Abstract
This study examines whether maternal vitamin D deficiency is a risk factor for infantile autism disease (IAD). We used epidemiologic data seasonal variation of birth rates and prevalence of IAD for cohorts born before 1985. For seven studies reporting spring-to-summer excess birth rates for IAD, the season progressed from broad near 30° N latitude, spring/summer in midlatitudes, to winter at the highest latitude. Also, using data from 10 studies, we found a strong effective latitudinal (related to wintertime solar ultraviolet B radiation) increase in IAD prevalence. These findings are consistent with maternal vitamin D deficiency’s being a risk factor for IAD, possibly by affecting fetal brain development as well as possibly by affecting maternal immune system status during pregnancy. Further investigation of this hypothesis is warranted.
Key words: autism, cathelicidin, ecological, maternal, season of birth, ultraviolet-B, viral infections, vitamin D
Introduction
The etiology of autism is still somewhat of an enigma. Autism is considered an autoimmune disease.1 It also appears to have important risk factors in utero, as evidenced by a highly significant increased frequency of congenital malformations;2 and those with autism have several characteristics associated with schizophrenia,3 which is also linked to in utero risk factors.4 Perinatal viral infection of mother or infant is a risk factor for both infantile autism and schizophrenia.5 Also, several studies have reported seasonality in excess births of those with autism, with March being a peak month in Sweden,6 Denmark7 and Boston,8 but without a good explanation for this seasonality.
Schizophrenia is another disease that exhibits excess of births in winter and a deficit in summer.9 This seasonality has sometimes been shown to be linked to influenza epidemics.10,11 Infectious disease during pregnancy has been found to adversely affect rodent brain development in a manner that can lead to schizophrenia as well as autism.12–18 The hypothesis that the seasonality was related to low levels of maternal serum 25-hydroxyvitamin D (calcidiol) was also advanced.19 Support for the maternal vitamin D deficiency hypothesis has been reported based on rat studies.20–22
It was recently proposed that the annual solar ultraviolet-B (UVB) and vitamin D cycles explained some of the seasonality of epidemic influenza, which peaks in winter.23 This hypothesis received experimental support in a randomized, prospective, placebo-controlled vitamin D study involving 204 postmenopausal black women living in the state of New York. Those taking 2,000 IU of vitamin D3 per day got 10% as many colds or influenza as those taking the placebo.24 More support came from a study of meteorological variables associated with incidence of respiratory syncytial virus that found an effect for solar UVB in addition to temperature and relative humidity, with greatest effect at lower latitude.25 Thus, serum calcidiol levels can affect risk of maternal viral disease during pregnancy.
This report examines the evidence supporting the hypothesis that maternal vitamin D deficiency is an important risk factor for the development of infantile autism disease (IAD), through both direct effect and indirect effects in reducing the risk of infectious diseases. The analysis is limited to the period prior to the mid-1980s. There has been a rapid rise in birth rates of autism in countries such as the UK and the US since the mid-1980s.26 While there is concern that increased rate of vaccinations, especially those containing Thimerosal, has led to the increases,27 there is no general agreement that vaccinations are a cause of the increases.28,29 Factors accounting for the increase include a broadening of diagnostic concepts and criteria, increased awareness and, therefore, better identification of children with pervasive developmental disorders in communities and epidemiologic surveys, and improved access to services.30
Results
Prevalence.
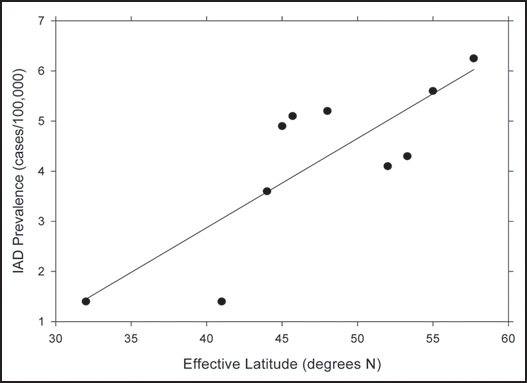
Table 1 gives the regression results for IAD and their difference versus latitude. Figure 1 also shows the regression results for IAD. Effective latitude was highly correlated with prevalence; i.e., prevalence increased at the higher latitudes, which are associated with lower vitamin D produced by solar UVB in summer. Actual latitude was also correlated with prevalence data, but at lower levels of significance.
Table 1.
