Abstract
Dizocilpine (MK-801), an extensively investigated drug possessing secondary amine and benzenoid functions, displays a wide array of biological properties, including anticonvulsant and anesthetic. There is scant discussion of biomechanism. A relevant, important finding is formation of oxidative metabolites in the hydroxylamine and phenolic categories. Analogy to cocaine metabolites suggests participation of redox entities, such as, hydroxylamine, nitroxide and nitrosonium, which can lead to electron transfer and radical formation. There is also similarity to metabolism by 3,3′-iminodipropionitrile and phencyclidine. Alternatively, the phenolic metabolites are well-known precursors of ET quinones. The review documents various physiological effects, mainly involving the central nervous system. Also of interest are the pro- and anti-oxidant properties. Considerable attention has been paid to MK-801 as an antagonist of the N-methyl-D-aspartate receptor in the glutamate category. This aspect is often associated with effects on the central nervous system. The review also provides recent literature dealing with MK-801/NMDA receptor in various areas of bioactivity. Studies were made of MK-801 involvement in working memory processing. Deficits in behavior were noted after administration of the drug. Treatment of mice with dizocilpine induced learning impairment. The influence of MK-801 on fear has been investigated. The substance is known to exert an analgesic effect in pain control. A number of reports deal with anesthetic properties.
Key words: dizocilpine (MK-801), mechanism, redox metabolites, radicals, bioactivity
Introduction
Dizocilpine (MK-801) (1) is an antagonist of the N-methyl-D-aspartate receptor in the glutamate category involved with the central nervous system (CNS). The drug displays a variety of physiological actions, many of which involve the CNS, such as anesthetic and anticonvulsant properties. The bioactivity is discussed within the framework of a unifying mechanistic theme which has been discussed in prior reviews.
The preponderance of bioactive substances and their metabolites incorporate electron transfer (ET) functionalities, which, we believe, play an important role in physiological responses. The main groups include quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derivatives), and conjugated imines (or iminium species). In vivo redox cycling with oxygen can occur giving rise to oxidative stress (OS) through generation of reactive oxygen species (ROS), such as hydrogen peroxide, hydroperoxides, alkylperoxides, and diverse radicals [hydroxyl, alkoxyl, hydroperoxyl and superoxide (SO)]. In some cases, ET results in interference with normal electrical effects, e.g., in respiration or neurochemistry. Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range, i.e., more positive than −0.5 V. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g., anti-infective agents,1 anticancer drugs,2 carcinogens,3 reproductive toxins,4 nephrotoxins,5 hepatotoxins,6 cardiovascular toxins,7 nerve toxins,8 mitochondrial toxins,9 abused drugs,10 ototoxins,11 pulmonary toxins,12 immune system toxins13 and various other categories of drugs and toxins, including human illnesses.14
There is a plethora of experimental evidence supporting the OS theoretical framework, including generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of antioxidants (AOs), and DNA oxidation and cleavage products, as well as electrochemical data. This comprehensive, unifying mechanism is in keeping with the frequent observations that many ET substances display a variety of activities, e.g., multiple drug properties, as well as toxic effects. Knowledge of events at the molecular level can result in practical application in medicine.
It is instructive to examine the basic biochemistry of ET functionalities in more detail. Redox cycling occurs between hydroquinone and p-benzoquinone, and between catechol and o-benzoquinone with generation of superoxide via ET to oxygen. Semiquinones act as intermediates. Various amino acids can operate as electron donors. Superoxide serves as precursor to a variety of other ROS. The quinones can belong in either the endogenous or exogenous category. In the case of aromatic nitro compounds, the reduced nitroso and hydroxylamine metabolites can similarly enter into redox cycling, including an oxy radical intermediate. This class is only in the exogenous group and is related to MK-801 metabolism. Less known are conjugated iminium compounds, of which paraquat is a predominant member.
This review demonstrates that the ET-ROS-OS unifying theme, which has been successful for many other classes of drugs and toxins, can also be applied to MK-801 which is a bicyclic secondary amine. Various biochemical properties of the drug are addressed, based on the ET-ROS-OS perspective. The in vivo activities include anesthetic, anticonvulsant, interaction in the brain, neurotoxicity, neuro protection, interaction with abused drugs, motor effects, receptor interaction, behavior, learning and memory. Metabolic evidence points to two main routes whereby ET may be induced, namely, hydroxylamine and phenol formation. Hydroxylamines can undergo redox interactions involving nitroxides and nitrosonium (oxoammonium) species. Analogy is provided based on similar transformations with cocaine, 3,3′-iminodipropionitrile, and phenylhydroxylamine. The phenolic metabolites are well known precursors of ET quinones. Also, receptors and pro-and anti-oxidant actions are treated.15
However, it should be emphasized that physiological activity of endogenous and exogenous substances is often complex and multifaceted. Our objective does not encompass extensive treatment of other modes of action. The citations are usually representative, rather than exhaustive. A number of original references may be found in the reviews and articles cited.
Cocaine Metabolism16
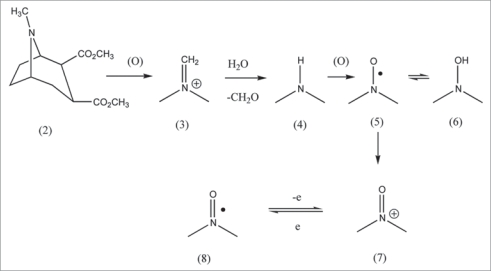
These data provide insight concerning MK-801 mechanism. Cocaine (2) is mainly metabolized by two distinct pathways in humans.17 The major transformation consists of hydrolysis of the ester groups, which is apparently not important in the toxic manifestations. The minor route (Scheme 1) is an oxidative one involving the amine moiety, which has attracted most attention relative to some toxic responses. Possible metabolites participating in the effects of (2), in addition to the drug itself, are norcocaine (4), norcocaine nitroxide (5), N-hydroxynorcocaine (6), norcocaine nitrosonium (7), cocaine iminium (3), and formaldehyde.
Scheme 1.
Cocaine metabolism. There is similarity to MK-801 in relation to involvement of a secondary-amine (4) with subsequent metabolism to a hydroxylamine (6). Various studies also report participation of nitroxide (3) and nitrosonium (7). There is evidence for redox cycling entailing ET process with subsequent formation of ROS in some cases. The CNS may be involved in the ET reactions. Toxicity might reflect harmful effects from ROS generation.
Oxidative metabolism of (2) yields norcocaine (4), evidently via the iminium derivative (3).17 Microsomes in the brain and liver further oxidize (4) to the nitroxide (5). This radical is stabilized by delocalization over O and N, and apparently by intramolecular interaction with n-electrons on the ester substituent. The transannular effect which has ample precedent, might well apply to other radical species involved in redox cycling in the cocaine system, as well as to magnitude of the reduction potential.
Another metabolite is the N-hydroxy derivative (5) (a hydroxylamine) which arises from (4) by a sequence comprising electron and proton uptake in reversible manner. The duo of (4) and (5) apparently operates as a couple.17,18 This system bears resemblance to the one comprising aromatic nitroso compounds (ArNO)-aromatic hydroxylamine (ArNHOH) which redox cycles with participation of the ArNHO· radical.3
An alternate redox cycle entails electron loss by (5) to form nitrosonium cation (7) (oxoammonium) reversibly. Which could redox cycle with (8). This aspect has been the object of less attention.
There have been numerous examples concerning involvement of iminiums in drug or toxic action by way of ET-OS.1–5 Bioactive tert-amines are believed to be widespread source of this functionality.19 Application to cocaine was made in 1988,17 followed more recently by speculation that long-term exposure to cyclic tert-amines, such as cocaine, may result in biochemical lesions via reactive metabolites, e.g., iminium types, thought to be primarily responsible for neurotoxicity.20 Usually, iminiums that participate in ET are conjugated types.1–5 Exceptions appear to be those that attain stabilization of the derived radical by intramolecular interaction with n-electrons, as in the case of cocaine or phencyclidine (PCP).21
Although electrochemistry can provide valuable mechanistic insight, unfortunately, it has received little attention in the medicinal chemistry area. If the reduction potential is more positive than −0.5 V, then there is the possibility of ET in the biological domain. Electrochemical studies with nitroxide (5) yielded a reduction potential of −0.48 V, indicating a conceivable role in redox cycling in vivo with production of OS via ROS.17 Only a small quantity of minor metabolites is required to generate large quantities of ROS since the operation is catalytic.
