgenics expressing AtALMT12 from the native promoter. Therefore, to localize AtALMT12, the AtALMT12 coding sequence was fused to GFP under the control of the cauliflower mosaic virus (CaMV) 35S promoter, and transiently expressed by particle bombardment in onion epidermal cells and in Vicia faba guard cells (Fig. 4). Fluorescence from the GFP fusion protein was observed on endomembranes, as seen by it surrounding the nucleus and co-localization with defined markers, and at the plasma membrane as judged by plasmolysis.
Fig. 4.
Localization of AtALMT12. The AtALMT12 coding sequence–GFP (ORF::GFP) construct under the control of the 35S promoter was transiently expressed in onion epidermal cells (A–O) and V. faba guard cells (P–V). As control, GFP alone was expressed in onion cells (A–C) or V. faba cells (P–R). Expression of GFP::AtALMT12 (N-terminal fusion, D–F) or AtALMT12::GFP (C-terminal fusion, G–I) shows similar localizations in onion cells. GFP fluorescence surrounds the nucleus, suggesting that AtALMT12 localized to both endomembranes and plasma membrane (J–L). Plasmolysis of AtALMT12::GFP-expressing cells with 1 M mannitol shows that the Hechtian strands attaching the plasma membrane to the cell wall are labeled, confirming that the protein is localized on the plasma membrane. Co-expression of AtALMT12::GFP with Cyt b5::mRFP as an ER marker (S–V). Fluorescence from AtALMT12::GFP co-localized with Cyt b5::mRFP suggests that AtALMT12 is targeted to the ER. Photographs show GFP fluorescence images (A, D, G, J, M, P, S), mRFP images (T), transmitted light images (C, I, L, O, R, V) and merged images (B, E, H, K, N, Q, U).
Electrophysiological analysis of AtALMT12
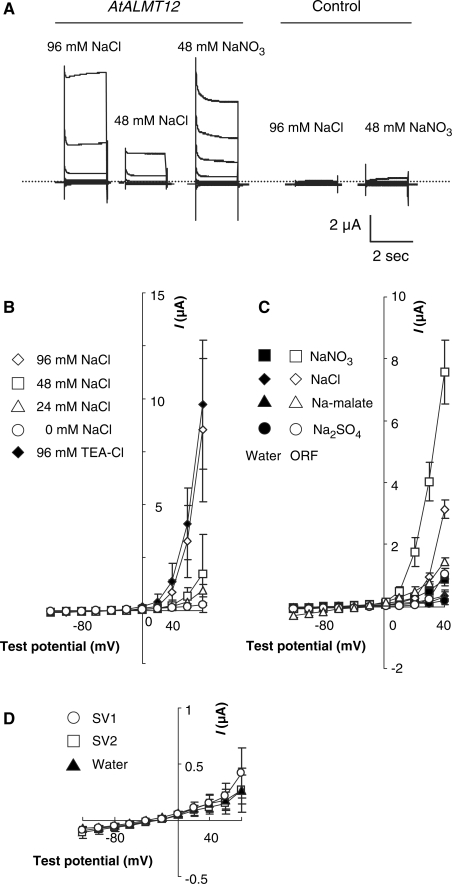
To determine the electrophysiological properties of AtALMT12, we used two-electrode voltage–clamp recording in a heterologous expression system (Xenopus laevis oocytes), where the AtALMT12 (coding sequence)::sGFP is predominantly localized to the plasma membrane (Supplementary Fig. S4A). AtALMT12-expressing oocytes showed large outward currents when sodium chloride was present in the bathing solution (Fig. 5A). Decreasing the extracellular concentration reduced the outward current, but replacing sodium chloride by other chloride salts did not affect the outward current (Fig. 5B and Supplementary Fig. S4B), demonstrating that AtALMT12 is an anion channel permeable to chloride. Although the related transporters, TaALMT1 and ZmALMT1, carry both inward and outward currents (Piñeros et al. 2008a, Piñeros et al. 2008b), AtALMT12 gave rise to little if any inward current as extracellular chloride concentrations were changed (Fig. 5A, B), implying that AtALMT12 is an outward rectifier, at least when expressed in oocytes.
Fig. 5.
Electrophysiological properties of AtALMT12 expressed in Xenopus oocyte plasma membranes. (A) Typical traces of anion currents across the plasma membrane in oocytes expressing AtALMT12 (ORF) (left) and water-injected controls (right). The dotted line indicates zero current level (±0 nA). (B) Mean current–voltage relationships in AtALMT12 (ORF)-expressing oocytes recorded with a range of extracelluar NaCl concentrations [96 mM (n = 23), 48 mM (n = 12), 24 mM (n = 11), 0 mM (n = 11) and 96 mM TEA-Cl (n = 11)]. (C) Current–voltage relationships for AtALMT12-expressing (ORF: open symbols) and water-injected control (water: filled symbols) oocytes recorded with various anions (n = 4 for each solution). (D) Current–voltage relationships for oocytes expressing the splicing variant SV1 (n = 10) and SV2 (n = 12) and water-injected controls (n = 10). All symbols plot the mean ± SEM.