Infantile autism disease (IAD) prevalence data for cohorts born before 1985
| Country, city | No. with autism; year of data collection | Diagnostic criteria | Prevalence per 10,000 (95% CI); age range | Reference |
| Israel | >200; 1960–82 | 1.4 | 31 | |
| USA, Minnesota | 1990–6 | 52 | 32 | |
| North Dakota, USA | 21; 1966–82 | DSM-III, infantile | 1.16 (1.4 age); 2–18 yrs | 33 |
| Wisconsin, USA | 69; <1970 | 3.1 (3.6 age); 3–12 yrs | 34 | |
| France (Rhone) | 61; 1977–82 | DSM-III, infantile | 5.1 (3.9–6.3); 5–9 yrs | 35 |
| France | 154; 1972, 76 | ICD-9 (1978); infantile | 4.9 (3.7–6.1); 9–13 yrs | 36 |
| UK | 32; <1966 | Kanner | 4.1; 8–10 yrs | 37 |
| UK | 17; <1976 | 4.9; 5–14 yrs | 38 | |
| Ireland | 28; 1968–70 | 4.3; 8–10 yrs | 39 | |
| Denmark | 20; <1970 | Kanner | 4.3 (5.2 age); 2–14 yrs | 40 |
| Sweden | 20 (urban), 15 (rural); 1975–84 | DSM-III; infantile | 4.5 (4.2 rural, 4.7 urban) (5.6, 6.3 age); 0–9 years | 41 |
Figure 1.
Prevalence of infantile autism disease for those born prior to 1985 vs. effective latitude.
However, people born between 1988 and 1995 showed little variation with latitude for IAD and an insignificant inverse correlation with latitude for PDD.
Season of birth.
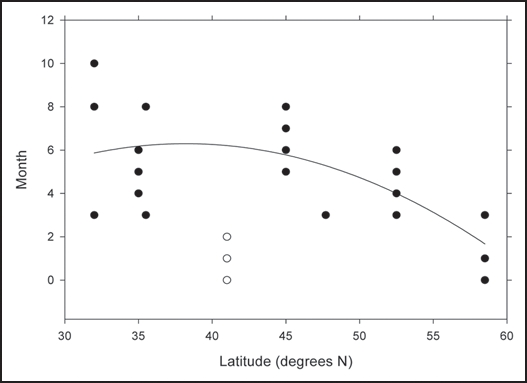
Figure 2 graphs the months of excess birth rate versus latitude, with December counted as month zero; when two adjacent quarters were listed as having excess birth rates, we omitted the two extreme months of these quarters. At the lower latitudes, there is a large spread, decreasing to 3–4 months for the other latitudes; also, there is a trend toward the beginning of the year with increasing latitude. New York does not fall into this pattern.
Figure 2.
Months of the year with excess birth rates for autism vs. latitude of the country.
Discussion
Prevalence and vitamin D deficiency.
The data for seasonal variation of prevalence of those born prior to 1985 are consistent with an increased risk during pregnancy in winter. From the timing, risk is most likely affecting the mother during the third trimester of pregnancy since serum calcidiol values are lowest in late winter/early spring, and risk of influenza and other viral diseases is highest then. The brain develops mostly in the later stages of pregnancy, so a vitamin D deficiency would exert more effect during this period.15 The prevalence of IAD quadruples in going from Israel to Sweden. The earlier period to the north is consistent with colder temperatures and lower solar UVB doses arriving earlier there than to the south. The fact that a similar effect was not found for the non-IAD portion of PDD, which develops after 30 months of age, is further evidence pointing to IAD’s association with a maternal vitamin D deficiency.
The IAD prevalence data for France are somewhat above the regression line in Figure 1, perhaps partly because food there is not vitamin D fortified. On this basis, the French appear the largest winter/spring drop in serum calcidiol level of any country studied.42 People living near the Arctic Circle are more likely to consume fish, an important source of vitamin D, and to take vitamin D supplements.
Infection and vitamin D deficiency.
Multiple sclerosis (MS) is another disease for which risk increases rapidly with latitude. 43–45 The geographic variation in the US is more highly correlated with latitude, an index for wintertime UVB and vitamin D, than with July UVB.43,46 Risk of MS is linked to the Epstein- Barr virus,47 and both solar UVB48 and vitamin D49 reduce the risk. It has also been proposed that higher calcidiol levels reduce the risk of MS by reducing the risk of Epstein-Barr infection.50
Correlation of risk indices.
