Abstract
A layer of lipids, which are of both sebaceous and keratinocyte origin, covers the surface of the skin. The apparent composition of surface lipids varies depending on the selected method of sampling. Lipids produced by the epidermal cells are an insignificant fraction of the total extractable surface lipid on areas rich in sebaceous glands. Due to the holocrine activity of the sebaceous gland, its product of secretion (sebum) is eventually released to the surface of the skin and coats the fur as well. Lipids of epidermal origin fill the spaces between the cells, like mortar or cement. The sebaceous lipids are primarily non polar lipids as triglycerides, wax esters and squalene, while epidermal lipids are a mixture of ceramides, free fatty acids and cholesterol. The composition of the sebaceous lipids is unique and intriguing and elevated sebum excretion is a major factor involved in the pathophysiology of acne. Recent studies have elucidated the roles that epidermal surface lipids have on normal skin functions and acne.
Key words: lipid, sebaceous, skin, fatty acid, desaturase, wax, squalene, ceramide
Introduction
The sebaceous gland is now considered to be an important endocrine organ. The holocrine eruption of the sebaceous cells results in the secretion and release of sebum, which eventually coats the surface of the skin and the fur. The majority of the epidermal surface lipids are in fact of sebaceous origin while the lipids produced by the epidermis are an insignificant fraction of the total extractable surface lipid.1 That is more apparent on areas rich in sebaceous glands, where the epidermal origin lipids average between 5 to 10 µg per sq cm, compared with average recoveries of 150 to 300 µg of sebum per sq cm from the forehead. Since this chapter is part of a sebaceous forum, the focus will include both classes of lipids on the surface of the skin. In addition, areas rich in sebaceous glands are the areas that acne lesions are manifested.
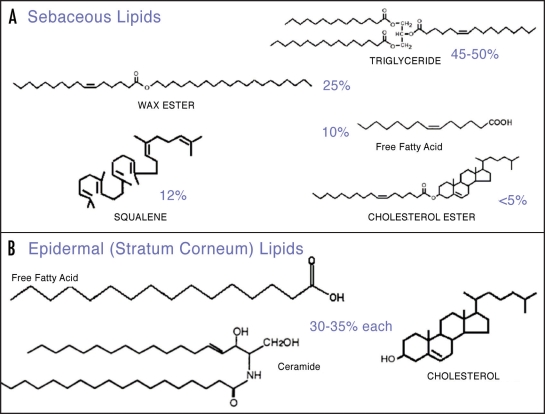
Human sebum is a mixture of non-polar lipids, mainly triglycerides, wax esters, squalene, fatty acids and smaller amounts of cholesterol, cholesterol esters and diglycerides.2–5 On the other hand, lipids produced by keratinocytes are a mixture of almost equal proportions of free fatty acids, cholesterol and ceramides.6 Figure 1 shows the representative structures of the various lipid classes of epidermal surface lipids.
Figure 1.
Representative structures of epidermal surface lipids.
Sebaceous Lipids
The sebaceous lipids are unique and intriguing. According to Nicolaides:7 “two key words characterize the uniqueness of skin lipids: complexity and perversity”. The relative composition of sebum depends on the sampling method used. In particular, if the major components of sebum, triglycerides, are sampled before or after their modification by bacteria, which hydrolyze them to free fatty acids and glycerol.7–11 The mean weight % that is often cited in the literature is given in Table 1.
Table 1.
