Abstract
The sebaceous gland is renewed throughout adult life and homeostasis of this particular organ is controlled by a precise interplay of hormones, cytokines, signalling molecules and mediators of the lipid metabolism. Although the true function of sebaceous glands has yet to be fully determined, recent evidence demonstrates that normal homeostasis of the sebaceous gland and functional lipid metabolism of sebocytes is crucial for maintenance of the skin barrier. In addition, analysis of mutant mouse models revealed a close interdependency of the sebaceous gland with hair follicles because abnormal morphogenesis of sebaceous glands often results in degeneration of hair follicle structures. Anomalous regulation of sebaceous glands is involved in the pathogenesis of acne, one of the most prevalent human diseases, or could lead to formation of sebaceous hyperplasia and tumours. This review highlights some of the recent findings on the importance of signalling pathways controlling morphogenesis and differentiation of the sebaceous gland in vivo.
Key words: sebaceous gland, differentiation, hedgehog, β-catenin, skin, sebocyte, epidermis, SCD1, c-myc
Introduction
The sebaceous gland and hair follicles constitute the pilosebaceous unit of the skin (Fig. 1). Development of the pilosebaceous unit starts at day 14.5 of mouse embryogenesis and involves an ordered set of developmental processes. In recent years formidable progress has been made in understanding how hair follicle development and regeneration are regulated.1–3 The formation of hair follicles occurs in well delineated stages and some of the crucial signalling molecules and factors controlling these developmental processes have been unraveled.4 In contrast, much less is known about the formation of the sebaceous gland. During late embryogenesis, developing hair follicles (hair peg stage) display several bulges of which one will give rise to the sebaceous gland and is located just above the hair follicle stem cell bulge and below the infundibulum of the developing follicle.
Figure 1.
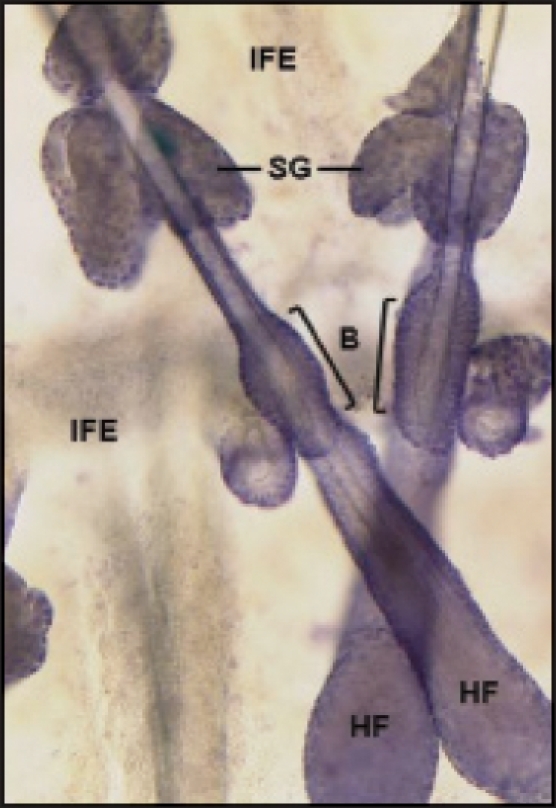
Wholemount of pilosebaceous unit isolated from mouse tail skin. Micrograph of wholemount stained with hematoxylin displaying two pilosebaceous units of mouse skin. B, bulge region of the hair follicle containing the stem cell compartment; HF, hair follicle; IFE, interfollicular epidermis; SG, sebaceous gland.
The sebaceous gland arises as an outgrowth of the outer root sheath of the hair follicle as undifferentiated sebocytes emerge from peripheral basal cells and then move centrally as first partially, and later fully, differentiated sebocytes. The fully developed sebaceous gland remains attached to the hair follicle throughout adult life. Differentiated sebocytes produce and secrete lipid-rich sebum into the hair canal that empties out to the skin surface.5 It has been demonstrated that normal development and proper maintenance of the sebaceous gland are required for skin homeostasis since atrophic sebaceous glands and disturbances in sebaceous lipid composition result in major defects of the physiological barrier of the skin.6
The sebaceous gland is constantly renewed throughout adult life by a population of progenitor cells responding to a coordinated interplay of various signals thereby controlling the balance between proliferation and differentiation of the tissue.7 Two models have been proposed to describe how sebaceous gland cells might arise and regenerate. One is that bulge stem cells of the hair follicle serve as multipotent progenitors which migrate upwards to generate the sebaceous gland.8,9 Another possibility is that self-renewing stem or progenitor cells reside within the sebaceous gland itself to generate and maintain the mature sebocytes.10,11 Recently, the molecular signatures characteristic for the hair follicle stem cell compartment have been unraveled.12,13 However, relevant signalling molecules important for sebaceous gland development and maintenance of sebocyte precursor cells have not been yet discovered.