Brain microsomal reduction of norcocaine nitroxide generates SO.22 Incubation of liver microsomes with either the nitroxide or the N-hydroxy derivative leads to lipid peroxidation, the degree of which is greater for the metabolites than for cocaine itself,23 indicating the crucial role of the metabolites in toxicity. Redox cycling involving the metabolites is believed to take place, accompanied by formation of SO and lipid peroxyl radicals.18 Immunotoxicity in rats is promoted by N-oxidative metabolism, evidently via OS, accompanied by depletion in GSH.25 Formaldehyde, an oxidative metabolite of 2 (Fig. 1)24 is reported to produce ROS.14
Figure 1.
Diazocilpine MK-801. This secondary-amine is addressed mainly in connection mechanism and physiological activity. Metabolism yields a hydroxylamine which may be associated with redox entities (Fig. 2), as in the case of cocaine. There is also similarity to 3,3′-iminodipropionitrile and phencyclidine. Mk-801 possesses a large number of physiological effects involving anesthesia, anticonvulsant, the CNS, memory, behavior, learning, fear and analgesia.
3,3′-Iminodipropionitrile Metabolism16
3,3′-Iminodipropionitrile (IDPN), HN(CH2CH2CN)2, an industrial intermediate, is used as an experimental neurotroxin.16 Which might be related to (2) mechanistically. Administration produced a significant increase in malondialdehyde and decrease in vitamin E and glutathione (GSH), suggesting a role for ROS in toxicity.26 Concurrent use of Se significantly inhibited IDPNinduced neurobehavioral changes in rats, and reduced ROS production, evidently through attenuation of toxicity by decreased lipid peroxidation.27 Co-treatment with cysteamine protected rats against dyskinesia by IDPN.28 Depletion of vitamin E and GSH through toxic action was alleviated by the thiolamine as AO. Similarly, adjuvant use of salicylate decreased abnormal neurobehavior and reversed GSH depletion, presumably by AO action of the phenol.29
Little is known about the action mechanism of IDPN. A clue is provided by one of several metabolites, namely the neurotoxic N-hydroxy derivative produced by flavin monooxygenase-mediated oxidation.30 The data suggest that metabolism is necessary for certain neurotoxicities. There is similarity between IDPN and cocaine, both in structure and metabolism. By analogy with (6), the N-hydroxy form could operate in a redox cycle with the nitroxide radical, or nitrosonium could be involved. The ester and cyano substituents in the two toxins may play similar roles in intramolecular stabilization of the nitroxide radical and other reactive intermediates.
Phencyclidine (PCP) Metabolism
MK-801 has been designated a PCP-type drug.31 Results suggest that mode of action involves PCP receptors, not opioid types. Other results suggest that MK-801 induced hyperlocomotion may be mediated by NMDA receptor antagonism.32 The data were similar to those for PCP. PCP-like drugs, such as MK-801, have many effects in common, including impact on learning and performance.33 Similarity of the two drugs in various studies may indicate a similar mechanism entailing ET.
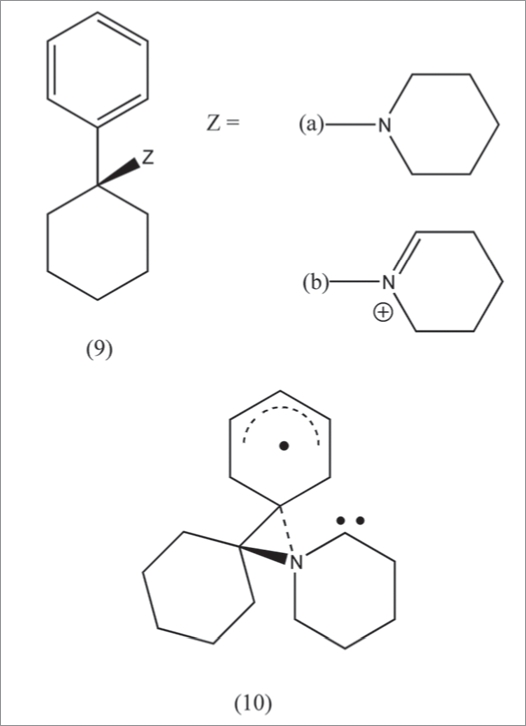
Theoretical studies on PCP provide useful insight.21 It is well established that oxidative metabolism of PCP (9a) leads to the iminium species 9b. Although 9b is not directly conjugated with the aromatic nucleus, cyclic voltammetry studies indicated interactive stabilization of the reduced species. Recently, computational studies were performed on the energetics of electron uptake by 9b with respect to conformation. In relation to the reduced species, thermodynamic preferences were found for conformations similar to that present in PCP bound to the active site. The through space delocalization is depicted in 9c. The calculations suggest that a factor in the biological responses may be ET by the iminium metabolite.
MK-801 Metabolism
A metabolic report provides important insight concerning the mechanism of MK-801 action.34 With labeled drug, radioactivity was widely distributed among various tissues. Major metabolites were the N-hydroxy derivative, and the 2- and 8-hydroxyl analogs involving oxidation at carbon. The N-OH (hydroxylamine) product is the focus of our attention. Related literature (see above) demonstrates that subsequent oxidation products, such as nitroxide and nitrosonium ion can play an important mechanistic role.
MK-801 ET Mechanisms
There are various means whereby the hydroxylamine-nitroxide redox coupling could operate. The main ones have been pointed out in the cocaine section. Other relevant reports exist. Results indicate that dismutation of superoxide is catalyzed by the oxoammonium/nitroxide redox couple for carbocyclic nitroxide derivatives.35 Complexes were studied in which nitrosonium served as acceptor with aromatic donors.36,37 The oxoammonium cation can be generated by reaction of nitroxide with various radicals.38 Nitroxides might act as both anti- and pro-oxidants. Other nitrosonium ions and nitroxide can be generated in vivo and might serve in cell signaling and as cytotoxic agents.39 Hence, there exists ample analogy for assigning a role for the hydroxylamine-nitroxide couple, together with associated species, in the bioactivity of MK-801. There is similarity to PCP in relation to nitrogen with radical character in the beta position to the aromatic ring. Alternatively, the phenolic metabolites formed at C2 and C8 may conceivably play a role. Phenols are well-known metabolic precursors of ET quinones.
Receptors
MK-801 binds at two sites on the NMDA receptor-ion channel complex, which is a glutamate (Glu) receptor.40 Glu is a main excitory neurotransmitter in the brain. The drug attaches to the ion channel at the PCP binding site of the receptor, requiring depolarizartion of the neuron. Receptor blockage by MK-801 occurs in a voltage-dependent manner. Our proposed ET mechanism with accompanying electrical effects is in keeping with the receptor phenomena.
In a recent publication, the sub-unit mechanisms and proton sensitivity of NMDA receptor channel block were discussed.41 The transient ischemia during occlusive stroke triggers changes in the nature of the extracellular milieu, including strong acidification of the ischemic core with more modest acidification of penumbral regions. NMDA receptors are inhibited by protons at pH 6.9–7.3, suggesting that even modest acidification could reduce or delay the contribution of NMDA receptors to neuronal death until pH gradients surrounding the ischemic insult dissipate. Mutagenesis studies of NMDA receptor subunits suggest that residues in the linker regions connect the agonist binding domain to the transmembrane pore-forming elements proton-sensitive gating. The authors show the binding capabilities of several proton-dependent channel blockers, kinetic modeling and single channel experiments using acidic extracellular pH, which reduce receptor open probability, increases the association rate of optical isomer (−)MK-801, but not isomer (+) MK-801.
Iron is thought to play a critical role in pathogenesis of neurodegenerative disorders, as Parkinson’s and Alzheimer’s diseases, by the generation of oxygen-free radicals in association with oxidative stress. Ferrous chloride markedly inhibited, in a concentration dependent manner, dizolcilpine binding to an open ion channel associated with NMDA receptor in rat brain synaptic membranes. Addition of an NMDA agonist, such as, spermidine, attenuated the inhibition of MK-801 binding. The results suggest that ferrous ions may interfere with opening processes of the native NMDA channel through molecular mechanisms peculiar to neuronal development in a manner associated with the polyamine recognition domain.42
Oxidative Stress
A study with MK-801 showed that induced neurotoxicity involved OS in the prefrontal cortex of rats.43 A protective effect was shown by caffeic acid phenethyl ester (CAPE) an inhibitor of ROS generation.44 Induction of schizophrenia by MK-801 resulted in significant OS, involving increases in malondialdehyde and protein carbonyl levels.45 Administration of CAPE reduced the detrimental histopathological changes. The drug influences lipid metabolism and the intensity of lipid peroxidation.45 MK-801-induced neurotoxicity causes OS in the prefrontal cortex of rats.43
Anesthesia
Results indicate that blockage of the central NMDA receptor may contribute to the production of anesthesia.46 The ability of the receptor antagonists to increase the potency of general anesthetics paralleled their potency as NMDA antagonists and their affinity for the PCP receptor site of the NMDA receptorionophore complex: MK-801 > PCP > ketamine. MK-801 is not used clinically as an anesthetic due to development of brain lesions.