The seasonal excess of autistic births has an interesting variation with latitude. The general coherence to the data indicates that there are seasonal influences on the risk of autism. The large spread in months of excess births for Israel—March, August and October—may be related to the fact that the latitude is low enough that there is not a pronounced seasonal variation of serum calcidiol levels. That the season of excess autistic birth is generally from December to April is consistent with a maternal vitamin D deficiency during the last 3–5 months of pregnancy. Serum calcidiol levels tend to be lowest in the spring at mid-to-higher latitudes as the body depletes vitamin D stores built up in the fatty tissues and photoproduction from solar UVB has not yet resumed.51 Curiously, however, serum calcidiol levels in Europe were found to increase with increasing latitude in winter,52 which is probably due to higher cold water fish consumption, increased vitamin D fortification of food and use of supplements.
Positive role of vitamin D.
Role of vitamin D in reducing the risk of IAD. Vitamin D could reduce the risk of IAD by (1) aiding proper development of the brain and/or (2) strengthening the immune system. The best evidence for a role of maternal calcidiol on brain development comes from the study of rat brain development for maternal vitamin D deficiency. Several adverse effects were found, such as reductions in brain content of nerve growth factor and glial cell line-derived neurotrophic factor, as well as structural differences of the cortex and lateral ventricles.20 Other effects on rat brain development were reported more recently.22,53
Role of vitamin D in resistance to infection. Recent reports indicate that vitamin D reduces the risk of respiratory diseases caused by viral infections,23–25 which are most common in winter. Other reports indicate that maternal influenza during pregnancy can adversely affect brain development.11,12 Edwards indicates that hyperthermia during infectious diseases such as influenza can adversely affect fetal brain development and lead to birth defects such as later-life schizophrenia.54 Taken together, these studies provide good evidence that vitamin D can affect development of the brain in utero by improving the immune system to reduce the risk of infectious diseases.
Possible role of infectious agents.
Maternal viral infection is known to increase the risk for schizophrenia and autism in the offspring.5 Animal models support this finding. Mice exposed to human influenza virus on Day 9 of pregnancy produced offspring with adverse effects on the developing brain including altered pyramidal and nonpyramidal cell density values; atrophy of pyramidal cells despite normal cell proliferation rate and final enlargement of brain.14 In a subsequent study, prenatal viral infection showed significant upregulation of 21 genes and downregulation of 18 genes in the affected neonatal brain homogenates spanning gene families affecting cell structure and function.14 Another study found that infection of mice with human influenza virus yielded offspring that displayed highly abnormal behavioral responses as adults. The effect was attributed to an effect of maternal immune response on the fetus.12
Application of these findings.
The finding that vitamin D can reduce the risk of infectious diseases and that seasonal and latitudinal variations in solar UVB doses seems to explain some of the epidemiology of IAD prior to 1985 suggests that increased calcidiol levels during pregnancy, breast feeding, and infancy could reduce the risk of autism. While the role of vaccinations in the etiology of autism is not clear, higher levels of calcidiol could reduce the risk of adverse reactions to vaccinations, based on reports that calcidiol reduces the risk of respiratory viral infections and that calcitriol enhances the effectiveness of vaccinations.55–58
Materials and Methods
We searched the literature for relevant reports relating to prevalence of and seasonality of birth of childhood autism. For prevalence, several reviews were.59–62 Several ideas were readily apparent in reviewing the published prevalence data:
both IAD (defined as developing autism prior to 30 months of age) and pervasive developmental disorders (PDD) were studied;
the criteria for determination changed several times since the original criteria developed by Kanner63 for “nuclear autism”;
the prevalence rates varied geographically;
the autism rates determined in Japan and other Asian countries were considerably higher than those determined in primarily white countries; and
the prevalence rates increased when the DSM-III criteria were replaced by the DSM-III-R criteria.64 This finding is related to a broadening of the diagnostic boundaries.65 On the basis of these observations, the prevalence data used to investigate a possible latitudinal variation were restricted to those gathered using DSM-III and earlier criteria. Some of the subsequent data were used separately.
Tabular parameters and adjustments.
Tables 2 and 3 give the prevalence data used in this study. We omitted data for Asian countries since the prevalence values are much higher than those in western developed countries. For Utah, an educated guess was to associate the prevalence stated as for “autism” with that for PDD since in Table 2 of that paper, many of the prevalence values from other reports are listed as associated with autism but are, in fact, associated with PDD. Also, the report states merely that DSM-III criteria were used. Also, some adjustments had to be made to account for different age ranges included in the various studies since even though IAD develops prior to 30 months of age, health officials often do not learn about student IAD status until the children attend school. The variation in observed prevalence rate versus age tabulated in Cialdella and Mamelle35 was used to determine an effective prevalence for those values that included those younger than 5 years, 1.2 starting at age 3 years, and 1.33 starting at 0 years.