Furthermore, TaALMT1 is permeable to several ions, including the eponymous malate, as well as chloride, nitrate and sulfate (Piñeros et al. 2008a, Zhang et al. 2008), whereas AtALMT12 was more permeable to nitrate than chloride but scarcely permeable to malate or sulfate (Fig. 5C). In addition, when cultured tobacco cells were transformed with the AtALMT12 coding sequence under the control of the 35S promoter, no malate exclusion was detected (data not shown), in contrast to TaALMT1 (Sasaki et al. 2004). These results confirm that the ion selectivity of AtALMT12 is distinct from that of TaALMT1.
We also examined the effect of aluminum on AtALMT12, since aluminum-dependent activation is a specific feature of both TaALMT1 and AtALMT1 (Sasaki et al. 2004, Hoekenga et al. 2006). Extracellular aluminum (100 μM AlCl3) slightly enhanced outward currents at +80 mV (8.6 ± 5.2%, n = 12), but this effect is far smaller than for TaALMT1 (139.3 ± 68.2%, n = 9), indicating that AtALMT12 possesses a negligible response to aluminum.
To examine whether the splice variants also encode active anion channels, we expressed them in Xenopus oocytes. Neither variant gave rise to currents distinguishable from water-injected control (Fig. 5D), despite their being detected at the oocyte crude membrane in immunoblots (Supplementary Fig. S4C). These results suggest that the splice variants are not functional as anion transporters.
Plasma membrane anion channels play essential roles in stomatal movement (Ward et al. 2009). For example, the slac1 mutation disrupts both calcium- and ABA-dependent S-type anion currents and stomatal responses, but alters currents from neither R-type anion channels nor calcium-permeable channels (Vahisalu et al. 2008). The fact that stomatal closure was impaired in atalmt12-1 led us to measure both R- and S-type anion currents in guard cells by the whole-cell patch–clamp technique (Mori et al. 2006, Munemasa et al. 2007). Elevated extracellular calcium (40 mM) or ABA (10 or 50 μM) activated S-type anion currents in atalmt12-1 guard cell protoplasts, to a similar extent as in the wild type (Fig. 6A–E). As a control, calcium activation of an S-type anion current was assayed in cpk6-1 and was absent, as reported previously (Mori et al. 2006). Similarly, significant differences in R-type anion currents between the wild type and atalmt12-1 were absent (Fig. 6F, G). These results imply that AtALMT12 encodes neither R-type nor S-type anion channels.
Fig. 6.
S-type and R-type anion currents in A. thaliana guard cell protoplasts. (A, B) Whole-cell S-type anion currents in response to a high extracellular calcium concentration (40 mM CaCl2 in the bath solution). (A) Representative traces of calcium-activated S-type anion currents. (B) Current–voltage relationships of the wild type (n = 6), atalmt12-1 (n = 6) and cpk6-1 (n = 3). (C–E) Whole-cell S-type anion currents with 10 μM (C, D) or 50 μM (E) ABA in the bath solution. (C) Representative traces of S-type anion currents. (D) Current–voltage relationships of the wild type (n = 5) and atalmt12-1 (n = 7). (E) Current–voltage relationship of the wild type and atalmt12-1 (n = 2). (F) Representative traces of R-type anion currents in the wild-type and atalmt12-1. (G) Average peak R-type anion channel current in the wild type (n = 4) and atalmt12-1 (n = 3). All data are the mean ± SEM. Significant differences (Student’s t-test) were not observed between the wild type and atalmt12-1 in both the S-type anion current (E; P = 0.587 at −115 mV) and the R-type anion current (F; P = 0.348 at negative peak values).
Discussion
The ALMT family was originally identified in wheat, with the characterization of TaALMT1 in conferring aluminum resistance (Sasaki et al. 2004). Subsequent reports demonstrated that TaALMT1 transported malate, chloride, nitrate and sulfate (Pineros et al. 2008a, Zhang et al. 2008). While the maize homolog, ZmALMT1, is permeable to the inorganic anions rather than malate, it is neither activated by aluminum nor related to aluminum resistance (Pineros et al. 2008b). In A. thaliana, AtALMT1 has been characterized as an aluminum-activated malate transporter and involved in aluminum resistance (Hoeckenga et al. 2006, Kobayashi et al. 2007), although it is still unknown whether it transports inorganic anions. Another family member in A. thaliana, AtALMT9, is a malate transporter localized on vacuolar membrane and involved in cytosolic malate homeostasis (Kovermann et al. 2007). These findings suggest that the ALMT proteins comprise a diverse family of anion channels and transporters, possessing multiple functions.