Relative sebum composition
| Lipid class | Range weight, % | Mean weight, % |
| Triglycerides | 20–60 | 45 |
| Wax esters | 23–29 | 25 |
| Squalene | 10–14 | 12 |
| Free Fatty Acids | 5–40 | 10 |
| Cholesterol and Sterol esters | 1–5 | 4 |
| Diglycerides | 1–2 | 2 |
Interestingly, human sebaceous lipids are significantly different in quantity and quality from sebaceous lipids of other species.12–14 The reason for such a unique sebum composition is not understood; however, one can also consider that human skin has a unique texture. In addition, acne is also unique to humans. These seem to be pieces of the same puzzle, which suggest that the unique sebaceous lipids are associated to this odd and human specific disease. Elevated sebum excretion is clearly a major factor involved in the pathophysiology of acne.15–17
Although the majority of lipids produced by all other organs of the human body are alike, the sebaceous gland produces some unique species that cannot be found in any other organ of the body. The synthesis of sapienic acid or wax esters, the accumulation of squalene and the presence of very long chain branched or hydroxylated fatty acids are uncommon in other organs and unique manifestations in sebum.7,12 In other mammals and rodents even higher levels of unique fatty acids exist and with either odd numbers of carbon atoms or branched chains. It is also possible that some of these molecules are in reality products of the resident skin micro flora, since they are more common to bacterial metabolism.18 However, another possibility is that they could be synthesized from branched precursors, products of essential branched amino acid catabolism.4
Sapienic acid.
The predominant fatty acid of sebum is the sapienic acid (16:1, Δ6), which has its single double bond at the sixth position from the carboxyl end.7,19 In nature, long chain fatty acids with similar chain length are abundant but there is a predominant preference for the first double bond to be inserted in the 9th position from the carboxyl end. The 16-carbon isomer with one double bond at the ninth position is the palmitoleic acid, which is naturally found in many tissues and organisms. Sapienic acid is truly unique to sebum and is not found anywhere else in the human body. In addition, humans do not obtain it from the diet since very few plant species have been reported to manufacture this unusual fatty acid.7,18 The elongation of sapienic acid by two carbons and then an additional insertion of another double bond between the fifth and sixth carbon yields sebaleic acid (18:2Δ5, 8), a reaction and metabolite that occurs only in human sebaceous cells. The levels of sapienic acid are multiple folds higher than any of its derivatives, isomers or other monounsaturated fatty acids found in sebum. However, the potential role of sapienic acid in the etiology of acne is still controversial. It has been argued that its presence in sebum correlates with elevated sebum levels,20 while others report that it can be potent against bacteria commonly associated with acne.21-23
Wax esters.
Wax esters are also unique to sebaceous cells and are not produced by any other cell in the body. They account for about 25% of the sebaceous gland lipids and their production correlates with sebaceous gland differentiation.5,19 Animal models demonstrated a strong correlation between atrophic sebaceous gland and impaired wax ester synthesis.24,25
Wax ester synthases26,27 have recently been discovered, however additional recent reports28,29 provided evidence that another family of enzymes can also synthesize waxes. Therefore there is not a unique wax synthase and it is apparent that wax ester biosynthesis is still unexplored in humans. Although active wax synthesis correlates with the differentiation of sebaceous cells, it is still unclear if they are the cause or the effect of the differentiation process. In vitro, this is the pathway that its’ expression is usually downregulated no matter if explanted sebaceous glands, tissues, cell preps or transformed cell lines are used. Age and sex related differences have been reported in wax ester synthesis, which also correlates with total sebum output and activity.30–32
In nature, waxes act as protective layers for leaves and fruits of plants, or skin, feathers and fur of animals. Additionally waxes are also found to coat bacteria, algae and fungi.33 Waxes are more resistant to oxidation, hydrolysis and heat than triglycerides or phospholipids. Besides protection they also serve as lubrication aim. Additionally, they are sealing in the internal moisture of tissues while they are preventing their excessive hydration.33 In certain instances the packing and physicochemical properties of the wax crystals demonstrate unusual surface self cleaning properties that repel not only moisture, but together with water any kind of physical or biological invader. This phenomenon has been termed as the “lotus effect”.34
Squalene.
There is nothing unique about the synthesis of squalene, which is a precursor of cholesterol. Most mammalian cells synthesize cholesterol, which is an essential molecule for membrane fluidity and structure. Squalene is a long unsaturated hydrocarbon which other tissues quickly convert to lanosterol and finally to cholesterol.6,7 The uniqueness in human sebum is that this cholesterol precursor accumulates in unusually high levels (∼12%) compared to the levels of any other tissue or organ. On the other hand cholesterol accounts for less than 2% of the total sebaceous gland lipids and about 30–35% of the total epidermal lipids. Squalene synthase is the enzyme responsible for the production of squalene and Squalene epoxidase or monooxygenase for its further metabolism. It is possible that in sebaceous cells the activity of these two enzymes is responsible for the accumulation of squalene.