In addition to cell autonomous regulators and signals guiding proliferation and maturation among sebaceous cells, the diverse cellular environment and components of the extracellular matrix surrounding the sebaceous gland might have a profound effect on homeostasis of the tissue. Given that a molecular cross-talk between dermis (dermal condensate, dermal papilla) and overlaying epithelial cells (outer root sheath, matrix cells) is a prerequisite for initiation and maintenance of hair follicle structures,14 it seems most likely that similar mechanisms of communication between basal keratinocytes of the sebaceous gland and the surrounding dermal tissue will apply.
Recent studies exploring mouse models are just beginning to elucidate the signalling pathways regulating homeostasis between proliferation and maturation of the sebaceous gland in vivo. This review will highlight important signalling molecules that have been identified as crucial modulators of the differentiation program experienced by sebocytes.
Canonical Wnt/β-Catenin Signalling
Several components of the canonical Wnt/β-catenin signalling pathway have been implicated in epidermal cell fate decisions, including regulating sebaceous lineage differentiation. Blocking canonical Wnt signalling during skin development by expression of a dominant negative mutant transcription factor Lef1 (ΔNLef1) under the control of a keratin14 promoter results in transdifferentiation of hair follicle keratinocytes into mature sebocytes.15,16 De novo sebocyte differentiation occurs along the length of the hair follicle and is not defined to a particular region of the pilosebaceous unit. One interesting question arising is if the change in cell fate towards sebaceous lineage is dependent on reprogramming stem cells of the hair follicle. This is particularly interesting because in addition to the newly recognised plasticity of adult stem cells, there is an increasing number of examples of cells changing fate after commitment to a particular program of differentiation.
Recently, it has been discovered that a high proportion of human sebaceous adenomas and sebaceomas have double nucleotide mutations within the β-catenin binding domain of the lef1 gene. These mutations within the NH2 terminus of Lef1 prevent β-catenin binding and inhibit expression of β-catenin target genes.17 Accordingly, transgenic mice expressing N-terminally deleted ΔNLef1 in the skin develop spontaneous sebaceous tumours.16 In an attempt to investigate how Lef1 mutations contribute to tumour formation it was discovered that keratinocytes of K14ΔNLef1 transgenic mice failed to upregulate p53 and p21 proteins during tumourigenesis and in response to UV irradiation, and this correlated with impaired p19Arf induction.18
The consequences of antagonising canonical Wnt/β-catenin signalling for sebaceous gland differentiation has been further explored utilising a different transgenic mouse model exhibiting manipulation of Smad signalling. Particularly, Smad signalling was ablated in the skin by overexpression of the Smad antagonist Smad7 under the control of the keratin5 promoter. Analyses of these mice pointed to a direct interaction between Smad7 and the Wnt/β-catenin pathway involving the recruitment of the E3 ligase Smurf2, thereby targeting cytoplasmic β-catenin for degradation. This suppression in Wnt/β-catenin signalling activity resulted in accelerated sebaceous gland morphogenesis and increase in sebocyte differentiation.19 Interestingly, aged skin often features enlarged sebaceous glands and exhibits upregulation of Smad7 expression20 associated with reduced β-catenin protein levels.19
There is good evidence that activation of c-myc in mouse skin results in enhanced sebaceous gland morphogenesis,21,22 and induction of sebocyte cell fate even within the interfollicular epidermis.23 The effect of myc is somewhat surprising because c-myc is reported to act downstream of β-catenin and to be a direct target gene of canonical Wnt signalling.24,25 In skin, c-myc and β-catenin exert opposing effects on sebocyte differentiation. Analysis of transgenic mice with simultaneous activation of c-myc and β-catenin revealed mutual antagonism: c-myc blocked β-catenin-mediated formation of ectopic hair follicles and β-catenin reduced c-myc-stimulated sebocyte differentiation.26
Taken together, these data implicate, that antagonising Wnt/β-catenin signalling constitutes an important prerequisite for normal sebaceous differentiation in postnatal skin tissue.