Our results add stomatal aperture control to the list of functions handled by aluminum-activated malate transporter family members. AtALMT12 functions in mediating stomatal closure rather than in conferring aluminum resistance. The loss-of-function mutant has a wilty phenotype and closes its stomata sluggishly in response to dehydration, calcium or ABA.
An ambiguity involving AtALMT12 function concerns its subcellular localization. We failed to detect the protein when driven by the native promoter in a homologous system, implying that its endogenous expression level is low. However, when driven by the 35S promoter in transient, heterologous systems, AtALMT12 was observed at both the plasma membrane and endomembranes. It is difficult to evaluate the significance of these observations for the function of AtALMT12 because the localization of transporters is often surprisingly dynamic, reflecting an important level of regulation. For example, in A. thaliana guard cells, KAT1, an inward-rectifying potassium channel, is triggered by ABA to relocate from the plasma membrane to endosomes, thereby quickly decreasing the capacity for potassium flux (Sutter et al. 2007). As another example, also from A. thaliana, trafficking of a boron transporter, BOR1, from the plasma membrane to endosomes is regulated by boron availability, presumably to preserve optimal boron status (Takano et al. 2005). Until AtALMT12 can be detected when expressed at endogenous levels, the role of subcellular localization changes for the regulation of its function must be conjectural.
When stomata close, turgor pressure decreases and this is accompanied by a major efflux of solutes (Ward et al. 2009). Here, AtALMT12 in the oocyte expression system catalyzed outward-rectifying currents, which reflects an influx of anions across the plasma membrane of the oocyte (Fig. 5A–C), a direction opposite to what is expected for stomatal closure. One of the possible explanations could be that the AtALMT12 transporter releases anions into the cytosol from the endoplasmic reticulum (ER) or vacuole by the outward-rectifying currents. It might be helping to provoke the massive solute efflux via the anion channels on the plasma membrane needed for stomatal closure. Another explanation is that the direction of current flow through AtALMT12 located on the plasma membrane is regulated by a protein factor missing from oocytes. Consistent with this idea, the SLAC1-mediated S-type anion channel activity is up-regulated by phosphorylation with the open stomata 1 (OST1) protein kinase and down-regulated by dephosphorylation with group A-type 2C protein phosphatases (Geiger et al. 2009, Lee et al. 2009). In Xenopus oocytes, co-expression of SLAC1 and OST1 correlates with the ability of chloride and nitrate to efflux across the plasma membrane. Whether these pathways also regulate AtALMT12 function, especially for inward-rectifying currents, remains to be determined.
It has been suggested that at least one signaling pathway, in addition to the one mediated by S-type anion channels such as SLAC1, is involved in ABA-induced stomatal closure (Pandey et al. 2007). We hypothesize that the target of this pathway is AtALMT12, which possesses a high capacity for the transport of nitrate and chloride and substantially abrogates stomatal aperture response when knocked-down. Further study of AtALMT12 together with SLAC1, AtABCB14 and AtNRT1.1/CHL1 will enhance our understanding of the molecular mechanisms for stomatal movements (Guo et al. 2003, Lee et al. 2008, Negi et al. 2008, Saji et al. 2008, Vahisalu et al. 2008), and the study of the ALMT transporter family might reveal how transporters evolve new functions.
Materials and Methods
Plant materials and growth conditions
Arabidopsis thaliana L. Heynh was used for experiments, and all lines are in the Columbia background. Knockdown mutant lines atalmt12-1 (WiscDsLox_329D04) and atalmt12-2 (SALK_098126) were obtained from the Arabidopsis Biological Resource Center (Ohio University). T-DNA insertion and homozygous lines were selected by PCR using the T-DNA left border-specific primers, p745: 5′-AACGTCCGCAATGTGTTA TTAAGTTGTC-3′ for the WiscDsLox line or LBa1: 5′-TGGTT CACGTAGTGGGCCATCG-3′ for the SALK line, and the gene-specific primers, forward primer: 5′-CTCAGTTCTCGATGTACC TAC-3′ and reverse primer: 5′-GAATCTCTTGTAGGTTCGA GT-3′. Plants were grown on soil or hydroponic culture medium [one-sixth Murashige and Skoog (MS) medium supplemented with 1% sucrose] under 16 or 8 h light conditions (40–100 μmol m−2 s−1) at 20–22°C. For hydroponics, seeds were surface-sterilized in 70% ethanol followed by 0.25% sodium hypochlorite containing 0.05% Tween-20.