Table 2.
Pervasive development disorder (PDD) prevalence data for cohorts born before 1985
| Country, city | Number with PDD; year of data collection | Diagnostic criteria | Prevalence per 10,000 (95% CI); age range | Reference |
| USA, Utah | 241; 1975–79, 1980–4 | DSM-III; PDD? | 4.0; 8–12 yrs | 66 |
| North Dakota, USA | 59; 1966–82 | PDD | 3.26 (4.0 age); 2–18 yrs | 33 |
| France (Rhone) | 125; 1977–82 | DSM-III | 9.2; 5–9 yrs | 35 |
| UK | 61; <1966 | Kanner | 7.8; 8–10 yrs | 37 |
| Denmark | Kanner | 6.2 (7.4 age); 2–14 yrs | 40 | |
| Sweden | 25 (urban), 20 (rural); 1975–84 | DSM-III; PDD | 6.55 (5.6 rural, 7.5 urban) (7.45, 9.8 age); 0–9 years | 41 |
Table 3.
Data relating to seasonality of autistic births
| Location | Latitude (°N) | Years | Period with highest rate of autistic births, in order | Enhancement ratio (to expeded) | Reference |
| Israel | 32 | 1964–86 | August, March, October | 2.2 (Aug.), 1.6 (Mar) | 67 |
| Japan | 35 | Second quarter | 68 | ||
| USA, North Carolina | 35.5 | March, August | 69 | ||
| USA, New York | 41 | Winter (January, February, March) | 70 | ||
| USA, Boston | 43.3 | March | 8 | ||
| Canada | 45 | Second quarter, third quarter | 71 | ||
| U.K. | 52 | 1947–80 | None found | 72 | |
| Denmark | 52.5 | 1945–80 | March | 7 | |
| Denmark, IQ <35 | 52.5 | 1923–92 | Second quarter | 1.3 | 73 |
| Sweden | 58.5 | 1962–84 | March, January, December | 2.2 (Mar.) | 6 |
Several reports have looked for seasonality in autistic births. Table 4 summarizes the results of searching the National Library of Medicine’s PubMed database. The winter and spring quarters had the highest rate of autistic births, with March being the month mentioned most often.
Table 4.
Regression results for IAD and PDD prevalence for those born prior to 1985
| Disease | Latitudes | Adjusted r2, F, p |
| IAD | Effective | 0.63, 16, 0.004 |
| Actual | 0.42, 7.4, 0.03 | |
| PDD | Effective | 0.59, 9.5, 0.03 |
| Actual | 0.23, 2.8, 0.15 | |
| PDD-IAD | Effective | −0.25, 0.01, 0.91 |
| Actual | −0.21, 0.12, 0.75 |
Hypothesis and test indices.
The approaches used here to test the hypothesis that maternal vitamin D deficiency is a risk factor for IAD and PDD include trying to find a link between vitamin D and IAD and PDD prevalence and interpreting the reported results regarding the seasonal variation in birth rates. Thus, a suitable index for vitamin D at the population level must be used. The simplest index is latitude. Solar UVB radiation is the primary source of vitamin D for many people on Earth, especially in tropical and temperate climate zones. The well-documented seasonal variation of serum calcidiol shows the largest variation in countries, such as France, that do not fortify foods with vitamin D or encourage the use of vitamin D supplements.74 However, in Europe in winter, serum calcidiol levels actually increase with latitude.52
There are two simple indices for serum calcidiol assuming that solar UVB is the primary source. The simplest one is latitude for latitude greater than about 30°. For example, those living near 30° can produce vitamin D from solar UVB during the entire year, whereas those living at 42° N cannot produce vitamin D from solar UVB for 4–5 months of the year.35 However, this simple index cannot explain the geographic variation in US cancer mortality rates:75,76 July UVB is much higher at the same latitude west of the Rocky Mountains than east of them.77 Both lower column ozone and higher surface elevation west of the range explain the difference. The shift west of the Rockies is approximately 7° (770 km).77 Also, from the finding that a state’s degree of urbanization was an added risk factor for seven types of cancer for which UVB is a risk-reduction factor,76 living in an urban versus a rural environment apparently reduces the total dose of UVB. Thus, latitude is used as the primary index for vitamin D, with slight adjustments for high summertime UVB levels and for living in a primarily rural region. Changes made to yield effective latitude for the prevalence data include the following:
North Dakota, from 47.5° to 41° N
Utah, from 41° to 35° N
Rural Bohuslän near Göteborg, from 57.7° N to 55° N
We ran multiple linear regressions with SigmaStat version 2.0, applying normality (Kolmogorov-Smirnov) and constant variance tests. For each linear regression, we give the values of the adjusted r2, F (accounts for the number of degrees of freedom), and p.