Squalene, as a long and highly unsaturated hydrocarbon, is a natural lubricant and has high penetration efficiency; therefore its role could be more than just a precursor of cholesterol. Past reports demonstrated possible roles of squalene oxidation products on UV protection35 but also irritation.36 These products, together with unsaturated free fatty acids, have been reported to be comedogenic. 37,38 Perhaps that is why human sebum transports lipophilic antioxidants as vitamin E39 or humectants as glycerol,40 which play important roles in protecting skin from lipid oxidation and proper barrier function, respectively.
Epidermal (Stratum Corneum) Lipids
The epidermal lipids of keratinocyte origin play an essential role in the skin’s barrier function. These lipids provide a barrier against the movement of water and electrolytes as well as a barrier against microorganism invasion.41 Especially the permeability barrier, which limits water and minerals, is localized to the outer layers of the epidermis, the stratum corneum (SC). The SC consists of the upper layers of corneocytes, which are terminally differentiated keratinocytes. These cells are imbedded in a lipophilic extra cellular medium composed of equal proportions of ceramides, cholesterol and free fatty acids, providing fundamental limitations to water and electrolyte movement. The above lipid mixture originates from unique structures found in epidermis, the lamellar bodies.
The epidermis has a very active synthesis of cholesterol, fatty acids and ceramides. Disruption of the skin’s barrier function results in a rapid and marked increase in epidermal cholesterol and fatty acid synthesis; furthermore inhibitors of these pathways delay the recovery of the barrier function. The increase of sphingolipid synthesis, which precedes ceramide synthesis, is more delayed than cholesterol and fatty acids, but is equally important for the restoration of skin’s barrier function.6,41
The epidermal surface lipids also include unique ceramide species that cannot be found at any other cell type of the human body. For instance the fatty acid esterified to the amide of the (phyto)sphingosine head group can be either an α-hydroxy or an unusually long chain fatty acid. In some instances the fatty acid chain length could be as long as 34 carbon atoms and in others a substantial portion of the epidermal ceramides contain ω-hydroxy long chain fatty acids, which can be in the form of linoleic acid acyl esters. These molecules are believed to have the best barrier properties.42,43
Importance of Epidermal Surface Lipids and Animal Models
Genetic knock out (KO) animal models of lipid synthesis have clearly demonstrated the importance of surface lipids in skin physiology and pathology. In these studies, skin and fur abnormalities became the common denominator, once a certain surface lipid pathway is disturbed. The melanocortin-5 receptor (MC5-R) KO resulted in severe defects in water repulsion and thermoregulation due to decreased production of sebaceous lipids.44 The effect of the MC5-R on sebaceous lipid metabolism shed more light to a different path, besides the anticipated role that melanocortins have on pigmentation, obesity or body weight regulation.
Two years later Zheng at al.45 demonstrated by positional cloning that the dramatic alopecia manifested in the asebia mouse is due to the lack of a functional Stearoyl-CoA desaturase (Scd1) enzyme activity. The absence of mature sebaceous glands demonstrated the apparent importance of the SCD1 gene and its products (monounsaturated fatty acids) to normal sebaceous gland function in addition to their role in hair development. The same findings were further confirmed in 2001 by the reverse experiment where the SCD1 KO mice were constructed and bared a similar phenotype.25 The revelation that both the asebia’s and the SCD1’s non-functional SCD1 is solely responsible for scant to absent hair and hypoplastic to absent sebaceous glands58 was further supported by the fact that sebaceous glands are scant in certain forms of alopecias.47 The skin of the SCD1 KO mice has also lower than normal levels of triglycerides, wax esters besides the expected lower than normal levels in monounsaturated fatty acids.