The discriminative stimulus effect of MK-801 in ketamine-trained rats was investigated.47 The results indicate that both drugs may share a common mechanism of action, which is related to the phencyclidine recognition site in the brain.
A recent report lends credence to the ET approach in anesthetic action involving propofol.48 Four years ago, a novel unifying hypothesis was advanced for addiction and toxicity by abused drugs involving ET.10 One of the principal ET agents is the quinone group which is often generated metabolically. Appreciable numbers of abused drugs appear to function by the quinone route, including amphetamine, methamphetamine, ecstasy, morphine, heroin, phenobarbital and aspirin. Recently, two members were added to the quinone category, namely, mescaline49 and psilocybin.50
Inflated rates of opioid addiction among anesthesiologists may be caused by chronic exposure to low doses of anesthetic agents, such as propofol (2,6-diisopropyl phenol), in the operating room.51 Such second-hand exposure results in neuro sensitization of the reinforcing effects of the anesthetic, making later addiction more likely. Second hand exposure could occur by inhalation or skin contact. Other factors may be involved.
Various reports on metabolism of propofol provide insight concerning action mechanism. Analysis of products from oxidative metabolism revealed the presence of 2,6-diisopropyl-1,4-quinone,52 in addition to the quinone dimer (diphenoquinone).53 More specifically, it is reasonable to focus on involvement of ET processes and electrical fields. The radical anion semiquinone provides molecular electrostatic potential. Since ET by quinone is often associated with generation of ROS, these radicals, at low levels, may play a role. At high concentrations, toxicity could result.10 There has been scant attention paid to action mode at the molecular level. These results provide additional support for the hypothesis based on ET by addictive agents.
Anticonvulsants
MK-801 is a potent anticonvulsant which displays high affinity for binding sites in rat brain membranes, particularly in the hippocampus.54 None of the major neurotransmitters was active at these sites. The only competitive substances were those that block the responses of excitatory amino acids mediated by NMDA receptors, including phencyclidine and ketamine. These findings provide a clue to the action mechanism.
Also, a 1992 review advanced the proposal that ET may be a possible mode of action for anticonvulsants.55 The conjugated iminium functionality is a common one among the active drugs. The resulting flow of electrons from ET would generate electrical fields, thereby affecting ion movement in membrane channels and nerve synapses.
Brain Electrochemistry
A study of brain field potentials revealed a particular pattern of charges which was very stable over time.56 Higher doses of MK-801 produced a continuous change from power decreases to increases, accompanied by strong behavioral effects involving impaired locomotor control. Changes in the frequency content of the field potentials occurred over time. The present authors believe that the electrical effects may be related to ET properties of the MK-801 metabolites.
Other Brain Effects
MK-801 failed to reduce infarct size in animals whose body temperature rose during ischemia. In contrast, the drug markedly reduced infarct volume in temperature controlled animals. The results suggest that amelioration of focal cerebral ischemia cannot be expected if body and brain temperature is allowed to rise above normal.57
Gao et al. showed phencyclidine and MK-801 exert timedependent effects on the expression of immediate early genes in rat brain.58 The mRNA expression pattern for four different immediate early genes was examined dynamically in rat brain after administration of PCP or MK-801. Following each treatment, the expression of mRNA changed. The authors suggest functional consequences of PCP- or MK-801-induced reduction in NMDA-sensitive glutamate transmission may be relevant to an understanding of animal NMDA pharmacology and/ or to clinical psychotomimetic side effects of antiglutamatergic treatments.
Bilateral injection of dizocilpine into the anterior thalamus of rat brain induced HSP70 protein formation in pyramidal neurons in deep layer III retrosplenial cortex. This bilateral blockade of NMDA receptors in the anterior thalamus by MK-801 injures neurons in retrosplenial cortex.59
Delta sleep-inducing peptide has been shown to increase the resistance of rats to stress. Increase in the expression of the early c-fos in the paraventricular nucleus of the hypothalamus is regarded as the primary response of animals in conditions of emotional stress. Injection of the peptide leads to decrease in stress-induced c-fos expression in the paraventricular nucleus of the hypothalamus. Exposure to MK-801 blocks the effects of delta sleep-inducing peptide-induced suppression of c-fos gene expression.60
CNS Effects: Neurotoxicity and Neuroprotection by MK-8018
NMDA antagonists, such as, MK-801, disrupt sensorimotor gating in rats, suggesting that the effects may provide a model of such deficits exhibited in schizophrenia.61 MK-801, a noncompetitive NMDA receptor antagonist, is a well known neuroprotectant in models of stroke, trauma, Parkinsonism and organophosphate-induced seizures. Despite this, MK-801, like other phencyclidine receptor ligands, such as phencyclidine, ketamine or tiletamine, induces psychotic behavior and neuronal degeneration. MK-801 exposure caused neuronal degeneration in rat axon terminals, microglia, retrospinal cortex, neurons in the pyriform, and entorhinal cortices, in amygdala in tenia tecti, and in the temporal dentate gyrus.62
MK-801 affected the nervous system of male and female rats differently.63 Female rats indicated higher sensitivity to MK-801 neurotoxicity; Authors suggest the possible involvement of 17β-estradiol in the sex differences of the sensitivity. The female rats also showed increased glial fibrillary acidic protein when treated with MK-801 vs. the male rats.
Non-competitive NMDA antagonist MK-801 and phencyclidine increase glucose metabolism in many brain areas and induce cytoplasmic vacuoles, heat shock protein and necrotic cell death in neurons of the rodent posterior cingulate and retrosplenial cortex. Administration of radical scavengers (dimethyl sulfoxide and α-tocopherol), produced marked attenuation of MK-801-induced neuronal necrosis. This supports the hypothesis that OS plays a role in MK-801-induced neuronal necrosis since pathological changes are attenuated by several AOs.64
Glutamate, the most widely used excitory amino acid transmitter in the brain, stimulates ionotropic and metabotropic receptors. There is an increased release of glutamate after cerebral ischemia or hypoxia which cause overstimulation of its receptors, leading to an increase in the cellular Ca2+ concentration. Elevation of Ca2+ is assumed to set various pathological processes into motion which degenerate neurons by activating proteases, lipases, endonucleases and nitric oxide synthases and by promoting the formation of free oxygen radicals. MK-801- treated cultures of rat hippocampal neurons showed a neuroprotective effect.65 The influence of MK-801 on release of inhibitory amino acids in the field of neuroprotection against ischemic injury was explored. The protection against brain damage induced by ischemia, at least partly, is related to inhibition of calcium influx and, more significantly, blockage of excitory amino acid release from ischemic synaptosomes.66 In a related study, paraquat or xanthine oxidase was shown to activate the NMDA receptor and the resultant excitory amino acid glutamate leads to excitotoxicity. Dizocilpine attenuates this oxidant injury, acting as an AO.67
In vitro studies have demonstrated that NMDA receptor activation rescues cerebellar granule neurons from apoptotic death. Mechanism involves MK-801 inhibiting the caspase activation.68 Nitroxyl anion (NO−), and/or its conjugate acid, HNO, may be formed in the cellular milieu by several routes under both physiological and pathophysiological conditions. Reactive nitrogen oxide species can contribute significantly to cerebral ischemic injury. A study using Angeli’s salt, a spontaneous HNO/NO− generating compound, showed time- and concentration-dependent increase in neural cell death. Addition of glutamate receptor antagonist MK-801 to the culture reduced the toxicity, acting as an antioxidant.69 In a related study, low doses of MK-801 were shown to protect iron-induced oxidative changes in a rat model of focal epilepsy, suggesting MK-801 acts as an antioxidant against both free radicals and excitory amino acids in epilepsy.70
L-2-Chloropropionic acid, when administered orally to rats, produces selective necrosis to the granule cell layer of the rat cerebellum, which is delayed in onset, after exposure. Administering MK-801 afforded partial protection against the toxicity.71
The effect of pro-inflammatory cytokine interleukin IL-1β on the hippocampal slices from neonatal Sprague Dawley rats was examined, and neuroprotective action by MK-801 and vitamin- E analog trilox was shown. The neuroprotective effects suggest that free radicals and NMDA receptor-mediated processes are involved in IL-1β induced neurodegeneration.72
Organophosphates and carbamate inhibitors acetylcholine-esterase produce seizures and lethality in mammals. MK-801 blocked these seizures. Results suggest that NMDA receptors seem critical to such seizures.73
Motor Activity, Learning and Memory
Glutamate transmission plays an important role in many behavioral systems, including motor activity, learning, and memory. The noncompetitive NMDA receptor antagonist MK-801 has been shown to increase motor activity and to impair learning and memory in a variety of tasks in rats, mice and other species.