RT–PCR
Total RNA was extracted from plant materials using RNeasy Plant Mini kits (Qiagen K.K., Tokyo, Japan) or TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Guard and mesophyll cell protoplasts were isolated enzymatically from leaves using a method described previously (Kwak et al. 2003). Total RNA from leaves, stems and flowers was prepared from plants grown on soil; the RNA from roots was prepared from seedlings grown on mesh in hydroponics. Prior to RT–PCR, RNA samples were treated with DNase (RQ1 RNase-Free DNase, Promega, Madison, WI, USA) to remove contaminating genomic DNA. First-strand cDNA synthesis was performed in a 20 μl reaction mixture containing 1 μg of total RNA and oligo(dT) primers using the SuperScript First-Strand Synthesis system for RT–PCR (Invitrogen). RT–PCR was performed using ExTaq polymerase (TAKARA BIO INC., Ohtsu, Japan). The primers for semi- quantitative amplification of AtALMT12 (249, 335 and 412 bp) are 5′-CAGATTCAAAAGACAGAATCTACG-3′ and 5′-GATC TTTAAAAAGCGCGCGAACGGAT-3′, which are designed for exon 4 and 6 (primer set #1 in Fig. 2A). The primers used as the guard cell-specific genes are: 5′-TGCTCGGATCAATTTCTTCA-3′, 5′-GATGCGACTCTTCCTCTGCT-3′ for SLAC1 (At1g12480, 377 bp) (Saji et al. 2008); 5′-AAGCATGGGATGGGAAGAG TGG-3′, 5′-CCATTAGAGCAGTGTCGGAAGT-3′ for KAT1 (At5g46240, 89 bp) (Mori et al. 2006); and 5′-GTTATATTAGT GGTCATGGGTCTTG-3′, 5′-CCTGTAACTCTTGTACACCTTTT GT-3′ for the mesophyll-specific gene, CBP (At4g33050, 378 bp) (Mori et al. 2006). The primers used for internal control genes are: 5′-CCTGATAACTTCGTCTTTGG-3′, 5′-GTGAACT CCATCTCGTCCAT-3′ for β-tubulins (968 bp, AT1G75780, AT5G62690, AT5G62700 and AT5G44340) (Knight et al. 1999); and 5′-GGCCGATGGTGAGGATATTCAGCCACTTG-3′, 5′-TCGATGGACCTGACTCATCGTACTCACTC-3′ for actin (At5g09810, 1,109 bp) (Mori et al. 2006). The amplified PCR products were resolved by agarose gel electrophoresis, and then imaged by ethidium bromide staining.
For quantitative real-time RT–PCR, levels of AtALMT12 and EF1α transcripts were determined on the LightCycler instrument (Roche Diagnostics, Mannheim, Germany) with the THUNDERBIRD SYBR qPCR Mix (TOYOBO, Osaka, Japan). The primers 5′-CATCTCCACGTGGCACTTCAAGAT-3′ and 5′- CAGTCCTAAAGCTTGAAAGTGAAAC-3′ amplified a 271 bp fragment of the AtALMT12 gene (primer set #2 in Fig. 2A). To amplify the EF1α (At5g60390) transcript (103 bp), primers 5′-CCTTGGTGTCAAGCAGATGA-3′ and 5′-TGAAGACACCTCC TTGATGATTT-3′ were designed as described previously (Takano et al. 2006). Reaction conditions for thermal cycling were: 95°C for 30 s, 40 cycles of 95°C for 5 s, and 60°C for 30 s. For each gene, a standard curve was prepared using a serial dilution of the reverse-transcribed cDNA sample. Taking into account the differences in total RNA present in each sample, the amount of AtALMT12 transcript was normalized to the amount of EF1α transcript detected in the same sample.