A similar phenotype was demonstrated in the Acyl CoA:diacylgylcerol acyltransferase 1(DGAT1) KO mouse, where sebaceous gland atrophy and hair loss were also apparent.24 DGAT is the primary triglyceride synthase and exists in two forms, DGAT1 and DGAT2. The two isoforms differ in sequence and localization. 48 DGAT1 is also involved in the synthesis of wax esters, unlike DGAT2,28 and is expressed in most tissues, including the sebaceous gland.24,48 The apparent involvement of DGAT1 in wax ester synthesis is consistent with the observation that there are no or little wax esters in the fur lipids of the DGAT1 KO mouse.
The DGAT2 KO animals,49 similarly to SCD2 KO mice,50 do not survive due to an impaired skin barrier function. The animals deficient in SCD-2 demonstrated abnormal lamellar bodies and epidermal maturation proving that the presence of monounsaturated fatty acids is vital also for skin’s barrier component besides the formation of the sebaceous glands.
Another animal model that demonstrated the importance of sebaceous and epidermal lipids to skin function is the ELOVL3 KO mouse.51 The Elovl3 gene product is involved in the formation of very long chain fatty acids (VLCFA) and has a distinct expression in the skin that is restricted to sebaceous glands and epithelial cells of hair follicles. Disruption of that gene impaired the formation of neutral lipids that are necessary for skin functions but also resulted in disturbed water barrier and increased trans epidermal water loss. This was caused partly from a disruption in normal lamellar body formation that the deficiency of Elovl3 has caused. The Elovl3-ablated mice had also sparse hair coats and hyperplastic sebaceous glands with unusual lipid content in monounsaturated fatty acid with 20 carbons.
Furthermore the elongation of fatty acids was recently further confirmed in another similar animal model. Mice deficient in elongation of very long chain fatty acid-like 4 (ELOVL4) displayed a scaly and wrinkled skin.43 In addition they demonstrated a severely compromised epidermal permeability barrier function, which results in death within a few hours after birth. Skin histology showed an abnormally compacted outer epidermis (SC) and electron microscopy revealed deficient epidermal lamellar body contents. The KO mice had decreased levels in VLCFA (>C28) in ceramide, glucosylceramide and the free fatty-acid fractions, demonstrating the necessity of VLCFA for the synthesis of skin ceramides. Omega-O-acylceramides, that are key hydrophobic components of the extracellular lamellar membranes in mammalian SC, were also fully depleted in the KO model.
That reinforces the notion that skin ceramides ought to have unique structures with either VLCFA or omega-hydroxylation that can be additionally esterified to linoleic acid.
Essential Fatty Acids
Linoleic (18:2, Δ9,12) and α-linolenic acid (18:2, Δ9,12,15) are well known essential fatty acids that come strictly form the diet. As vitamins and minerals do, they survive the digestive tract and are delivered via the systemic circulation to various organs. Sebum analysis demonstrates that the essential fatty acids that come strictly from the diet and their derivatives constitute small amounts in surface lipid samplings.7 However, two intriguing studies, previously unnoticed by the skin research community,52,53 revealed a tight association of essential fatty acids and skin. When guinea pigs were dosed with radioactively labeled linoleic or linolenic acids, skin and fur were the most heavily labeled tissue, suggesting a necessary role for essential fatty acids in sebaceous gland biology.
There is very little known about how these essential nutrients are utilized in human sebaceous cells. However, one study has claimed that many acne patients had a linoleic acid deficiency.54 On the other hand, there is substantial evidence that linoleic acid is an essential structural component of skin ceramides. A recent study55 revealed that linoleic acid undergoes a rapid oxidation and degradation in sebaceous cells. Consequently, this activity will allow palmitic acid to be available as the sole substrate to the delta 6 desaturase of sebaceous cells. This is the predominant desaturase of the human sebaceous cells56 that otherwise would have catalyzed the synthesis of more omega-6 derivatives from linoleic acid, since it is the enzyme’s preferred substrate. In fact, linoleic acid was selectively subjected to β-oxidation and this activity correlated with the ability of sebaceous cells to synthesize wax esters, which as noted above is a differentiation marker for the sebaceous cells. Thus, oxidation of linoleic acid is specific to sebaceous cells and correlates with their differentiation and production of sapienate.