Hedgehog Signalling Pathway
There is good evidence for a function of the Hedgehog signalling pathway controlling the equation of proliferation and differentiation of sebocytes.27 Inhibition of Hedgehog signalling in the skin by overexpressing a dominant-negative transcription factor Gli2 (K5-Gli2ΔC4) leads to suppression of sebocyte development. In contrast, ectopic Hedgehog signalling by overexpression of a gain of function mutant receptor smoothened (K5-M2Smo) induced an increase in number and size of the sebaceous glands.28 Interestingly, transgenic mice overexpressing Sonic hedgehog under the control of the keratin14 promoter do not show an enlargement of sebaceous glands.29 Instead, it has been shown that a different hedgehog ligand, Indian hedgehog, is expressed in mature sebocytes and could play an important role in regulating proliferation and differentiation of the sebaceous gland in skin.30 This hypothesis is supported by experiments demonstrating that treatment of human sebocyte cells with cyclopamine, an antagonist of hedgehog signalling, reduces proliferation and stimulated sebocyte differention.30 These data exploring human sebocyte cultures suggest that hedgehog signalling could be a positive regulator of proliferation of progenitor cells. Interestingly, the canonical Wnt/β-catenin pathway may also be linked to Indian hedgehog signalling in sebocytes, as overexpression of mutant ΔNLef1 leads to upregulation of Indian hedgehog in human sebocyte cells in culture.30,31 This suggests that a tight balance of both signalling cascades is crucial for proper sebaceous gland proliferation and differentiation.
More recently, hedgehog signalling has been shown to be involved in regulating duct fate in sebaceous glands. Transgenic mice with constitutive expression of the hedgehog effector molecule Gli2 develop prominent sebaceous ducts and additional pairs of highly branched sebaceous glands.32 The authors identify keratin6 as a marker for sebaceous duct cells and present a direct link between keratin6 expression and hedgehog signalling activity in sebaceous duct cells in vivo. These results now shed light on one potential mechanism for how mutations affecting keratin6 and keratin partner genes lead to defects of the pilosebaceous unit that are often associated with abnormal expansion of sebaceous glands in patients.32
Lipid Metabolism
Constituents of the lipid metabolism have been shown to be important for sebaceous gland morphogenesis. Targeted deletion of stearoyl-CoA desaturase 1 (SCD1) or mutations within the gene coding for this key enzyme of mammalian lipid metabolism (asebia mouse) result in atrophy of sebaceous glands of the skin and Meibomian glands of the eyelid as well as abnormal hair growth.6,33–35 SCD1 converts saturated fatty acids into monounsaturated fatty acids using palmitoyl- and stearoyl-CoA as preferred substrates to generate palmitoleic (Δ9-16:1) and oleic acid (Δ9-18:1), respectively. Both are essential components of triglycerides and more complex lipids found in lipoproteins and eukaryotic membranes. Interestingly, SCD1 deficiency leads to disruption of the epidermal lipid barrier most likely caused by the strong reduction of epidermis-specific ceramides, one of the crucial lipid fraction in mammalian skin.6 The dramatic phenotype seen in SCD1 mutants highlights the importance of unsaturated fatty acids for sebaceous gland formation and functional skin barrier. Furthermore, the SCD1 mouse mutants are representative for the interdependence of hair follicle structures and sebaceous glands. Particularly, it has been observed that damaging one of the two organs results in disturbance of homeostasis of the other one. In the future, it will be important to analyse crucial signals responsible for this tight interaction between hair follicle and sebaceous gland and their mutual dependence for tissue homeostasis.
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor family and emerged as important mediators of the lipid metabolism in adipocytes but also in sebaceous glands.5,36 Analysis of mutant mice chimeric for PPARγ expression revealed that PPARγ is required for proper sebaceous gland differentiation.37 A set of different in vitro studies demonstrated the important function of PPARs for cell differentiation, lipid synthesis and fatty acid uptake into the cells.5,36 Additionally, like SCD1, PPARs have also been implicated in the regulation of keratinocyte differentiation and the formation of a functional skin barrier.38,39 These interesting results provoke the speculation that the effects observed following PPAR activation are accomplished in close coordination between PPARs and signalling pathways known to be important for regulating skin development and epidermal homeostasis.