Construction of binary plasmids and transformation of plants
For construction of plasmids, PCR was performed using the high fidelity enzyme Prime STAR HS or GXL DNA polymerases (TAKARA BIO INC.). To amplify the 3,157 bp genomic sequence upstream of AtALMT12, the primers were used as follows. Forward primer: 5′-aaccaattcagtcgacGCAGTCTTGCAGACATATTAGCGAG-3′ and reverse primers: 5′-aagctgggtctagatatctctagaTTTGAGGGAGAGAAATTGGTACTCTC-3′ or 5′-aagctgggtctagatatccctaggTTTGAGGGAGAGAAATTGGTACTCTC-3′. These primers are designed for In-Fusion cloning and include restriction endonuclease site sequences (underlined). The fragments were cloned into the pENTR 3C entry plasmid (Invitrogen) using the In-Fision 2.0 Dry-Down PCR cloning kit (Clontech–TAKARA BIO INC.). The construction of the AtALMT12 promoter::GUS reporter gene was performed using the pGWB3 plasmid (Nakagawa et al. 2007) via the Gateway Cloning system (Invitorgen). The AtALMT12 genomic fragment of 2,241 bp and the 1,684 bp ORF (coding sequence) were amplified using primers 5′-GCgtcgactctagaATGTCCAATAAGGTTCACGTAGGGAGC-3′ and 5′-CCgagctcTCATTCCGCGGCACCGACACTG ATCGT-3′ (for the stop codon), and 5′-CGccatggACCCTCCG CCACCTTCCGCGGCACCGACACTGATCGT-3′ (for fusion of C-terminal GFP), which include restriction sites (underlined). Binary plasmids of the AtALMT12 promoter fused to the coding sequence, genomic sequence or genomic sequence::GFP were modified from the AtALMT12 promoter::GUS reporter gene plasmid using pGWB3 by replacing the GUS gene with the AtALMT12 fragments. The binary plasmids under the control of the CaMV 35S promoter were constructed using pIG121-Hm (Ohta et al. 1990). For transient expression of AtALMT12::GFP or GFP::AtALMT12 constructs under the control of the CaMV 35S promoter, the plasmids pTH2 (Chiu et al. 1996) or pGWB2 (Nakagawa et al. 2007) were used, with modification.
The binary plasmids were introduced into Agrobacterium tumefaciens strain EHA101 (Hood et al. 1986). Transformations were performed as described previously (Clough and Bent 1998). Transgenic plants (T1) were selected on Murashige–Skoog (MS) medium containing 20 μg ml−1 kanamycin and 100 μg ml−1 carbenicillin, and then grown in soil. The self-pollinated progeny (T2) were selected by kanamycin resistance and analyzed for expression of ALMT1 and measurement of stomatal apertures. Transformation of tobacco cells was carried out as described previously with minor modifications (An 1985, Sasaki et al. 2004).
The AtALMT12::GFP fusion under the CaMV 35S promoter was transiently transformed into onion epidermal cells and V. faba guard cells by particle bombardment (PDS-1000, Bio-Rad Laboratories, Hercules, CA, USA). For co-localization analysis in V. faba cells, the construct of the Cyt b5::monomeric red fluorescent protein (mRFP) was used as an ER marker (Toyooka et al. 2006). Fluorescence was observed on a Zeiss confocal microscope (LSM510 Carl Zeiss, Oberkochen, Germany), with 488 nm excitation from an argon laser and a 505–530 nm bandpass filter for GFP, and 543 nm excitation from an He–Ne laser and a 560–615 nm bandpass filter for mRFP.
GUS staining
A stable transgenic plant expressing GUS under the AtALMT12 native promoter control was used for GUS staining by a method described previously (Weigel and Glazebrook 2002).
Stomatal aperture measurements
The width of stomatal apertures was measured as described previously (Mori et al. 2006). Excised rosette leaves from 4- to 5-week-old plants were floated on opening buffer (5 mM KCl, 50 μM CaCl2 and 10 mM MES-Tris, pH 5.7) for 2 h at 22°C under illumination from a fluorescent lamp at a photon flux of 100 μmol m−2 s−1, to open stomata. Leaves were floated for another 2 h after addition of ABA to the opening buffer. For measuring calcium-induced stomatal closure, CaCl2 was omitted from the opening buffer. For dark-induced stomatal closure, the Petri dish containing leaves was wrapped with aluminum foil at 2 h, then further incubated for the indicated periods.
Electrophysiology using X. laevis oocytes
For expression in oocytes, cRNAs were prepared using a MEGA script kit with cap analog (Ambion, Austin, TX, USA) from linearized (BamHI-digested) pXBG-AtALMT12 plasmid DNA, which also contained the coding region of a Xenopus β-globin gene (Preston et al. 1992). Female X. laevis were purchased from Hamamatsu Seibutsu Kyozai Co. (Hamamatsu, Japan). Stage V–VI defolliculated oocytes were isolated and used for experiments. Oocytes were injected with 50 nl of water containing 40 ng of cRNA (or 50 nl of water for controls) using a microinjection system (NANOJECT II, Drummond Scientific Co., Broomall, PA, USA). The oocytes were stored at 18°C in modified Barth’s solution [88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 20 mM HEPES-Tris (pH 7.5), 50 μg ml−1 gentamycin] before and after microinjection. Recordings were obtained 2 d after microinjection with MEZ-7200 and CEZ-1200 amplifiers equipped with the SET-1201 step pulse generator (Nihon Kohden, Tokyo, Japan) to measure net currents across the oocyte membrane at different membrane voltages. The recording electrodes were filled with 3 M KCl. The basic bath solution contained 96 mM NaCl, 1 mM KCl, 1.8 mM CaCl2, 0.1 mM LaCl3 and 5 mM HEPES-NaOH (pH 7.5). The extracellular chloride concentration was adjusted by decreasing the NaCl, and the osmolarities of each test solution were adjusted to 210 mOsm kg−1 (equivalent to that of Barth’s solution) with sorbitol. Currents were measured in response to voltage pulses from −120 to +80 mV in 20 mV increments, stepped from a holding potential of −60 mV with each 3 s interval. Current–voltage curves were constructed from the steady-state currents.