Many agents, e.g., MK-801, that antagonize the NMDA receptor-channel complex also cause disturbances of motor coordination.74 The effects of MK-801 upon motor activity and memory of rats were assessed in behavior testing. The results indicated MK-801 can produce profound effects upon motor activity and memory, and that these two effects can be dissociated.75
The long-lasting effects of a single high dose treatment with MK-801 on water maze performance of rats was reported. The drug-treated rats learned to find the escape platform at a slower rate than control animals and showed increased thigmotaxis during acquisition of the task.76 A study demonstrated that MK-801 impairs working memory of conscious monkeys. In addition, acute and chronic MK-801 produces different effects on receptor D1, D2 and generally lowered glutamate and dopamine levels in prefrontal cortex.77 The effects of MK-801 on learning ability in non-human primates were investigated. MK-801 impaired acquisition of visuo-spatial tasks requiring spatial responses to colored objects, and perceptually difficult visual discrimination tasks.78
MK-801 Induced Behavior
MK-801 in rats induced characteristic behavioral syndrome with ataxia, stereotypies and hyperlocomotion. Part of the behavioral syndrome is thought to be related to interactions between glutamatergic and dopaminergic neurotransmission, suggesting serotonin involvement.79 In a related study involving psychoticlike behavior induced by MK-801 and serotonin receptors, the authors conclude that there is an interaction between NMDA and seratonin receptors.80
With zebrafish as a neurobehavioral model, MK-801 increases circling behavior, alters swimming activity, and impairs place preference.81 Data indicate that systemic administration of a relatively small dosage of MK-801 facilitates performance when reward is small. Facilitation may be due to the reinforcement of mechanisms that work in opposition to response bias.82
Circadian Behavior and Sleep Induction
The NMDA receptor antagonist MK-801 blocks the phase shifting effects of light on the circadian rhythm in hamsters.83 A dosedependent blockade of both light-induced phase advances and delays occurred. The data indicated an important role for excitatory amino acid receptor in transmission of light information from the retina to the circardian system.
A commonly used sleep inducer is zolpidem (Ambien).84 A proposed action mechanism comprises protonation to a conjugated pyridinium (iminium) structure which may function as an ET agent. Electrochemical phenomena may be responsible for sleep induction and adverse side effects.
Abused Drugs
Upon repeated exposure to drugs of abuse, the gradual development of tolerance, dependence and sensitization is thought to play a crucial role in the psychopathology of drug additiction.85 In recent years, NMDA receptor antagonist MK-801 has been widely used to investigate the involvement of NMDA receptors in drug-induced neuroadaptations. Dizocilpine shares stimulus properties with phencyclidine and, to lesser extent, with ethanol. Dizocilpine was found to potentiate the reinforcing properties of morphine and cocaine in models. Regarding the cerebral sites where dizocilpine might exert its reinforcing effects, several areas were designated as likely candidates. However, dizocilpine and morphine were found to dramatically enhance lethality. Dizocilpine was shown to prevent, as well as enhance, the development of drug-induced behavioral effects, and appears to induce sensitization to its own behavioral effects, as well as cross-sensitization to drugs of abuse.
Studies were carried out on rats implanted with morphine.86,87 The naloxane-precipitated abstinence syndrome in rats exposed to MK-801 was more intense than controls, whereas the abstinence syndrome in rats that received MK-801 before naloxane injection was less intense. The intensification is attributed to increase in upregulation and supersensitivity of NMDA receptors caused by morphine. The attenuation is explained based on blockage by MK-801 of NMDA receptors. Related studies are reported for morphine and MK-801. The present authors believe ET events may be involved in line with the recent unifying hypothesis for abused drugs.10
In a related study, results indicate that MK801 not only blocks the development of morphine-induced conditioned place preference, but it is also able to block the expression of a conditioned response that has been acquired.88
Glutamate receptor activation participates in mediation of neurotoxic effects in the striatum induced by the psychomotor stimulant amphetamine. MK-801 inhibits amphetamine- induced formation of nitric oxide, lipid peroxidation and amphetamine-induced release of amino acids and acetylcholine in rat brain. Part of the effect reflects action as an AO.89
Methamphetamine causes long-term toxicity to dopamine nerve endings of the striatum. In vivo experiments show that MK-801 ability to protect against methamphetamine neurotoxicity is related to their common property as blockers of microglial activation.90
Another drug in this category is mescaline (peyote) whose mode of action has been discussed recently.49 The proposed mechanism entails demethylation to a catechol moiety which then has the potential of redox cycling with an ET o-quinone. Electrochemical effects may be involved in the hallucinogenic and other effects.
MK-801/NMDA Receptor and Bioactivity
The prior sections illustrate the variety of this drug in the biological realm. The following portion provides recent literature from 2008–2009 dealing with memory, behavior, learning, fear, pain control and anesthesia.
Various literature reports address the role of MK-801 in memory. Inhibition of NMDA Glu receptors during food aversion conditioning reactivation initiated disruption of long-term memory.91 Injection of MK-801 produced a decrease in the number of conditioned food aversions. The long-term memory reactivation disruption induced two stages of amnesia development. The NMDA Glu receptor is implicated in memory formation and consolidation.92 Administration of MK-801 immediately after training impaired inhibitory avoidance performance, suggesting induction of state-dependent recall. NMDA receptor antagonists induce amnesia.93 Data revealed that MK-801 produced anterograde and retrograde amnesia. Pharmacological disruption of reconsolidation of alcohol-associated memories can be achieved by use of NMDA antagonists, such as MK-801.94 This may be a therapeutic strategy for prevention of relapse in alcohol addiction. Studies were made of MK-801 involvement in working memory processing in the medical prefrontal cortex.95
MK-801 has been employed in behavioral studies. Deficits in behavior were noted following treatment by NMDA antagonists, such as MK-801. More specifically, there was increased anxiety-like behavior.96 The effects of MK-801 treatment on rat behavior were observed after neonatal lesions of the entorhinal cortex.97 The combined treatment may serve to model certain aspects of psychiatric behavior. A genetically-inbred mouse strain shows heightened sensitivity to the ability of MK-801 to raise the threshold voltage necessary to precipitate tonic hindlimb extension and elicit irregular episodes of intense jumping behavior.98 The heightened behavioral sensitivity to MK-801 does not appear to result from alterations of expression of the NMDA receptor protein subunits. Behavioral flexibility refers to the ability to modify ongoing behavior in response to changing goals or environmental contingencies.99 Administration of MK-801 significantly impaired task performance. The amygdala appears to be a primary locus in mediating the effects of drug stimuli on subsequent drug-seeking behavior.100 The NMDA subtype of the Glu receptor within the amygdala is important for consolidation of associations between environmental conditioned stimuli and the effects of additive drugs. Systemic antagonism of NMDA receptors with MK-801 before memory reactivation reduced acquired drug-seeking behavior that depends on drug-associated cues acting as conditioned reinforcers. Such drugs may be useful in treatment of relapse prevention in drug addiction.
Effect on learning has been examined for MK-801. Food aversion conditioning consolidation was investigated with various substances in snails.101 The conditioning was absent after molecular mechanisms evoked disruption of long-term memory consolidation during learning. Treatment of mice with MK-801 induced learning impairment, accompanied by inhibition of NMDA-stimulated phosphorylation.102 Ginsenoside Rg 1 improves spatial learning capacity impaired by morphine, and restores the morphine-inhibited long-term potentiation.103 This effect is NMDA receptor dependent, as evidenced by experiments with MK-801. Systemic administration of the drug impairs reversal learning in rats.104 The study entailed spatial discrimination. Administration of MK-801 before exposure to ethanol significantly inhibited ethanol state-dependent learning.105 Dorsal hippocampal NMDA receptors are involved in mediating the learning process.
The influence of MK-801 on fear has been investigated. A study addressed whether Ro-64-6198 impairs acquisition of fear conditioning through glutamatergic mechanisms.106 Administration of Ro-64-6198 and MK-801, either separately or concomitantly, reduced the facilitating effects of context exposure. The findings demonstrate the existence of functional antagonism between NMDA and nociceptin opioid peptide receptors that predominantly contributes to modulation of conditional fear learning that involves spatial-processing demands. The role of conditioned stimulus familiarity in determining the effects of MK-801 on fear extinction was shown to be an important factor.107 The drug impaired extinction learning about novel stimuli, but spared extinction learning about familiar stimuli. Long-term fear memory can be extinguished by disruption of reconsolidation of specific memories associated with the fear response.108 The effects of MK-801 on memory reconsolidation were studied. Neurotrsansmitters that are important for extinction of conditioned fear in adult rats are not important for extinction in young rats.109 Pre-extinction injection of MK-801 has no effect on extinction in young rats, whereas it impairs long-term extinction in older rats.
