Abstract
The principal activity of mature sebaceous glands is producing and secreting sebum, which is a complex mixture of lipids. Sebum composition is different among species and this difference is probably due to the function that sebum has to absolve. In human sebum there are unique lipids, such as squalene and wax esters not found anywhere else in the body nor among the epidermal surface lipids. Moreover, they correspond to major components supplying the skin with protection. However, the ultimate role of human sebum, as well the metabolic pathways regulating its composition and secretion rate, are far from a complete understanding. Increased sebum secretion is considered, among all features, the major one involved in the pathophysiology of acne. Along with increased sebum secretion rate, quali- and quantitative modifications of sebum are likely to occur in this pathology. Understanding the factors and mechanisms that regulate sebum production is needed in order to identify new targets that can be addressed to achieve a selective modulation of lipid biosynthesis as a novel therapeutic strategy to correct lipid disregulations in acne and other disorders of the pilosebaceous unit.
Key words: sebum, squalene peroxide, acne, diet
Sebum
The principal activity of mature sebaceous glands is producing and secreting sebum, which is a complex mixture of lipids. This is a holocrine secretion formed by the complete disintegration of glandular cells into the follicular duct of pilosebaceous unit. Sebum discharge represents a major step in the final stages of differentiation of sebaceous specialized cells, namely sebocytes, and it is the result of accumulation of cytoplasmic lipid droplets and subsequent cell disintegration and release of their content into the follicle.
Sebum composition is different among species and this difference is probably due to the function that sebum has to absolve. Among the functions attributed to sebum in humans there is photoprotection, antimicrobial activity, delivery of fat-soluble anti-oxidants to the skin surface and pro- and anti-inflammatory activity exerted by specific lipids.1 However, the ultimate role of human sebum, as well as the metabolic pathways regulating its composition and secretion rate, are far from a complete understanding.
Human sebum consists of squalene, esters of glycerol, wax and cholesterol, as well as free cholesterol and fatty acids (Table 1). Triglycerides and fatty acids, taken together, account for the predominant proportion (57.5%), followed by wax esters (26%) and squalene (12%). The least abundant lipid in sebum is cholesterol, which with its esters, accounts for the 4.5% of total lipids.2 The most characteristic products of sebaceous secretion are squalene and wax esters. They are unique to sebum and not found anywhere else in the body nor among the epidermal surface lipids. Moreover, they correspond to major components supplying the skin with protection. Squalene is a linear intermediate preceding cholesterol in its biosynthesis. Interestingly, in the sebaceous gland, the squalene produced is not converted into lanosterol, halting the completion of the biosynthetic pathway leading to cholesterol. The reason cholesterol is not synthesized in the sebaceous gland, favoring squalene accumulation, is still unclear. Possible explanation for the squalene buildup in the sebaceous gland may be linked to an overexpression or an increase in the activity of squalene-synthase in the cells; or it may be related to decreased level or activity of the enzymes involved in the conversion to cholesterol.3 In addition, taking into account the peculiarity of squalene, it may be considered as a marker for sebocytes differentiation and thus for sebogenesis.4 Other features unique to sebum are the branched chain fatty acids and lipids with particular pattern of unsaturation. The Δ6 desaturase enzyme (fatty acid desaturase-2) catalyzes a “sebaceous-type” reaction of desaturation that leads to particular compounds.5 It is the major desaturase found in the sebaceous gland, and it is detectable mainly in differentiated sebocytes, which occupy the suprabasal layers of the sebaceous gland and have reached a full lipid synthetic capacity, providing a functional marker of activity and differentiation of sebocytes.5 Δ6 desaturase preferentially converts palmitic acid (16:0) to sapienic acid (16:1, Δ6), which is unique to the human sebum and represents ca. 25% of the total fatty acids. Elongation of sapienic acid by 2-carbon unit and further unsaturation leads to the formation of sebaleic acid (18:2, Δ5,8), which is also peculiar of human sebum. The ratio between Δ6 and Δ9 unsaturated fatty acids has been proposed as an index of maturation of sebaceous cells and of metabolic process associated to it.6
Table 1.
Components of skin surface lipids
| Sebum % | Epidermal lipids % | |
| Glycerides | 30–50 | 305 |
| FFA | 15–30 | 8–16 |
| WE | 26–30 | – |
| SQ | 12–20 | – |
| CE | 3.0–6.0 | 15–20 |
| CH | 1.5–2.5 | 20–25 |
Glycerides; Free Fatty Acids (FFA); Wax Esters (WE); Squalene (SQ); Cholesterol Esters (CE); Cholesterol (CH).