Summary and Conclusion
The results presented here for season of birth and prevalence variation with effective latitude are consistent with maternal vitamin D’s being a risk factor for development of IAD. However, in the late 1990s, the rates of IAD prevalence became several times higher than those prior to 1985, to the point where maternal vitamin D deficiency is no longer discernible in the epidemiologic data. Further work is required to determine whether vitamin D could be used to reduce the risk of autism, perhaps by reducing the risk of infectious disease or adverse reactions to vaccinations.
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/9500
Note
This paper was largely written in 2004 and updated in 2008. Since that time, John J. Cannell has added further evidence for a role of vitamin D in reducing the risk of autism.78,79 See also his ongoing discussion of the topic at his web site, http://vitamindcouncil.org/.
References
- 1.Krause I, He XS, Gershwin ME, Shoenfeld Y. Brief report: immune factors in autism: a critical review. J Autism Dev Disord. 2002;32:337–345. doi: 10.1023/a:1016391121003. [DOI] [PubMed] [Google Scholar]
- 2.Lauritsen MB, Mors O, Mortensen PB, Ewald H. Medical disorders among inpatients with autism in Denmark according to ICD-8: a nationwide register-based study. J Autism Dev Disord. 2002;32:115–119. doi: 10.1023/a:1014840622023. [DOI] [PubMed] [Google Scholar]
- 3.Konstantareas MM, Hewitt T. Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord. 2001;31:19–28. doi: 10.1023/a:1005605528309. [DOI] [PubMed] [Google Scholar]
- 4.Mackay-Sim A, Feron F, Eyles D, Burne T, McGrath J. Schizophrenia, vitamin D, and brain development. Int Rev Neurobiol. 2004;59:351–380. doi: 10.1016/S0074-7742(04)59014-1. [DOI] [PubMed] [Google Scholar]
- 5.Libbey JE, Sweeten TL, McMahon WM, Fujinami RS. Autistic disorder and viral infections. J Neurovirol. 2005;11:1–10. doi: 10.1080/13550280590900553. [DOI] [PubMed] [Google Scholar]
- 6.Gillberg C. Do children with autism have March birthdays? Acta Psychiatr Scand. 1990;82:152–156. doi: 10.1111/j.1600-0447.1990.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 7.Mouridsen SE, Nielsen S, Rich B, Isager T. Season of birth in infantile autism and other types of childhood psychoses. Child Psychiatry Hum Dev. 1994;25:31–43. doi: 10.1007/BF02251098. [DOI] [PubMed] [Google Scholar]
- 8.Stevens MC, Fein DH, Waterhouse LH. Season of birth effects in autism. J Clin Exp Neuropsychol. 2000;22:399–407. doi: 10.1076/1380-3395(200006)22:3;1-V;FT399. [DOI] [PubMed] [Google Scholar]
- 9.Davies G, Welham J, Chant D, Torrey EF, McGrath J. A systematic review and metaanalysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29:587–593. doi: 10.1093/oxfordjournals.schbul.a007030. [DOI] [PubMed] [Google Scholar]
- 10.Adams W, Kendell RE, Hare EH, Munk-Jorgensen P. Epidemiological evidence that maternal influenza contributes to the aetiology of schizophrenia. An analysis of Scottish, English, and Danish data. Br J Psychiatry. 1993;163:522–534. doi: 10.1192/bjp.163.4.522. [DOI] [PubMed] [Google Scholar]
- 11.McGrath JJ, Pemberton MR, Welham JL, Murray RM. Schizophrenia and the influenza epidemics of 1954, 1957 and 1959: a southern hemisphere study. Schizophr Res. 1994;14:1–8. doi: 10.1016/0920-9964(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 12.Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, et al. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- 15.Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- 17.Meyer U, Murray PJ, Urwyler A, Yee BK, Schedlowski M, Feldon J. Adult behavioral and pharmacological dysfunctions following disruption of the fetal brain balance between pro-inflammatory and IL-10-mediated anti-inflammatory signaling. Mol Psychiatry. 2008;13:208–221. doi: 10.1038/sj.mp.4002042. [DOI] [PubMed] [Google Scholar]