In relation to pain control, the mechanism of orthodontic pain is poorly understood.110 By use of a behavioral model, application of both systemic and peripheral MK-801 and morphine exerted an analgesic effect. Face-grooming behavior is a reliable measure for tooth pain in rats. Cyclooxygenase-2 (COX-2) is upregulated and plays an important role in pain and hyperalgesia induced by nociceptive stimulation.111 Activation of the spinal NMDA receptor might contribute to the upregulation of COX-2 spinal expression. Results show that injection of MK-801 suppressed the upregulation of the COX-2 expression and characteristic pain behavior responses.
In a study of anesthetics, classical ones of the gamma-aminobutyric acid (GABA) type A receptor-enhancing class, e.g., phenobarbital and chloral hydrate, produce analgesia and unconsciousness (sedation).112 Dissociative anesthetics that antagonize the NMDA receptor, e.g., MK-801, ketamine and PCP, produce analgesia, but do not induce complete loss of consciousness. From a mechanistic study, the authors hypothesize that neural substrates of sleep-wake behavior are engaged by low-dose sedative anesthetics, and that the mesopontine descending noradrenergic cell groups contribute to the analgesic effects of both NMDA receptor antagonists and GABA (A) receptor-enhancing anesthetics.
Figure 2.
Phencyclidine and metabolites. Phencyclidine (PCP) (9a) has been extensively investigated as an abused drug. Mechanistic insight is gained from metabolic studies that show formation of iminium (9b). The electroreduction of 9b is facilitated by the aromatic nucleus despite lack of conjugation, indicating through space delocalization of the radical product. A computational report also supports this type of stabilization. MK-801 bears analogy by the presence ofa beta-nitrogen possessing radical character from electroreduction of nitrosonium.
Acknowledgements
Editorial assistance by Thelma Chavez is acknowledged.
Abbreviations
- ET
electron transfer
- ROS
reactive oxygen species
- OS
oxidative stress
- AO
antioxidant
- PCP
phencyclidine
- GSH
glutathione
- IDPN
3,3′-iminodipropionitrile
- CAPE
caffeic acid phenethyl ester
- NMDA
N-methyl-D-aspartate
- CNS
central nervous system
- SO
superoxide
- Glu
glutamate
- GABA
gamma-aminobutyric acid
- COX-2
cyclooxygenase-2
Footnotes
Previously published online: www.landesbioscience.com/journals/oximed/article/10028
References
- 1.Kovacic P, Becvar LE. Mode of action of anti-infective agents: emphasis on oxidative stress and electron transfer. Curr Pharm Des. 2000;6:143–167. doi: 10.2174/1381612810006020143. [DOI] [PubMed] [Google Scholar]
- 2.Kovacic P, Osuna JA. Mechanisms of anticancer agents: emphasis on oxidative stress and electron transfer. Curr Pharm Des. 2000;6:277–309. doi: 10.2174/1381612003401046. [DOI] [PubMed] [Google Scholar]
- 3.Kovacic P, Jacintho JD. Mechanism of carcinogenesis: focus on oxidative stress and electron transfer. Curr Med Chem. 2001;8:773–796. doi: 10.2174/0929867013373084. [DOI] [PubMed] [Google Scholar]
- 4.Kovacic P, Jacintho JD. Reproductive toxins: pervasive theme of oxidative stress and electron transfer. Curr Med Chem. 2001;8:863–892. doi: 10.2174/0929867013372878. [DOI] [PubMed] [Google Scholar]
- 5.Kovacic P, Sacman A, Wu-Weis M. Nephrotoxins: widespread role of oxidative stress and electron transfer. Curr Med Chem. 2002;9:823–847. doi: 10.2174/0929867024606803. [DOI] [PubMed] [Google Scholar]
- 6.Poli G, Cheeseman GH, Dianzani MU, Slater TF. Free Radicals in the Pathogenesis of Liver Injury. Oxford: Pergamon Pree,; 1989. pp. 1–330. [Google Scholar]
- 7.Kovacic P, Thurn LA. Cardiovascular toxins from the perspective of oxidative stress and electron transfer. Curr Vasc Pharmacol. 2005;3:107–117. doi: 10.2174/1570161053586912. [DOI] [PubMed] [Google Scholar]
- 8.Kovacic P, Somanathan R. Neurotoxicity: the broad framework of electron transfer, oxidative stress and protection by antioxidants. Curr Med Chem-CNS Agents. 2005;5:249–258. [Google Scholar]
- 9.Kovacic P, Pozos RS, Somanathan R, Shangari N, O’Brien PG. Mechanism of mitochondrial uncouplers, inhibitors and toxins: focus on electron transfer, free radicals and structure-activity relationships. Curr Med Chem. 2005;5:2601–2623. doi: 10.2174/092986705774370646. [DOI] [PubMed] [Google Scholar]
- 10.Kovacic P, Cooksy AL. Unifying mechanism for toxicity and addiction by abused drugs: electron transfer and reactive oxygen species. Med Hypotheses. 2005;64:366–367. doi: 10.1016/j.mehy.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 11.Kovacic P, Somanathan R. Ototoxicity and noise trauma: electron transfer, reactive oxygen species, cell signaling, electrical effects and protection by antioxidants: practical medical aspect. Med Hypotheses. 2008;70:914–923. doi: 10.1016/j.mehy.2007.06.045. [DOI] [PubMed] [Google Scholar]
- 12.Kovacic P, Somanathan R. Pulmonary toxicity and environmental contamination: radicals, electron transfer and protection by antioxidants. In: Whitacre WD, editor. Reviews of Environmental Contamination and Toxicology. Vol. 201. New York: Springer,; 2009. pp. 41–69. [DOI] [PubMed] [Google Scholar]
- 13.Kovacic P, Somanathan R. Intergrated approach to immunotoxicity: electron transfer, reactive oxygen species, antioxidants, cell signaling and receptors. J Recept Signal Transduct. 2008;28:323–346. doi: 10.1080/10799890802305217. [DOI] [PubMed] [Google Scholar]
- 14.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press,; 1999. pp. 1–936. [Google Scholar]
- 15.Kovacic P, Somanathan R. Beneficial Effects of Antioxidants in Relation to Carcinogens, Toxins and Various Illnesses. In: Panglossi HV, editor. Antioxidants: New Research. Vol. 1. NY: Nova Science Publishers Inc.,; 2006. pp. 1–37. [Google Scholar]
- 16.Kovacic P. Role of oxidative metabolites of cocaine in toxicity and addiction: oxidative stress and electron transfer. Med Hypotheses. 2005;64:350–356. doi: 10.1016/j.mehy.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Kovacic P, Ames JR, Jawdosiuk M, Ryan MD. Electron transfer mechanism for cocaine action. In: Dryhurst G, Niki K, editors. Redox Chemistry and Interfacial Behavior of Biological Molecules. New York: Plenum Press,; 1988. pp. 323–331. [Google Scholar]
- 18.Lloyd RV, Shuster L, Mason RP. Reexamination of the microsomal transformation of N-hydroxynorcocaine to norcocaine nitroxide. Mol Pharmacol. 1993;43:645–648. [PubMed] [Google Scholar]
- 19.Sayre LM, Engelhart DA, Nadkarni DV, Manoj Babu MK, Flammang AM, McCoy GD. The role of iminium-enamine species in the toxication and detoxication of cyclic tertiary amines. Pharmacokinetics, metabolism and pharmaceutics of drugs of abuse, 173. Natl Inst of Drug Abuse. 1997. pp. 106–127. [PubMed]
- 20.Rapaka RS, Chiang N, Martin BR. Introduction. Pharmacokinetics, metabolism and pharmaceutics of drugs of abuse, 173. Natl Inst of Drug Abuse. 1997. pp. 1–5.