Sebum Alterations in Acne
Acne vulgaris is a disease of the pilosebaceous unit resulting from the interplay of different factors: seborrhoea, P. acnes colonization, hyperkeratinization of the follicular duct and release of inflammatory mediators. Increased sebum secretion is considered, among all features, the major one involved in the pathophysiology of acne. On average, acne subjects excrete more sebum than normal ones and secretion rates correlate well with the severity of clinical manifestations.7 Along with increased sebum secretion rate, quali- and quantitative modifications of sebum are likely to occur. Decreased concentration of linoleic acid has been observed in skin surface lipids of acne patients. In particular, its level has been found significantly reduced in wax esters making it reasonable to assume that linoleic acid is directly involved in the sebaceous lipid synthesis.8
Moreover, experimental data suggest that it is incorporated in epidermal lipids of the infundibulum. In experimental models, linoleic acid is preferentially transformed into two carbons precursors in the sebaceous gland by entering the β-oxidation reaction at the acylside chain, which yields to acetyl-CoA. The latter product feeds the biosynthetic pathway leading to squalene and wax esters synthesis.9 It seems that β-oxidation of linoleic acid is specific of sebocytes and that it is correlated with their differentiation. A diminished amount of linoleic acid has been proposed as a factor predisposing to comedones formation.10 Moreover, low level of linoleic acid also produces impairment of the epidermal barrier function, which might account for increased permeability of comedonal wall to inflammatory substances.11 Other lipids have been proposed as having involvement in the development of comedone lesions. In particular the attention has been pointed to the increase of other fatty acid and by-products of squalene peroxidation.12–14 Following UV exposure, squalene undergoes massive photodegradation. Irradiation of human skin leads to a squalene decomposition of about 60% similar to that observed in vitro.15 Upon oxidative challenge, squalene is readily oxidized giving rise to different squalene peroxidation by-products exerting harmful activities in skin cell cultures and in vivo, including keratinocytes cytotoxicity,15 histologic changes and immunosuppressison.16 According to Kohno et al. the primary peroxidation product in human skin surface lipids is squalene monohydroperoxide.17 Squalene peroxide has been demonstrated to be comedogenic: in animal experiments, comedones have been triggered by exposing rabbit ears to irradiated squalene. A positive correlation was found between degree of squalene peroxidation and size of the comedones elicited. In addition, marked hyperplasia and hyperkeratosis of the epithelium in follicular infundibulum and marked proliferation of sebaceous glands were observed.13 The all saturated squalene form (squalane), squalene itself and synthetic peroxides with different backbone structures, exerted a negligible comedogenic effect and did not lead to skin roughness and wrinkling, indicating that squalene monohydroperoxide is specifically involved in the observed skin alterations.14,18 In addition, in vitro data showed that squalene peroxide beyond induction of HaCaT keratinocytes proliferation, led also to the upregulation and release of inflammatory mediators, which indicate a pro-inflammatory activity of by-products of squalene oxidation.19 The strategy that skin adopts to limit the potentially harmful effects of peroxidated squalene relies on the vitamin E supply to the skin surface. Vitamin E is found in the skin surface lipids as a significant constituent of human sebum. In sites with elevated sebaceous glands density continuous secretion of vitamin E is detected, which is in tight correlation with the levels of cosecreted squalene.20 The role played by squalene peroxidation in acne development is strengthened by the observation that skin surface and comedones lipids collected from acne patients are enriched in polar lipids mainly derived from squalene oxidation.21,22
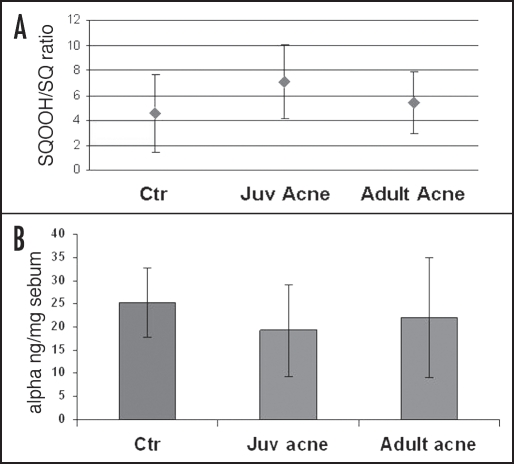
More recent data collected in vivo have confirmed these findings and indicated significant differences in sebum composition of acne patients in comparison with healthy subjects with regard to squalene and vitamin E amount. In particular, higher amounts of squalene peroxide and consequently decreased levels of vitamin E level have been detected in acne subjects23 further supporting the role of squalene peroxidation,24 and, in general, of lipid peroxidation in acne development (Figure 1).25 A lower 16:0/16:1 ratio in triglycerides and wax esters has been also found, underlying another kind of alteration characteristic of sebum from acne patients.23
Figure 1.