- 18.Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.McGrath J. Hypothesis: is low prenatal vitamin D a risk-modifying factor for schizophrenia? Schizophr Res. 1999;40:173–177. doi: 10.1016/s0920-9964(99)00052-3. [DOI] [PubMed] [Google Scholar]
- 20.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience. 2003;118:641–653. doi: 10.1016/s0306-4522(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 21.Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, et al. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol. 2007;103:538–545. doi: 10.1016/j.jsbmb.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 22.Cui X, McGrath JJ, Burne TH, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci. 2007;25:227–232. doi: 10.1016/j.ijdevneu.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res. 2008;187:343–350. doi: 10.1016/j.bbr.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 24.Aloia JF, Li-Ng M. Re: epidemic influenza and vitamin D. Epidemiol Infect. 2007;135:1095–1096. doi: 10.1017/S0950268807008308. author reply 1097–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yusuf S, Piedimonte G, Auais A, Demmler G, Krishnan S, Van Caeseele P, et al. The relationship of meteorological conditions to the epidemic activity of respiratory syncytial virus. Epidemiol Infect. 2007;135:1077–1090. doi: 10.1017/S095026880600776X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blaxill MF. What’s going on? The question of time trends in autism. Public Health Rep. 2004;119:536–551. doi: 10.1016/j.phr.2004.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geier DA, Geier MR. A meta-analysis epidemiological assessment of neurodevelopmental disorders following vaccines administered from 1994 through 2000 in the United States. Neuro Endocrinol Lett. 2006;27:401–413. [PubMed] [Google Scholar]
- 28.Parker SK, Schwartz B, Todd J, Pickering LK. Thimerosal-containing vaccines and autistic spectrum disorder: a critical review of published original data. Pediatrics. 2004;114:793–804. doi: 10.1542/peds.2004-0434. [DOI] [PubMed] [Google Scholar]
- 29.Schechter R, Grether JK. Continuing increases in autism reported to California’s developmental services system: mercury in retrograde. Arch Gen Psychiatry. 2008;65:19–24. doi: 10.1001/archgenpsychiatry.2007.1. [DOI] [PubMed] [Google Scholar]
- 30.Fombonne E, Zakarian R, Bennett A, Meng L, McLean-Heywood D. Pervasive developmental disorders in Montreal, Quebec, Canada: prevalence and links with immunizations. Pediatrics. 2006;118:e139–150. doi: 10.1542/peds.2005-2993. [DOI] [PubMed] [Google Scholar]
- 31.Ticher A, Ring A, Barak Y, Elizur A, Weizman A. Circannual pattern of autistic births: reanalysis in three ethnic groups. Hum Biol. 1996;68:585–592. [PubMed] [Google Scholar]
- 32.Gurney JG, Fritz MS, Ness KK, Sievers P, Newschaffer CJ, Shapiro EG. Analysis of prevalence trends of autism spectrum disorder in Minnesota. Arch Pediatr Adolesc Med. 2003;157:622–627. doi: 10.1001/archpedi.157.7.622. [DOI] [PubMed] [Google Scholar]
- 33.Burd L, Fisher W, Kerbeshian J. A prevalence study of pervasive developmental disorders in North Dakota. J Am Acad Child Adolesc Psychiatry. 1987;26:700–703. doi: 10.1097/00004583-198709000-00014. [DOI] [PubMed] [Google Scholar]
- 34.Treffert DA. Epidemiology of infantile autism. Arch Gen Psychiatry. 1970;22:431–438. doi: 10.1001/archpsyc.1970.01740290047006. [DOI] [PubMed] [Google Scholar]
- 35.Cialdella P, Mamelle N. An epidemiological study of infantile autism in a French department (Rhone): a research note. J Child Psychol Psychiatry. 1989;30:165–175. doi: 10.1111/j.1469-7610.1989.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 36.Fombonne E, du Mazaubrun C. Prevalence of infantile autism in four French regions. Soc Psychiatry Psychiatr Epidemiol. 1992;27:203–210. doi: 10.1007/BF00789007. [DOI] [PubMed] [Google Scholar]
- 37.Lotter V. Epidemiology of autistic conditions in young children. Soc Psychiatry Psychiatric Epidemiol. 1966;1:124–135. [Google Scholar]