- 21.Kirschner KN, Van Dyke C, Kovacic P, Bowen JP. Computational studies on electron transfer by iminium metabolite of phencyclidine (PCP) Theochem. 2000;498:167–179. [Google Scholar]
- 22.Kloss MW, Rosen GM, Rauckman EJ. Biotransformation of norcocaine to norcocaime nitroxide by rat brain microsomes. Psychopharmacol. 1984;84:221–224. doi: 10.1007/BF00427449. [DOI] [PubMed] [Google Scholar]
- 23.Kloss MW, Rosen GM, Rauckman EJ. Evidence of enhanced in vivo lipid peroxidation after acute cocaine administration. Toxicol Lett. 1983;15:65–70. doi: 10.1016/0378-4274(83)90171-6. [DOI] [PubMed] [Google Scholar]
- 24.Dahl AR, Hadley WM. Formaldehyde production promoted by rat nasal cytochrome P-450-dependent monooxygenases with nasal decongestants, essences, solvents, air pollutants, nicotine and cocaine as substrates. Toxicol Appl Pharm. 1983;67:200–205. doi: 10.1016/0041-008x(83)90225-9. [DOI] [PubMed] [Google Scholar]
- 25.Pacifici R, Fiaschi AI, Micheli L, Centini F, Giorgi G, Zuccaro P. Immunosuppression and oxidative stress induced by acute and chronic exposure to cocaine administration. Int Immunopharmacol. 2003;3:581–592. doi: 10.1016/S1567-5769(03)00050-X. [DOI] [PubMed] [Google Scholar]
- 26.Tariq M, Khan HA, Rehana Z, Al Moutaery K, Al Deeb S. Proglumide, a cholecystokinin receptor antagonist, exacerbates β,β′-iminodipropionitileinduced dyskinetic syndrome in rats. Neurotoxicol Teratol. 1998;20:571–579. doi: 10.1016/s0892-0362(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 27.Al-Deeb S, Al-Moutaery K, Bruyn GW, Tariq MJ. Neuroprotective effect of selenium on iminodipropionitrile-induced toxicity. J Psych Neurosci. 1995;20:189–192. [PMC free article] [PubMed] [Google Scholar]
- 28.Tariq M, Al Deeb S, Al Moutaery K, Ahmad Khan H. Cysteamine attenuates iminodipropionitrile (IDPN) induced dyskinesia in rats. Int J Neurosci. 1995;83:165–175. doi: 10.3109/00207459508986336. [DOI] [PubMed] [Google Scholar]
- 29.Tariq M, Khan HA, Al Moutaery K, Al-Deeb S. Attenuation of iminodipropionitrile induced behavioral syndrome by sodium salicylate in rats. Pharmacol Biochem Behav. 2002;73:647–654. doi: 10.1016/s0091-3057(02)00858-4. [DOI] [PubMed] [Google Scholar]
- 30.Nace CG, Genter MB, Sayre LM, Crofton KM. Effect of methimazole, an FMO substrate and competitive inhibitor, on the neurotoxicity of 3,3′-iminodipropionitrile in male rats. Fund Appl Toxicol. 1997;37:131–140. doi: 10.1006/faat.1997.2307. [DOI] [PubMed] [Google Scholar]
- 31.Koek W, Colpaert FC, Vignon J. Effects of phencyclidine-type drugs in rats discriminating fentanyl from saline: pharmacological and behavioral characterization of intermediate levels of drug lever selection. J Pharmacol Exp Ther. 1993;264:746–756. [PubMed] [Google Scholar]
- 32.Irifune M, Shimizu T, Nomoto M, Fukuda T. Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav. 1995;51:291–296. doi: 10.1016/0091-3057(94)00379-w. [DOI] [PubMed] [Google Scholar]
- 33.France CP, Moerschbaecher JM, Woods JH. MK-801 and related compounds in monkeys: discriminative stimulus effects and effects on a conditional discrimination. J Pharmacol Exp Ther. 1991;257:727–734. [PubMed] [Google Scholar]
- 34.Hucker HB, Hutt JE, White SD, Arison BH, Zacchei AG. Disposition and metabolism of (+)-5-methyl-10,11-dihydro-5H-dibenzo[a,b] cyclohepten-5,10-imine in rats, dogs and monkeys. Drug Metab Dispos. 1983;11:54–58. [PubMed] [Google Scholar]
- 35.Krishna MC, Grahame DA, Samuni A, Mitchell JB, Russo A. Oxoammonium cation intermediate in the nitroxide-catalyzed dismutation of superoxide. Proc Natl Acad Sci USA. 1992;89:5537–5541. doi: 10.1073/pnas.89.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosokha SV, Kochi JK. Mechanism of inner-sphere electron transfer via charge-transfer (precursor) complexes. Redox energetics of aromatic donors with the nitrosonium acceptor. J Am Chem Soc. 2001;123:8985–8999. doi: 10.1021/ja010859w. [DOI] [PubMed] [Google Scholar]
- 37.Botta B, D’Acquarica I, Delle Monache G, Nevola L, Tullo D, Ugozzoli F, Pierini M. Nitrosonium complexes of resorc[4]arenes: spectra, kinetic and theoretical studies. J Am Chem Soc. 2007;129:11202–11212. doi: 10.1021/ja072855i. [DOI] [PubMed] [Google Scholar]
- 38.Israeli A, Patt M, Oron M, Samuni A, Kohen R, Goldstein S. Kinetics and mechanism of the comproportionation reaction between oxoammonium cation and hydroxylamine derived from cyclic nitroxides. Free Rad Biol Med. 2005;38:317–324. doi: 10.1016/j.freeradbiomed.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 39.Butler AR, Flitne FW, Williams DL. NO, nitrosonium ions, nitroxide ions, nitrosothiols and ironnitrosyls in biology: a chemist’s perspective. Trends Pharmacol Sci. 1995;16:18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- 40.Kornhuber J, Mack-Burkhardt F, Kornhuber ME, Riederer P. [3H]MK-801 binding sites in post-mortem human frontal cortex. Eur J Pharmacol. 1989;162:483–490. doi: 10.1016/0014-2999(89)90339-7. [DOI] [PubMed] [Google Scholar]
- 41.Dravid SM, Erreger K, Yuan H, Nicholson K, Le P, Lyuboslavsky P, et al. Subunit-specific mechanisms and proton sensitivity of NMDA receptor channel block. J Physiol. 2007;581:107–128. doi: 10.1113/jphysiol.2006.124958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogita K, Shuto M, Kuramoto N, Manabe T, Hinoi R, Kitayama T, et al. Differential inhibition by ferrous ions of [3H]MK-801 binding to native N-methyl-D-aspartate channel in neonatal and adult brains. Brain Res. 1999;818:548–552. doi: 10.1016/s0006-8993(98)01300-6. [DOI] [PubMed] [Google Scholar]
- 43.Ozyurt B, Ozyurt H, Akpolat N, Erdogan H, Sarsilmaz M. Oxidative stress in prefrontal cortex of rat exposed to MK-801 and protective effects of CAPE. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:832–838. doi: 10.1016/j.pnpbp.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 44.Ozyurt B, Parlaktas BS, Ozyurt H, Aslan H, Ekici F, Atis O. A preliminary study of the levels of testis oxidative stress parameters after MK-801-induced experimental psychosis model: protective effects of CAPE. Toxicol. 2007;230:83–89. doi: 10.1016/j.tox.2006.11.047. [DOI] [PubMed] [Google Scholar]
- 45.Abidova SS, Ishankulova GF. Effect of the joint administration of ketamine and propofol on the lipid metabolism and peroxidation in rats. Eksp Klin Farmakol. 2004;67:45–47. [PubMed] [Google Scholar]
- 46.Daniell LC. The noncompetitative N-methyl-Daspartate antagonists, MK-801, phencyclidine and ketamine, increase the potency of general anesthetics. Pharmacol Biochem Behav. 1990;36:111–115. doi: 10.1016/0091-3057(90)90134-4. [DOI] [PubMed] [Google Scholar]
- 47.Benvenga MJ, Wing AV, Del Vecchio RA. The discriminative stimulus effect of MK-801 in ketaminetrained rats. Pharmcol Biochem Behav. 1991;38:211–213. doi: 10.1016/0091-3057(91)90613-7. [DOI] [PubMed] [Google Scholar]
- 48.Kovacic P. Unifying electron transfer mechanism for addiction involvement bt the anesthestic propofol. Med Hypotheses. 2010;74:206. doi: 10.1016/j.mehy.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Kovacic P, Somanathan R. Novel unifying mechanism for mescaline in the central nervous system: electrochemistry, catechol redox metabolite, receptor, cell signaling and structure activity relationship. Oxid Med Cell Longev. 2009;2:1–10. doi: 10.4161/oxim.2.4.9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kovacic P. Unifying electron transfer mechanism for psilocybin and psilocin. Med Hypotheses. 2009;73:626. doi: 10.1016/j.mehy.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 51.Merlo LG, Goldberger BA, Kolodner D, Fritzgerald K, Gold MS. Fentanyl and propofol exposure in the operating room: sensitization hypothesis. J Addict Dis. 2008;27:67–76. doi: 10.1080/10550880802122661. [DOI] [PubMed] [Google Scholar]