Acne sebum presents a higher level of squalene peroxide when compared with the level found in sebum of control subjects (A). Moreover, a decreased amount of vitamin E was associated with the increase of squalene peroxidation degree (B). These findings are emphasized for juvenile acne with respect to adult acne. Juvenile acne has, in fact, a greater inflammatory character.
Recently it has been reported that acne subjects differ significantly from unaffected subjects for a different ratio between saturated and monounsaturated fatty acids in skin surface triglycerides.26 Higher sebum outflow, as well as clinical manifestations, seemed to be associated with an increase in the proportion of monounsaturated fatty acids suggesting a possible role for desaturase enzyme in the sebaceous lipogenesis and acne onset. Low glycemic load diet has been demonstrated to be able to correct the increased sebum production and compositional changes proper of acne, indicating the need to point to diet habits as possible concurrent factors influencing sebaceous gland physiology.26
Sebum, Diet and Acne
There is evidence indicating that dietary factors alter sebaceous gland output. It has been demonstrated that sebum production can be increased by the consumption of dietary fat or carbohydrate.27 Variations in carbohydrates can also affect sebum composition.28,29 In turn caloric restriction has been shown to dramatically decrease the sebum secretion rate.30,31 All these findings suggest that dietary habits, supplying substrates for the sebaceous lipid synthesis, can be involved in the sebum production mechanism.32 Considering that increased sebum production is a primary component in acne, dietary factors have long been implicated in its pathogenesis. So far data concerning this issue are still controversial. Epidemiological studies have shown that increasing the intake of ω-3 fatty acids through a diet rich in fish and seafood results in a lower rates of acne.33 Intake of ω-3 polyunsaturated fatty acids (PUFAs) may affect the inflammatory pathways activation through their inhibitory activity on the pro-inflammatory cytokines secretion and the leukotriene B4 (LTB4) synthesis, mechanisms demonstrated beneficial in acne.25,34,35 The western diet typically provides a higher supply of ω-6 over ω-3 PUFAs, with a ratio between 10:1 and 20:1,33,36 which is higher than the 2:1 ratio found in the non-westernized diet.37
From a limited study conducted in ten subjects studied in time frames longer than two months, it resulted that the fluctuations observed in the composition of sebum fatty acids, including branched ones, were unlikely due to changes in the dietary habits or in the metabolism. Instead, variability was observed between the investigated subjects suggesting an interindividual difference in the processing of this particular class of sebaceous lipids.38 A larger twin study investigating sebum secretion in 40 sets of adolescent acne twins found that differently from dizygotic twins, the sebum secretion rate was omogeneous between monozygotic twins.39 Differences in the iso-even fatty acids proportion were very small in identical twins, whereas inter-pairs differences were comparable to the nontwin population, suggesting that the synthesis of branched fatty acids is under genetic control.40
Conclusion
Human sebum is a complex and specific mixture of lipids. Its uniqueness, when compared with the sebum of other mammals, could be attributable to the different functions it has to accomplish among species. Currently, the ultimate role of human sebum is not completely understood and the metabolic pathways regulating its composition and secretion rate are far from completely understood. In particular, the pathways leading to the formation of lipids, which are typically sebaceous, such as branched fatty acids and fatty acids with unshared unsaturation positions, remain to be elucidated. New insights regarding modifications in the amount, composition and arrangement of fatty acids assembled in complex lipids of sebum could improve our knowledge on the function of sebum and on the role of alteration of sebum organization in the pathogenesis of acne and of different sebaceous gland disorders. Moreover, understanding the factors and mechanisms that regulate sebum production is needed in order to identify new targets that could be addressed to achieve a selective modulation of lipid biosynthesis as a novel therapeutic strategy to correct lipid disregulations in acne and other disorders of the pilosebaceous unit.