Patch–clamp experiments
For whole-cell patch–clamp recordings of S-type and R-type anion currents, A. thaliana guard cell protoplasts were prepared from rosette leaves of 4- to 6-week-old plants by an enzymatic method, as previously described (Pei et al. 1997). Whole-cell currents were recorded as previously described (Munemasa et al. 2007). For S-type anion current measurements, the patch–clamp solutions contained 150 mM CsCl, 2 mM MgCl2, 6.7 mM EGTA, 5.58 mM CaCl2 (free Ca2+ concentration: 2 μM), 5 mM ATP and 10 mM HEPES-Tris (pH 7.1) in the pipet and 30 mM CsCl, 2 mM MgCl2, 1 mM CaCl2 and 10 mM MES-Tris (pH 5.6) in the bath (Pei et al. 1997). The concentration of free calcium was calculated using the ‘CALCIUM’ program (Foehr et al. 1993). To measure R-type anion channel currents, the pipet solution contained 75 mM K2SO4, 2 mM MgCl2, 5 mM EGTA, 2.5 mM CaCl2 and 10 mM HEPES-Tris (pH 7.1). The bath solution contained 50 mM CaCl2, 2 mM MgCl2 and 10 mM MES-Tris (pH 5.6) (Mori et al. 2006). Osmolality was adjusted to 500 mmol kg−1 (pipet solutions) and 485 mmol kg−1 (bath solutions) with sorbitol. In S-type anion channel recording, membrane voltage was clamped from +35 to −145 mV (for calcium- activated currents) or to −115 mV (for ABA-activated currents) with 30 mV decrements. The holding potential was +30 mV. For R-type anion channel recording, the voltage was ramped from the holding potential of 0 mV to −200 mV with a ramp speed of −20 mV s−1.
Immunoblot analysis
Crude microsomal membrane fractions of guard cells and oocytes were prepared as described previously (Sasaki et al. 2004, Yamaguchi et al. 2005, Ledc-Nadeau et al. 2007). The proteins were separated by SDS–PAGE and electroblotted onto a polyvinylidene difluoride filter. The filter was incubated with the anti-AtALMT12 antiserum raised against the synthetic polypeptide (C-EKTDSKDRIYEGYQA) or an anti-GFP antibody (TOYOBO). The antibody-bound antigen was detected using a protocol described previously (Sasaki et al. 2004, Yamaguchi et al. 2005).
Supplementary data
Supplementary data are available at PCP online.
Funding
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan [Grant-in-Aid for Scientific Research (No. 17078007 to T.S., No. 17078006 to Y.M., Nos. 17380049, 1820800821580078 to Y.Y.)]; the Nissan Science Foundation [to I.C.M.]; Ohara Foundation for Agricultural Science.
Supplementary Material
Acknowledgments
We are grateful to the following for providing plasmids: T. Nakagawa (pGWB2, pGWB3), Y. Niwa (pTH2 plasmids), H. Sano and K. Nakamura (pIG121-Hm plasmid and Agrobacterium EHA101). We also thank M. Ariyoshi, Y. Tsuchiya, E. Himi and M. Fujii for experimental assistance, and T.I. Baskin for critical reading of the manuscript.
Glossary
Abbreviations
- ALMT
aluminum-activated malate transporter
- CaMV
cauliflower mosaic virus
- CBP
calmodulin-binding family protein
- GFP
green fluorescent protein
- GUS
β-glucuronidase
- mRFP
monomeric red fluorescent protein
- ORF
open reading frame
- OST1
open stomata 1
- RT–PCR
reverse transcription–PCR
- SLAC1
SLOW ANION CHANNEL-ASSOCIATED 1.