- 38.Wing L, Yeates SR, Brierley LM, Gould J. The prevalence of early childhood autism: comparison of administrative and epidemiological studies. Psychol Med. 1976;6:89–100. doi: 10.1017/s0033291700007522. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy P, Fitzgerald M, Smith MA. Prevalence of childhood autism in Ireland. Ir Med J. 1984;77:129–130. [PubMed] [Google Scholar]
- 40.Brask BH. A prevalence investigation of childhood psychoses. Nordic Symposium on the Care of Psychotic Children. 1970 [Google Scholar]
- 41.Steffenburg S, Gillberg C. Autism and autistic-like conditions in Swedish rural and urban areas: a population study. Br J Psychiatry. 1986;149:81–87. doi: 10.1192/bjp.149.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Guillemant J, Taupin P, Le HT, et al. Vitamin D status during puberty in French healthy male adolescents. Osteoporos Int. 1999;10:222–225. doi: 10.1007/s001980050219. [DOI] [PubMed] [Google Scholar]
- 43.Kurtzke JF. Geographic distribution of multiple sclerosis: An update with special reference to Europe and the Mediterranean region. Acta Neurol Scand. 1980;62:65–80. doi: 10.1111/j.1600-0404.1980.tb03006.x. [DOI] [PubMed] [Google Scholar]
- 44.van der Mei IA, Ponsonby AL, Blizzard L, Dwyer T. Regional variation in multiple sclerosis prevalence in Australia and its association with ambient ultraviolet radiation. Neuroepidemiology. 2001;20:168–174. doi: 10.1159/000054783. [DOI] [PubMed] [Google Scholar]
- 45.Wallin MT, Page WF, Kurtzke JF. Multiple sclerosis in US veterans of the Vietnam era and later military service: race, sex, and geography. Ann Neurol. 2004;55:65–71. doi: 10.1002/ana.10788. [DOI] [PubMed] [Google Scholar]
- 46.Grant WB, Holick MF. Benefits and requirements of vitamin D for optimal health: a review. Altern Med Rev. 2005;10:94–111. [PubMed] [Google Scholar]
- 47.Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part I: the role of infection. Ann Neurol. 2007;61:288–299. doi: 10.1002/ana.21117. [DOI] [PubMed] [Google Scholar]
- 48.Ponsonby AL, Lucas RM, van der Mei IA. UVR, vitamin D and three autoimmune diseases-multiple sclerosis, type 1 diabetes, rheumatoid arthritis. Photochem Photobiol. 2005;81:1267–1275. doi: 10.1562/2005-02-15-IR-441. [DOI] [PubMed] [Google Scholar]
- 49.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 50.Holmoy T. Vitamin D status modulates the immune response to Epstein Barr virus: Synergistic effect of risk factors in multiple sclerosis. Med Hypotheses. 2008;70:66–69. doi: 10.1016/j.mehy.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 51.Webb AR, Engelsen O. Calculated ultraviolet exposure levels for a healthy vitamin D status. Photochem Photobiol. 2006;82:1697–1703. doi: 10.1562/2005-09-01-RA-670. [DOI] [PubMed] [Google Scholar]
- 52.van der Wielen RP, Lowik MR, van den Berg H, et al. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 53.Eyles DW, Cui X, Kesby JP, Harms LH, Ko P, McGrath JJ, et al. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology. 2009 doi: 10.1016/j.psyneuen.2009.04.015. In press. [DOI] [PubMed] [Google Scholar]
- 54.Edwards MJ. Hyperthermia in utero due to maternal influenza is an environmental risk factor for schizophrenia. Congenit Anom (Kyoto) 2007;47:84–89. doi: 10.1111/j.1741-4520.2007.00151.x. [DOI] [PubMed] [Google Scholar]
- 55.Reinhardt TA, Stabel JR, Goff JP. 1,25-dihydroxyvitamin D3 enhances milk antibody titers to Escherichia coli J5 vaccine. J Dairy Sci. 1999;82:1904–1909. doi: 10.3168/jds.S0022-0302(99)75425-1. [DOI] [PubMed] [Google Scholar]
- 56.Hayes CE, Nashold FE, Spach KM, Pedersen LB. The immunological functions of the vitamin D endocrine system. Cell Mol Biol (Noisy-le-grand) 2003;49:277–300. [PubMed] [Google Scholar]
- 57.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–1720. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 58.Ivanov AP, Dragunsky EM, Chumakov KM. 1,25-dihydroxyvitamin d3 enhances systemic and mucosal immune responses to inactivated poliovirus vaccine in mice. J Infect Dis. 2006;193:598–600. doi: 10.1086/499970. [DOI] [PubMed] [Google Scholar]