Epilogue. Epidermal surface lipids contribute to normal skin functions as the barrier function and the maintenance of healthy skin and fur. Consequently, they contribute to aging and to the conditioning and defense of this organ. The idea that the unusual lipids found on skin’s surface make the skin unfriendly to fungi and bacteria has gained more attention. Even if the major component of sebum, the triglycerides, are hydrolyzed by bacteria to fatty acids; these are unusual enough to orchestrate together with other perverse lipids a unique mechanism that will select, which organism is an enemy and which is desirable on our skin. Additional studies are required before we have a complete understanding of the roles of the epidermal surface lipids on acne. Better analytical techniques would help to increase our understanding on their role and to clarify their complexity. Few years ago the classes of ceramides were expanded from six to nine and with new and modern analytical techniques there is sound evidence that there are more classes of ceramides than previously believed.57,58 The field of epidermal surface lipids is open for many new discoveries and is constantly enhanced by advances in analytical techniques. It is not by coincidence that in an era that genomics is the past, proteomics is the present and metabolomics is the near future the term lipidomics is still a challenging term to most biologists. Undoubtedly future scientists will incorporate all the acquired learning from the various “-omics” fields to advance into the new era of lipidomics. This will eventually shed light on intriguing dermatological diseases such as acne, atopic dermatitis, ichthyosis and many others that scientists do not currently associate with the epidermal surface lipids.
Abbreviations
- SC
stratum corneum
- KO
knock out
- VLCFA
very long chain fatty acids
Footnotes
Previously published online as a Dermato-Endocrinology E-publication: http://www.landesbioscience.com/journals/dermatoendocrinology/article/7811
References
- 1.Greene RS, Downing DT, Pochi PE, Strauss JS. Anatomical variation in the amount and composition of human skin surface lipid. J Invest Dermatol. 1970;54:240–247. doi: 10.1111/1523-1747.ep12280318. [DOI] [PubMed] [Google Scholar]
- 2.Downing DT, Stewart ME, Strauss JS. Changes in sebum secretion and the sebaceous gland. Clin Geriatr Med. 1989;5:109–114. [PubMed] [Google Scholar]
- 3.Smith KR, Thiboutot DM. Sebaceous gland lipids: Friend or foe? J Lipid Res. 2008;49:271–281. doi: 10.1194/jlr.R700015-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Stewart ME. Sebaceous glands lipids. Seminars in Dermatology. 1992;11:100–105. [PubMed] [Google Scholar]
- 5.Strauss JS, Downing DT, Ebling JF, Stewart ME. Sebaceous glands. In: Goldsmith LA, editor. Physiology, Biochemistry and Molecular Biology of the Skin. New York,: Oxford University Press, Inc; 1991. pp. 712–740. [Google Scholar]
- 6.Elias PM, Feingold KR. Lipids and the epidermal water barrier: Metabolism, regulation and pathophysiology. Semin Dermatol. 1992;11:176–182. [PubMed] [Google Scholar]
- 7.Nicolaides N. Skin lipids: Their biochemical uniqueness. Science. 1974;186:19–26. doi: 10.1126/science.186.4158.19. Review. [DOI] [PubMed] [Google Scholar]
- 8.Downing DT, Strauss JS, Pochi PE. Variability in the chemical composition of human skin surface lipids. J Invest Dermatol. 1969;53:322–327. doi: 10.1038/jid.1969.157. [DOI] [PubMed] [Google Scholar]