Abbreviations
- Sq
squalene
- PUFAs
polyunsaturated fatty acids
- 16:0
palmitic acid
- 16:1 Δ6
sapienic acid
- 18:2 Δ5,8
sebaleic acid
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/8472
References
- 1.Zouboulis CC, Baron JM, Bohm M, Kippenberger S, Kurzen H, Reichrath J, Thielitz A. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551. doi: 10.1111/j.1600-0625.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- 2.Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54:240–247. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- 3.Smith KR, Thiboutot DM. Sebaceous Gland lipids: Friends or foe? J Lip Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Zouboulis CC, Xia L, Akamatsu H, Seltmann H, Fritsch M, Hornemann S, et al. The human sebocytes culture model provides new insights into development and management of seborrhea and acne. Dermatology. 1998;196:21–31. doi: 10.1159/000017861. [DOI] [PubMed] [Google Scholar]
- 5.Ge L, Gordon J, Hsuan C, Stenn K, Prouty S. Identification of the d-6 desaturase of human sebaceous glands: Expression and enzyme activity. J Invest Dermatol. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- 6.Zheng Y, Eliertsen K, Ge L, Zhang L, Sundberg J, Prouty S, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 7.Cunliffe WJ. In: Acne. Dunitz M, editor. London: Martin Dunitz; 1989. [Google Scholar]
- 8.Stewart ME, Grahek MO, Cambier LS, Wertz PW, Downing DT. Dilutional effect of increased sebaceous gland activity on the proportion of linoleic acid in sebaceous wax esters and in epidermal acylceramides. J Invest Dermatol. 1986;87:733–736. doi: 10.1111/1523-1747.ep12456856. [DOI] [PubMed] [Google Scholar]
- 9.Pappas A, Anthonavage M, Gordo JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002;118:164–171. doi: 10.1046/j.0022-202x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- 10.Downing DT, Stewart ME, Wertz PW, Strauss J. Essential fatty acids in acne. J Am Acad Dermatol. 1986;14:221–225. doi: 10.1016/s0190-9622(86)70025-x. [DOI] [PubMed] [Google Scholar]
- 11.Cunliffe WJ, Holland DB, Jeremy A. Comedone formation: Etiology, clinical presentation and treatment. Clinics in Dermatol. 2004;22:367–374. doi: 10.1016/j.clindermatol.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Kanaar P. Follicular-keratogenic properties of fatty acids in the external ear canal of the rabbit. Dermatologica. 1971;142:14–22. doi: 10.1159/000252365. [DOI] [PubMed] [Google Scholar]
- 13.Motoyoshi K. Enhanced comedo formation in rabbit ear skin by squalene and oleic acid peroxides. Br J Dermatol. 1983;109:191–198. doi: 10.1111/j.1365-2133.1983.tb07080.x. [DOI] [PubMed] [Google Scholar]
- 14.Chiba K, Yoshizawa K, Makino I, Kawakami K, Onoue M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J Toxicol Sci. 2000;25:77–83. doi: 10.2131/jts.25.77. [DOI] [PubMed] [Google Scholar]
- 15.Picardo M, Zompetta C, De Luca C, Amantea A, Faggioni A, Nazzaro-Porro M, Passi S. Squalene peroxides may contribute to ultraviolet ligh-induced immunological effects. Photodermatol Photoimmunol Photomed. 1991;8:105–110. [PubMed] [Google Scholar]
- 16.Picardo M, Zompetta C, De Luca C, et al. Role of skin surface lipids in UV-induced epidermal cell changes. Arch Dermatol Res. 1991;283:191–197. doi: 10.1007/BF00372061. [DOI] [PubMed] [Google Scholar]
- 17.Kohno Y, Sakamoto O, Nakamura T, Miyazawa T. Determination of human skin surface lipid peroxides by chemiluminescence-HPLC. J Jpn Oil Chem Soc. 1993;40:715–718. [Google Scholar]
- 18.Chiba K, Sone T, Kawakami K, Onoue M. Skin roughness and wrinkle formation induced by repeated application of squalene monohydroperoxide to the hairless mouse. Exp Dermatol. 1999;8:471–479. doi: 10.1111/j.1600-0625.1999.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 19.Ottaviani M, Alestas T, Flori E, Mastrofrancesco A, Zouboulis C, Picardo M. Peroxidated squalene induces the production of inflammatory mediators in HaCaT keratinocytes: a possible role in Acne vulgaris. J Invest Dermatol. 2006;136:2430–2437. doi: 10.1038/sj.jid.5700434. [DOI] [PubMed] [Google Scholar]