Summary and Outlook
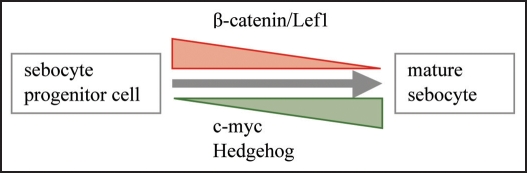
Over the last years we have begun to characterise some of the crucial factors controlling sebaceous gland morphogenesis and differentiation. Recent studies exploring mouse models have identified canonical Wnt/β-catenin and hedgehog pathways as well as c-myc activation as important modulators of proliferation and maturation of sebocytes in vitro and in vivo (Fig. 2). However, the exact molecular mechanism how these signals govern sebocyte function has not been elucidated yet. Particularly, it is tantalising to unravel how the different signalling events are connected and regulate each other. Although some progress has been made in our understanding how sebocyte differentiation is regulated, hardly anything is known about essential inductive events responsible for the morphogenesis of the sebaceous gland during embryonal and neonatal development. It will be challenging to determine the molecular mechanism by which hormones, lipid metabolism and signalling pathways/cytokines regulate sebocyte function in vivo and how an imbalance of one of these important modulators affects the others causing abnormal sebaceous gland differentiation and skin diseases. In the future, it will be important to unravel how sebaceous gland function impacts different compartments and cell types of the skin. Furthermore, to conceive how sebaceous glands contribute to skin pathology we need to increase our understanding of basic molecular and cellular mechanisms governing development and morphogenesis of the sebaceous gland.
Figure 2.
Signals controlling sebocyte differentiation in vivo. Summary of major signalling pathways regulating differentiation of the sebaceous gland in vivo. Repression of β-catenin signalling and activation of c-myc and hedgehog signalling cascade are important for the differentiation and maturation process experienced by sebocytes.
Acknowledgements
The author would like to thank Susan John for critically reading the manuscript and Monika Petersson for providing the micrograph.
Abbreviations
- Lef1
lymphoid enhancer-binding factor1
- PPAR
peroxisome proliferator-activated receptor
- SCD1
stearoyl-CoA desaturase 1
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/8486
References
- 1.Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- 2.Niemann C, Watt FM. Designer skin: Lineage commitment in postnatal skin. Trends in Cell Biology. 2002;12:185–192. doi: 10.1016/s0962-8924(02)02263-8. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 4.Blanpain C, Fuchs E. Epidermal stem cells of the skin. Annu Rev Cell Dev Biol. 2006;22:339–373. doi: 10.1146/annurev.cellbio.22.010305.104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 6.Binczek E, Jenke B, Holz B, Gunter RH, Thevis M, Stoffel W. Obesity resistance of the stearoyl-CoA desaturase-deficient (scd1−/−) mouse results from disruption of the epidermal lipid barrier and adaptive thermoregulation. Biol Chem. 2007;388:405–418. doi: 10.1515/BC.2007.046. [DOI] [PubMed] [Google Scholar]
- 7.Blanpain C, Fuchs E. Epidermal homeostasis: A balancing act of stem cells in the skin. Nat Rev Mol Cell Biol. 2009;10:207–217. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Panteleyev AA, Rosenbach T, Paus R, Christiano AM. The bulge is the source of cellular renewal in the sebaceous gland of mouse skin. Arch Dermatol Res. 2000;292:573–576. doi: 10.1007/s004030000182. [DOI] [PubMed] [Google Scholar]
- 9.Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000;102:451–461. doi: 10.1016/s0092-8674(00)00050-7. [DOI] [PubMed] [Google Scholar]
- 10.Ghazizadeh S, Taichman LB. Multiple classes of stem cells in cutaneous epithelium: A lineage analysis of adult mouse skin. EMBO J. 2001;20:1215–1222. doi: 10.1093/emboj/20.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horsley V, O’Carroll D, Tooze R, Ohinata Y, Saitou M, Obukhanych T, et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell. 2006;126:597–609. doi: 10.1016/j.cell.2006.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, et al. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- 13.Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rendl M, Lewis L, Fuchs E. Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 2005;3:331. doi: 10.1371/journal.pbio.0030331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill BJ, Gat U, DasGupta R, Fuchs E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 2001;15:1688–1705. doi: 10.1101/gad.891401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niemann C, Owens DM, Hülsken J, Birchmeier W, Watt FM. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicle keratinocytes into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- 17.Takeda H, Lyle S, Lazar AFJ, Zouboulis CC, Smyth I, Watt FM. Human sebaceous tumors harbour inactivating mutations in Lef1. Nat Med. 2006;12:395–397. doi: 10.1038/nm1386. [DOI] [PubMed] [Google Scholar]