- 9.Haahti E, Horning EC. Isolation and characterization of saturated and unsaturated fatty acids and alcohols of human skin surface lipids. Scandinavian journal of clinical and laboratory investigation. 1963;15:73–78. doi: 10.3109/00365516309051313. [DOI] [PubMed] [Google Scholar]
- 10.James AT, Wheatley VR. Studies of sebum 6. The determination of the component fatty acids of human forearm sebum by gas-liquid chromatography. The Biochemical journal. 1956;63:269–273. doi: 10.1042/bj0630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knags H. Cell biology of the Pilosebaceous Unit. In: Webster GF, Rawlings AV, editors. Acne and its therapy. Informa Healthcare USA; 2007. pp. 9–36. [Google Scholar]
- 12.Nicolaides N, Ansari MN. Fatty acids of unusual double-bond positions and chain lengths found in rat skin surface lipids. Lipids. 1968;3:403–410. doi: 10.1007/BF02531278. [DOI] [PubMed] [Google Scholar]
- 13.Nikkari T. Comparative chemistry of sebum. J Invest Dermatol. 1974;62:257–267. doi: 10.1111/1523-1747.ep12676800. [DOI] [PubMed] [Google Scholar]
- 14.Stewart ME, Downing DT. Chemistry and function of mammalian sebaceous lipids. Adv Lipid Res. 1991;24:263–301. doi: 10.1016/b978-0-12-024924-4.50013-4. [DOI] [PubMed] [Google Scholar]
- 15.Cunliffe WJ. Acne. London: Martin Dunitz; 1989. [Google Scholar]
- 16.Thiboutot D. Regulation of human sebaceous glands. J Invest Dermatol. 2004;123:1–12. doi: 10.1111/j.1523-1747.2004.t01-2-.x. [DOI] [PubMed] [Google Scholar]
- 17.Zouboulis CC. Acne and sebaceous gland function. Clin Dermatol. 2004;22:360–366. doi: 10.1016/j.clindermatol.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaides N. The structures of the branched fatty acids in the wax esters of vernix caseosa. Lipids. 1971;6:901–905. doi: 10.1007/BF02531172. [DOI] [PubMed] [Google Scholar]
- 19.Wertz PW. Sebum secretions and acne. In: Webster GF, Rawlings AV, editors. Acne and its therapy. Informa Healthcare USA; 2007. pp. 37–43. [Google Scholar]
- 20.Smith RN, Braue A, Varigos GA, Mann NJ. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008:3. doi: 10.1016/j.jdermsci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Drake DR, Brogden KA, Dawson DV, Wertz PW. Thematic Review Series: Skin Lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- 22.Georgel P, Crozat K, Lauth X, Makrantonaki E, Seltmann H, Sovath S, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immunol. 2005;73:4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wille JJ, Kydonieus A. Palmitoleic acid isomer (C16:1delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol Appl Skin Physiol. 2003;16:176–187. doi: 10.1159/000069757. [DOI] [PubMed] [Google Scholar]
- 24.Chen HC, Smith SJ, Tow B, Elias PM, Farese RV., Jr Leptin modulates the effects of acyl CoA:diacylglycerol acyltransferase deficiency on murine fur and sebaceous glands. J Clin Invest. 2002;109:175–181. doi: 10.1172/JCI13880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki M, Man WC, Ntambi JM. Targeted disruption of stearoyl-CoA desaturase1 gene in mice causes atrophy of sebaceous and meibomian glands and depletion of wax esters in the eyelid. J Nutr. 2001;131:2260–2268. doi: 10.1093/jn/131.9.2260. [DOI] [PubMed] [Google Scholar]