References
- An G. High efficiency transformation of cultured tobacco cells. Plant Physiol. 1985;79:568–570. doi: 10.1104/pp.79.2.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu W-I, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Curr. Biol. 1996;6:325–330. doi: 10.1016/s0960-9822(02)00483-9. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–745. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Gruber BD, Ryan PR. The roles of organic anion permeases in aluminium resistance and mineral nutrition. FEBS Lett. 2007;581:2255–2262. doi: 10.1016/j.febslet.2007.03.057. [DOI] [PubMed] [Google Scholar]
- Foehr KJ, Worchol W, Gratzel M. Calculation and control of free divalent cations in solutions used for membrane fusion studies. Methods Enzymol. 1993;221:149–157. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, et al. Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase–phosphatase pair. Proc. Natl Acad. Sci. USA. 2009;106:21425–21430. doi: 10.1073/pnas.0912021106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F-Q, Young J, Crawford NM. The nitrate tansporter AtNRT1.1 (CHL1) functions in stomatal opening and contributes to drought susceptibility in Arabidopsis. Plant Cell. 2003;15:107–117. doi: 10.1105/tpc.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R, Busch H, Raschke K. Ca2+ and nucleotide dependent regulation of voltage dependent anion channels in the plasma membrane of guard cells. EMBO J. 1990;9:3889–3892. doi: 10.1002/j.1460-2075.1990.tb07608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekenga OA, Maron LG, Piñeros MA, Cançado GMA, Shaff J, Kobayashi Y, et al. AtALMT1, which encodes a malate transporter, is identified as one of several genes critical for aluminum tolerance in Arabidopsis. Proc. Natl Acad Sci USA. 2006;103:9738–9743. doi: 10.1073/pnas.0602868103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood EE, Helmer GL, Fraley RT, Chilton M-D. The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 1986;168:1291–1301. doi: 10.1128/jb.168.3.1291-1301.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosy E, Vavasseur A, Mouline K, Dreyer I, Gaymard F, Porée F, et al. The Arabidopsis outward K+ channel GORK is involved in regulation of stomatal movements and plants transpiration. Proc. Natl Acad. Sci. USA. 2003;100:5549–5554. doi: 10.1073/pnas.0733970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight H, Veale EL, Warren GJ, Knight MR. The sfr6 mutation in Arabidopsis suppresses low-temperature induction of genes dependent on the CRT/DRE sequence motif. Plant Cell. 1999;11:875–886. doi: 10.1105/tpc.11.5.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Hoekenga OA, Itoh H, Nakashima M, Saito S, Shaff JE, et al. Characterization of AtALMT1 expression in aluminum-induced malate release and its role for rhizotoxic stress tolerance in Arabidopsis. Plant Physiol. 2007;145:843–852. doi: 10.1104/pp.107.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu. Rev. Plant Biol. 2004;55:459–493. doi: 10.1146/annurev.arplant.55.031903.141655. [DOI] [PubMed] [Google Scholar]
- Kovermann P, Meyer S, Hörtensteiner S, Picco C, Scholz-Starke J, Ravera S, et al. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007;52:1169–1180. doi: 10.1111/j.1365-313X.2007.03367.x. [DOI] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei Z-M, Leonhardt N, Torres MA, Dangl JL, et al. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledc-Nadeau A, Lahjouji K, Bissonnette P, Lapoinate J-Y, Bichet DG. Elaboration of a novel technique for purification of plasma membrane from Xenopus laevis oocytes. Amer. J. Physiol. Cell Physiol. 2007;292:C1132–C1136. doi: 10.1152/ajpcell.00136.2006. [DOI] [PubMed] [Google Scholar]
- Lee M, Choi Y, Burla B, Kim Y-Y, Jeon B, Maeshima M, et al. The ABC transporter AtABCB14 is a malate importer and modulates stomatal response to CO2. Nature Cell Biol. 2008;10:1217–1223. doi: 10.1038/ncb1782. [DOI] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. A protein kinase–phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc. Natl Acad. Sci. USA. 2009;106:21419–21424. doi: 10.1073/pnas.0910601106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001;6:273–278. doi: 10.1016/s1360-1385(01)01961-6. [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S Wang, Andreoli Y-FS, et al. CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol. 2006;4:e327. doi: 10.1371/journal.pbio.0040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemasa S, Oda K, Watanabe-Sugimoto M, Nakamura Y, Shimoishi Y, Murata Y. The coronatine-insensitive 1 mutation reveals the hormonal signaling interaction between abscisic acid and methyl jasmonate in Arabidopsis guard cells. Specific impairment of ion channel activation and second messenger production. Plant Physiol. 2007;143:1398–1407. doi: 10.1104/pp.106.091298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, et al. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007;104:34–41. doi: 10.1263/jbb.104.34. [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, et al. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature. 2008;452:487–491. doi: 10.1038/nature06720. [DOI] [PubMed] [Google Scholar]