- 59.Sponheim E, Skjeldal O. Autism and related disorders: epidemiological findings in a Norwegian study using ICD-10 diagnostic criteria. J Autism Dev Disord. 1998;28:217–227. doi: 10.1023/a:1026017405150. [DOI] [PubMed] [Google Scholar]
- 60.Fombonne E. The epidemiology of autism: a review. Psychol Med. 1999;29:769–786. doi: 10.1017/s0033291799008508. [DOI] [PubMed] [Google Scholar]
- 61.Gillberg C, Wing L. Autism: not an extremely rare disorder. Acta Psychiatr Scand. 1999;99:399–406. doi: 10.1111/j.1600-0447.1999.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 62.Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- 63.Kanner L. Autistic disturbances of affective contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- 64.American Psychiatric Assoc, authors. Diagnostic and statistical manual of mental disorders. revised 3rd edn. Washington, DC.: American Psychiatric Assoc; 1987. [Google Scholar]
- 65.Charman T. Autism: neural basis and treatment possibilities. Chichester: Wiley,; 2003. Epidemiology and early identification of autism: research challenges and opportunities; pp. 10–25. (Novartis Foundation Symposium 251). [PubMed] [Google Scholar]
- 66.Ritvo ER, Freeman BJ, Pingree C, et al. The UCLA-University of Utah epidemiologic survey of autism: prevalence. Am J Psychiatry. 1989;146:194–199. doi: 10.1176/ajp.146.2.194. [DOI] [PubMed] [Google Scholar]
- 67.Barak Y, Ring A, Sulkes J, Gabbay U, Elizur A. Season of birth and autistic disorder in Israel. Am J Psychiatry. 1995;152:798–800. doi: 10.1176/ajp.152.5.798. [DOI] [PubMed] [Google Scholar]
- 68.Tanoue Y, Oda S, Asano F, Kawashima K. Epidemiology of infantile autism in southern Ibaraki, Japan: differences in prevalence in birth cohorts. J Autism Dev Disord. 1988;18:155–166. doi: 10.1007/BF02211943. [DOI] [PubMed] [Google Scholar]
- 69.Bartlik BD. Monthly variation in births of autistic children in North Carolina. J Am Med Womens Assoc. 1981;36:363–368. [PubMed] [Google Scholar]
- 70.Atlas JA. Birth seasonality in developmentally disabled children. Psychol Rep. 1989;64:1213–1214. doi: 10.2466/pr0.1989.64.3c.1213. [DOI] [PubMed] [Google Scholar]
- 71.Konstantareas MM, Hauser P, Lennox C, Homatidis S. Season of birth in infantile autism. Child Psychiatry Hum Dev. 1986;17:53–65. doi: 10.1007/BF00707913. [DOI] [PubMed] [Google Scholar]
- 72.Bolton P, Pickles A, Harrington R, Macdonald H, Rutter M. Season of birth: issues, approaches and findings for autism. J Child Psychol Psychiatry. 1992;33:509–530. doi: 10.1111/j.1469-7610.1992.tb00888.x. [DOI] [PubMed] [Google Scholar]
- 73.Yeates-Frederikx MH, Nijman H, Logher E, Merckelbach HL. Birth patterns in mentally retarded autistic patients. J Autism Dev Disord. 2000;30:257–262. doi: 10.1023/a:1005500803764. [DOI] [PubMed] [Google Scholar]
- 74.Ovesen L, Andersen R, Jakobsen J. Geographical differences in vitamin D status, with particular reference to European countries. Proc Nutr Soc. 2003;62:813–821. doi: 10.1079/PNS2003297. [DOI] [PubMed] [Google Scholar]
- 75.Grant WB. An estimate of premature cancer mortality in the United States due to inadequate doses of solar ultraviolet-B radiation. Cancer. 2002;94:1867–1875. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 76.Grant WB, Garland CF. The association of solar ultraviolet B (UVB) with reducing risk of cancer: multifactorial ecologic analysis of geographic variation in age-adjusted cancer mortality rates. Anticancer Res. 2006;26:2687–2699. [PubMed] [Google Scholar]
- 77.Leffell DJ, Brash DE. Sunlight and skin cancer. [Accessed June 30, 2009];Sci Am. 1996 275:52–53. doi: 10.1038/scientificamerican0796-52. http://toms.gsfc.nasa.gov/ery_uv/dna_exp.gif. [DOI] [PubMed] [Google Scholar]
- 78.Cannell JJ. Autism and vitamin D. Med Hypotheses. 2008;70:750–759. doi: 10.1016/j.mehy.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 79.Cannell JJ, Hollis BW. Use of vitamin D in clinical practice. Altern Med Rev. 2008;13:6–20. [PubMed] [Google Scholar]