- 26.Cheng JB, Russell DW. Mammalian wax biosynthesis II. Expression cloning of wax synthase cDNAs encoding a member of the acyltransferase enzyme family. J Biol Chem. 2004;279:37798–37807. doi: 10.1074/jbc.M406226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lardizabal KD, Metz JG, Sakamoto T, Hutton WC, Pollard MR, Lassner MW. Purification of a jojoba embryo wax synthase, cloning of its cDNA and production of high levels of wax in seeds of transgenic arabidopsis. Plant Physiol. 2000;122:645–655. doi: 10.1104/pp.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yen CL, Monetti M, Burri BJ, Farese RV., Jr The triacylglycerol synthesis enzyme DGAT1 also catalyzes the synthesis of diacylglycerols, waxes and retinyl esters. J Lipid Res. 2005;46:1502–1511. doi: 10.1194/jlr.M500036-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Yen CL, Brown CH, 4th, Monetti M, Farese RV., Jr A human skin multifunctional O-acyltransferase that catalyzes the synthesis of acylglycerols, waxes and retinyl esters. J Lipid Res. 2005;46:2388–2397. doi: 10.1194/jlr.M500168-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downing DT, Stewart ME, Strauss JS. Changes in sebum secretion and the sebaceous gland. Clin Geriatr Med. 1989;5:109–114. [PubMed] [Google Scholar]
- 31.Jacobsen E, Billings JK, Frantz RA, Kinney CK, Stewart ME and, Downing DT. Age-related changes in sebaceous wax ester secretion rates in men and women. J Invest Dermatol. 1985;85:483–485. doi: 10.1111/1523-1747.ep12277224. [DOI] [PubMed] [Google Scholar]
- 32.Stewart ME, Quinn MA, Downing DT. Variability in the fatty acid composition of wax esters from vernix caseosa and its possible relation to sebaceous gland activity. J Invest Dermatol. 1982;78:291–295. doi: 10.1111/1523-1747.ep12507228. [DOI] [PubMed] [Google Scholar]
- 33.Kolattukudy PE. Cutn, suberin and waxes. In: Stumpf PK, editor. Comprehensive Biochemistry of Plants. IV. London, Academic Press: 1980. pp. 600–645. [Google Scholar]
- 34.Koch K, Dommisse A, Barthlott W, Gorb SN. The use of plant waxes as templates for micro- and nanopatterning of surfaces. Acta Biomater. 2007;3:905–909. doi: 10.1016/j.actbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Ohsawa K, Watanabe T, Matsukawa R, Yoshimura Y, Imaeda K. The possible role of squalene and its peroxide of the sebum in the occurrence of sunburn and protection from the damage caused by U.V. irradiation. J Toxicol Sci. 1984;9:151–159. doi: 10.2131/jts.9.151. [DOI] [PubMed] [Google Scholar]
- 36.Chiba K, Yoshizawa K, Makino I, Kawakami K, Onoue M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. J Toxicol Sci. 2000;25:77–83. doi: 10.2131/jts.25.77. [DOI] [PubMed] [Google Scholar]
- 37.Kligman AM, Wheatley VR, Mills OH. Comedogenicity of human sebum. Arch Dermatol. 1970;102:267–275. [PubMed] [Google Scholar]
- 38.Motoyoshi K. Enhanced comedo formation in rabbit ear skin by squalene and oleic acid peroxides. Br J Derm. 1983;109:191–198. doi: 10.1111/j.1365-2133.1983.tb07080.x. [DOI] [PubMed] [Google Scholar]
- 39.Thiele JJ, Weber SU, Packer L. Sebaceous gland secretion is a major physiologic route of vitamin E delivery to skin. J Invest Dermatol. 1999;113:1006–1010. doi: 10.1046/j.1523-1747.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 40.Fluhr JW, Mao-Qiang M, Brown BE, Wertz PW, Crumrine D, Sundberg JP, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120:728–737. doi: 10.1046/j.1523-1747.2003.12134.x. [DOI] [PubMed] [Google Scholar]
- 41.Feingold KR. The outer frontier: the importance of lipid metabolism in the skin. J Lipid Res. 2008 doi: 10.1194/jlr.R800039-JLR200. DOI: 10.1194/jlr.R 800039-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ijzerman AP, Ponec M. Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res. 1998;39:186–196. [PubMed] [Google Scholar]