- 18.Niemann C, Owen DM, Schettina P, Watt FM. Dual role of inactivating Lef1 mutations in epidermis: tumour promotion and specification of tumour type. Cancer Res. 2007;67:2916–2921. doi: 10.1158/0008-5472.CAN-06-3427. [DOI] [PubMed] [Google Scholar]
- 19.Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced β-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Ultraviolet irradiation alters transforming growth factor beta/smad pathway in human skin in vivo. J Invest Dermatol. 2002;119:499–506. doi: 10.1046/j.1523-1747.2002.01834.x. [DOI] [PubMed] [Google Scholar]
- 21.Arnold I, Watt FM. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr Biol. 2001;11:558–568. doi: 10.1016/s0960-9822(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 22.Waikel RL, Kawachi Y, Waikel PA, Wang XJ, Roop DR. Deregulated expression of c-Myc depletes epidermal stem cells. Nat Genet. 2001;28:165–168. doi: 10.1038/88889. [DOI] [PubMed] [Google Scholar]
- 23.Braun KM, Niemann C, Jensen UB, Sundberg JP, Watt FM. Manipulation of stem cell proliferation and lineage commitment in mouse epidermis: Visualisation of label-retaining cells in whole mounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- 24.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 25.Clevers H. Wnt/beta-catenin signalling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Lo Celso C, Berta MA, Braun KM, Frye M, Lyle S, Zouboulis CC, Watt FM. Characterization of bipotent epidermal progenitors derived from human sebaceous gland: Contrasting roles of c-myc and β-catenin. Stem Cells. 2008;26:1241–1252. doi: 10.1634/stemcells.2007-0651. [DOI] [PubMed] [Google Scholar]
- 27.Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C. Human skin stem cells and the aging process. Exp Gerontol. 2008;43:986–997. doi: 10.1016/j.exger.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Allen M, Grachtchouk M, Sheng H, Grachtchouk V, Wang A, Wei L, et al. Hedgehog signaling regulates sebaceous gland development. Am J Pathol. 2003;163:2173–2178. doi: 10.1016/S0002-9440(10)63574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oro AE, Higgins KM, Hu Z, Bonifas JM, Epstein EH, Jr, Scott MP. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science. 1997;276:817–821. doi: 10.1126/science.276.5313.817. [DOI] [PubMed] [Google Scholar]
- 30.Niemann C, Unden AB, Lyle S, Zouboulis CC, Toftgård R, Watt FM. Indian hedgehog and β-catenin signalling: Role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci. 2003;100:11873–11880. doi: 10.1073/pnas.1834202100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zouboulis CC, Seltmann H, Neitzel H, Orfanos CE. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
- 32.Gu LH, Coulombe PA. Hedgehog signalling, keratin6 induction and sebaceous gland morphogenesis: Implications for pachyonychia congenital and related conditions. Am J Pathol. 2008;173:752–761. doi: 10.2353/ajpath.2008.071089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 34.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase 1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 35.Sundberg JP, Boggess D, Sundberg BA, Eilertsen K, Parimoo S, Filippi M, Stenn K. Asebia-2J (Scd1(ab2J)): A new allele and a model for scarring alopecia. Am J Pathol. 2002;156:2067–2075. doi: 10.1016/S0002-9440(10)65078-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith KR, Thiboutot DM. Sebaceous gland lipids: Friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPARγ is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- 38.Hanley K, Jiang Y, Crumrine D, Bass NM, Appel R, Elias PM, et al. Activators of the nuclear hormone receptors PPARα and FXR accelerate the development of the fetal epidermal permeability barrier. J Clin Invest. 1997;100:705–712. doi: 10.1172/JCI119583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanley K, Kömüves LG, Bass NM, He SS, Jiang Y, Crumrine D, et al. Fetal epidermal differentiation and barrier development in vivo is accelerated by nuclear hormone receptor activators. J Invest Dermatol. 1999;113:788–795. doi: 10.1046/j.1523-1747.1999.00743.x. [DOI] [PubMed] [Google Scholar]