- 20.Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major route of vitamin E delivery to the skin. J Invest Dermatol. 1999;113:1006–1010. doi: 10.1046/j.1523-1747.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 21.Saint-Leger D, Bague A, Cohen E, Chivot M. A possible role for squalene in the pathogenesis of acne I. In vitro study of squalene oxidation. Br J Dermatol. 1986;114:535–542. doi: 10.1111/j.1365-2133.1986.tb04060.x. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Leger D, Bague A, Lefebvre E, Cohen E, Chivot M. A possible role for squalene in the pathogenesis of acne II. In vivo study of squalene oxides in skin surface and intra-comedonal lipids of acne patients. Br J Dermatol. 1986;114:543–552. doi: 10.1111/j.1365-2133.1986.tb04061.x. [DOI] [PubMed] [Google Scholar]
- 23.Picardo M. Sebaceous gland lipids. 2nd International Conference “Sebaceous gland, acne, rosacea and related disorders Basic and clinical research, clinical entities and treatment”; 2008; Rome. [Google Scholar]
- 24.Capitanio B, Sinagra JL, Ottaviani M, Bordignon V, Amantea A, Picardo M. “Smoker’s acne”: A new clinical entity? Br J Dermatol. 2007;157:1070–1071. doi: 10.1111/j.1365-2133.2007.08164.x. [DOI] [PubMed] [Google Scholar]
- 25.Zouboulis CC, Nestoris S, Adler YD, Orth M, Orfanos CE, Picardo M, et al. A new concept for acne therapy: A pilot study with zileuton, an oral 5-lipoxygenase inhibitor. Arch Dermatol. 2003;139:668–670. doi: 10.1001/archderm.139.5.668. [DOI] [PubMed] [Google Scholar]
- 26.Smith RN, Braue A, Varigos GA, Mann NJ. The effect of low glycemic load diet on Acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41–52. doi: 10.1016/j.jdermsci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Llewellyn A. Variations in the composition of skin surface lipid associated with dietary carbohydrates. Proc Nutr Soc. 1967;26:11. [Google Scholar]
- 28.MacDonald L. Changes in the fatty acid composition of sebum associated with high carbohydrate diets. Nature. 1964;203:1067–1068. doi: 10.1038/2031067b0. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald L. Dietary carbohydrates and skin lipids. Br J Dermatol. 1967;79:119–121. [PubMed] [Google Scholar]
- 30.Downing D, Strauss J, Pochi P. Changes in skin surface lipid composition induced by severe caloric restriction in man. Am J Clin Nutr. 1972;25:365–367. doi: 10.1093/ajcn/25.4.365. [DOI] [PubMed] [Google Scholar]
- 31.Pochi P, Downing D, Strauss J. Sebaceous gland response in man to prolonged total caloric deprivation. J Invest Dermatol. 1970;55:303–309. doi: 10.1111/1523-1747.ep12260136. [DOI] [PubMed] [Google Scholar]
- 32.Rasmussen J. Diet and acne. Int J Dermatol. 1977;16:488–491. doi: 10.1111/j.1365-4362.1977.tb01861.x. [DOI] [PubMed] [Google Scholar]
- 33.Kris-Etherton PM, Taylor DS, Yu-Poth S, Huth P, Moriarty K, Fishell V, et al. Polyunsaturated fatty acids in the food chain in the United States. Am J Clin Nutr. 2000;71:179–188. doi: 10.1093/ajcn/71.1.179S. [DOI] [PubMed] [Google Scholar]
- 34.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71:343–348. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 35.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, et al. Inhibition of tumour necrosis factor-a and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. Br J Nutr. 2003;90:405–412. doi: 10.1079/bjn2003892. [DOI] [PubMed] [Google Scholar]
- 36.Logan AC. Omega-3 fatty acids and acne. Arch Dermatol. 2003;139:941–942. doi: 10.1001/archderm.139.7.941-b. [DOI] [PubMed] [Google Scholar]
- 37.Cordain L, Lindeberg S, Hurtado M, Hill K, Eaton SB, Brand-Miller J. Acne Vulgaris. A Disease of Western Civilization. Arch Dermatol. 2002;138:1584–1590. doi: 10.1001/archderm.138.12.1584. [DOI] [PubMed] [Google Scholar]
- 38.Green SC, Stewart ME, Downing DT. Vatiation in sebum fatty acid composition among adult humans. J Invest Dermatol. 1984;83:114–117. doi: 10.1111/1523-1747.ep12263287. [DOI] [PubMed] [Google Scholar]
- 39.Walton S, Wyatt EH, Cunliffe WJ. Genetic control of sebum excretion and acne—A twin study. Br J Dermatol. 1988;118:393–396. doi: 10.1111/j.1365-2133.1988.tb02433.x. [DOI] [PubMed] [Google Scholar]
- 40.Stewart ME, Downing DT. Proportions of various straight and branched fatty acid chain types in the sebaceous wax esters of young children. J Invest Dermatol. 1985;84:501–503. doi: 10.1111/1523-1747.ep12273469. [DOI] [PubMed] [Google Scholar]