- 52.Favetta P, Guitton J, Deguote CS, Van Daele L, Boilieu R. High-performance chromatographic assays to detect hydroxylate and conjugate metabolites of propofol in human urine. J Chromatogr B Biomed Sci Appl. 2000;742:25–35. doi: 10.1016/s0378-4347(00)00097-9. [DOI] [PubMed] [Google Scholar]
- 53.Baker MT, Gregerson MS, Martin SM, Buettner GR. Free radical and drug oxidation products in an intensive care unit sedative: propofol with sulfite. Crit Care Med. 2003;31:787–792. doi: 10.1097/01.CCM.0000053560.05156.73. [DOI] [PubMed] [Google Scholar]
- 54.Wong EH, Kemp JA, Priestley T, Knight AR, Woodruff GN, Iversen LL. The anticonvulsant MK-801 is a potent N-methyl-D-aspartate antagonist. Proc Natl Acad Sci USA. 1986;83:7104–7108. doi: 10.1073/pnas.83.18.7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ames JR, Kovacic P, Kadaba PK, Kiser PF. Electrochemistry of anticonvulsants: electron transfer as a possible mode of action. Epilepsia. 1992;33:936–943. doi: 10.1111/j.1528-1157.1992.tb02204.x. [DOI] [PubMed] [Google Scholar]
- 56.Dimpfel W, Spüler M. Dizocilpine (MK-801), ketamine and phencyclidine: low doses affect brain field potentials in the freely moving rat in the same way as activation of dopaminergic transmission. Psychopharmacol. 1990;101:317–323. doi: 10.1007/BF02244048. [DOI] [PubMed] [Google Scholar]
- 57.Memezawa H, Zhao Q, Smith M-L. Siesj Hyperthermia nullifies the ameliorating effect of dizocilpine maleate (MK-801) in focal cerebral ischemia. Brain Res. 1995;670:48–52. doi: 10.1016/0006-8993(94)01251-c. [DOI] [PubMed] [Google Scholar]
- 58.Gao X-M, Hashimoto T, Tamminga CA. Phencyclidine (PCP) and dizocilpine (MK801) exert time-dependent effects on the expression of immediate early genes in rat brain. SYNAPSE. 1998;29:14–28. doi: 10.1002/(SICI)1098-2396(199805)29:1<14::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 59.Tomitaka S, Tomitaka M, Tolliver BK, Sharp FR. Bilateral blockade of NMDA receptors in anterior thalamus by dizocilpine (MK801) injures pyramidal neurons in rat retrosplenial cortex. Eur J Neurosci. 2001;12:1420–1430. doi: 10.1046/j.1460-9568.2000.00018.x. [DOI] [PubMed] [Google Scholar]
- 60.Umrykhin PE, Anokhin KV, Raevskii KS. Dizocilpine blocks the effect of delta sleep-inducing peptide-induced suppression of c-fos gene expression in the paraventricular nucleus of the hypothalamus in rats. Neurosci Behavioral Physiol. 2004;34:501–503. doi: 10.1023/b:neab.0000022637.57852.e0. [DOI] [PubMed] [Google Scholar]
- 61.Mansbach RS, Geyer MA. Effects of phencyclidine and phencyclidine biologs on sensorimotor gating in the rat. Neuropsychopharmacol. 1989;2:299–308. doi: 10.1016/0893-133x(89)90035-3. [DOI] [PubMed] [Google Scholar]
- 62.Horvàth ZC, Czopf J, Buzsàki G. MK-801-induced neuronal damage in rats. Brain Res. 1997;753:181–195. doi: 10.1016/s0006-8993(96)01290-5. [DOI] [PubMed] [Google Scholar]
- 63.Hur G-H, Son W-C, Shin S, Kang J-K, Kim Y-B. Sex differences in diazocilpine (MK-801) neurotoxicity in rats. Environ Toxicol Pharmacol. 1999;7:143–146. doi: 10.1016/s1382-6689(99)00003-4. [DOI] [PubMed] [Google Scholar]
- 64.Wills CL, Ray DE. Antioxidants attenuate MK-801-induced cortical neurotoxicity in the rat. Neuro Toxicol. 2007;28:161–167. doi: 10.1016/j.neuro.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Kreieglstein J. Excitotoxicity and neuroprotection. Eur J Pharmaceut Sci. 1997;5:181–187. [Google Scholar]
- 66.Jiang Q, Xu S, Zhou Q, Yang J. Effects of dizocilpin on release of amino acids and free calcium from ischemic-synatosomes. Zhongguo Yaolixue Yu Dulixue Zazhi. 2002;16:161–164. [Google Scholar]
- 67.Said SA, Pakbaz H, Berisha HI, Raza S. NMDA receptor activation: critical role in oxidant tissue injury. Free Rad Biol Med. 2000;28:1300–1302. doi: 10.1016/s0891-5849(00)00289-6. [DOI] [PubMed] [Google Scholar]
- 68.Alavez S, Blancas S, Moran J. Effect of N-methyl- D-aspartate receptor blockade on caspase activation and neuronal death in the developing rat cerebellum. Neurosci Lett. 2006;404:176–181. doi: 10.1016/j.neulet.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 69.Hewett SJ, Espey MG, Uliasz TF, Wink DA. Neurotoxicity of nitroxyl: insights into HNO and NO biochemical imbalance. Free Rad Biol Med. 2005;39:1478–1488. doi: 10.1016/j.freeradbiomed.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Küçükkaya B, Alker R, Yüksel M, Onat F, Yalçin AS. Low dose MK-801 protects against iron-induced oxidative changes in a rat model of focal epilepsy. Brain Res. 1998;788:133. doi: 10.1016/s0006-8993(97)01544-8. [DOI] [PubMed] [Google Scholar]
- 71.Sturgess NC, Rustad A, Fonnum F, Lock EA. Neurotoxic effect of L-2-chloropropionic acid on primary cultures of rat cerebellar granule cells. Arch Toxicol. 2000;74:153–160. doi: 10.1007/s002040050668. [DOI] [PubMed] [Google Scholar]
- 72.Radesäter A-C, Johansson S, Öberg C, Luthman J. The vitamin-E analog trilox and the NMDA antagonist MK-801 protect pyramidal neurons in hippocampal slice cultures from IL-1β-induced neurodegeneration. Neurotox Res. 2003;5:433–442. doi: 10.1007/BF03033173. [DOI] [PubMed] [Google Scholar]
- 73.Dekundy A, Kaminski RM, Zielinska E, Turski WA. NMDA antagonists exert distinct effects in experimental organophosphate or carbamate poisoning in mice. Toxicol Appl Pharmacol. 2007;219:114–121. doi: 10.1016/j.taap.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 74.Carter AJ. Many agents that antagonize the NMDA receptor-channel complex in vivo also cause disturbances of motor coordination. J Pharmacol Exp Ther. 1994;269:573–580. [PubMed] [Google Scholar]
- 75.Carey RJ, Dai H, Gui J. Effects of dizocilpine (MK-801) on motor activity and memory. Psychopharmacol. 1998;137:241–246. doi: 10.1007/s002130050616. [DOI] [PubMed] [Google Scholar]
- 76.Lukoyanov NV, Paula-Barbosa MM. A single high dose of dizocilpine produces long-lasting impairment of the water maze performance in adult rats. Neurosci Lett. 2000;285:139–142. doi: 10.1016/s0304-3940(00)01053-3. [DOI] [PubMed] [Google Scholar]
- 77.Tsukada H, Nishiyama S, Fukumoto D, Sato K, Kakiuchi T, Domino EF. Chronic NMDA antagonism impairs working memory, decreases extracellular dopamine, and increases D1 receptor binding in prefrontal cortex of conscious monkeys. Neuropsychopharmacol. 2005;30:1861–1869. doi: 10.1038/sj.npp.1300732. [DOI] [PubMed] [Google Scholar]
- 78.Harder JA, Aboobaker AA, Hodgetts TC, Ridley RM. Learning impairment induced by glutamate blockade using dizocilpine (MK-801) in monkeys. Brit J Pharmacol. 1998;125:113–1018. doi: 10.1038/sj.bjp.0702178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loscher W, Honack D. The behavioural effects of MK-801 in rats: involvement of dopaminergic, seratonergic and nonadreanergic systems. Eur J Pharmacol. 1992;14:199–208. doi: 10.1016/0014-2999(92)90029-4. [DOI] [PubMed] [Google Scholar]
- 80.Bubenikova-Valesova V, Votava M, Palenicek T, Horacek J. The opposite effect of a low and a high dose of serotonin-1A agonist on behavior induced by MK-801. Neuropharmacol. 2007;52:1071–1078. doi: 10.1016/j.neuropharm.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 81.Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity and place preference in zebra fish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Wisnewski RG, Lauweryns J. Systemic dizocilpine (MK-801) facilitates performance in opposition to response bias. Behav Brain Funct. 2007;3:48. doi: 10.1186/1744-9081-3-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effect of light on circadian behavior? Brain Res. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- 84.Kovacic P, Somanathan R. Zolpidem, a clinical hypnotic that effects electron transfer, alters synaptic activity through potential GABA receptors in the nervous system without significant free radical generation. Oxid Med Cell Longev. 2009;2:52–57. doi: 10.4161/oxim.2.1.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vanderschuren LJMJ, Schoffelmeer ANM, Mulder AH, De Vries TJ. Dizocilpine (MK 801): use or abuse? TIPS. 1998;19:79–81. doi: 10.1016/s0165-6147(97)01164-4. [DOI] [PubMed] [Google Scholar]