- 43.Vasireddy V, Uchida Y, Salem N, Jr, Kim SY, Mandal MN, Reddy GB, et al. Loss of functional ELOVL4 depletes very long-chain fatty acids (> or =C28) and the unique omega-Oacylceramides in skin leading to neonatal death. Hum Mol Genet. 2007;16:471–482. doi: 10.1093/hmg/ddl480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: Evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 45.Zheng Y, Eilertsen KJ, Ge L, Zhang L, Sundberg JP, Prouty SM, et al. Scd1 is expressed in sebaceous glands and is disrupted in the asebia mouse. Nat Genet. 1999;23:268–270. doi: 10.1038/15446. [DOI] [PubMed] [Google Scholar]
- 46.Sundberg JP. The asebia (ab, ab1) mutations, chromosome 19. In: Sundberg JP, editor. Handbook of mouse mutations with skin and hair abnormlities. Bar Harbor,: CRC Press; 1994. pp. 171–178. [Google Scholar]
- 47.Headington JT. Cicatricial alopecia. Dermatologic clinics. 1996;14:773–782. doi: 10.1016/s0733-8635(05)70403-4. [DOI] [PubMed] [Google Scholar]
- 48.Cases S, Smith SJ, Zheng YW, Myers HM, Lear SR, Sande E, et al. Identification of a gene encoding an acyl CoA:diacylglycerol acyltransferase, a key enzyme in triacylglycerol synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:13018–13023. doi: 10.1073/pnas.95.22.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone SJ, Myers HM, Watkins SM, Brown BE, Feingold KR, Elias PM, et al. Lipopenia and skin barrier abnormalities in DGAT2-deficient mice. J Biol Chem. 2004;279:11767–11776. doi: 10.1074/jbc.M311000200. [DOI] [PubMed] [Google Scholar]
- 50.Miyazaki M, Dobrzyn A, Elias PM, Ntambi JM. Stearoyl-CoA desaturase-2 gene expression is required for lipid synthesis during early skin and liver development. Proc Natl Acad Sci USA. 2005;102:12501–12506. doi: 10.1073/pnas.0503132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westerberg R, Tvrdik P, Undén AB, Månsson JE, Norlén L, Jakobsson A, et al. Role for ELOVL3 and fatty acid chain length in development of hair and skin function. J Biol Chem. 2004;279:5621–5629. doi: 10.1074/jbc.M310529200. [DOI] [PubMed] [Google Scholar]
- 52.Fu Z, Sinclair AJ. Increased alpha-linolenic acid intake increases tissue a-linolenic acid content and apparent oxidation with little effect on tissue docosahexaenoic acid in the guinea pig. Lipids. 2000;35:395–400. doi: 10.1007/s11745-000-537-7. [DOI] [PubMed] [Google Scholar]
- 53.Fu Z, Attar-Bashi NM, Sinclair AJ. 1-14C-linoleic acid distribution in various tissue lipids of guinea pigs following an oral dose. Lipids. 2001;36:255–260. doi: 10.1007/s11745-001-0715-7. [DOI] [PubMed] [Google Scholar]
- 54.Downing DT, Stewart ME, Wertz PW, Strauss JS. Essential fatty acids and acne. J Am Acad Dermatol. 1986;14:221–225. doi: 10.1016/s0190-9622(86)70025-x. [DOI] [PubMed] [Google Scholar]
- 55.Pappas A, Anthonavage M, Gordon JS. Metabolic fate and selective utilization of major fatty acids in human sebaceous gland. J Invest Dermatol. 2002;118:164–171. doi: 10.1046/j.0022-202x.2001.01612.x. [DOI] [PubMed] [Google Scholar]
- 56.Ge L, Gordon JS, Hsuan C, Stenn K, Prouty SM. Identification of the delta-6 desaturase of human sebaceous glands: expression and enzyme activity. J Invest Dermatol. 2003;120:707–714. doi: 10.1046/j.1523-1747.2003.12123.x. [DOI] [PubMed] [Google Scholar]
- 57.Farwanah H, Wohlrab J, Neubert RH, Raith K. Profiling of human stratum corneum ceramides by means of normal phase LC/APCI-MS. Anal Bioanal Chem. 2005;383:632–637. doi: 10.1007/s00216-005-0044-3. [DOI] [PubMed] [Google Scholar]
- 58.Farwanah H, Pierstorff B, Schmelzer CE, Raith K, Neubert RH, Kolter T, et al. Separation and mass spectrometric characterization of covalently bound skin ceramides using LC/ APCI-MS and Nano-ESI-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852:562–570. doi: 10.1016/j.jchromb.2007.02.030. [DOI] [PubMed] [Google Scholar]