- Ohta S, Mita S, Hattori T, Nakamura K. Construction and expression in tobacco of a β-glucuronidase (GUS) reporter gene containing an intron within the coding sequence. Plant Cell Physiol. 1990;31:805–813. [Google Scholar]
- Pandey S, Zhang W, Assmann SM. Roles of ion channels and transporters in guard cell signal transduction. FEBS Lett. 2007;581:2325–2336. doi: 10.1016/j.febslet.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell. 1997;9:409–423. doi: 10.1105/tpc.9.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot G, Lacombe B, Gaymard F, Chérel I, Boucherez J, Thibaud J-B, et al. Guard cell inward K+ channel activity in Arabidopsis involves expression of the twin channel subunits KAT1 and KAT2. J. Biol. Chem. 2001;276:3215–3221. doi: 10.1074/jbc.M007303200. [DOI] [PubMed] [Google Scholar]
- Piñeros MA, Cançado GMA, Kochian LV. Novel properties of the wheat aluminum tolerance organic acid transporter (TaALMT1) revealed by electrophysiological characterization in Xenopus oocytes: functional and structural implications. Plant Physiol. 2008a;147:2131–2146. doi: 10.1104/pp.108.119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piñeros MA, Cançado GMA, Maron LG, Lyi SM, Menassi M, Kochian LV. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: the case of ZmALMT1—an anion-selective transporter. Plant J. 2008b;53:352–367. doi: 10.1111/j.1365-313X.2007.03344.x. [DOI] [PubMed] [Google Scholar]
- Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256:385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- Reddy ASN. Alternative splicing of pre-messenger RNAs in plants in the genomic era. Annu. Rev. Plant Biol. 2007;58:267–94. doi: 10.1146/annurev.arplant.58.032806.103754. [DOI] [PubMed] [Google Scholar]
- Saji S, Bathula S, Kubo A, Tamaoki M, Kanna M, Aono M, et al. Disruption of a gene encoding C4-dicarboxylate transporter-like protein increases ozone sensitivity through deregulation of the stomatal response in Arabidopsis thaliana. Plant Cell Physiol. 2008;49:2–10. doi: 10.1093/pcp/pcm174. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, et al. A wheat gene encoding an aluminum-activated malate transporter. Plant J. 2004;37:645–653. doi: 10.1111/j.1365-313x.2003.01991.x. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Schelle I, Liao Y-J, Schroeder JI. Strong regulation of slow anion channels and abscisic acid signaling in guard cells by phosphorylation and dephosphorylation events. Proc. Natl Acad. Sci. USA. 1995;92:9535–9539. doi: 10.1073/pnas.92.21.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Keller BU. Two types of anion channel currents in guard cells with distinct voltage regulation. Proc. Natl Acad. Sci. USA. 1992;89:5025–5029. doi: 10.1073/pnas.89.11.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter J-U, Sieben C, Hartel A, Eisenach C, Thiel G, Blatt MR. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007;17:1396–1402. doi: 10.1016/j.cub.2007.07.020. [DOI] [PubMed] [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl Acad. Sci. USA. 2005;102:12276–12281. doi: 10.1073/pnas.0502060102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell. 2006;18:1498–1509. doi: 10.1105/tpc.106.041640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Moriyasu Y, Goto Y, Takeuchi M, Fukuda H, Matsuoka K. Protein aggregates are transported to vacuoles by a macroautophagic mechanism in nutrient-starved plant cells. Autophagy. 2006;2:96–106. doi: 10.4161/auto.2.2.2366. [DOI] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G, et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signaling. Nature. 2008;452:483–486. doi: 10.1038/nature06608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J, Mäser P, Schroeder JI. Plant ion channels: gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel D, Glazebrook J. Arabidopsis: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2002. [Google Scholar]
- Yamaguchi M, Sasaki T, Sivaguru M, Yamamoto Y, Osawa H, Ahn SJ, et al. Evidence for the plasma membrane localization of Al-activated malate transporter (ALMT1) Plant Cell Physiol. 2005;46:812–816. doi: 10.1093/pcp/pci083. [DOI] [PubMed] [Google Scholar]
- Zhang W-H, Ryan PR, Sasaki T, Yamamoto Y, Sullivan W, Tyerman SD. Characterization of the TaALMT1 protein as an Al3+-activated anion channel in transformed tobacco (Nicotiana tabacum L.) cells. Plant Cell Physiol. 2008;49:1316–1330. doi: 10.1093/pcp/pcn107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.