- 86.Koyuncuo lu H, Dizdar Y, Aricio lu F, Sayin U. Effects of MK801 on morphine physical dependence: attenuation and intensification. Pharmacol Biochem Behav. 1992;43:487–490. doi: 10.1016/0091-3057(92)90181-e. [DOI] [PubMed] [Google Scholar]
- 87.Wong CS, Chering CH, Luk HN, Ho ST, Tung CS. Effects of NMDA receptor antagonist on inhibition of morphine tolerance in rats: binding at mu-opioid receptors. Eur J Pharmacol. 1996;297:27–33. doi: 10.1016/0014-2999(95)00728-8. [DOI] [PubMed] [Google Scholar]
- 88.Tzschentke TM, Schmidt WJ. Interactions of MK-801 and GYKI 52466 with morphine and amphetamine in place preference conditioning and behavioural sensitization. Behav Brain Res. 1997;84:99–107. doi: 10.1016/s0166-4328(97)83329-3. [DOI] [PubMed] [Google Scholar]
- 89.Michaela K, Bashkatova M, Valentina V, Anatoly P, Prast H. Dizocilpine inhibits amphetamine-induced formation of nitric oxide and amphetamine-induced release of amino acids and acetylcholine in rat brain. Neurochem Res. 2002;27:229–235. doi: 10.1023/a:1014836621717. [DOI] [PubMed] [Google Scholar]
- 90.Thomas DM, Kuhn DM. Mk-801 and dextromethorphan block microglial activation and protect against methamphetamine-induced neurotoxicity. Brain Res. 2005;1050:190–198. doi: 10.1016/j.brainres.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 91.Solntseva SV, Nikitin VP. Reversible and irreversible stages of amnesia development after disruption of associative memory reactivation in snail. Zh Vyssh Nerv Deiat Im I P Pavlova. 2009;59:344–352. [PubMed] [Google Scholar]
- 92.Ceretta AP, Camera K, Mello CF, Rubin MA. Arcaine and MK-801 make recall state-dependent in rats. Psychopharmacol (Berl) 2008;201:405–411. doi: 10.1007/s00213-008-1304-7. [DOI] [PubMed] [Google Scholar]
- 93.Saraf MK, Prabhakar S, Anand A. Bacopa monniera alleviates N(omega)-nitro-L-arginine arginine-induced but not MK-801-induced amnesia: a mouse Morris watermaze study. Neuroscience. 2009;160:149–155. doi: 10.1016/j.neuroscience.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 94.von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R. Cueinduced alcohol behavior is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacol (Berl) 2009;205:389–397. doi: 10.1007/s00213-009-1544-1. [DOI] [PubMed] [Google Scholar]
- 95.Rios Valentim SJ, Jr, Gontijo AV, Peres MD, Rodrigues LC, Nakamura Palacios EM. D1 dopamine and NMDA receptors interactions in the medial prefrontal cortex: modulation of spatial working memory in rats. Behav Brain Res. 2009;2004:124–128. doi: 10.1016/j.bbr.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 96.Coleman LG, Jr, Jarskog LF, Moy SS, Crews FT. Deficits in adult prefrontal cortex neurons and behavior following early post-natal NMDA antagonist treatment. Pharmacol Biochem Behav. 2009;93:322–330. doi: 10.1016/j.pbb.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Harich S, Koch M, Schwaba K. Effects of repeated dizocilpine treatment on adult rat behavior after neonatal lesions of the entorhinal cortex. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:816–827. doi: 10.1016/j.pnpbp.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 98.Perera PY, Lichy JH, Mastropaola J, Rosse RB, Deutsch SI. Expression of NR1, NR2A and NR2B NMDA receptor subunits is not altered in the genetically-inbred Balb/c mouse strain with heightened behavioral sensitivity to MK-801, a noncompetitive NMDA receptor antagonist. Eur Neuropsychopharmacol. 2008;18:814–819. doi: 10.1016/j.euroneuro.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 99.Darrah JM, Stefani MR, Moghaddam B. Interaction of N-methyl-D-aspartate and group 5 metabotropic glutamate receptors on behavioral flexibility using a novel operant set-shift paradigm. Behav Pharmacol. 2008;19:225–234. doi: 10.1097/FBP.0b013e3282feb0ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ. Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behavior. J Neurosci. 2008;28:8230–8237. doi: 10.1523/JNEUROSCI.1723-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Solntseva SV, Nikitin P. Neurochemical mechanisms of food aversion conditioning consolidation in snail Helix lucorum. Ross Fiziol Zh Im I M Sechenova. 2008;94:1259–1269. [PubMed] [Google Scholar]
- 102.Al Rahim M, Nakajima A, Saigusa D, Tetsu N, Maruyama Y, Shibuya M, et al. 4′-Demethylnobiletin, a bioactive metabolite of nobiletin enhancing PKA/ ERK/CREB signaling, rescues learning impairment associated with NMDA receptor antagonism via stimulation of ERK cascade. Biochemistry. 2009;48:7713–7721. doi: 10.1021/bi901088w. [DOI] [PubMed] [Google Scholar]
- 103.Qi D, Zhu Y, Wen L, Liu Q, Qiao H. Ginsenoside Rg1 restores the impairment of learning induced by chronic morphine administration in rats. J Psychopharmacol. 2009;23:74–83. doi: 10.1177/0269881107082950. [DOI] [PubMed] [Google Scholar]
- 104.Watson DJ, Stanton ME. Intrahippocampal administration of an NMDA-receptor antagonist impairs spatial discrimination reversal learning in weaning rats. Neurobiol Learn Mem. 2009;92:89–98. doi: 10.1016/j.nlm.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rezayof A, Sharifi K, Zarrindast MR, Rassouli Y. Modulation of ethanol state-dependent learning by dorsal hippocampal NMDA receptors in mice. Alcohol. 2008;42:667–674. doi: 10.1016/j.alcohol.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Goeldner C, Reiss D, Wichmann J, Kieffer BL, Ouagazzal AM. Activation of nociceptin opioid peptide (NOP) receptor impairs contrextual fear learning in mice through glutamatergic mechanisms. Neurobiol Learn Mem. 2009;91:393–401. doi: 10.1016/j.nlm.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chan WY, McNally GP. Conditioned stimulus familiarity determines effects of MK-801 on fear extinction. Behav Neurosci. 2009;123:303–314. doi: 10.1037/a0014988. [DOI] [PubMed] [Google Scholar]
- 108.Itzhak Y. Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann NY Acad Sci. 2008;1139:350–357. doi: 10.1196/annals.1432.051. [DOI] [PubMed] [Google Scholar]
- 109.Kim JH, Richardson R. The effect of the mu-opioid receptor antagonist naloxone on extinction of conditioned fear in the developing rat. Learn Mem. 2009;16:161–166. doi: 10.1101/lm.1282309. [DOI] [PubMed] [Google Scholar]
- 110.Yang Z, Luo W, Hou J, Zhao Z, Jian F, Wamalwa P, et al. Development of behavior model of pain induced by experimental tooth movement in rats. Eur J Oral Sci. 2009;117:380–384. doi: 10.1111/j.1600-0722.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- 111.Li SQ, Xing YL, Chen WN, Yue SL, Li L, Li WB. Activation of NMDA receptor is associated with upregulation of COX-2 expression in the spinal dorsal horn during nociceptive inputs in rats. Neurochem Res. 2009;34:1451–1463. doi: 10.1007/s11064-009-9932-9. [DOI] [PubMed] [Google Scholar]
- 112.Lu J, Nelson LE, Franks N, Maze M, Chamberlin NL, Saper CB. Role of endogenous sleep-wake and analgesic systems in anesthesia. J Comp Neurol. 2008;508:648–662. doi: 10.1002/cne.21685. [DOI] [PMC free article] [PubMed] [Google Scholar]